Integration of Metabolomics and Transcriptomics to Reveal the Antitumor Mechanism of Dendrobium officinale Polysaccharide-Based Nanocarriers in Enhancing Photodynamic Immunotherapy in Colorectal Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of DOP@3BCP NPs
2.3. Cells and Animals
2.4. Non-Targeted Metabolomic Analysis Based on UPLC-ESI-MS/MS
2.5. Transcriptome Sequencing
2.6. H&E, Ki67, and TUNEL Staining
2.7. Quantitative Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of DOP@3BCP NPs
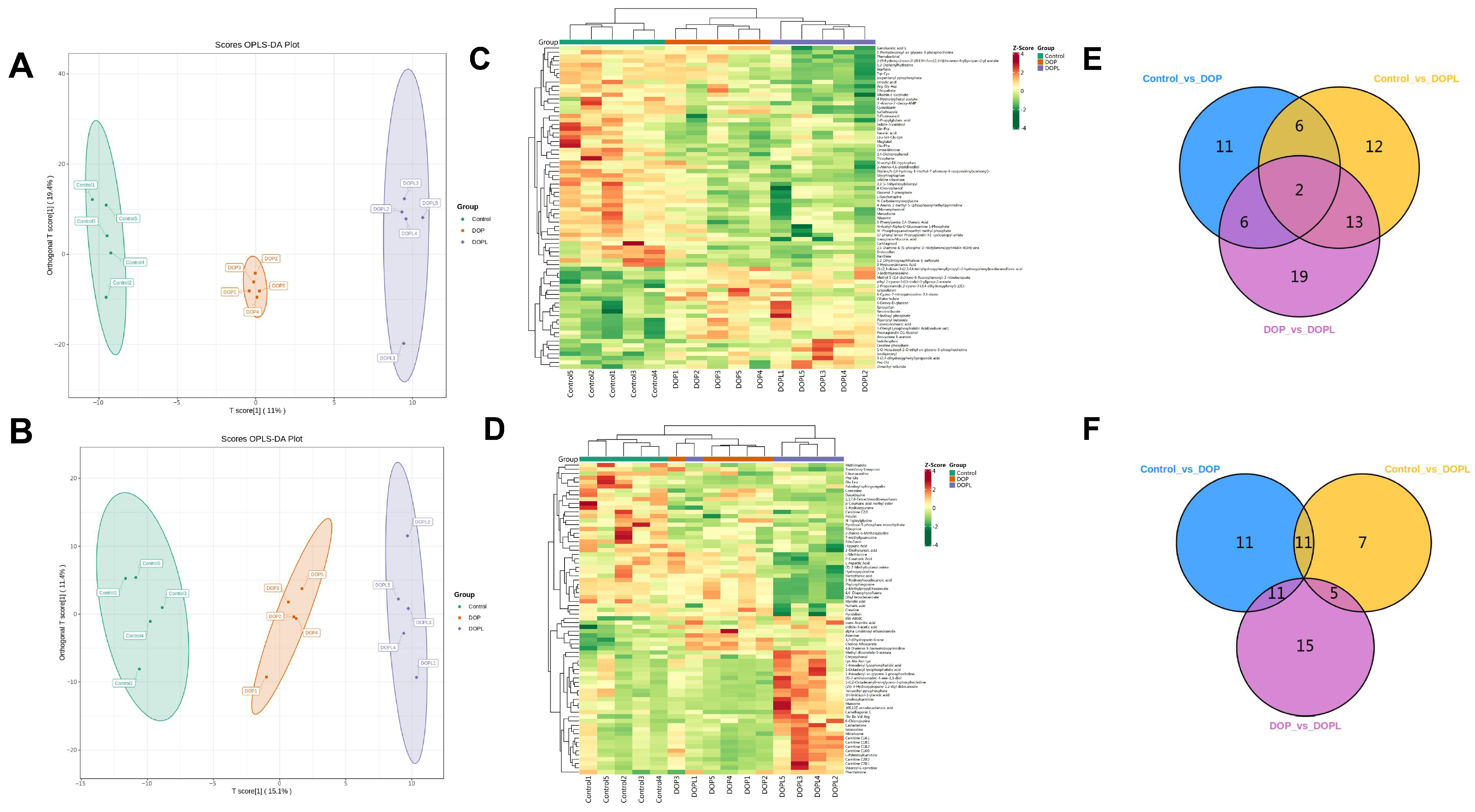
3.2. Effects of DOP@3BCP NPs on the Metabolome of the Tumors
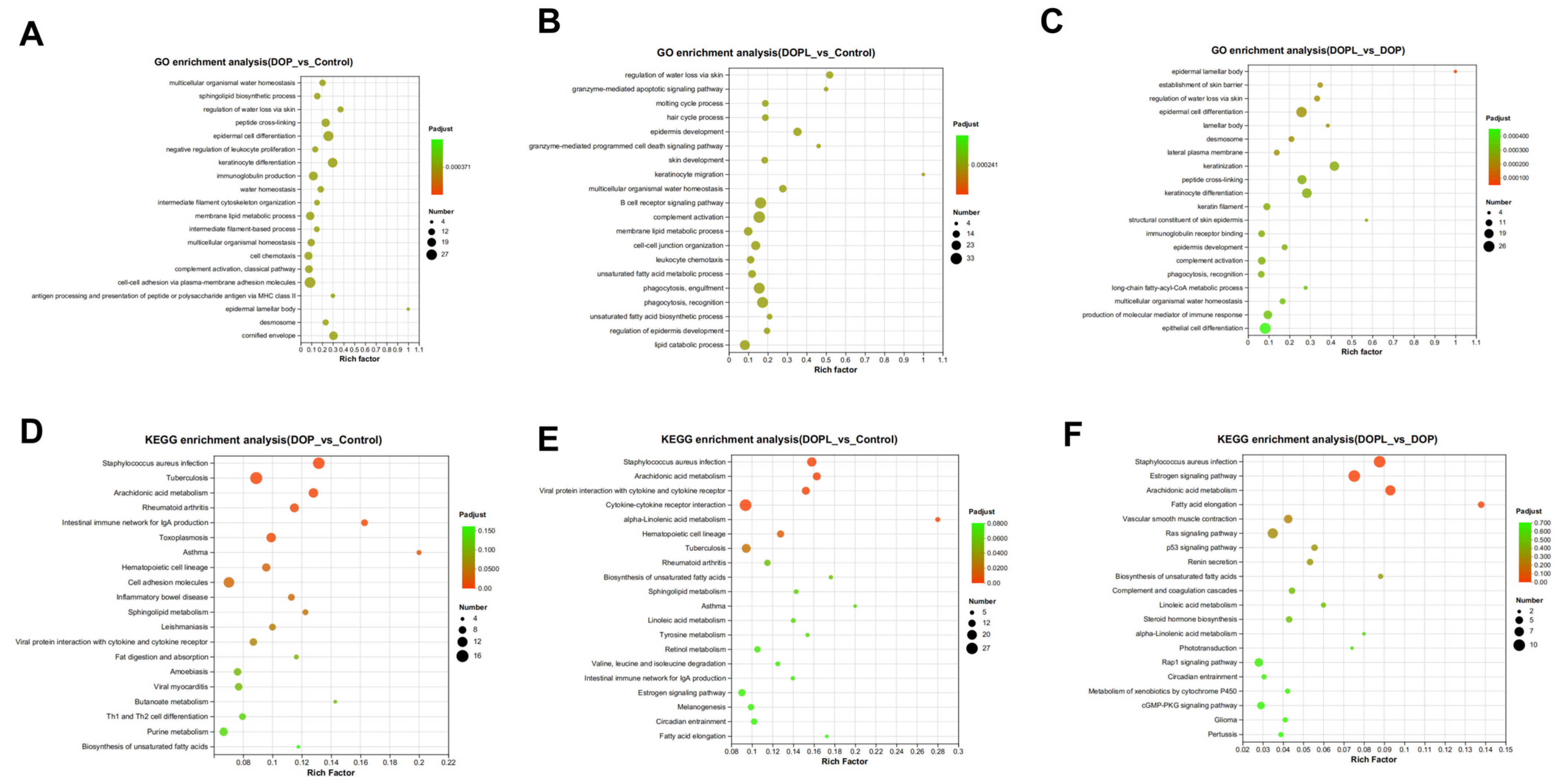
3.3. Metabolic Pathway Analysis of DEMs
3.4. Transcriptomic Analysis
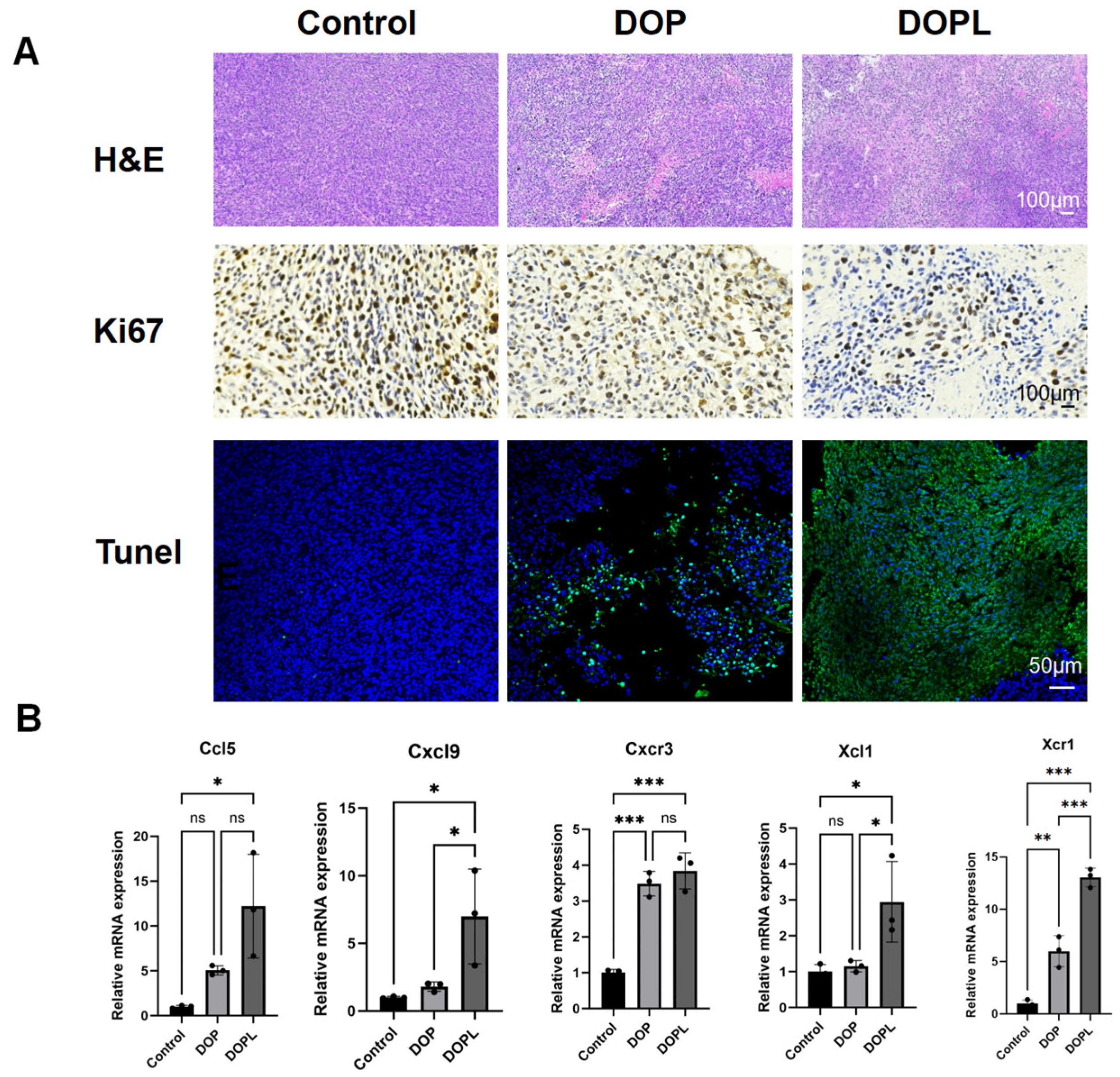
3.5. H&E, Ki67, and TUNEL Staining of Tumors
3.6. Verification of the Gene Expression Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer: Lyon, France. Available online: https://gco.iarc.who.int/today (accessed on 11 December 2024).
- Li, J.X.; Ma, X.D.; Chakravarti, D.; Shalapour, S.; DePinho, R.A. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021, 35, 787–820. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Eng, C.; Yoshino, T.; Ruíz-García, E.; Mostafa, N.; Cann, C.G.; O’Brian, B.; Benny, A.; Perez, R.O.; Cremolini, C. Colorectal cancer. Lancet 2024, 404, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Saini, V.; Venkatesh, V. AIE material for photodynamic therapy. In Advances in Aggregation Induced Emission Materials in Biosensing and Imaging for Biomedical Applications—Part B; Academic Press: Cambridge, MA, USA, 2021; pp. 45–73. [Google Scholar]
- Zhou, Y.; Zhang, W.; Wang, B.; Wang, P.; Li, D.; Cao, T.; Zhang, D.; Han, H.; Bai, M.; Wang, X.; et al. Mitochondria-targeted photodynamic therapy triggers GSDME-mediated pyroptosis and sensitizes anti-PD-1 therapy in colorectal cancer. J. ImmunoTher. Cancer 2024, 12, e008054. [Google Scholar] [CrossRef] [PubMed]
- She, T.; Shi, Q.; Li, Z.; Feng, Y.; Yang, H.; Tao, Z.; Li, H.; Chen, J.; Wang, S.; Liang, Y.; et al. Combination of long-acting TRAIL and tumor cell-targeted photodynamic therapy as a novel strategy to overcome chemotherapeutic multidrug resistance and TRAIL resistance of colorectal cancer. Theranostics 2021, 11, 4281–4297. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Song, Y.; Ding, S.; He, R.; Shi, Q.; Hu, F. Dendrobium officinale polysaccharide-based carrier to enhance photodynamic immunotherapy. Carbohydr. Polym. 2023, 317, 121089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Duan, S.; Tao, S.; Huang, J.; Liu, C.; Xing, S.; Ren, Z.; Lei, Z.; Li, Y.; Wei, G. Polysaccharides from Dendrobium officinale inhibit proliferation of osteosarcoma cells and enhance cisplatin-induced apoptosis. J. Funct. Foods 2020, 73, 104143. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, P.; Tian, H. Recent updates in the polysaccharides-based Nano-biocarriers for drugs delivery and its application in diseases treatment: A review. Int. J. Biol. Macromol. 2021, 182, 115–128. [Google Scholar] [CrossRef]
- Plucinski, A.; Lyu, Z.; Schmidt, B.V.K.J. Polysaccharide nanoparticles: From fabrication to applications. J. Mater. Chem. B 2021, 9, 7030–7062. [Google Scholar] [CrossRef] [PubMed]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef] [PubMed]
- Babu, M.; Snyder, M. Multi-Omics Profiling for Health. Mol. Cell. Proteom. 2023, 22, 100561. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Cao, X.-Y.; Chen, F.; Zhu, Y.; Sun, D.-D.; Cheng, H.-B.; Duan, J.-A.; Su, S.-L. Xianlian Jiedu Decoction alleviates colorectal cancer by regulating metabolic profiles, intestinal microbiota and metabolites. Phytomedicine 2024, 128, 155385. [Google Scholar] [CrossRef]
- Kang, W.; Qiu, X.; Luo, Y.; Luo, J.; Liu, Y.; Xi, J.; Li, X.; Yang, Z. Application of radiomics-based multiomics combinations in the tumor microenvironment and cancer prognosis. J. Transl. Med. 2023, 21, 598. [Google Scholar] [CrossRef]
- Yang, S.; Qian, L.; Li, Z.; Li, Y.; Bai, J.; Zheng, B.; Chen, K.; Qiu, X.; Cai, G.; Wang, S.; et al. Integrated Multi-Omics Landscape of Liver Metastases. Gastroenterology 2023, 164, e17.407–e17.423. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Metabolomics and Metabolic Diseases: Where Do We Stand? Cell Metab. 2017, 25, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Tao, S.; Zhang, L.; Diao, L.-T.; Huang, X.; Huang, S.; Xie, S.-J.; Xiao, Z.-D.; Zhang, H. RNA sequencing: New technologies and applications in cancer research. J. Hematol. Oncol. 2020, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Casamassimi, A.; Federico, A.; Rienzo, M.; Esposito, S.; Ciccodicola, A. Transcriptome Profiling in Human Diseases: New Advances and Perspectives. Int. J. Mol. Sci. 2017, 18, 1652. [Google Scholar] [CrossRef]
- Cui, H.; Jin, Y.; Wang, N.; Liu, H.; Shu, R.; Wang, J.; Wang, X.; Jia, B.; Wang, Y.; Bian, Y.; et al. Mechanic evaluation of Wu-Mei-Pill on colitis-associated colorectal cancer: An integrated transcriptomics, metabolomics, and experimental validation study. Phytomedicine 2024, 128, 155509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Qiang, Y.; Wu, Y.; Tong, X.; Tie, Y.; Sun, Z.; Guan, S.; Xu, L.; Xu, P.; Li, X.; et al. Multi-omic approaches provide insights into the molecular mechanisms of Sojae semen germinatum water extract against overactive bladder. Food Res. Int. 2024, 175, 113746. [Google Scholar] [CrossRef]
- Klopfenstein, D.V.; Zhang, L.; Pedersen, B.S.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.J.; Yunes, J.M.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Meth. 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Leone, R.D.; Powell, J.D. Metabolism of immune cells in cancer. Nat. Rev. Cancer 2020, 20, 516–531. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.V.G.K.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40-41, 48–81. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Jarczak, D.; Nierhaus, A. Cytokine Storm—Definition, Causes, and Implications. Int. J. Mol. Sci. 2022, 23, 11740. [Google Scholar] [CrossRef]
- Opal, S.M.; DePalo, V.A. Anti-Inflammatory Cytokines. Chest 2000, 117, 1162–1172. [Google Scholar] [CrossRef]
- Koon, H.-K.; Lo, K.-W.; Leung, K.-N.; Lung, M.L.; Chang, C.C.-K.; Wong, R.N.-S.; Leung, W.-N.; Mak, N.-K. Photodynamic therapy-mediated modulation of inflammatory cytokine production by Epstein–Barr virus-infected nasopharyngeal carcinoma cells. Cell. Mol. Immunol. 2010, 7, 323–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Märkl, F.; Huynh, D.; Endres, S.; Kobold, S. Utilizing chemokines in cancer immunotherapy. Trends Cancer 2022, 8, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. The Chemokine Superfamily Revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Mollica Poeta, V.; Massara, M.; Capucetti, A.; Bonecchi, R. Chemokines and Chemokine Receptors: New Targets for Cancer Immunotherapy. Front. Immuno. 2019, 10, 379. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.T.; Ozga, A.J.; Servis, R.L.; Frederick, D.T.; Lo, J.A.; Fisher, D.E.; Freeman, G.J.; Boland, G.M.; Luster, A.D. Intratumoral Activity of the CXCR3 Chemokine System Is Required for the Efficacy of Anti-PD-1 Therapy. Immunity 2019, 50, e5.1498–e5.1512. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, e14.1022–e14.1037. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.J.; Chow, M.T.; Luster, A.D. Chemokines and the immune response to cancer. Immunity 2021, 54, 859–874. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Wang, S.; Agarwal, V.; Marcisak, E.F.; Zuo, A.; Jablonski, S.A.; Loth, M.; Fertig, E.J.; MacDougall, J.; Zhukovsky, E.; et al. DPP inhibition alters the CXCR3 axis and enhances NK and CD8+ T cell infiltration to improve anti-PD1 efficacy in murine models of pancreatic ductal adenocarcinoma. J. ImmunoTher. Cancer 2021, 9, e002837. [Google Scholar] [CrossRef] [PubMed]



| Gene Name | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| GAPDH | GGCAAATTCAACGGCACAGT | AGATGGTGATGGGCTTCCC |
| Ccl5 | GCATCCCTCACCGTCATCCTC | GCACTTGCTGCTGGTGTAAAA |
| Cxcl9 | AATGCACGATGCTCCTGCA | AGGTCTTTGAGGGATTTGTAGTGG |
| Cxcr3 | TACGATCAGCGCCTCAATGCCA | AGCAGGAAACCAGCCACTAGCT |
| Xcl1 | CTTTCCTGGGAGTCTGCTGC | CAGCCGCTGGGTTTGTAAGT |
| Xcr1 | AGAGACACCGAACAGTCAGGCT | TGTCCAGTTGCTGAAGGCTCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, S.; Wang, H.; Ji, Q.; Yang, Y.; Wei, G.; Li, R.; Zhou, B. Integration of Metabolomics and Transcriptomics to Reveal the Antitumor Mechanism of Dendrobium officinale Polysaccharide-Based Nanocarriers in Enhancing Photodynamic Immunotherapy in Colorectal Cancer. Pharmaceutics 2025, 17, 97. https://doi.org/10.3390/pharmaceutics17010097
Tao S, Wang H, Ji Q, Yang Y, Wei G, Li R, Zhou B. Integration of Metabolomics and Transcriptomics to Reveal the Antitumor Mechanism of Dendrobium officinale Polysaccharide-Based Nanocarriers in Enhancing Photodynamic Immunotherapy in Colorectal Cancer. Pharmaceutics. 2025; 17(1):97. https://doi.org/10.3390/pharmaceutics17010097
Chicago/Turabian StyleTao, Shengchang, Huan Wang, Qiufeng Ji, Yushan Yang, Gang Wei, Ruiming Li, and Benjie Zhou. 2025. "Integration of Metabolomics and Transcriptomics to Reveal the Antitumor Mechanism of Dendrobium officinale Polysaccharide-Based Nanocarriers in Enhancing Photodynamic Immunotherapy in Colorectal Cancer" Pharmaceutics 17, no. 1: 97. https://doi.org/10.3390/pharmaceutics17010097
APA StyleTao, S., Wang, H., Ji, Q., Yang, Y., Wei, G., Li, R., & Zhou, B. (2025). Integration of Metabolomics and Transcriptomics to Reveal the Antitumor Mechanism of Dendrobium officinale Polysaccharide-Based Nanocarriers in Enhancing Photodynamic Immunotherapy in Colorectal Cancer. Pharmaceutics, 17(1), 97. https://doi.org/10.3390/pharmaceutics17010097








