Niosome Preparation Techniques and Structure—An Illustrated Review
Abstract
:1. Introduction
2. Niosomes Structure
3. Hydrophile–Lipophile Balance (HLB)
4. Bilayer-Inducting Agents in Niosomes
5. Charge Inducer Agents in Niosomes
6. Types of Niosomes
7. Methods of Niosome Preparation
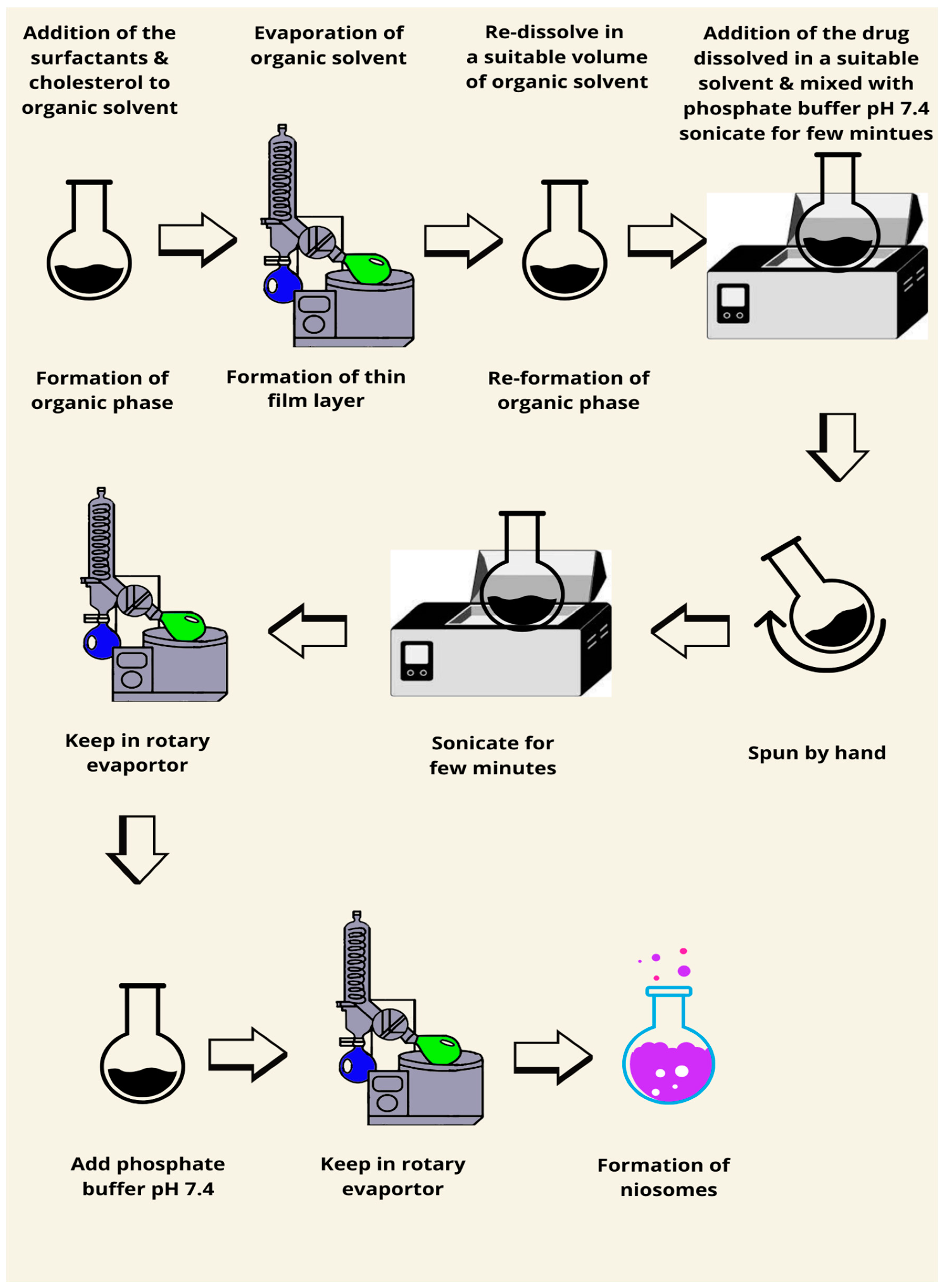
7.1. Thin Film Hydration Method
7.2. Reverse Phase Evaporation
7.3. Microfluidics Method
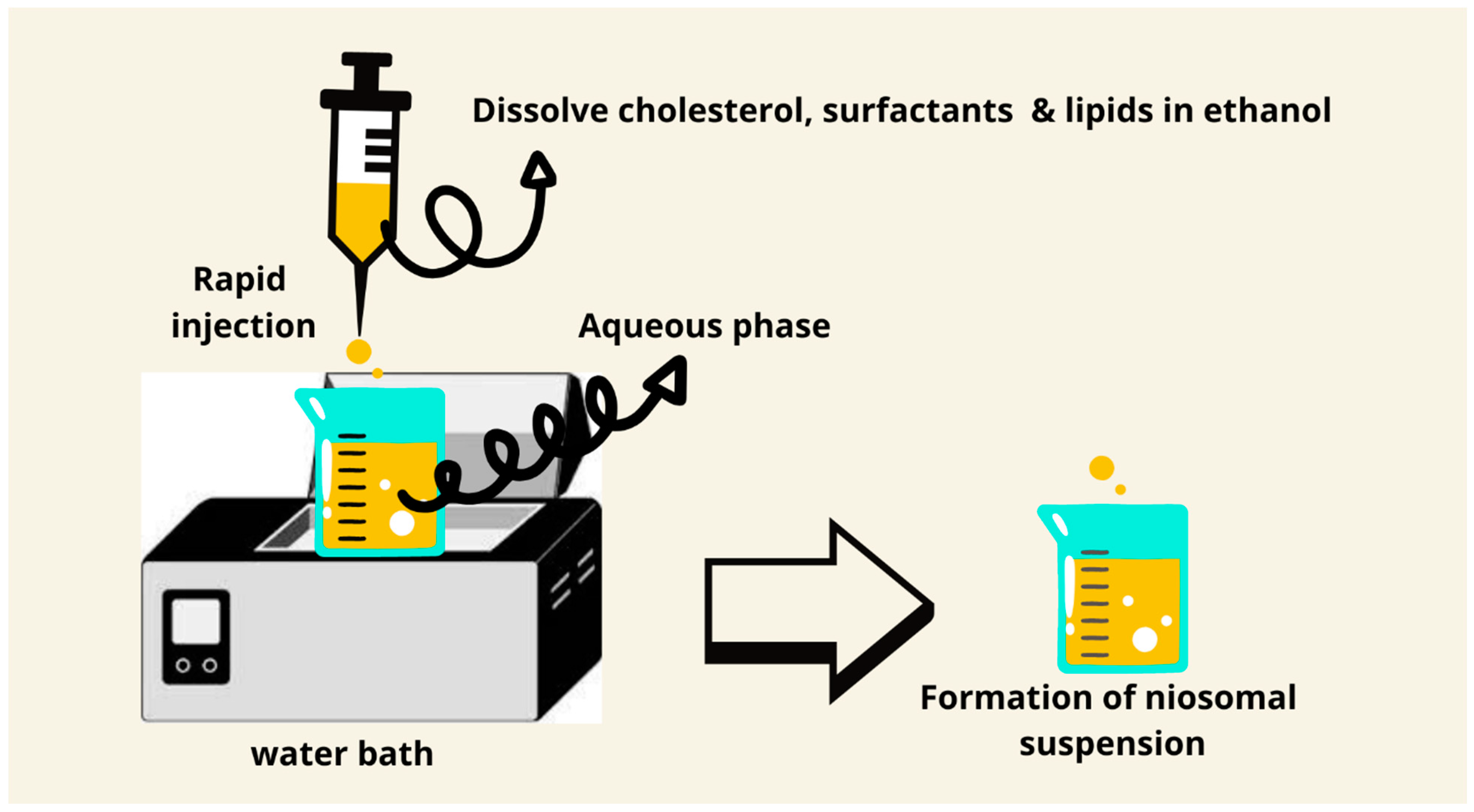
7.4. Ethanol Injection Method
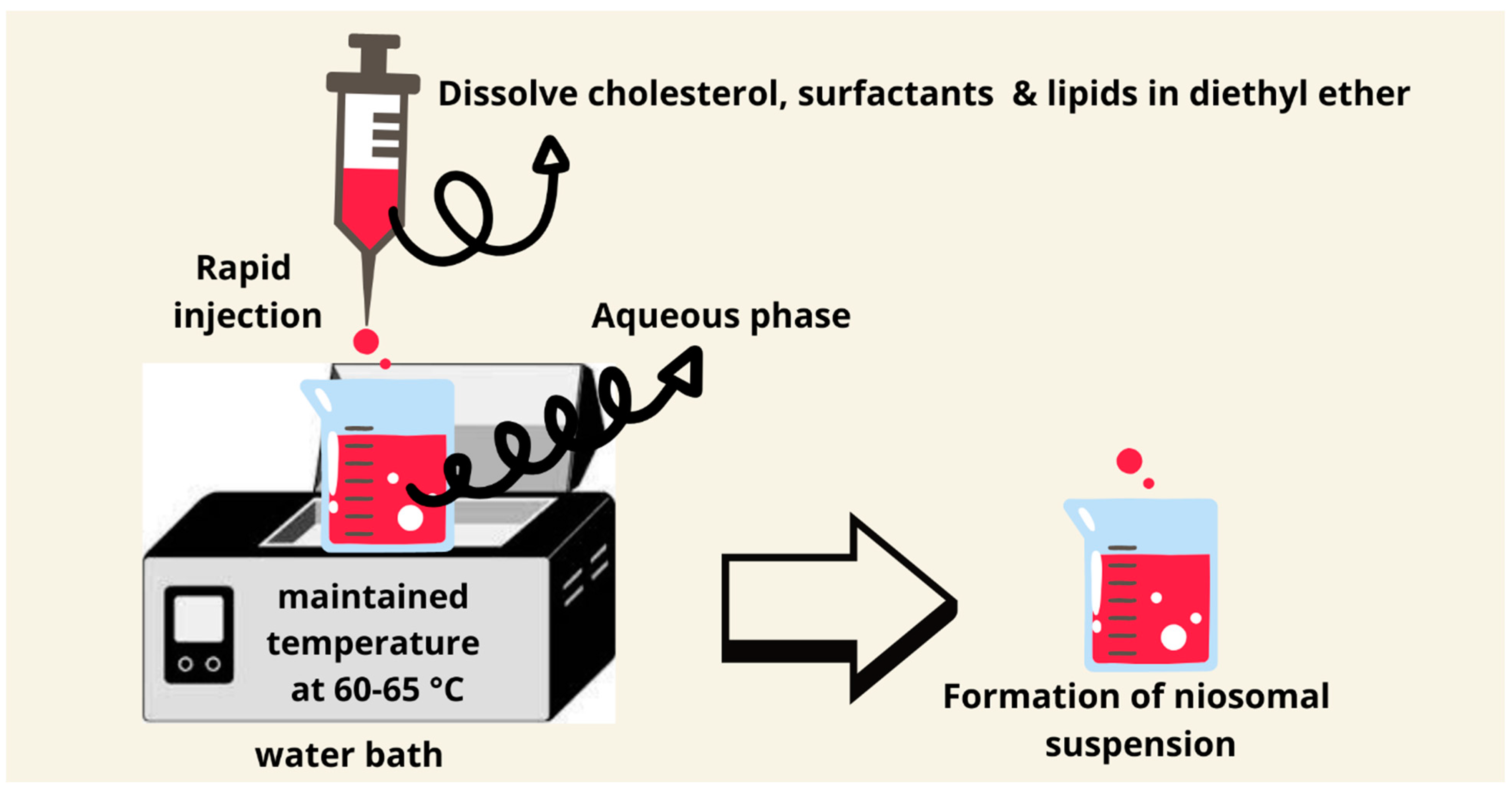
7.5. Ether Injection Method
7.6. The Bubble Method
7.7. Sonication Method
7.8. Transmembrane pH Gradient Method
7.9. Heating Method
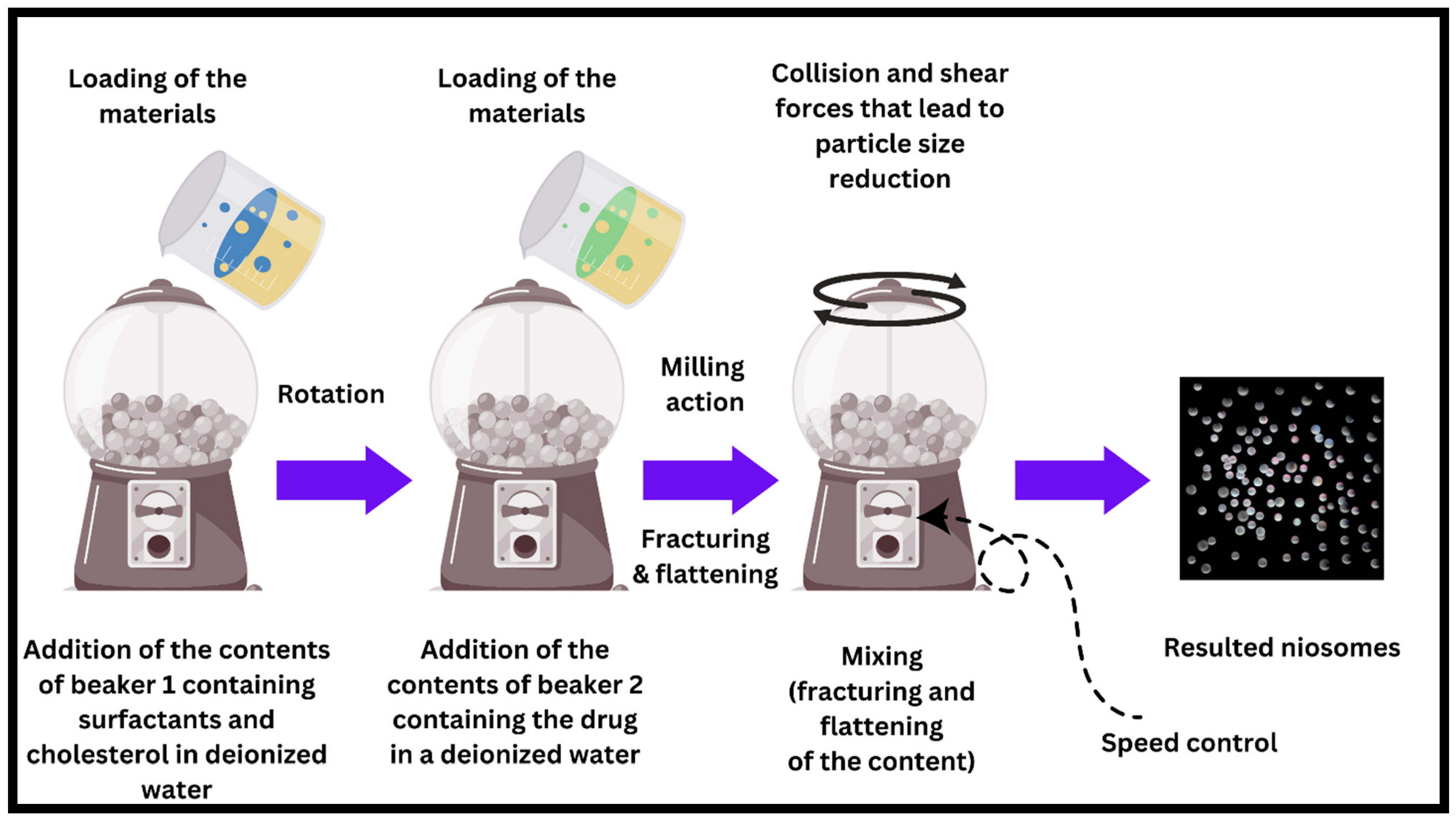
7.10. The Novel Ball Milling Method
8. Applications of Niosomes
| Preparation Technique | Particle Size Range (nm) | Encapsulation Efficiency | Advantages | Limitations | Applications | Possibility of Scale-Up | Reference Number |
|---|---|---|---|---|---|---|---|
| Thin Film Hydration | 18–29 and can go up to 450 nm | 77–92% | High reproducibility; cost-effective; easy to prepare with optimized parameters such as hydration temperature and polymer ratio | Limited stability in solution form; requires freeze-drying for long-term storage | Delivery of hydrophobic drugs, e.g., luteolin for anticancer applications | High potential for scaling up due to the simplicity of the method and reproducibility | [100,101] |
| Reverse Phase Evaporation | 55–120 | Up to 85.5% | High encapsulation efficiency, stability during freeze-drying, and good inhalation properties. | Toxicity potential of cationic surfactants, limited scalability. | Curcumin delivery for non-small cell lung cancer via the inhalation route. | Moderate: Requires optimization of solvent evaporation and vesicle stabilization for large-scale production. | [102] |
| Microfluidics Method | 237.97–281.73 | >90% |
|
|
|
| [101] |
| Ball Milling (BM) Method | Customizable, 250–600 | 85.09–86.69% | Precise size control, high stability, and encapsulation efficiency | Requires specialized equipment | Custom drug delivery profiles, advanced nanocarriers | High, scalable with suitable equipment | [90] |
| Ethanol Injection | 186–256 | 87.6–98.2% |
|
|
| High potential due to simple injection setup and ability to adapt operating conditions for larger batches. | [80] |
| Ether Injection | 129–319 | 72–96% |
|
|
| Moderate: The method is scalable with proper control over solvent evaporation and injection rate. | [103] |
| Sonication Method | 165–893 nm | 95–99% |
|
|
|
| [85] |
| Bubble Method | 200–500 | 50–75% |
|
|
|
| [84] |
| Transmembrane pH Gradient | 150–300 | Up to 47.7% |
|
|
|
| [104] |
| Heating Method | 73.85–186 | 61–98% |
|
|
|
| [105] |
9. Mechanism of Drug Delivery
9.1. Transdermal Drug Delivery
9.2. Oral Drug Delivery
9.3. Cellular Drug Delivery
10. Patent Information Related to the Niosomes Method of Preparations
11. Preclinical Validation and Clinical Application
12. Future Directions and Recommendations
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238-IN27. [Google Scholar] [CrossRef]
- Vanlerberghe, G.; Handjani-Vila, R.M.; Berthelot, C.; Sebag, H. Synthese et activite de surface comparee d’une serie de nouveaux derives non-ioniques. In Proceedings of the 6th International Congress on Surface Activity, Zurich, Switzerland, 11–15 September 1972; Carl Hanser Verlag: Munich, Germany, 1972. [Google Scholar]
- Handjani-Vila, R.M.; Ribier, A.; Rondot, B.; Vanlerberghie, G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int. J. Cosmet. Sci. 1979, 1, 303–314. [Google Scholar] [CrossRef]
- Parthasarathi, G.; Udupa, N.; Pillai, G.K. Formulation and in vitro evaluation of vincristine encapsulated Niosomes. Indian J. Pharm. Sci. 1994, 56, 90–94. [Google Scholar]
- Mohanty, D.; Rani, M.J.; Haque, M.A.; Bakshi, V.; Jahangir, M.A.; Imam, S.S.; Gilani, S.J. Preparation and evaluation of transdermal naproxen niosomes: Formulation optimization to preclinical anti-inflammatory assessment on murine model. J. Liposome Res. 2020, 30, 377–387. [Google Scholar] [CrossRef]
- Hasan, A.A.; Madkor, H.; Wageh, S. Formulation and evaluation of metformin hydrochloride-loaded niosomes as controlled release drug delivery system. Drug Deliv. 2013, 20, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Alam, G.; Singh, A.P.; Verma, N.K. Niosomes: An approach to current drug delivery-A Review. Int. J. Adv. Pharm. 2017, 6, 41–48. [Google Scholar]
- Azmin, M.N.; Florence, A.T.; Handjani-Vila, R.M.; Stuart, J.F.B.; Vanlerberghe, G.; Whittaker, J.S. The effect of non-ionic surfactant vesicle (niosome) entrapment on the absorption and distribution of methotrexate in mice. J. Pharm. Pharmacol. 1985, 37, 237–242. [Google Scholar] [CrossRef]
- Brewer, J.M.; Alexander, J. The adjuvant activity of non-ionic surfactant vesicles (niosomes) on the BALB/c humoral response to bovine serum albumin. Immunology 1992, 75, 570. [Google Scholar]
- Jayaraman, S.C.; Ramachandran, C.; Weiner, N. Topical delivery of erythromycin from various formulations: An in vivo hairless mouse study. J. Pharm. Sci. 1996, 85, 1082–1084. [Google Scholar] [CrossRef]
- Moser, P.; Marchand-Arvier, M.; Labrude, P.; Handjani Vila, R.M.; Vigneron, C. Niosomes d’hémoglobine. I. Preparation, proprietes physicochimiques et oxyphoriques, stabilite. Pharm. Acta Helv. 1989, 64, 192–202. [Google Scholar] [PubMed]
- Usman, M.R.M.; Ghuge, P.R.; Jain, B. V Niosomes: A novel trend of drug delivery. Eur. J. Biomed. Pharm. Sci. 2017, 4, 436–442. [Google Scholar]
- Marianecci, C.; Di Marzio, L.; Rinaldi, F.; Celia, C.; Paolino, D.; Alhaique, F.; Esposito, S.; Carafa, M. Niosomes from 80 s to present: The state of the art. Adv. Colloid Interface Sci. 2014, 205, 187–206. [Google Scholar] [CrossRef]
- Uchegbu, I.F.; Florence, A.T. Non-ionic surfactant vesicles (niosomes): Physical and pharmaceutical chemistry. Adv. Colloid Interface Sci. 1995, 58, 1–55. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Van Hal, D.A.; Hofland, H.E.J.; Junginger, H.E. Preparation and characterization of nonionic surfactant vesicles. Colloids Surfaces A Physicochem. Eng. Asp. 1997, 123–124, 71–80. [Google Scholar] [CrossRef]
- Pardakhty, A.; Varshosaz, J.; Rouholamini, A. In vitro study of polyoxyethylene alkyl ether niosomes for delivery of insulin. Int. J. Pharm. 2007, 328, 130–141. [Google Scholar] [CrossRef]
- Bayindir, Z.S.; Yuksel, N. Characterization of niosomes prepared with various nonionic surfactants for paclitaxel oral delivery. J. Pharm. Sci. 2010, 99, 2049–2060. [Google Scholar] [CrossRef]
- Rajera, R.; Nagpal, K.; Singh, S.K.; Mishra, D.N. Niosomes: A controlled and novel drug delivery system. Biol. Pharm. Bull. 2011, 34, 945–953. [Google Scholar] [CrossRef]
- Park, E.-S.; Chang, S.-Y.; Hahn, M.; Chi, S.-C. Enhancing effect of polyoxyethylene alkyl ethers on the skin permeation of ibuprofen. Int. J. Pharm. 2000, 209, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Tripathi, P.; Gupta, R.; Pandey, S. Niosomes: A review on niosomal research in the last decade. J. Drug Deliv. Sci. Technol. 2020, 56, 101581. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of non-ionic surfactant vesicles (niosomes) and their application in drug delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Bartelds, R.; Nematollahi, M.H.; Pols, T.; Stuart, M.C.A.; Pardakhty, A.; Asadikaram, G.; Poolman, B. Niosomes, an alternative for liposomal delivery. PLoS ONE 2018, 13, e0194179. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Academic Press: New York, NY, USA, 1985. [Google Scholar]
- Moghassemi, S.; Hadjizadeh, A. Nano-niosomes as nanoscale drug delivery systems: An illustrated review. J. Control. Release 2014, 185, 22–36. [Google Scholar] [CrossRef] [PubMed]
- Corin, K.C.; O’connor, C.T. A proposal to use excess Gibbs energy rather than HLB number as an indicator of the hydrophilic–liphophilic behavior of surfactants. Miner. Eng. 2014, 58, 17–21. [Google Scholar] [CrossRef]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Zheng, Y.; Zheng, M.; Ma, Z.; Xin, B.; Guo, R.; Xu, X. Sugar Fatty Acid Esters. In Polar Lipids: Biology, Chemistry, and Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 215–243. ISBN 9781630670450. [Google Scholar]
- Premlal Ranjith, H.M.; Wijewardene, U. Lipid emulsifiers and surfactants in dairy and bakery products. In Modifying Lipids for Use in Food; Elsevier Inc.: Amsterdam, The Netherlands, 2006; pp. 393–428. ISBN 9781855739710. [Google Scholar]
- Ruckmani, K.; Jayakar, B.; Ghosal, S.K. Nonionic surfactant vesicles (niosomes) of cytarabine hydrochloride for effective treatment of leukemias: Encapsulation, storage, and in vitro release. Drug Dev. Ind. Pharm. 2000, 26, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Das, M.K.; Palei, N.N. Sorbitan Ester Niosomes for Topical Delivery of Rofecoxib. 2011. Available online: https://nopr.niscpr.res.in/handle/123456789/11742 (accessed on 1 September 2024).
- Yoshioka, T.; Sternberg, B.; Florence, A.T. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85). Int. J. Pharm. 1994, 105, 1–6. [Google Scholar] [CrossRef]
- Khazaeli, P.; Pardakhty, A.; Shoorabi, H. Caffeine-loaded niosomes: Characterization and in vitro release studies. Drug Deliv. 2007, 14, 447–452. [Google Scholar] [CrossRef]
- Kamboj, S.; Saini, V.; Bala, S. Formulation and characterization of drug loaded nonionic surfactant vesicles (niosomes) for oral bioavailability enhancement. Sci. World J. 2014, 2014, 959741. [Google Scholar] [CrossRef]
- Gugleva, V.; Titeva, S.; Rangelov, S.; Momekova, D. Design and in vitro evaluation of doxycycline hyclate niosomes as a potential ocular delivery system. Int. J. Pharm. 2019, 567, 118431. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Raish, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Sorbitane monostearate and cholesterol based niosomes for oral delivery of telmisartan. Curr. Drug Deliv. 2018, 15, 260–266. [Google Scholar] [CrossRef]
- Ruckmani, K.; Sankar, V. Formulation and optimization of zidovudine niosomes. AAPS PharmSciTech 2010, 11, 1119–1127. [Google Scholar] [CrossRef]
- Arunachalam, A.; Jeganath, S.; Yamini, K.; Tharangini, K. Niosomes: A novel drug delivery system. Int. J. Nov. Trends Pharm. Sci. 2012, 2, 25–31. [Google Scholar]
- Tangri, P.; Khurana, S. Niosomes: Formulation and evaluation. Int. J. 2011, 2229, 7499. [Google Scholar]
- Moghddam, S.R.M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater. Sci. Eng. C 2016, 69, 789–797. [Google Scholar] [CrossRef]
- Shah, A.; Boldhane, S.; Pawar, A.; Bothiraja, C. Advanced development of a non-ionic surfactant and cholesterol material based niosomal gel formulation for the topical delivery of anti-acne drugs. Mater. Adv. 2020, 1, 1763–1774. [Google Scholar] [CrossRef]
- Gregoriadis, G. Liposome Technology; CRC Press: Boca Raton, FL, USA, 1992; Volume 3, ISBN 0849367093. [Google Scholar]
- Devaraj, G.N.; Parakh, S.R.; Devraj, R.; Apte, S.S.; Rao, B.R.; Rambhau, D. Release studies on niosomes containing fatty alcohols as bilayer stabilizers instead of cholesterol. J. Colloid Interface Sci. 2002, 251, 360–365. [Google Scholar] [CrossRef]
- Qumbar, M.; Imam, S.S.; Ali, J.; Ahmad, J.; Ali, A. Formulation and optimization of lacidipine loaded niosomal gel for transdermal delivery: In-vitro characterization and in-vivo activity. Biomed. Pharmacother. 2017, 93, 255–266. [Google Scholar] [CrossRef]
- Abdelbary, G.; El-Gendy, N. Niosome-encapsulated gentamicin for ophthalmic controlled delivery. AAPS PharmSciTech 2008, 9, 740–747. [Google Scholar] [CrossRef]
- Ghadi, Z.S.; Dinarvand, R.; Asemi, N.; Amiri, F.T.; Ebrahimnejad, P. Preparation, characterization and in vivo evaluation of novel hyaluronan containing niosomes tailored by Box-Behnken design to co-encapsulate curcumin and quercetin. Eur. J. Pharm. Sci. 2019, 130, 234–246. [Google Scholar] [CrossRef]
- Manconi, M.; Sinico, C.; Valenti, D.; Loy, G.; Fadda, A.M. Niosomes as carriers for tretinoin. I. Preparation and properties. Int. J. Pharm. 2002, 234, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B. Effect of cholesterol and temperature on the elastic properties of niosomal membranes. Int. J. Pharm. 2005, 300, 95–101. [Google Scholar] [CrossRef]
- Barani, M.; Nematollahi, M.H.; Zaboli, M.; Mirzaei, M.; Torkzadeh-Mahani, M.; Pardakhty, A.; Karam, G.A. In silico and in vitro study of magnetic niosomes for gene delivery: The effect of ergosterol and cholesterol. Mater. Sci. Eng. C 2019, 94, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Pencer, J.; Nieh, M.-P.; Harroun, T.A.; Krueger, S.; Adams, C.; Katsaras, J. Bilayer thickness and thermal response of dimyristoylphosphatidylcholine unilamellar vesicles containing cholesterol, ergosterol and lanosterol: A small-angle neutron scattering study. Biochim. Biophys. Acta (BBA)-Biomembr. 2005, 1720, 84–91. [Google Scholar] [CrossRef]
- Machado, N.D.; García-Manrique, P.; Fernández, M.A.; Blanco-López, M.C.; Matos, M.; Gutiérrez, G. Cholesterol free niosome production by microfluidics: Comparative with other conventional methods. Chem. Eng. Res. Des. 2020, 162, 162–171. [Google Scholar] [CrossRef]
- Kalsin, A.M.; Grzybowski, B.A. Controlling the growth of “ionic” nanoparticle supracrystals. Nano Lett. 2007, 7, 1018–1021. [Google Scholar] [CrossRef]
- Elci, S.G.; Jiang, Y.; Yan, B.; Kim, S.T.; Saha, K.; Moyano, D.F.; Yesilbag Tonga, G.; Jackson, L.C.; Rotello, V.M.; Vachet, R.W. Surface charge controls the suborgan biodistributions of gold nanoparticles. ACS Nano 2016, 10, 5536–5542. [Google Scholar] [CrossRef]
- Ag Seleci, D.; Seleci, M.; Walter, J.-G.; Stahl, F.; Scheper, T. Niosomes as nanoparticular drug carriers: Fundamentals and recent applications. J. Nanomater. 2016, 2016, 7372306. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Yuksel, N. Investigation of formulation variables and excipient interaction on the production of niosomes. AAPS PharmSciTech 2012, 13, 826–835. [Google Scholar] [CrossRef]
- Blazek–Welsh, A.I.; Rhodes, D.G. SEM imaging predicts quality of niosomes from maltodextrin-based proniosomes. Pharm. Res. 2001, 18, 656–661. [Google Scholar] [CrossRef]
- Sun, D.; Zhang, H. Electrochemical determination of acetaminophen using a glassy carbon electrode coated with a single-wall carbon nanotube-dicetyl phosphate film. Microchim. Acta 2007, 158, 131–136. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Supaperm, T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS PharmSciTech 2008, 9, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Sammour, O.A.; Hammad, M.A.; Megrab, N.A. Effect of some formulation parameters on flurbiprofen encapsulation and release rates of niosomes prepared from proniosomes. Int. J. Pharm. 2008, 361, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Weston, N.; Coombes, A.G.A.; Fitzgerald, M.; Perrie, Y. Liposome formulation of poorly water soluble drugs: Optimisation of drug loading and ESEM analysis of stability. Int. J. Pharm. 2004, 285, 23–34. [Google Scholar] [CrossRef]
- Jaehnig, F.; Harlos, K.; Vogel, H.; Eibl, H. Electrostatic interactions at charged lipid membranes. Electrostatically induced tilt. Biochemistry 1979, 18, 1459–1468. [Google Scholar] [CrossRef]
- Jeon, H.S.; Seo, J.E.; Kim, M.S.; Kang, M.H.; Oh, D.H.; Jeon, S.O.; Jeong, S.H.; Choi, Y.W.; Lee, S. A retinyl palmitate-loaded solid lipid nanoparticle system: Effect of surface modification with dicetyl phosphate on skin permeation in vitro and anti-wrinkle effect in vivo. Int. J. Pharm. 2013, 452, 311–320. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A promising nanocarrier for natural drug delivery through blood-brain barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Kumar, S. Niosomes: Present scenario and future aspects. J. Drug Deliv. Ther. 2018, 8, 35–43. [Google Scholar] [CrossRef]
- Abdelkader, H.; Farghaly, U.; Moharram, H. Effects of surfactant type and cholesterol level on niosomes physical properties and in vivo ocular performance using timolol maleate as a model drug. J. Pharm. Investig. 2014, 44, 329–337. [Google Scholar] [CrossRef]
- Nasir, A.; Harikumar, S.L.; Amanpreet, K. Niosomes: An excellent tool for drug delivery. Int. J. Res. Pharm. Chem. 2012, 2, 479–487. [Google Scholar]
- Agarwal, R.; Katare, O.P.; Vyas, S.P. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int. J. Pharm. 2001, 228, 43–52. [Google Scholar] [CrossRef]
- Yeo, L.K.; Chaw, C.S.; Elkordy, A.A. The effects of hydration parameters and co-surfactants on methylene blue-loaded niosomes prepared by the thin film hydration method. Pharmaceuticals 2019, 12, 46. [Google Scholar] [CrossRef]
- Yeo, L.K.; Olusanya, T.O.B.; Chaw, C.S.; Elkordy, A.A. Brief effect of a small hydrophobic drug (cinnarizine) on the physicochemical characterisation of niosomes produced by thin-film hydration and microfluidic methods. Pharmaceutics 2018, 10, 185. [Google Scholar] [CrossRef] [PubMed]
- Ravalika, V.; Sailaja, A. Formulation and evaluation of etoricoxib niosomes by thin film hydration technique and ether injection method. Nano Biomed. Eng. 2017, 9, 242–248. [Google Scholar] [CrossRef]
- Hashim, I.I.A.; El-Dahan, M.S.; Yusif, R.M.; Abd-ElGawad, A.-E.H.; Arima, H. Potential use of niosomal hydrogel as an ocular delivery system for atenolol. Biol. Pharm. Bull. 2014, 37, 541–551. [Google Scholar] [CrossRef]
- Gupta, M.; Vaidya, B.; Mishra, N.; Vyas, S.P. Effect of surfactants on the characteristics of fluconazole niosomes for enhanced cutaneous delivery. Artif. Cells Blood Substit. Biotechnol. 2011, 39, 376–384. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; Nasongkla, N. Layer-by-layer nanocoating of antibacterial niosome on orthopedic implant. Int. J. Pharm. 2018, 547, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.D.; Silva, O.F.; de Rossi, R.H.; Fernández, M.A. Cyclodextrin modified niosomes to encapsulate hydrophilic compounds. RSC Adv. 2018, 8, 29909–29916. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Miller, N. Phospholipid model membranes. I. Structural characteristics of hydrated liquid crystals. Biochim. Biophys. Acta (BBA)-Biomembr. 1967, 135, 624–638. [Google Scholar] [CrossRef]
- Szoka, F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Guinedi, A.S.; Mortada, N.D.; Mansour, S.; Hathout, R.M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005, 306, 71–82. [Google Scholar] [CrossRef]
- Alle, M.; Samed, N.; Kim, J.-C. Niosomes: A Smart Drug Carrier Synthesis, Properties and Applications. In Smart Nanomaterials in Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 449–486. [Google Scholar]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Garcia-Manrique, P.; Gutiérrez, G.; Matos, M.; Cristaldi, A.; Mosayyebi, A.; Carugo, D.; Zhang, X.; Blanco-López, M.C. Continuous flow production of size-controllable niosomes using a thermostatic microreactor. Colloids Surf. B Biointerfaces 2019, 182, 110378. [Google Scholar] [CrossRef] [PubMed]
- Estupiñan, O.R.; Garcia-Manrique, P.; Blanco-Lopez, M.d.C.; Matos, M.; Gutiérrez, G. Vitamin D3 Loaded Niosomes and Transfersomes Produced by Ethanol Injection Method: Identification of the Critical Preparation Step for Size Control. Foods 2020, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Hunter, C.A.; Dolan, T.F.; Coombs, G.H.; Baillie, A.J. Vesicular systems (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J. Pharm. Pharmacol. 1988, 40, 161–165. [Google Scholar] [CrossRef]
- Arora, R. Advances in niosome as a drug carrier: A review. Asian J. Pharm. 2016, 1, 11. [Google Scholar]
- Lohumi, A. A novel drug delivery system: Niosomes review. J. Drug Deliv. Ther. 2012, 2, 130–134. [Google Scholar] [CrossRef]
- Talsma, H.; Van Steenbergen, M.J.; Borchert, J.C.H.; Crommelin, D.J.A. A novel technique for the one-step preparation of liposomes and nonionic surfactant vesicles without the use of organic solvents. Liposome formation in a continuous gas stream: The ‘Bubble’method. J. Pharm. Sci. 1994, 83, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.H.; Bashir, S.; Khan, M.I.; Figueiredo, P.; Santos, H.A.; Peltonen, L. Formulation optimization and in vitro characterization of rifampicin and ceftriaxone dual drug loaded niosomes with high energy probe sonication technique. J. Drug Deliv. Sci. Technol. 2020, 58, 101763. [Google Scholar] [CrossRef]
- Khan, M.I.; Madni, A.; Hirvonen, J.; Peltonen, L. Ultrasonic processing technique as a green preparation approach for diacerein-loaded niosomes. AAPS PharmSciTech 2017, 18, 1554–1563. [Google Scholar] [CrossRef]
- Asaithambi, K.; Muthukumar, J.; Chandrasekaran, R.; Ekambaram, N.; Roopan, M. Synthesis and characterization of turmeric oil loaded non-ionic surfactant vesicles (niosomes) and its enhanced larvicidal activity against mosquito vectors. Biocatal. Agric. Biotechnol. 2020, 29, 101737. [Google Scholar] [CrossRef]
- Bhaskaran, S.; Panigrahi, L. Formulation and evaluation of niosomes using different non-ionic surfactants. Indian J. Pharm. Sci. 2002, 64, 63. [Google Scholar]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. In Liposomes; Springer: Berlin/Heidelberg, Germany, 2010; pp. 29–50. [Google Scholar]
- Temprom, L.; Krongsuk, S.; Thapphasaraphong, S.; Priperm, A.; Namuangruk, S. A novel preparation and characterization of melatonin loaded niosomes based on using a ball milling method. Mater. Today Commun. 2022, 31, 103340. [Google Scholar] [CrossRef]
- Varshosaz, J.; Pardakhty, A.; Hajhashemi, V.; Najafabadi, A.R. Development and physical characterization of sorbitan monoester niosomes for insulin oral delivery. Drug Deliv. 2003, 10, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Eid, R.K.; Essa, E.A.; El Maghraby, G.M. Essential oils in niosomes for enhanced transdermal delivery of felodipine. Pharm. Dev. Technol. 2019, 24, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, Q.; Hu, H.; He, Z.; Wu, T.; Guo, T.; Feng, N. Sodium dodecyl sulfate improved stability and transdermal delivery of salidroside-encapsulated niosomes via effects on zeta potential. Int. J. Pharm. 2020, 580, 119183. [Google Scholar] [CrossRef]
- Vyas, J.; Vyas, P.; Raval, D.; Paghdar, P. Development of topical niosomal gel of benzoyl peroxide. Int. Sch. Res. Not. 2011, 2011, 503158. [Google Scholar] [CrossRef]
- Pamornpathomkul, B.; Niyomtham, N.; Yingyongnarongkul, B.-E.; Prasitpuriprecha, C.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Cationic Niosomes for Enhanced Skin Immunization of Plasmid DNA-Encoding Ovalbumin via Hollow Microneedles; Springer: Berlin/Heidelberg, Germany, 2018; Volume 19, pp. 481–488. [Google Scholar]
- Nayak, A.S.; Chodisetti, S.; Gadag, S.; Nayak, U.Y.; Govindan, S.; Raval, K. Tailoring solulan C24 based niosomes for transdermal delivery of donepezil: In vitro characterization, evaluation of pH sensitivity, and microneedle-assisted Ex vivo permeation studies. J. Drug Deliv. Sci. Technol. 2020, 60, 101945. [Google Scholar] [CrossRef]
- Dwivedi, R.K. Development of Novel Formulation for Intranasal Delivery Containing Antidepressant Agent. Saudi J. Med. Pharm. Sci. 2021, 7, 358–367. [Google Scholar]
- Moghaddam, F.D.; Akbarzadeh, I.; Marzbankia, E.; Farid, M.; Reihani, A.H.; Javidfar, M.; Mortazavi, P. Delivery of melittin-loaded niosomes for breast cancer treatment: An in vitro and in vivo evaluation of anti-cancer effect. Cancer Nanotechnol. 2021, 12, 14. [Google Scholar] [CrossRef]
- Demirbolat, G.M.; Aktas, E.; Coskun, G.P.; Erdogan, O.; Cevik, O. New Approach to Formulate Methotrexate-Loaded Niosomes: In Vitro Characterization and Cellular Effectiveness. J. Pharm. Innov. 2021, 17, 622–637. [Google Scholar] [CrossRef]
- Mod Razif, M.R.F.; Chan, S.Y.; Widodo, R.T.; Chew, Y.-L.; Hassan, M.; Hisham, S.A.; Rahman, S.A.; Ming, L.C.; Tan, C.S.; Lee, S.-K. Optimization of a Luteolin-Loaded TPGS/Poloxamer 407 Nanomicelle: The Effects of Copolymers, Hydration Temperature and Duration, and Freezing Temperature on Encapsulation Efficiency, Particle Size, and Solubility. Cancers 2023, 15, 3741. [Google Scholar] [CrossRef]
- Kanpipit, N.; Mattariganont, S.; Janphuang, P.; Rongsak, J.; Daduang, S.; Chulikhit, Y.; Thapphasaraphong, S. Comparative Study of Lycopene-Loaded Niosomes Prepared by Microfluidic and Thin-Film Hydration Techniques for UVB Protection and Anti-Hyperpigmentation Activity. Int. J. Mol. Sci. 2024, 25, 11717. [Google Scholar] [CrossRef]
- Jyoti, K.; Pandey, R.S.; Madan, J.; Jain, U.K. Inhalable cationic niosomes of curcumin enhanced drug delivery and apoptosis in lung cancer cells. Indian J. Pharm. Educ. Res. 2016, 50, S21–S31. [Google Scholar]
- AMREEN, A.; KV, R. Formulation and evaluation of tramadol hydrochloride-loaded niosomal gel by ether injection method. Asian J. Pharm. Clin. Res. 2023, 16, 170–173. [Google Scholar] [CrossRef]
- Dehaghi, M.H.; Haeri, A.; Keshvari, H.; Abbasian, Z.; Dadashzadeh, S. Dorzolamide loaded niosomal vesicles: Comparison of passive and remote loading methods. Iran. J. Pharm. Res. 2017, 16, 413. [Google Scholar]
- Basiri, L.; Rajabzadeh, G.; Bostan, A. α-Tocopherol-loaded niosome prepared by heating method and its release behavior. Food Chem. 2017, 221, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration: A review. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef]
- Alves, M.P.; Scarrone, A.L.; Santos, M.; Pohlmann, A.R.; Guterres, S.S. Human skin penetration and distribution of nimesulide from hydrophilic gels containing nanocarriers. Int. J. Pharm. 2007, 341, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Sharma, P.K.; Narasimha Murthy, S. Effect of mild hyperthermia on transdermal absorption of nicotine from patches. AAPS PharmSciTech 2019, 20, 77. [Google Scholar] [CrossRef]
- Bird, D.; Ravindra, N.M. Transdermal drug delivery and patches—An overview. Med. Devices Sens. 2020, 3, e10069. [Google Scholar] [CrossRef]
- El-Ridy, M.S.; Badawi, A.A.; Safar, M.M.; Mohsen, A.M. Niosomes as a novel pharmaceutical formulation encapsulating the hepatoprotective drug silymarin. Int. J. Pharm. Pharm. Sci. 2012, 4, 549–559. [Google Scholar]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef]
- Khoee, S.; Yaghoobian, M. Niosomes: A Novel Approach in Modern Drug Delivery Systems; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Xu, Y.-Q.; Chen, W.-R.; Tsosie, J.K.; Xie, X.; Li, P.; Wan, J.-B.; He, C.-W.; Chen, M.-W. Niosome encapsulation of curcumin: Characterization and cytotoxic effect on ovarian cancer cells. J. Nanomater. 2016, 2016, 6365295. [Google Scholar] [CrossRef]
- Sezgin-Bayindir, Z.; Onay-Besikci, A.; Vural, N.; Yuksel, N. Niosomes encapsulating paclitaxel for oral bioavailability enhancement: Preparation, characterization, pharmacokinetics and biodistribution. J. Microencapsul. 2013, 30, 796–804. [Google Scholar] [CrossRef]
- Garbati, P.; Picco, C.; Magrassi, R.; Signorello, P.; Cacopardo, L.; Dalla Serra, M.; Faticato, M.G.; De Luca, M.; Balestra, F.; Scavo, M.P. Targeting the Gut: A Systematic Review of Specific Drug Nanocarriers. Pharmaceutics 2024, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Prosperi, D.; Colombo, M.; Zanoni, I.; Granucci, F. Drug nanocarriers to treat autoimmunity and chronic inflammatory diseases. Semin.Immunol. 2017, 34, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Tavano, L.; Muzzalupo, R.; Mauro, L.; Pellegrino, M.; Andò, S.; Picci, N. Transferrin-conjugated pluronic niosomes as a new drug delivery system for anticancer therapy. Langmuir 2013, 29, 12638–12646. [Google Scholar] [CrossRef]
- Tavano, L.; Infante, M.R.; Riya, M.A.; Pinazo, A.; Vinardell, M.P.; Mitjans, M.; Manresa, M.A.; Pérez, L. Role of aggregate size in the hemolytic and antimicrobial activity of colloidal solutions based on single and gemini surfactants from arginine. Soft Matter 2013, 9, 306–319. [Google Scholar] [CrossRef]
- Francis, M. Study of the Mechanisms of Destabilization of Niosomes and Liposomes by a PH-Sensitive N-Isopropylacrylamide Copolymer. 2001. Available online: https://papyrus.bib.umontreal.ca/xmlui/bitstream/handle/1866/31102/Francis_Mira_2001_memoire.pdf?sequence=1 (accessed on 15 September 2024).
- Setenay, Ö.; Kerimoğlu, O.; Uğurlu, T. Nanocarriers: Novel approaches to oral delivery of insulin. Clin. Exp. Health Sci. 2017, 7, 115–122. [Google Scholar]
- Al Gailani, M.; Liu, M.; Wen, J. Ligands for oral delivery of peptides across the blood-brain-barrier. Acta Mater. Medica 2022, 1, 106–123. [Google Scholar] [CrossRef]
- Argenziano, M.; Arpicco, S.; Brusa, P.; Cavalli, R.; Chirio, D.; Dosio, F.; Gallarate, M.; Peira, E.; Stella, B.; Ugazio, E. Developing actively targeted nanoparticles to fight cancer: Focus on Italian research. Pharmaceutics 2021, 13, 1538. [Google Scholar] [CrossRef]
- Saharkhiz, S.; Nasri, N.; Naderi, N.; Dini, G.; Ghalehshahi, S.S.; Firoozbakht, F. Evaluating a targeted Palbociclib-Trastuzumab loaded smart niosome platform for treating HER2 positive breast cancer cells. Int. J. Pharm. X 2024, 7, 100237. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bahadure, S.; Chilamakuri, S.N.; Dadhale, A.; Gulbake, A. Multifunctional nanocarrier-mediated codelivery for targeting and treatment of prostate cancer. In Multifunctional Nanocomposites for Targeted Drug Delivery in Cancer Therapy; Elsevier: Amsterdam, The Netherlands, 2024; pp. 81–111. [Google Scholar]
- Damrongrungruang, T.; Paphangkorakit, J.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Davies, M.J.; Sungthong, B.; Priprem, A. Anthocyanin complex niosome gel accelerates oral wound healing: In vitro and clinical studies. Nanomed.Nanotechnol. Biol. Med. 2021, 37, 102423. [Google Scholar] [CrossRef]
- Obeid, M.A.; Gany, S.A.S.; Gray, A.I.; Young, L.; Igoli, J.O.; Ferro, V.A. Niosome-encapsulated balanocarpol: Compound isolation, characterisation, and cytotoxicity evaluation against human breast and ovarian cancer cell lines. Nanotechnology 2020, 31, 195101. [Google Scholar] [CrossRef]
- Pando, D.; Matos, M.; Gutiérrez, G.; Pazos, C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surfaces B Biointerfaces 2015, 128, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, D.; Garg, A.; Kaur, I.P. Development of a topical niosomal preparation of acetazolamide: Preparation and evaluation. J. Pharm. Pharmacol. 2004, 56, 1509–1517. [Google Scholar] [CrossRef]
- Khan, D.H.; Bashir, S.; Correia, A.; Khan, M.I.; Figueiredo, P.; Santos, H.A.; Peltonen, L. Utilization of green formulation technique and efficacy estimation on cell line studies for dual anticancer drug therapy with niosomes. Int. J. Pharm. 2019, 572, 118764. [Google Scholar] [CrossRef] [PubMed]











| Method | Patented (Yes/No) | Patent Number (If Applicable) | Title | Preparation Details | Applications | Key Features |
|---|---|---|---|---|---|---|
| Thin Film Hydration | Yes | US20080050445A1 | Niosome-Hydrogel Drug Delivery System | Lipid and surfactant dissolved in organic solvent; hydrated with aqueous phase; vesicles formed under sonication. | Hydrophilic and lipophilic drug delivery; prolonged release in hydrogel systems. | Simple, cost-effective, high encapsulation efficiency for hydrophilic drugs. |
| Ball Milling | Yes | CN114632936 | Multistage Gradient Ball Milling Method for Composite Preparation of Nanophase | Nanophase powders are dispersed and reduced in size using mechanical milling with controlled speeds. | Nanophase composites in aerospace, automotive, and electronics industries (not applicable to niosomes). | Produces nanophase powders with uniformity and scalability; not applicable to liquid-based niosome preparation. |
| Microfluidics | Yes | WO2021030268A1 | Microfluidic Apparatus and Methods of Use Thereof | Controlled flow rates of lipid/surfactant solutions through microchannels for precise vesicle formation. | Precision drug delivery (e.g., RNA vaccines, targeted therapeutics). | High reproducibility, uniform particle size, scalability challenges. |
| Ethanol Injection | Yes | IN201711038841 | Development and Optimization of Niosomes for Skin Warts | Lipid and surfactant dissolved in ethanol; injected into aqueous phase with stirring to form niosomes. | Topical drug delivery (e.g., treatment of skin warts). | High encapsulation efficiency, small vesicle size, sustained drug release. |
| Ether Injection | Yes | IN202211001758 | Non-Ionic Nanostructured Vesicles for Controlled Release of Curcumin | Lipid and surfactant dissolved in ether; injected into aqueous phase, forming vesicles via ether vaporization. | Controlled delivery of hydrophobic drugs (e.g., curcumin) for CNS disorders via nasal route. | Produces monodisperse vesicles (50–200 nm) with sustained drug release. |
| Sonication Modified with Thin Film | Yes | IN3417/MUM/2010 | Niosomal Gel for Topical Delivery of Lornoxicam | Thin film hydration followed by probe sonication to reduce particle size; vesicles incorporated into carbomer-based gel. | Localized drug delivery for inflammatory conditions (e.g., arthritis). | High entrapment efficiency (52.38%), sustained release, and increased skin penetration. |
| Reverse Phase Evaporation | No | N/A | Not Patented | Lipids dissolved in the organic phase emulsified with aqueous phase; solvent evaporated to form vesicles. | Delivery of both hydrophilic and lipophilic drugs. | High encapsulation efficiency, vesicles with large unilamellar structure. |
| Bubble Method | No | N/A | Not Patented | Vesicles are formed by bubbling nitrogen gas through an aqueous lipid solution. | Exploratory drug delivery systems. | Solvent-free, simple one-step process; scalability limited by experimental status. |
| Transmembrane pH Gradient | No | N/A | Not Patented | pH gradient drives drug encapsulation in vesicles; suitable for weakly basic drugs. | Targeted release in pH-sensitive environments (e.g., tumors). | High encapsulation efficiency for pH-sensitive drugs; complex preparation process. |
| Heating Method | No | N/A | Not Patented | Lipid and surfactant mixed in a phosphate buffer at elevated temperatures to form vesicles. | General drug encapsulation applications. | Simple, cost-effective, but unsuitable for heat-sensitive drugs. |
| Preparation Method | Clinical Trial/Preclinical Study Type | Study Model | Encapsulated Drug/Compound | Key Findings | Relevance to Clinical Applications | References |
|---|---|---|---|---|---|---|
| Thin Film Hydration | Human Trial (Double-blind, placebo-controlled) | Volunteers with oral inflammatory lesions | Anthocyanin complex (AC) | Enhanced wound healing, reduced pain, and improved quality of life with AC-loaded niosome gel compared to non-niosomal and placebo gels. | Demonstrates potential for topical niosomal gels in treating oral lesions and wounds with localized delivery and prolonged therapeutic effects. | [126] |
| Ball Milling | No report available | N/A | N/A | N/A | Lacks evidence for niosomal drug delivery. Primarily used for nanopowder preparation but not liquid-phase vesicular systems like niosomes. | No preclinical or clinical research has been conducted. |
| Microfluidics | Preclinical (In vitro study) | A2780 (ovarian carcinoma), ZR-75-1 (breast carcinoma) | Balanocarpol | Improved cytotoxicity (2.8-fold) and drug efficacy with balanocarpol-loaded niosomes. Small, uniform particles (<175 nm) ensured higher cellular uptake. | Demonstrates high precision and efficacy for hydrophobic drug delivery using the microfluidic method, highlighting scalability for personalized cancer treatments. | [127] |
| Ethanol Injection | Preclinical (Ex vivo) study | Newborn pig skin model | Resveratrol | Improved skin penetration, sustained drug release (over 24 h), and high entrapment efficiency (up to 48%) compared to the thin film hydration method. | Validates the method for topical delivery of hydrophobic drugs, especially in dermatology, with enhanced skin retention and minimal systemic absorption. | [128] |
| Ether Injection | Preclinical (In vitro) study | Porcine corneal model | Acetazolamide | Ether injection produced small unilamellar vesicles with higher entrapment efficiency (39.62%) compared to thin film hydration and comparable to the reverse phase. | Demonstrated enhanced corneal permeability and drug delivery efficiency, suitable for topical ocular formulations of poorly soluble drugs | [129] |
| Sonication Modified with Thin Film | Preclinical (In vitro) study | MCF-7 (breast cancer), PC3-MM2 (prostate cancer) | Doxorubicin (DOX), Paclitaxel (PXT) | Dual drug therapy achieved synergistic effects, improved antiproliferative activity, and enhanced cell uptake. Sustained drug release over 24 h. | Ideal for dual-drug delivery systems targeting resistant cancer cells, leveraging sonication to achieve uniform particles and high drug entrapment efficiency. | [130] |
| Reverse Phase Evaporation | Preclinical (In vitro) study | A549 (lung carcinoma) | Curcumin | Cationic niosomes with curcumin demonstrated higher cellular uptake, apoptosis induction, and sustained release for 72 h. Zeta potential ~−30 mV enhanced stability. | Suitable for inhalable drug delivery targeting lung cancer. Cationic charge promotes better cellular interactions, improving drug delivery to negatively charged cancer cells. | [102] |
| Bubble Method | No report available | N/A | N/A | N/A | The solvent-free method is primarily in the experimental stages. Lacks evidence for drug delivery applications, requiring further studies to establish clinical relevance. | No preclinical or clinical research has been conducted. |
| Transmembrane pH Gradient | No report available | N/A | N/A | N/A | Used for encapsulating pH-sensitive drugs. However, no specific clinical or preclinical trials have been reported for niosomal systems prepared by this method. | No preclinical or clinical research has been conducted. |
| Heating Method | No report available | N/A | N/A | N/A | Simple and cost-effective but unsuitable for heat-sensitive drugs. No clinical or cell line studies reported for this method in niosome preparation. | No preclinical or clinical research has been conducted. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mawazi, S.M.; Ge, Y.; Widodo, R.T. Niosome Preparation Techniques and Structure—An Illustrated Review. Pharmaceutics 2025, 17, 67. https://doi.org/10.3390/pharmaceutics17010067
Mawazi SM, Ge Y, Widodo RT. Niosome Preparation Techniques and Structure—An Illustrated Review. Pharmaceutics. 2025; 17(1):67. https://doi.org/10.3390/pharmaceutics17010067
Chicago/Turabian StyleMawazi, Saeid Mezail, Yi Ge, and Riyanto Teguh Widodo. 2025. "Niosome Preparation Techniques and Structure—An Illustrated Review" Pharmaceutics 17, no. 1: 67. https://doi.org/10.3390/pharmaceutics17010067
APA StyleMawazi, S. M., Ge, Y., & Widodo, R. T. (2025). Niosome Preparation Techniques and Structure—An Illustrated Review. Pharmaceutics, 17(1), 67. https://doi.org/10.3390/pharmaceutics17010067





