Fabrication and Characterization of Curcumin-Complexed Nanoparticles Using Coconut Protein Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Coconut Protein Concentrate
2.3. Coconut Protein Nanoparticle Formation
2.4. Scanning Electron Microscopy (SEM)
2.5. Preparation of Cur-Coconut Protein Nanoparticle Complexes
2.6. Measurement of Entrapment Efficiency (EE) and Loading Capacity (LC)
2.7. Fluorescence Spectroscopy Analysis
2.8. Particle Size, Polydispersity Index, and Zeta Potential Measurement
2.9. FT-IR Spectroscopy Methodology
2.10. X-ray Diffraction Spectroscopy (XRD)
2.11. ABTS Radical Scavenging Assay Method
2.12. In Vitro Controlled Release
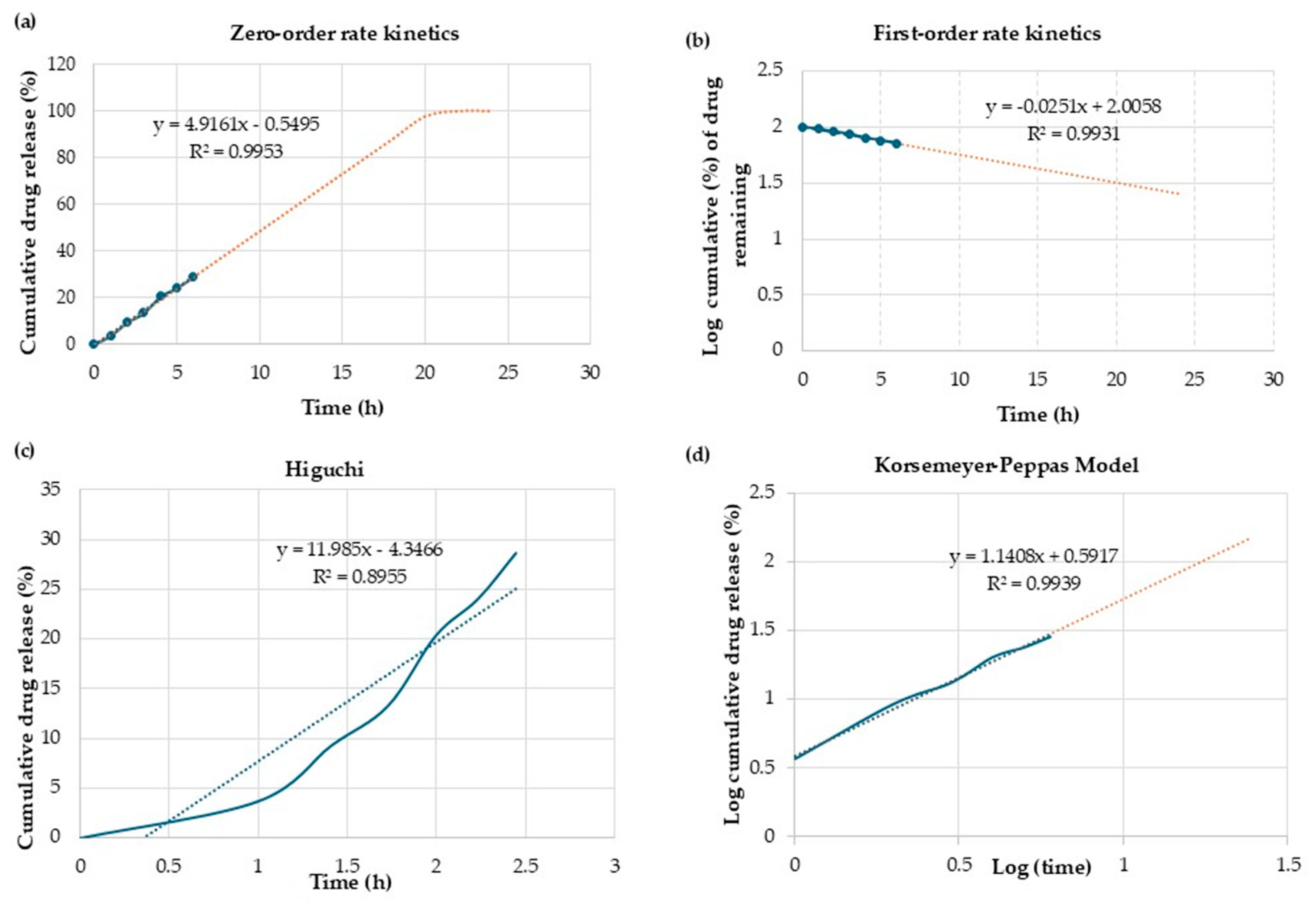
2.13. Mathematical Modeling and Drug Release Kinetics
- n ≤ 0.45 Fickian diffusion
- 0.45 < n< 0.89 Anomalous transport
- n > 0.89 Super case II transport mechanism (erosion)
2.14. Statistical Analysis
3. Results and Discussion
3.1. The Fabrication of Coconut Protein Nanoparticles and Characterization Using Scanning Electron Microscopy
3.2. Entrapment Efficiency (EE) and Loading Capacity (LC)
3.3. Fluorescence Spectroscopy
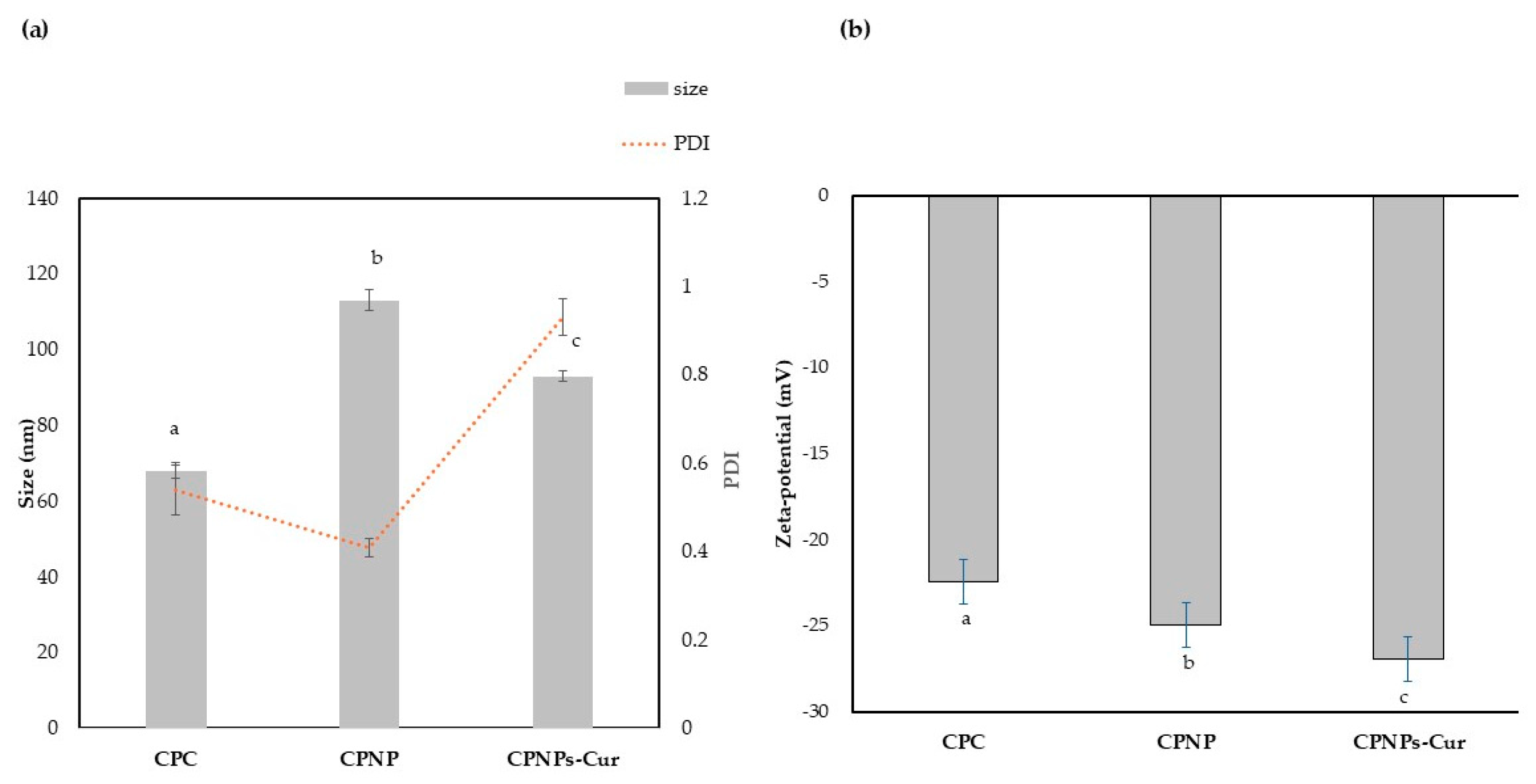
3.4. Average Particle Size and Polydispersity Index (PDI) and Zeta Potential
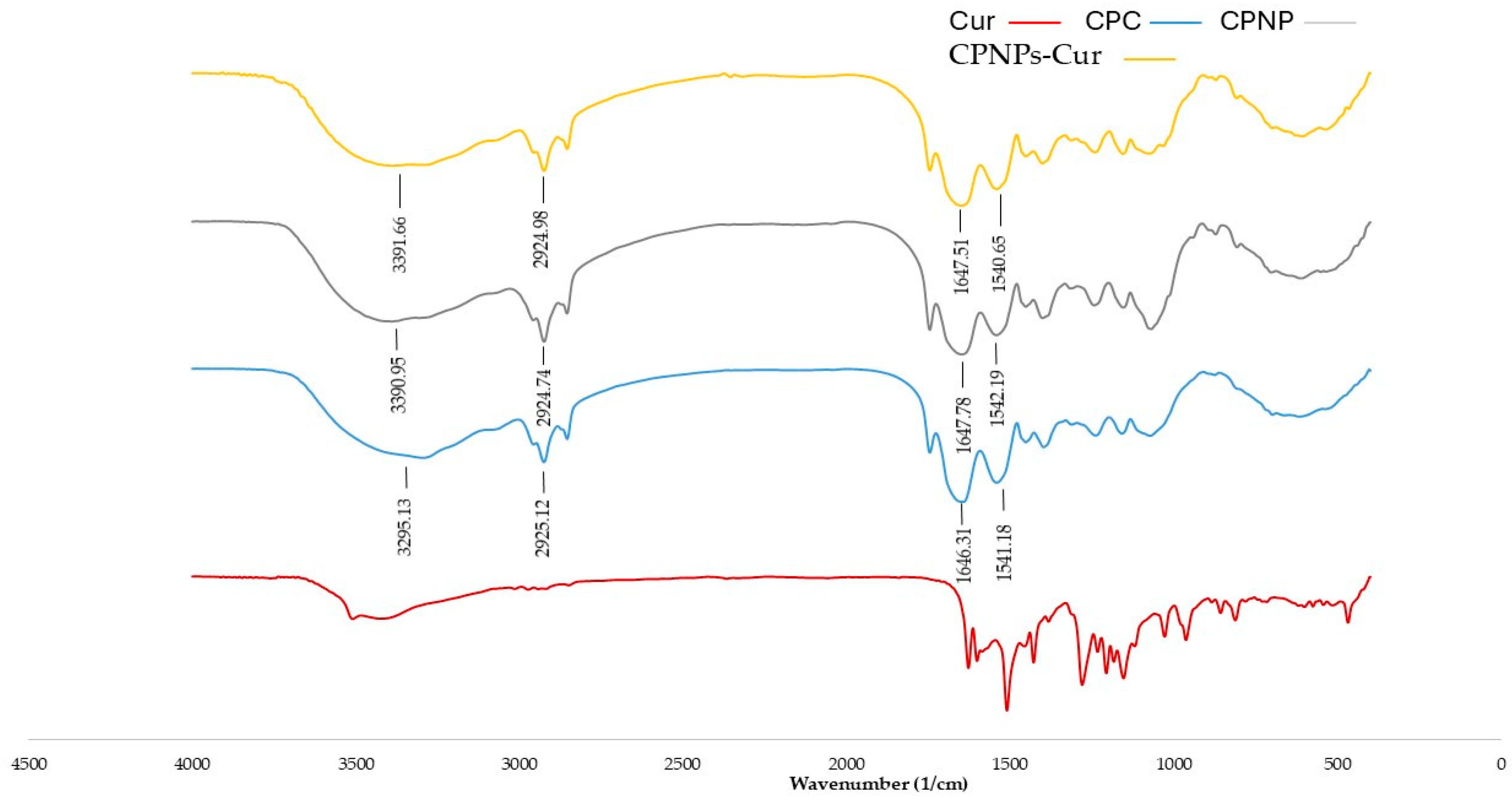
3.5. FT-IR Spectroscopy
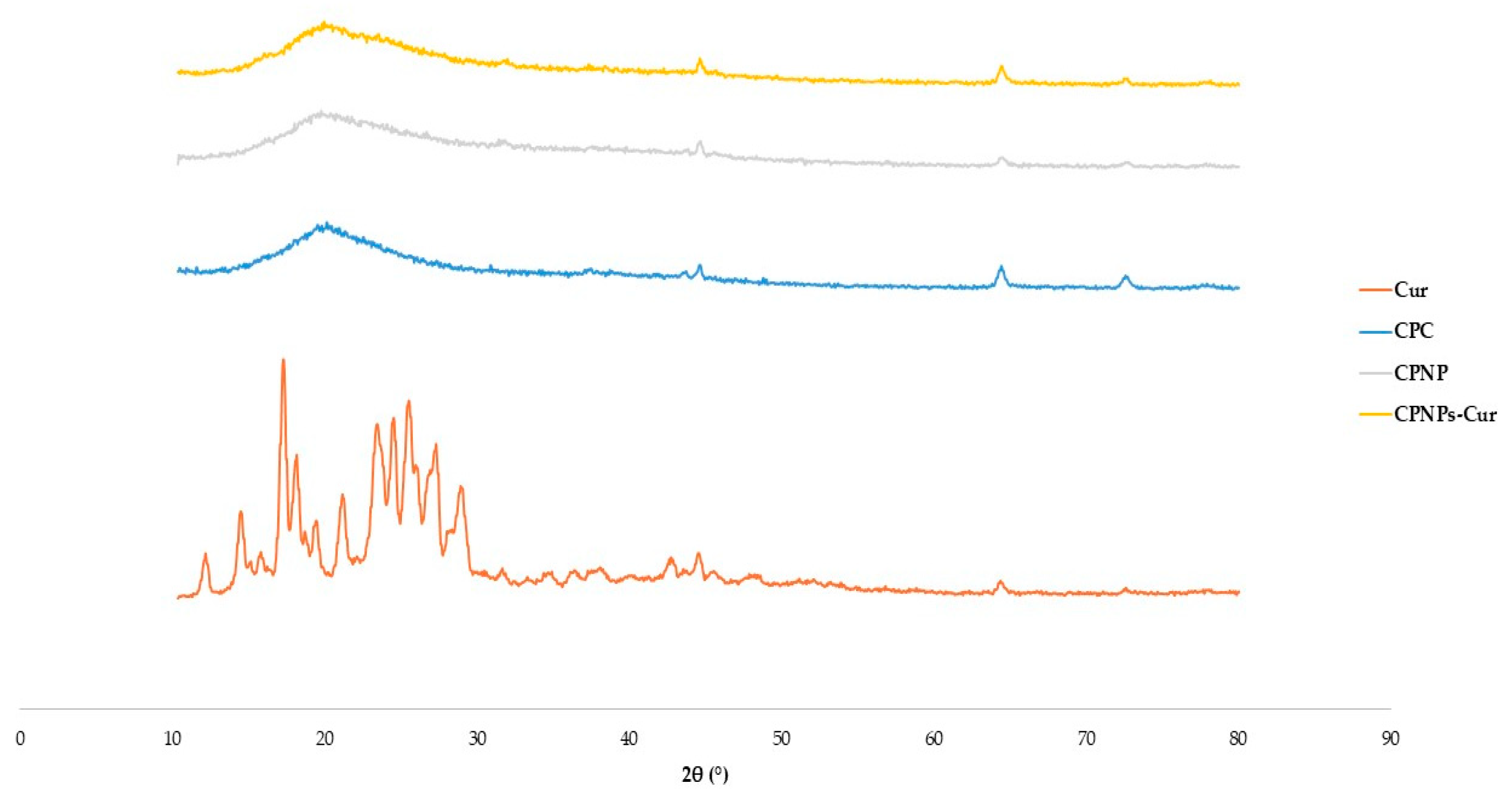
3.6. X-ray Diffraction Analysis
3.7. ABTS Antiradical Activity
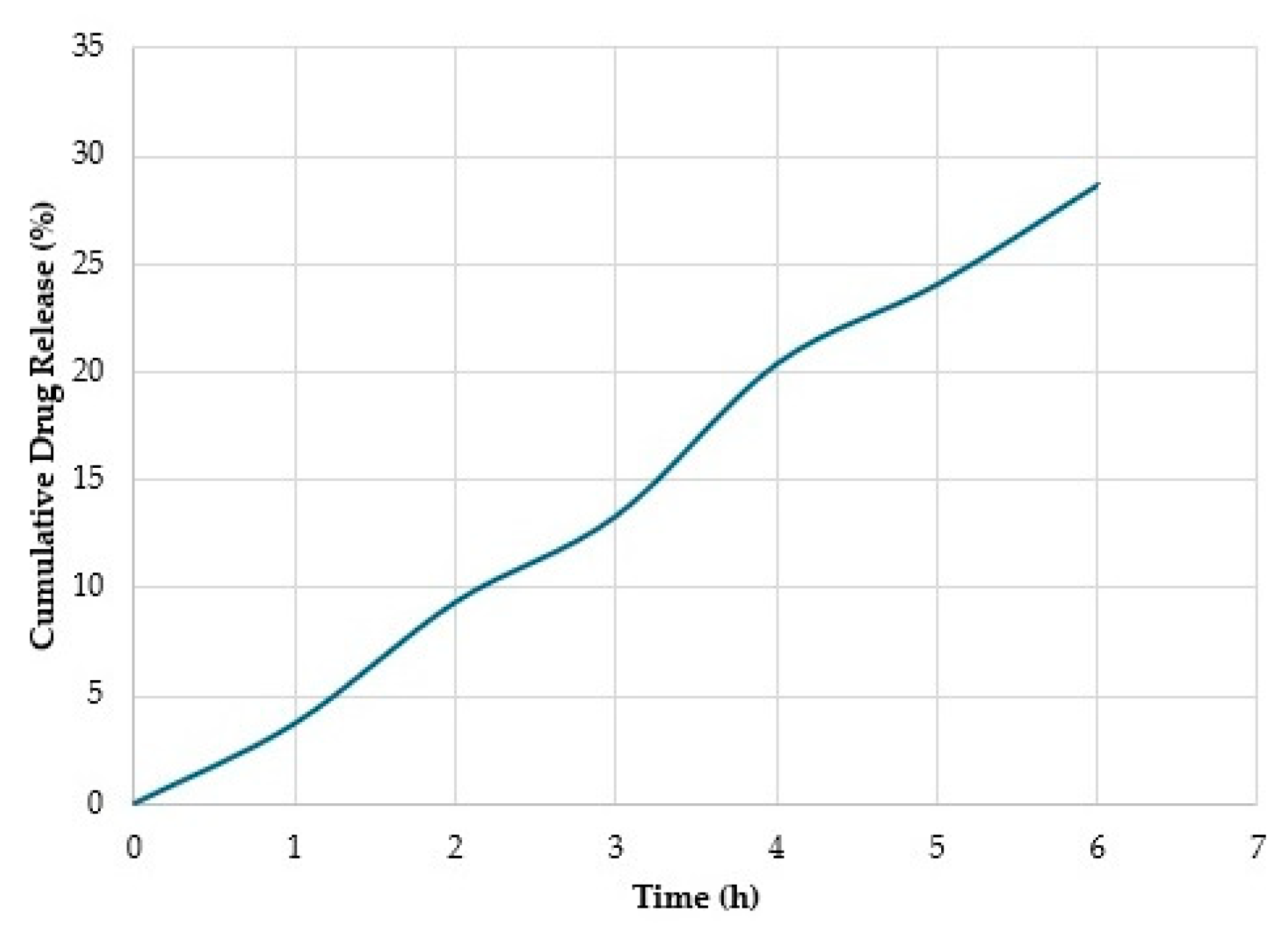
3.8. In Vitro Release Studies
3.9. Mathematical Modeling and Drug Release Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of Cur in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]
- Tang, C.H. Nanocomplexation of proteins with Cur: From interaction to nanoencapsulation (A review). Food Hydrocoll. 2020, 109, 106106. [Google Scholar] [CrossRef]
- Peng, H.; Gan, Z.; Xiong, H.; Luo, M.; Yu, N.; Wen, T.; Wang, R.; Li, Y. Self-assembly of protein nanoparticles from rice bran waste and their use as delivery system for Cur. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating Cur. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Wei, X.Q.; Ba, K. Construction a long-circulating delivery system of liposomal curcumin by coating albumin. ACS Omega 2020, 5, 16502–16509. [Google Scholar] [CrossRef]
- Marcon, H.; Griss, L.G.; Molosse, V.L.; Cecere, B.G.; Alba, D.F.; Leal, K.W.; Galli, G.; Souza, C.F.; Baldissera, M.D.; Gundel, S.; et al. Dietary supplementation with Cur-loaded nanocapsules in lambs: Nanotechnology as a new tool for nutrition. Anim. Nutr. 2021, 7, 521–529. [Google Scholar] [CrossRef]
- Barros, C.H.; Hiebner, D.W.; Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Synthesis and self-assembly of Cur-modified amphiphilic polymeric micelles with antibacterial activity. J. Nanobiotechnol. 2021, 19, 104. [Google Scholar] [CrossRef]
- Liu, K.; Zha, X.Q.; Li, Q.M.; Pan, L.H.; Luo, J.P. Hydrophobic interaction and hydrogen bonding driving the self-assembling of quinoa protein and flavonoids. Food Hydrocoll. 2021, 118, 106807. [Google Scholar] [CrossRef]
- Mehryar, L.; Esmaiili, M.; Zeynali, F.; Imani, M.; Sadeghi, R. Fabrication and characterization of sunflower protein isolate nanoparticles, and their potential for encapsulation and sustainable release of curcumin. Food Chem. 2021, 355, 129572. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gulati, P.; Santra, D.; Rose, D.; Zhang, Y. Nanoparticles prepared by proso millet protein as novel Cur delivery system. Food Chem. 2018, 240, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Lonnie, M.; Johnstone, A.M. The public health rationale for promoting plant protein as an important part of a sustainable and healthy diet. Nutr. Bull. 2020, 45, 281–293. [Google Scholar] [CrossRef]
- Xu, G.; Li, L.; Bao, X.; Yao, P. Curcumin, casein and soy polysaccharide ternary complex nanoparticles for enhanced dispersibility, stability and oral bioavailability of curcumin. Food Biosci. 2020, 35, 100569. [Google Scholar] [CrossRef]
- Ren, J.; Wu, H.; Lu, Z.; Qin, Q.; Jiao, X.; Meng, G.; Liu, W.; Li, G. pH-driven preparation of pea protein isolate-Cur nanoparticles effectively enhances antitumor activity. Int. J. Biol. Macromol. 2024, 256, 128383. [Google Scholar] [CrossRef]
- Qiao, X.; Liu, F.; Kong, Z.; Yang, Z.; Dai, L.; Wang, Y.; Sun, Q.; Mc Celement, D.J.; Xu, X. Pickering emulsion gel stabilized by pea protein nanoparticle induced by heat-assisted pH-shifting for Cur delivery. J. Food Eng. 2023, 350, 111504. [Google Scholar] [CrossRef]
- Kong, Z.; Li, Z.; Zhang, L.; Dai, L.; Wang, Y.; Sun, Q.; McClements, D.J.; Cheng, Y.; Zhang, Z.; Wang, C.; et al. Development of pea protein nanoparticle/hydrolyzed rice glutelin fibril emulsion gels for encapsulation of curcumin. Int. J. Biol. Macromol. 2024, 276, 133640. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Xu, J.; Ji, F.; Luo, S.; Zhong, X.; Zhao, Y.; Zheng, Z. Self-assembled pea vicilin nanoparticles as nanocarriers for improving the antioxidant activity, environmental stability and sustained-release property of curcumin. J. Sci. Food Agric. 2024, 104, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Meiguni, M.S.M.; Salami, M.; Rezaei, K.; Aliyari, M.A.; Ghaffari, S.B.; Emam-Djomeh, Z.; Kennedy, J.F.; Ghasemi, A. Fabrication and characterization of a succinyl mung bean protein and arabic gum complex coacervate for Cur encapsulation. Int. J. Biol. Macromol. 2023, 224, 170–180. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Moghadam, M.; Amirsalehi, A.; Emam-Djomeh, Z. Mung bean protein as a promising biopolymeric vehicle for loading of Cur: Structural characterization, antioxidant properties, and in vitro release kinetics. J. Drug Deliv. Sci. Technol. 2021, 61, 102148. [Google Scholar] [CrossRef]
- Onsaard, E.; Vittayanont, M.; Srigam, S.; McClements, D.J. Comparison of properties of oil-in-water emulsions stabilized by coconut cream proteins with those stabilized by whey protein isolate. Food Res. Int. 2006, 39, 78–86. [Google Scholar] [CrossRef]
- Chambal, B.; Bergenståhl, B.; Dejmek, P. Edible proteins from coconut milk press cake; one step alkaline extraction and characterization by electrophoresis and mass spectrometry. Food Res. Int. 2012, 47, 146–151. [Google Scholar] [CrossRef]
- Salil, G.; Nevin, K.G.; Rajamohan, T. Arginine rich coconut kernel protein modulates diabetes in alloxan treated rats. Chem.-Biol. Interact. 2011, 189, 107–111. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Xu, J.; Gao, G. Antioxidant activity of coconut (Cocos nucifera L.) protein fractions. Molecules 2018, 23, 707. [Google Scholar] [CrossRef]
- Ma, J.; Pan, C.; Chen, H.; Chen, W.; Pei, J.; Zhang, M.; Zhong, Q.; Chen, W.; Zeng, G. Effects of protein concentration, ionic strength, and heat treatment on the interfacial and emulsifying properties of coconut (Cocos nucifera L.) globulins. Food Chem. X 2023, 20, 100984. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, M.; Shen, Y. Effects of ultrasound combined with preheating treatment to improve the thermal stability of coconut milk by modifying the physicochemical properties of coconut protein. Foods 2022, 11, 1042. [Google Scholar] [CrossRef]
- Kurniawati, A.D.; Hidayat, C.; Setiowati, A.D. Formation of Coconut Oil By–Product Protein Concentrate–Pectin Through Electrostatic Interaction to Improve Emulsifying Properties. AgriHealth J. Agri-Food Nutr. Public Health 2023, 4, 1–13. [Google Scholar] [CrossRef]
- Meenmanee, S.; Rattananukrom, A.; Thaiphanit, S.; Suppavorasatit, I. Improvement of solubility, foaming, and emulsification properties of coconut (Cocos nucifera L.) protein by non-enzymatic deamidation. LWT 2022, 153, 112493. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Jiang, L.; Yang, Z.; Fang, Y.; Zhang, W. Shear emulsification condition strategy impact high internal phase Pickering emulsions stabilized by coconut globulin-tannic acid: Structure of protein at the oil-water interface. LWT 2023, 187, 115283. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Jiang, L.; Huang, Z.; Zhang, W.; Yun, Y. Improvement of emulsifying stability of coconut globulin by noncovalent interactions with coffee polyphenols. Food Chem. X 2023, 20, 100954. [Google Scholar] [CrossRef] [PubMed]
- Rodsamran, P.; Sothornvit, R. Bioactive coconut protein concentrate films incorporated with antioxidant extract of mature coconut water. Food Hydrocoll. 2018, 79, 243–252. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Physicochemical and functional properties of protein concentrate from by-product of coconut processing. Food Chem. 2018, 241, 364–371. [Google Scholar] [CrossRef]
- Yan, J.; Jia, X.; Qu, Y.; Yan, W.; Li, Y.; Yin, L. Development of sorghum arabinoxylan-soy protein isolate composite nanoparticles for delivery of curcumin: Effect of polysaccharide content on stability and in vitro digestibility. Int. J. Biol. Macromol. 2024, 262, 129867. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Zhao, H.; Fu, S.; Li, R.; Wang, Z.; Wang, Y.; Lu, W.; Yang, X. Nanoparticle encapsulation using self-assembly abietic acid to improve oral bioavailability of curcumin. Food Chem. 2024, 436, 137676. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, I.F.; Pamunuwa, G.K.; Karunaratne, D.N. Efficacy of alginate and chickpea protein polymeric matrices in encapsulating curcumin for improved stability, sustained release and bioaccessibility. Food Hydrocoll. Health 2023, 3, 100119. [Google Scholar] [CrossRef]
- Blanco-Padilla, A.; López-Rubio, A.; Loarca-Piña, G.; Gómez-Mascaraque, L.G.; Mendoza, S. Characterization, release and antioxidant activity of Cur-loaded amaranth-pullulan electrospun fibers. LWT-Food Sci. Technol. 2015, 63, 1137–1144. [Google Scholar] [CrossRef]
- Kulandaivelu, K.; Mandal, A.K.A. Improved bioavailability and pharmacokinetics of tea polyphenols by encapsulation into gelatin nanoparticles. IET Nanobiotechnol. 2017, 11, 469–476. [Google Scholar] [CrossRef]
- Yuan, D.; Zhou, F.; Shen, P.; Zhang, Y.; Lin, L.; Zhao, M. Self-assembled soy protein nanoparticles by partial enzymatic hydrolysis for pH-driven encapsulation and delivery of hydrophobic cargo curcumin. Food Hydrocoll. 2021, 120, 106759. [Google Scholar] [CrossRef]
- Perović, M.N.; Knežević Jugović, Z.D.; Antov, M.G. Heat-induced nanoparticles from pumpkin leaf protein for potential application as β-carotene carriers. Future Foods 2024, 9, 100310. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef]
- He, W.; Tian, L.; Zhang, S.; Pan, S. A novel method to prepare protein-polysaccharide conjugates with high grafting and low browning: Application in encapsulating Cur. LWT 2021, 145, 111349. [Google Scholar] [CrossRef]
- Asadi, M.; Salami, M.; Hajikhani, M.; Emam-Djomeh, Z.; Aghakhani, A.; Ghasemi, A. Electrospray production of Cur-walnut protein nanoparticles. Food Biophys. 2021, 16, 15–26. [Google Scholar] [CrossRef]
- Sneharani, A.H. Cur–sunflower protein nanoparticles—A potential antiinflammatory agent. J. Food Biochem. 2019, 43, e12909. [Google Scholar] [CrossRef]
- Xu, P.; Qian, Y.; Wang, R.; Chen, Z.; Wang, T. Entrapping Cur in the hydrophobic reservoir of rice proteins toward stable antioxidant nanoparticles. Food Chem. 2022, 387, 132906. [Google Scholar] [CrossRef] [PubMed]
- Du, C.X.; Xu, J.J.; Luo, S.Z.; Li, X.J.; Mu, D.D.; Jiang, S.T.; Zheng, Z. Low-oil-phase emulsion gel with antioxidant properties prepared by soybean protein isolate and curcumin composite nanoparticles. LWT 2022, 161, 113346. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Ren, F.; Liu, N.; Tong, Y.; Li, Y.; Liu, A.; Wu, L.; Wang, P. Delivery of curcumin using Zein-Gum Arabic-Tannic Acid composite particles: Fabrication, characterization, and In Vitro release properties. Front. Nutr. 2022, 9, 842850. [Google Scholar] [CrossRef]
- Guo, Q.; Bayram, I.; Zhang, W.; Su, J.; Shu, X.; Yuan, F.; Mao, L.; Gao, Y. Fabrication and characterization of Cur-loaded pea protein isolate-surfactant complexes at neutral pH. Food Hydrocoll. 2021, 111, 106214. [Google Scholar] [CrossRef]
- Yan, S.; Wang, Q.; Zhang, S.; Huang, Y.; Zhu, H.; Qi, B.; Li, Y. Oxidized dextran improves the stability and effectively controls the release of Cur loaded in soybean protein nanocomplexes. Food Chem. 2024, 431, 137089. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Rong, S.; Guo, T.; Zhang, R.; Xu, D.; Han, Y.; Liu, F.; Su, J.; Hu, H.; Chen, S. Fabrication of compact zein-chondroitin sulfate nanocomplex by anti-solvent co-precipitation: Prevent degradation and regulate release of curcumin. Food Chem. 2024, 430, 137110. [Google Scholar] [CrossRef]
- Cheng, Y.; Chen, X.; Lin, Q.; Wang, J.; Chen, Y.; Mo, H.; Chen, M.; Xu, Z.; Wen, L.; Jiao, Y. Improvement effect of different polysaccharides on the functional properties of Cur-loaded rice protein hydrolysate nanoparticles. LWT 2024, 204, 116471. [Google Scholar] [CrossRef]
- Weng, Q.; Cai, X.; Zhang, F.; Wang, S. Fabrication of self-assembled Radix Pseudostellariae protein nanoparticles and the entrapment of Cur. Food Chem. 2019, 274, 796–802. [Google Scholar] [CrossRef]
- Vijayan, U.K.; Shah, N.N.; Muley, A.B.; Singhal, R.S. Complexation of Cur using proteins to enhance aqueous solubility and bioaccessibility: Pea protein vis-à-vis whey protein. J. Food Eng. 2021, 292, 110258. [Google Scholar] [CrossRef]
- Dai, L.; Wei, Y.; Sun, C.; Mao, L.; McClements, D.J.; Gao, Y. Development of protein-polysaccharide-surfactant ternary complex particles as delivery vehicles for Cur. Food Hydrocoll. 2018, 85, 75–85. [Google Scholar] [CrossRef]
- Dong, H.; Wang, P.; Yang, Z.; Li, R.; Xu, X.; Shen, J. Dual improvement in Cur encapsulation efficiency and lyophilized complex dispersibility through ultrasound regulation of Cur–protein assembly. Ultrason. Sonochem. 2022, 90, 106188. [Google Scholar] [CrossRef]
- Ghobadi, M.; Koocheki, A.; Varidi, M.J.; Varidi, M. Encapsulation of Cur using Grass pea (Lathyrus sativus) protein isolate/Alyssum homolocarpum seed gum complex nanoparticles. Innov. Food Sci. Emerg. Technol. 2021, 72, 102728. [Google Scholar] [CrossRef]
- Du, X.; Jing, H.; Wang, L.; Huang, X.; Mo, L.; Bai, X.; Wang, H. pH-shifting formation of goat milk casein nanoparticles from insoluble peptide aggregates and encapsulation of curcumin for enhanced dispersibility and bioactivity. LWT 2022, 154, 112753. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.K.; Ling, J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for Cur encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Momen, S.; Alavi, F.; Emam-Djomeh, Z.; Moosavi-Movahedi, A. Enhancing the aqueous solubility of curcumin at acidic condition through the complexation with whey protein nanofibrils. Food Hydrocoll. 2019, 87, 902–914. [Google Scholar] [CrossRef]
- Xie, H.; Xiang, C.; Li, Y.; Wang, L.; Zhang, Y.; Song, Z.; Ma, X.; Lu, X.; Lei, Q.; Fang, W. Fabrication of ovalbumin/κ-carrageenan complex nanoparticles as a novel carrier for Cur delivery. Food Hydrocolloid 2019, 89, 111–121. [Google Scholar] [CrossRef]
- Dai, L.; Sun, C.; Wei, Y.; Zhan, X.; Mao, L.; Gao, Y. Formation and characterization of zein-propylene glycol alginate-surfactant ternary complexes: Effect of surfactant type. Food Chem. 2018, 258, 321–330. [Google Scholar] [CrossRef]
- Qiao, X.; Jiang, Y.; Duan, R.; Li, Z.; Kong, Z.; Zhang, L.; Dai, L.; Wang, Y.; Sun, Q.; Mc Celement, D.J.; et al. Fabrication of Cur-loaded pea protein isolate-quillaja saponin-tannic acid self-assembled nanoparticles by tuning non-covalent interactions: Enhanced physicochemical, interfacial and emulsifying properties. Food Hydrocoll. 2024, 148, 109436. [Google Scholar] [CrossRef]
- Yuan, D.; Qin, L.; Niu, Z.; Zhou, F.; Zhao, M. Maintained particulate integrity of soy protein nanoparticles during gastrointestinal digestion via genipin crosslinking enhancing stability and bioavailability of curcumin. Int. J. Biol. Macromol. 2024, 274, 133213. [Google Scholar] [CrossRef]
- Xie, Y.; Gong, X.; Jin, Z.; Xu, W.; Zhao, K. Cur encapsulation in self-assembled nanoparticles based on amphiphilic palmitic acid-grafted-quaternized chitosan with enhanced cytotoxic, antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2022, 222, 2855–2867. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, Y.; Lin, H.; Zhang, H.; Zhang, W.; He, J. Ternary nanoparticles of sodium caseinate-zein-hexaglycerol monooleate: Fabrication, characterization, and Cur encapsulation. LWT 2024, 203, 116376. [Google Scholar] [CrossRef]
- Niu, F.; Hu, D.; Gu, F.; Du, Y.; Zhang, B.; Ma, S.; Pan, W. Preparation of ultra-long stable ovalbumin/sodium carboxymethylcellulose nanoparticle and loading properties of Cur. Carbohydr. Polym. 2021, 271, 118451. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, D.; Shen, P.; Zhou, F.; Zhao, Q.; Zhao, M. pH-Driven formation of soy peptide nanoparticles from insoluble peptide aggregates and their application for hydrophobic active cargo delivery. Food Chem. 2021, 355, 129509. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Shang, M.; Ji, H.; Li, X.; Sang, S.; Jiao, A.; Jin, Z.; Qiu, C. The construction of whey protein-coated OSA debranched starch particles used for Cur steady-state delivery and pH-sensitive sustained release. Food Hydrocoll. 2024, 147, 109425. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Zou, Y.; Liang, X.; Peng, Y.; McClements, D.J.; Hu, K. Encapsulation of resveratrol in zein/pectin core-shell nanoparticles: Stability, bioaccessibility, and antioxidant capacity after simulated gastrointestinal digestion. Food Hydrocoll. 2019, 93, 261–269. [Google Scholar] [CrossRef]
- Gupta, S.; Ghoshal, G. Plant protein hydrogel as a delivery system of curcumin: Characterization and in vitro release Kinetics. Food Bioprod. Process. 2024, 143, 66–79. [Google Scholar] [CrossRef]
- Ganguly, S.C.; Mahanti, B.; Ganguly, S.; Majumdar, S. Bovine serum albumin as a nanocarrier for efficient encapsulation of hydrophobic garcinol-A strategy for modifying the in vitro drug release kinetics. Int. J. Biol. Macromol. 2024, 278, 134651. [Google Scholar] [CrossRef] [PubMed]
- Jenifer, J.; Upputuri, R.T.P. In Vitro release mechanism and cytotoxic behavior of Cur loaded casein nanoparticles. Braz. J. Pharm. Sci. 2022, 58, e19801. [Google Scholar] [CrossRef]
- Türkeş, E.; Açıkel, Y.S. Folic acid-conjugated cancer drug Cur-loaded albumin nanoparticles: Investigation of Cur release kinetics. J. Drug Deliv. Sci. Technol. 2024, 91, 105178. [Google Scholar] [CrossRef]



| Plant Protein Source | Curcumin-Loaded Nanoparticle Size (nm) | EE (%) | LC (%) | References |
|---|---|---|---|---|
| Coconut protein nanoparticles | 94 | 96.6 | 1.932 | (This study) |
| Soybean protein nanoparticle (coating by dextran dialdehyde) | 199.2 | 92.37 | 0.924 | [46] |
| Zein (complexed with Chondroitin sulfate) | 94.7 | 3.8 | [47] | |
| Pea protein nanoparticle | 867.4 | 93 | 0.32 | [33] |
| Pea protein nanoparticle (using different surfactants) | 160.23–191.20 | 89.91–93.69 | ~0.5–1.5 | [45] |
| Rice protein hydrolysate | 132.16–150.28 | 55.5 | 5.6 | [48] |
| Rice bran waste | 384 | 93.56 | 26.20 | [3] |
| Polyelectrolytic complex of acylated rapeseed cruciferin and chitosan | 217–454 | 72–79 | 5.4–6.2 | [33] |
| Sunflower protein nanoparticle | 174.64 | 95.4 | [9] |
| Sample | Zero-Order | First-Order | Higuchi | Korsmeyer Peppas | |
|---|---|---|---|---|---|
| R2 | R2 | R2 | R2 | n | |
| CPNP-Cur | 0.99 | 0.99 | 0.89 | 0.88 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziaeifar, L.; Salami, M.; Askari, G.; Emam-Djomeh, Z.; Loebenberg, R.; Serpe, M.J.; Davies, N.M. Fabrication and Characterization of Curcumin-Complexed Nanoparticles Using Coconut Protein Nanoparticles. Pharmaceutics 2025, 17, 1247. https://doi.org/10.3390/pharmaceutics17101247
Ziaeifar L, Salami M, Askari G, Emam-Djomeh Z, Loebenberg R, Serpe MJ, Davies NM. Fabrication and Characterization of Curcumin-Complexed Nanoparticles Using Coconut Protein Nanoparticles. Pharmaceutics. 2025; 17(10):1247. https://doi.org/10.3390/pharmaceutics17101247
Chicago/Turabian StyleZiaeifar, Leila, Maryam Salami, Gholamreza Askari, Zahra Emam-Djomeh, Raimar Loebenberg, Michael J Serpe, and Neal M. Davies. 2025. "Fabrication and Characterization of Curcumin-Complexed Nanoparticles Using Coconut Protein Nanoparticles" Pharmaceutics 17, no. 10: 1247. https://doi.org/10.3390/pharmaceutics17101247
APA StyleZiaeifar, L., Salami, M., Askari, G., Emam-Djomeh, Z., Loebenberg, R., Serpe, M. J., & Davies, N. M. (2025). Fabrication and Characterization of Curcumin-Complexed Nanoparticles Using Coconut Protein Nanoparticles. Pharmaceutics, 17(10), 1247. https://doi.org/10.3390/pharmaceutics17101247










