Unique Features and Collateral Immune Effects of mRNA-LNP COVID-19 Vaccines: Plausible Mechanisms of Adverse Events and Complications
Abstract
1. Introduction
2. Essentials of Natural Immunogenicity and the Mode of Action of Conventional Vaccines Versus mRNA-LNP
3. The Rise, Impact, and Reassessment of the mRNA-LNP Vaccine Platform
4. Distinct Structural Features of mRNA-LNP Vaccines Contributing to Collateral Immune Effects and Adverse Phenomena
4.1. Ribosomal Translation May Alter Antigen Fate and Function
4.1.1. Diversification of SP Processing and Presentation
4.1.2. Proteasome Digestion, Resulting in Cross-Presentation of SP Peptides on Class I Molecules Triggering Autoimmunity
4.1.3. Spike Protein Secretion
4.1.4. Spike Protein Expression on Cell Surfaces
4.1.4.1. Antibody-Dependent Cellular Cytotoxicity
4.1.4.2. Complement Activation
4.1.4.3. Antigen-Independent Polyclonal T Cell Activation Due to Superantigen Activity of the SP
4.1.5. Exosomal Excretion of the Spike Protein
4.1.6. Excessive Somatic Hypermutation in B Cells
4.1.7. Polymorphism Due to Frameshift Mutation
4.1.8. Reverse Transcription of the mRNA with Insertion Mutagenesis
4.2. The Spike Protein Can Cause Systemic Toxicity
4.2.1. The Structure and Toxicities of the Spike Protein
4.2.2. Vaccinology Perspectives of Spike Protein Toxicity
4.3. Multiple Chemical Modifications of the mRNA and SP Increase Their Function and Stability
4.3.1. Replacement of mRNA Uridine with Pseudouridine (ψ)
4.3.2. Codon Optimization
4.3.3. Methylation of the 5′ Cap
4.3.4. UTR Stabilization
4.3.5. Poly(A) Tail Optimization
4.3.6. GC Enrichment of the mRNA
4.3.7. Proline Enrichment of the Spike Protein
4.4. The LNP Is a Strong Stimulant of Innate Immune Responses, Also Enabling mRNA Transfection
4.4.1. Activation of the Cellular Arm of Innate Immunity
4.4.2. Triggering of Humoral Immune Response: Complement Activation
4.4.3. The LNP Is a Superadjuvant
4.4.4. The LNP Is a Fusogenic Transfecting Agent
4.5. The PEG on the LNP Surface Is Immune Reactive and Immunogenic
4.5.1. True and Pseudoallergic Reactogenicity
4.5.2. Anti-PEG Immunogenicity
4.6. The LNP Is Unstable in Water
4.7. The Injectable Vaccines May Contain Contaminations with Plasmid DNA and Inorganic Elements
5. Turbo Cancer
6. Outlook
6.1. The Pendulum Swing of mRNA Vaccines
6.2. Novel Medical Perspectives from the mRNA Vaccine Era
6.3. A Unifying Message Amidst Abundant Data and Conclusions
6.4. Recommendations for the Future
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center of Disease Control and Prevention (CDC): Our World in Data. Available online: https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer?country=European+Union~HUN~USA (accessed on 22 September 2025).
- Krumholz, H.M.; Wu, Y.; Sawano, M.; Shah, R.; Zhou, T.; Arun, A.S.; Khosla, P.; Kaleem, S.; Vashist, A.; Bhattacharjee, B.; et al. Post-Vaccination Syndrome: A Descriptive Analysis of Reported Symptoms and Patient Experiences after COVID-19 Immunization. medRxiv 2023, 10, 1–32. [Google Scholar] [CrossRef]
- Palmer, M.; Bhakdi, S.; Hooker, B.; Holland, M.; DesBois, M.; Rasnick, D.; Fitts, C.A. mRNA Vaccine Toxicity. D4ce.Org. Available online: https://d4ce.org/mRNA-vaccine-toxicity/ (accessed on 22 September 2025).
- Costa, C.; Moniati, F. The Epidemiology of COVID-19 Vaccine-Induced Myocarditis. Adv. Med. 2024, 2024, 4470326. [Google Scholar] [CrossRef]
- Novak, N.; Tordesillas, L.; Cabanillas, B. Adverse Rare Events to Vaccines for COVID-19: From Hypersensitivity Reactions to Thrombosis and Thrombocytopenia. Int. Rev. Immunol. 2022, 41, 438–447. [Google Scholar] [CrossRef]
- Oueijan, R.I.; Hill, O.R.; Ahiawodzi, P.D.; Fasinu, P.S.; Thompson, D.K. Rare Heterogeneous Adverse Events Associated with mRNA-Based COVID-19 Vaccines: A Systematic Review. Medicines 2022, 9, 43. [Google Scholar] [CrossRef]
- Padilla-Flores, T.; Sampieri, A.; Vaca, L. Incidence and Management of the Main Serious Adverse Events Reported after COVID-19 Vaccination. Pharmacol. Res. Perspect. 2024, 12, e1224. [Google Scholar] [CrossRef]
- Law, B. Priority List of COVID-19 Adverse Events of Special Interest: Quarterly Update December 2020. Safety Platform for Emergency Vaccines (SPEAC). Available online: https://brightoncollaboration.org/wp-content/uploads/2023/08/SO2_D2.1.2_V1.2_COVID-19_AESI-update_V1.3-1.pdf (accessed on 22 September 2025).
- Fraiman, J.; Erviti, J.; Jones, M.; Greenland, S.; Whelan, P.; Kaplan, R.M.; Doshi, P. Serious Adverse Events of Special Interest Following mRNA COVID-19 Vaccination in Randomized Trials in Adults. Vaccine 2022, 40, 5798–5805. [Google Scholar] [CrossRef]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 Vaccines and Adverse Events of Special Interest: A Multinational Global Vaccine Data Network (GVDN) Cohort Study of 99 Million Vaccinated Individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef]
- Slater, K.M.; Buttery, J.P.; Crawford, N.W.; Cheng, D.R. Letter to the Editor: A Comparison of Post-COVID Vaccine Myocarditis Classification Using the Brighton Collaboration Criteria Versus (United States) Centers for Disease Control Criteria: An Update. Commun. Dis. Intell. 2024, 48. [Google Scholar] [CrossRef]
- Levitan, B.; Hadler, S.C.; Hurst, W.; Izurieta, H.S.; Smith, E.R.; Baker, N.L.; Bauchau, V.; Chandler, R.; Chen, R.T.; Craig, D.; et al. The Brighton Collaboration Standardized Module for Vaccine Benefit-Risk Assessment. Vaccine 2024, 42, 972–986. [Google Scholar] [CrossRef]
- Szebeni, J. Expanded Spectrum and Increased Incidence of Adverse Events Linked to COVID-19 Genetic Vaccines: New Concepts on Prophylactic Immuno-Gene Therapy, Iatrogenic Orphan Disease, and Platform-Inherent Challenges. Pharmaceutics 2025, 17, 450. [Google Scholar] [CrossRef]
- Wucher, M.B.; Bridle, B.; Hatfill, S. COVID-19 mRNA “Vaccine” Harms Research Collection (Version 2). In Toxic Shot: Facing the Dangers of the COVID “Vaccines”; Sass, E., Ed.; Zenodo: Geneva, Switzerland, 2025. [Google Scholar] [CrossRef]
- Szebeni, J.; Kiss, B.; Bozo, T.; Turjeman, K.; Levi-Kalisman, Y.; Barenholz, Y.; Kellermayer, M. Insights into the Structure of Comirnaty COVID-19 Vaccine: A Theory on Soft, Partially Bilayer-Covered Nanoparticles with Hydrogen Bond-Stabilized mRNA-Lipid Complexes. ACS Nano 2023, 17, 13147–13157. [Google Scholar] [CrossRef] [PubMed]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of Antigen Processing. Annu. Rev. Immunol. 2013, 31, 443–473. [Google Scholar] [CrossRef]
- Sarri, C.A.; Papadopoulos, G.E.; Papa, A.; Tsakris, A.; Pervanidou, D.; Baka, A.; Politis, C.; Billinis, C.; Hadjichristodoulou, C.; Malwest Project; et al. Amino Acid Signatures in the Hla Class Ii Peptide-Binding Region Associated with Protection/Susceptibility to the Severe West Nile Virus Disease. PLoS ONE 2018, 13, e0205557. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.A.; Furuta, K. The Ins and Outs of MHC Class Ii-Mediated Antigen Processing and Presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic Liposome-Mediated Rna Transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077–6081. [Google Scholar] [CrossRef]
- Holland, J.W.; Madden, T.D.; Pieter, R.C. Bilayer Stabilizing Components and Their Use in Forming Programmable Fusogenic Liposomes; U.S. Patent and Trademark Office: Alexandria, VA, USA, 1999.
- Dalby, B.; Cates, S.; Harris, A.; Ohki, E.C.; Tilkins, M.L.; Price, P.J.; Ciccarone, V.C. Advanced Transfection with Lipofectamine 2000 Reagent: Primary Neurons, Sirna, and High-Throughput Applications. Methods 2004, 33, 95–103. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Darjuan, M.M.; Mercer, J.E.; Chen, S.; van der Meel, R.; Thewalt, J.L.; Tam, Y.Y.C.; Cullis, P.R. On the Formation and Morphology of Lipid Nanoparticles Containing Ionizable Cationic Lipids and Sirna. ACS Nano 2018, 12, 4787–4795. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Horejs, C. From Lipids to Lipid Nanoparticles to mRNA Vaccines. Nat. Rev. Mater. 2021, 6, 1075–1076. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for mRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Pateev, I.; Seregina, K.; Ivanov, R.; Reshetnikov, V. Biodistribution of RNA Vaccines and of Their Products: Evidence from Human and Animal Studies. Biomedicines 2023, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Cullis, P.R.; Felgner, P.L. The 60-Year Evolution of Lipid Nanoparticles for Nucleic Acid Delivery. Nat. Rev. Drug Discov. 2024, 23, 709–722. [Google Scholar] [CrossRef]
- Kariko, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of Rna Recognition by Toll-Like Receptors: The Impact of Nucleoside Modification and the Evolutionary Origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine into mRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Kariko, K. Incorporation of Pseudouridine into mRNA Enhances Translation by Diminishing Pkr Activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef]
- Anderson, B.R.; Muramatsu, H.; Jha, B.K.; Silverman, R.H.; Weissman, D.; Kariko, K. Nucleoside Modifications in Rna Limit Activation of 2′-5′-Oligoadenylate Synthetase and Increase Resistance to Cleavage by Rnase L. Nucleic Acids Res. 2011, 39, 9329–9338. [Google Scholar] [CrossRef]
- Kariko, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the Optimal mRNA for Therapy: Hplc Purification Eliminates Immune Activation and Improves Translation of Nucleoside-Modified, Protein-Encoding mRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef]
- Pardi, N.; Muramatsu, H.; Weissman, D.; Kariko, K. In Vitro Transcription of Long mRNA Containing Modified Nucleosides. Methods Mol. Biol. 2013, 969, 29–42. [Google Scholar]
- Sahin, U.; Kariko, K.; Tureci, O. mRNA-Based Therapeutics--Developing a New Class of Drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef] [PubMed]
- Pancer, Z.; Cooper, M.D. The Evolution of Adaptive Immunity. Annu. Rev. Immunol. 2006, 24, 497–518. [Google Scholar] [CrossRef] [PubMed]
- Litman, G.W.; Rast, J.P.; Fugmann, S.D. The Origins of Vertebrate Adaptive Immunity. Nat. Rev. Immunol. 2010, 10, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F.; Kasahara, M. Origin and Evolution of the Adaptive Immune System: Genetic Events and Selective Pressures. Nat. Rev. Genet. 2010, 11, 47–59. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Lee, A.; Grigoryan, L.; Arunachalam, P.S.; Scott, M.K.D.; Trisal, M.; Wimmers, F.; Sanyal, M.; Weidenbacher, P.A.; Feng, Y.; Adamska, J.Z.; et al. Mechanisms of Innate and Adaptive Immunity to the Pfizer-Biontech BNT 162b2 Vaccine. Nat. Immunol. 2022, 23, 543–555. [Google Scholar]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef]
- Heath, W.R.; Carbone, F.R. Cross-Presentation, Dendritic Cells, Tolerance and Immunity. Annu. Rev. Immunol. 2001, 19, 47–64. [Google Scholar] [CrossRef]
- Mbongue, J.; Nicholas, D.; Firek, A.; Langridge, W. The Role of Dendritic Cells in Tissue-Specific Autoimmunity. J. Immunol. Res. 2014, 2014, 857143. [Google Scholar] [CrossRef]
- Kurts, C.; Heath, W.R.; Carbone, F.R.; Kosaka, H.; Miller, J.F. Cross-Presentation of Self Antigens to Cd8+ T Cells: The Balance between Tolerance and Autoimmunity. Novartis Found. Symp. 1998, 215, 172–181; discussion 81–90. [Google Scholar]
- Iberg, C.A.; Jones, A.; Hawiger, D. Dendritic Cells as Inducers of Peripheral Tolerance. Trends Immunol. 2017, 38, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Nguyen, D.; Bhadra, P.; Jung, M.; Helms, V.; Zimmermann, R. Signal Peptide Features Determining the Substrate Specificities of Targeting and Translocation Components in Human ER Protein Import. Front. Physiol. 2022, 13, 833540. [Google Scholar] [CrossRef] [PubMed]
- Blizard, G.S.; Dwivedi, G.; Pan, Y.G.; Hou, C.; Etersque, J.M.; Said, H.; Chevrier, A.; Lavertu, M.; Ni, H.; Davis, B.; et al. Monitoring mRNA Vaccine Antigen Expression in Vivo Using Pet/Ct. Nat. Commun. 2025, 16, 2234. [Google Scholar] [CrossRef] [PubMed]
- Burkova, E.E.; Bakhno, I.A. Sequences in the Cytoplasmic Tail Contribute to the Intracellular Trafficking and the Cell Surface Localization of SARS-CoV-2 Spike Protein. Biomolecules 2025, 15, 280. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Inoue, T.; Inoue, M.; Murae, M.; Fukasawa, M.; Kaneko, M.K.; Kato, Y.; Noguchi, K. SARS-CoV-2 Spike Protein Mutation at Cysteine-488 Impairs Its Golgi Localization and Intracellular S1/S2 Processing. Int. J. Mol. Sci. 2022, 23, 15834. [Google Scholar] [CrossRef]
- Cattin-Ortola, J.; Welch, L.G.; Maslen, S.L.; Papa, G.; James, L.C.; Munro, S. Sequences in the Cytoplasmic Tail of SARS-CoV-2 Spike Facilitate Expression at the Cell Surface and Syncytia Formation. Nat. Commun. 2021, 12, 5333. [Google Scholar] [CrossRef]
- Bakos, T.; Meszaros, T.; Kozma, G.T.; Berenyi, P.; Facsko, R.; Farkas, H.; Dezsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. mRNA-Lnp COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef]
- Cheng, M.H.; Zhang, S.; Porritt, R.A.; Rivas, M.N.; Paschold, L.; Willscher, E.; Binder, M.; Arditi, M.; Bahar, I. Superantigenic Character of an Insert Unique to SARS-CoV-2 Spike Supported by Skewed Tcr Repertoire in Patients with Hyperinflammation. Proc. Natl. Acad. Sci. USA 2020, 117, 25254–25262. [Google Scholar] [CrossRef]
- White, J.; Herman, A.; Pullen, A.M.; Kubo, R.; Kappler, J.W.; Marrack, P. The V Beta-Specific Superantigen Staphylococcal Enterotoxin B: Stimulation of Mature T Cells and Clonal Deletion in Neonatal Mice. Cell 1989, 56, 27–35. [Google Scholar] [CrossRef]
- Rivas, N.; Porritt, R.A.; Cheng, M.H.; Bahar, I.; Arditi, M. Multisystem Inflammatory Syndrome in Children and Long COVID: The SARS-CoV-2 Viral Superantigen Hypothesis. Front. Immunol. 2022, 13, 941009. [Google Scholar]
- Schwartz, N.; Ratzon, R.; Hazan, I.; Zimmerman, D.R.; Singer, S.R.; Wasser, J.; Dweck, T.; Alroy-Preis, S. Multisystemic Inflammatory Syndrome in Children and the Bnt162b2 Vaccine: A Nationwide Cohort Study. Eur. J. Pediatr. 2024, 183, 3319–3326. [Google Scholar] [CrossRef] [PubMed]
- Bittmann, S.; Weissenstein, A.; Luchter, E.; Moschüring-Alieva, E.; Villalon, G. Multisystem Inflammatory Syndrome in Children (Mis-C): The Role of Viral Superantigens in COVID-19 Disease. J. Allergy Infect. Dis. 2020, 1, 18–20. [Google Scholar]
- Hamdy, A.; Leonardi, A. Superantigens and SARS-CoV-2. Pathogens 2022, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Matthew, B.; Bhardwaj, N. Super(Antigen) Target for SARS-CoV-2. Nat. Rev. Immunol. 2021, 21, 72. [Google Scholar] [CrossRef]
- Lubinski, B.; Whittaker, G.R. The SARS-CoV-2 Furin Cleavage Site: Natural Selection or Smoking Gun? Lancet Microbe 2023, 4, e570. [Google Scholar] [CrossRef]
- Valleriani, F.; Di Pancrazio, C.; Spedicato, M.; Di Teodoro, G.; Malatesta, D.; Petrova, T.; Profeta, F.; Colaianni, M.L.; Berjaoui, S.; Puglia, I.; et al. A Cell-Adapted SARS-CoV-2 Mutant, Showing a Deletion in the Spike Protein Spanning the Furin Cleavage Site, Has Reduced Virulence at the Lung Level in K18-Hace2 Mice. Virology 2024, 592, 109997. [Google Scholar] [CrossRef]
- Li, L.; Gao, M.; Li, J.; Xie, X.; Zhao, H.; Wang, Y.; Xu, X.; Zu, S.; Chen, C.; Wan, D.; et al. Identification of an Immunogenic Epitope and Protective Antibody against the Furin Cleavage Site of SARS-CoV-2. EBioMedicine 2023, 87, 104401. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, X.M.; Xing, X.; Xu, Z.; Zhang, C.; Song, J.W.; Fan, X.; Xia, P.; Fu, J.L.; Wang, S.Y.; et al. Single-Cell Landscape of Immunological Responses in Patients with COVID-19. Nat. Immunol. 2020, 21, 1107–1118. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Xia, H.; Wang, C.; Tan, C.Y.; Cai, X.; Liu, Y.; Ji, F.; Xiong, P.; Liu, R.; et al. Transcriptional and Proteomic Insights into the Host Response in Fatal COVID-19 Cases. Proc. Natl. Acad. Sci. USA 2020, 117, 28336–28343. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501.e1415. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Gychka, S.G. SARS-CoV-2 Spike Protein Elicits Cell Signaling in Human Host Cells: Implications for Possible Consequences of COVID-19 Vaccines. Vaccines 2021, 9, 36. [Google Scholar] [CrossRef]
- Hamad Saied, M.; van der Griend, L.; van Straalen, J.W.; Wulffraat, N.M.; Vastert, S.; Jansen, M.H.A. The Protective Effect of COVID-19 Vaccines on Developing Multisystem Inflammatory Syndrome in Children (Mis-C): A Systematic Literature Review and Meta-Analysis. Pediatr. Rheumatol. Online J. 2023, 21, 80. [Google Scholar] [CrossRef] [PubMed]
- Alije, K.-S.; Ramosaj, A.; Baloku, A.; Zogaj, L.; Gjaka, P. Multisystem Inflammatory Syndrome in Children (Mis-C), Possibly Due to COVID-19 mRNA Vaccination. Vaccines 2023, 11, 956. [Google Scholar]
- Li, H.; Llera, A.; Malchiodi, E.L.; Mariuzza, R.A. The Structural Basis of T Cell Activation by Superantigens. Annu. Rev. Immunol. 1999, 17, 435–466. [Google Scholar] [CrossRef] [PubMed]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem Inflammatory Syndrome in Children Related to COVID-19: A Systematic Review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Ruvinsky, S.; Voto, C.; Roel, M.; Fustinana, A.; Veliz, N.; Brizuela, M.; Rodriguez, S.; Ulloa-Gutierrez, R.; Bardach, A. Multisystem Inflammatory Syndrome Temporally Related to COVID-19 in Children from Latin America and the Caribbean Region: A Systematic Review with a Meta-Analysis of Data from Regional Surveillance Systems. Front. Pediatr. 2022, 10, 881765. [Google Scholar] [CrossRef]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. COVID-Investigators Overcoming, and CDC COVID- Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef]
- Lauster, D.; Osterrieder, K.; Haag, R.; Ballauff, M.; Herrmann, A. Respiratory Viruses Interacting with Cells: The Importance of Electrostatics. Front. Microbiol. 2023, 14, 1169547. [Google Scholar] [CrossRef]
- Cotten, M.; Phan, M.V.T. Evolution of Increased Positive Charge on the SARS-CoV-2 Spike Protein May Be Adaptation to Human Transmission. iScience 2023, 26, 106230. [Google Scholar] [CrossRef]
- Sinha, A.; Sangeet, S.; Roy, S. Evolution of Sequence and Structure of SARS-CoV-2 Spike Protein: A Dynamic Perspective. ACS Omega 2023, 8, 23283–23304. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello, C.; Gaio, M.; Zinzi, A.; Sullo, M.G.; Liguori, V.; Ferraro, M.; Petronzelli, F.; Felicetti, P.; Marchione, P.; Marra, A.R.; et al. Disentangling a Thorny Issue: Myocarditis and Pericarditis Post COVID-19 and Following mRNA COVID-19 Vaccines. Pharmaceuticals 2022, 15, 525. [Google Scholar] [CrossRef] [PubMed]
- Massari, M.; Alegiani, S.S.; Morciano, C.; Spuri, M.; Marchione, P.; Felicetti, P.; Belleudi, V.; Poggi, F.R.; Lazzeretti, M.; Ercolanoni, M.; et al. Vax TheShin, and COVID Surveillance Group. Postmarketing Active Surveillance of Myocarditis and Pericarditis Following Vaccination with COVID-19 mRNA Vaccines in Persons Aged 12 to 39 Years in Italy: A Multi-Database, Self-Controlled Case Series Study. PLoS Med. 2022, 19, e1004056. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-Lasting, Biochemically Modified mRNA, and Its Frameshifted Recombinant Spike Proteins in Human Tissues and Circulation after COVID-19 Vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar] [CrossRef]
- Yonker, L.M.; Swank, Z.; Bartsch, Y.C.; Burns, M.D.; Kane, A.; Boribong, B.P.; Davis, J.P.; Loiselle, M.; Novak, T.; Senussi, Y.; et al. Circulating Spike Protein Detected in Post-COVID-19 mRNA Vaccine Myocarditis. Circulation 2023, 147, 867–876. [Google Scholar] [CrossRef]
- Amormino, C.; Tedeschi, V.; Paldino, G.; Arcieri, S.; Fiorillo, M.T.; Paiardini, A.; Tuosto, L.; Kunkl, M. SARS-CoV-2 Spike Does Not Possess Intrinsic Superantigen-Like Inflammatory Activity. Cells 2022, 11, 2526. [Google Scholar] [CrossRef]
- Paiardi, G.; Richter, S.; Oreste, P.; Urbinati, C.; Rusnati, M.; Wade, R.C. The Binding of Heparin to Spike Glycoprotein Inhibits SARS-CoV-2 Infection by Three Mechanisms. J. Biol. Chem. 2022, 298, 101507. [Google Scholar] [CrossRef]
- Cheng, M.H.; Porritt, R.A.; Rivas, M.N.; Krieger, J.M.; Ozdemir, A.B.; Garcia, G., Jr.; Arumugaswami, V.; Fries, B.C.; Arditi, M.; Bahar, I. A Monoclonal Antibody against Staphylococcal Enterotoxin B Superantigen Inhibits SARS-CoV-2 Entry in Vitro. Structure 2021, 29, 951–962.e3. [Google Scholar] [CrossRef]
- Dima, F.; Salvagno, G.L.; Lippi, G. Effects of Recombinant SARS-CoV-2 Spike Protein Variants on Red Blood Cells Parameters and Red Blood Cell Distribution Width. Biomed. J. 2024, 47, 100787. [Google Scholar] [CrossRef]
- Jung, F.; Connes, P. Morphology and Function of Red Blood Cells in COVID-19 Patients: Current Overview 2023. Life 2024, 14, 460. [Google Scholar] [CrossRef]
- Grau, M.; Ibershoff, L.; Zacher, J.; Bros, J.; Tomschi, F.; Diebold, K.F.; Predel, H.G.; Bloch, W. Even Patients with Mild COVID-19 Symptoms after SARS-CoV-2 Infection Show Prolonged Altered Red Blood Cell Morphology and Rheological Parameters. J. Cell. Mol. Med. 2022, 26, 3022–3030. [Google Scholar] [CrossRef]
- Boschi, C.; Scheim, D.E.; Bancod, A.; Militello, M.; Bideau, M.L.; Colson, P.; Fantini, J.; Scola, B. SARS-CoV-2 Spike Protein Induces Hemagglutination: Implications for COVID-19 Morbidities and Therapeutics and for Vaccine Adverse Effects. Int. J. Mol. Sci. 2022, 23, 15480. [Google Scholar] [CrossRef]
- Scheim, D.E.; Vottero, P.; Santin, A.D.; Hirsh, A.G. Sialylated Glycan Bindings from SARS-CoV-2 Spike Protein to Blood and Endothelial Cells Govern the Severe Morbidities of COVID-19. Int. J. Mol. Sci. 2023, 24, 17039. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tian, Z.; Zhu, M.; Zhang, B.; Li, Y.; Zheng, Y.; Mao, Y.; Zhao, Y.; Yang, Y. SARS-CoV-2 Spike Protein Potentiates Platelet Aggregation Via Upregulating Integrin Alphaiibbeta3 Outside-in Signaling Pathway. J. Thromb. Thrombolysis 2024, 57, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.C.; Basnet, N.; Bodakuntla, S.; Alvarez-Brecht, P.; Nichols, S.; Martinez-Sanchez, A.; Agostini, L.; Soh, Y.M.; Takagi, J.; Biertumpfel, C.; et al. Direct Cryo-Et Observation of Platelet Deformation Induced by SARS-CoV-2 Spike Protein. Nat. Commun. 2023, 14, 620. [Google Scholar] [CrossRef] [PubMed]
- Luzak, B.; Rozalski, M.; Przygodzki, T.; Boncler, M.; Wojkowska, D.; Kosmalski, M.; Watala, C. SARS-CoV-2 Spike Protein and Neutralizing Anti-Spike Protein Antibodies Modulate Blood Platelet Function. Int. J. Mol. Sci. 2023, 24, 5312. [Google Scholar] [CrossRef] [PubMed]
- Degenfeld-Schonburg, L.; Sadovnik, I.; Smiljkovic, D.; Peter, B.; Stefanzl, G.; Gstoettner, C.; Jaksch, P.; Hoetzenecker, K.; Aigner, C.; Radtke, C.; et al. Coronavirus Receptor Expression Profiles in Human Mast Cells, Basophils, and Eosinophils. Cells 2024, 13, 173. [Google Scholar] [CrossRef]
- Shouman, S.; El-Kholy, N.; Hussien, A.E.; El-Derby, A.M.; Magdy, S.; Abou-Shanab, A.M.; Elmehrath, A.O.; Abdelwaly, A.; Helal, M.; El-Badri, N. SARS-CoV-2-Associated Lymphopenia: Possible Mechanisms and the Role of Cd147. Cell Commun. Signal. 2024, 22, 349. [Google Scholar] [CrossRef]
- Faghihi, H. Cd147 as an Alternative Binding Site for the Spike Protein on the Surface of SARS-CoV-2. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11992–11994. [Google Scholar]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. Cd147-Spike Protein Is a Novel Route for SARS-CoV-2 Infection to Host Cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-Spike Protein Interaction in COVID-19: Get the Ball Rolling with a Novel Receptor and Therapeutic Target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Brodowski, M.; Pierpaoli, M.; Janik, M.; Kowalski, M.; Ficek, M.; Slepski, P.; Trzaskowski, B.; Swain, G.; Ryl, J.; Bogdanowicz, R. Enhanced Susceptibility of SARS-CoV-2 Spike RBD Protein Assay Targeted by Cellular Receptors ACE2 and CD147: Multivariate Data Analysis of Multisystem Impedimetric Response. Sens. Actuators B Chem. 2022, 370, 132427. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.M.; Nigh, G.; PA, M.; Seneff, S. Autoimmune and Neoplastic Outcomes After the mRNA Vaccination: The Role of T Regulatory Cell Responses. Int. J. Vaccine Theory Pract. Res. 2024, 3, 1395–1433. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and microRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT-162b2 (Pfizer-BioNtech) Vaccination Prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-Mediated mRNA Delivery in Vivo Is Safe and Can Be Used to Induce SARS-CoV-2 Immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar] [CrossRef]
- Cacciottolo, M.; Nice, J.B.; Li, Y.; LeClaire, M.J.; Twaddle, R.; Mora, C.L.; Adachi, S.Y.; Chin, E.R.; Young, M.; Angeles, J.; et al. Exosome-Based Multivalent Vaccine: Achieving Potent Immunization, Broadened Reactivity, and Strong T-Cell Responses with Nanograms of Proteins. Microbiol. Spectr. 2023, 11, e0050323. [Google Scholar] [CrossRef]
- Bansal, S.; Fleming, T.; Mohanakumar, T. Response to Comment on “Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT-162b2 (Pfizer-Biontech) Vaccination Prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines”. J. Immunol. 2022, 208, 1833–1834. [Google Scholar] [CrossRef]
- Giannotta, G.; Murrone, A.; Giannotta, N. COVID-19 mRNA Vaccines: The Molecular Basis of Some Adverse Events. Vaccines 2023, 11, 747. [Google Scholar] [CrossRef]
- Bellavite, P.; Ferraresi, A.; Isidoro, C. Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines 2023, 11, 451. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-Modified mRNA Vaccines Induce Potent T Follicular Helper and Germinal Center B Cell Responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Suurmond, J.; Diamond, B. Autoantibodies in Systemic Autoimmune Diseases: Specificity and Pathogenicity. J. Clin. Investig. 2015, 125, 2194–2202. [Google Scholar] [CrossRef] [PubMed]
- Alriyami, M.; Polychronakos, C. Somatic Mutations and Autoimmunity. Cells 2021, 10, 2056. [Google Scholar] [CrossRef] [PubMed]
- Zan, H.; Zhang, J.; Ardeshna, S.; Xu, Z.; Park, S.R.; Casali, P. Lupus-Prone Mrl/Faslpr/Lpr Mice Display Increased Aid Expression and Extensive DNA Lesions, Comprising Deletions and Insertions, in the Immunoglobulin Locus: Concurrent Upregulation of Somatic Hypermutation and Class Switch DNA Recombination. Autoimmunity 2009, 42, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Smith, D.; Aviszus, K.; Detanico, T.; Heiser, R.A.; Wysocki, L.J. Somatic Hypermutation as a Generator of Antinuclear Antibodies in a Murine Model of Systemic Autoimmunity. J. Exp. Med. 2010, 207, 2225–2237. [Google Scholar] [CrossRef]
- Sakuma, H.; Okumura, F.; Miyabe, S.; Sugiura, M.; Joh, T.; Shimozato, K.; Inagaki, H. Analysis of Vh Gene Rearrangement and Somatic Hypermutation in Sjogren’s Syndrome and Igg4-Related Sclerosing Sialadenitis. Scand. J. Immunol. 2010, 72, 44–49. [Google Scholar] [CrossRef]
- Zuckerman, N.S.; Hazanov, H.; Barak, M.; Edelman, H.; Hess, S.; Shcolnik, H.; Dunn-Walters, D.; Mehr, R. Somatic Hypermutation and Antigen-Driven Selection of B Cells Are Altered in Autoimmune Diseases. J. Autoimmun. 2010, 35, 325–335. [Google Scholar] [CrossRef]
- Okumura, F.; Sakuma, H.; Nakazawa, T.; Hayashi, K.; Naitoh, I.; Miyabe, K.; Yoshida, M.; Yamashita, H.; Ohara, H.; Inagaki, H.; et al. Analysis of Vh Gene Rearrangement and Somatic Hypermutation in Type 1 Autoimmune Pancreatitis. Pathol. Int. 2012, 62, 318–323. [Google Scholar] [CrossRef]
- Schroeder, K.; Herrmann, M.; Winkler, T.H. The Role of Somatic Hypermutation in the Generation of Pathogenic Antibodies in Sle. Autoimmunity 2013, 46, 121–127. [Google Scholar] [CrossRef]
- Mulroney, T.E.; Poyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N(1)-Methylpseudouridylation of mRNA Causes +1 Ribosomal Frameshifting. Nature 2024, 625, 189–194. [Google Scholar] [CrossRef]
- Safra, M.; Tamari, Z.; Polak, P.; Shiber, S.; Matan, M.; Karameh, H.; Helviz, Y.; Levy-Barda, A.; Yahalom, V.; Peretz, A.; et al. Altered Somatic Hypermutation Patterns in COVID-19 Patients Classifies Disease Severity. Front. Immunol. 2023, 14, 1031914. [Google Scholar] [CrossRef] [PubMed]
- Jacob-Dolan, C.; Lifton, M.; Powers, O.C.; Miller, J.; Hachmann, N.P.; Vu, M.; Surve, N.; Mazurek, C.R.; Fisher, J.L.; Rodrigues, S.; et al. B Cell Somatic Hypermutation Following COVID-19 Vaccination with Ad26.Cov2.S. iScience 2024, 27, 109716. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, E.; Morioka, H.; Kikuchi, T.; Fukushima, M. Behavioral and Health Outcomes of mRNA COVID-19 Vaccination: A Case-Control Study in Japanese Small and Medium-Sized Enterprises. Cureus 2024, 16, e75652. [Google Scholar] [CrossRef] [PubMed]
- Fay, M.M.; Lyons, S.M.; Ivanov, P. Rna G-Quadruplexes in Biology: Principles and Molecular Mechanisms. J. Mol. Biol. 2017, 429, 2127–2147. [Google Scholar] [CrossRef]
- Zhang, L.; Richards, A.; Barrasa, M.I.; Hughes, S.H.; Young, R.A.; Jaenisch, R. Reverse-Transcribed SARS-CoV-2 RNA Can Integrate into the Genome of Cultured Human Cells and Can Be Expressed in Patient-Derived Tissues. Proc. Natl. Acad. Sci. USA 2021, 118, e2105968118. [Google Scholar] [CrossRef]
- Alden, M.; Falla, F.O.; Yang, D.; Barghouth, M.; Luan, C.; Rasmussen, M.; De Marinis, Y. Intracellular Reverse Transcription of Pfizer Biontech COVID-19 mRNA Vaccine BNT-162b2 in Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- Chandramouly, G.; Zhao, J.; McDevitt, S.; Rusanov, T.; Hoang, T.; Borisonnik, N.; Treddinick, T.; Lopezcolorado, F.W.; Kent, T.; Siddique, L.A.; et al. Poltheta Reverse Transcribes Rna and Promotes Rna-Templated DNA Repair. Sci. Adv. 2021, 7, eabf1771. [Google Scholar] [CrossRef] [PubMed]
- Boros, L.G.; Krüger, T.P.J.; Letoha, T.; Tuszynski, J.A.; Dorfsman, P.D.; Lech, J.C. To Stabilize or Not to Stabilize RNA—That Is Still the Question. Sci. Adv. 2021, 7, eabf1771. [Google Scholar]
- Kruchinin, A.A.; Makarova, A.V. Multifaceted Nature of DNA Polymerase Theta. Int. J. Mol. Sci. 2023, 24, 3619. [Google Scholar] [CrossRef]
- Feng, W.; Simpson, D.A.; Carvajal-Garcia, J.; Price, B.A.; Kumar, R.J.; Mose, L.E.; Wood, R.D.; Rashid, N.; Purvis, J.E.; Parker, J.S.; et al. Genetic Determinants of Cellular Addiction to DNA Polymerase Theta. Nat. Commun. 2019, 10, 4286. [Google Scholar] [CrossRef]
- Kyriakopoulos, A.; McCullough, P.; Nigh, G.; Seneff, S. Potential Mechanisms for Human Genome Integration of Genetic Code from SARS-CoV-2 mRNA Vaccination Potential Mechanisms for Human Genome Integration of Genetic Code from SARS-CoV-2 Mrna Vaccination. J. Neurol. Disord. 2022, 10, 1–41. [Google Scholar]
- Smits, N.; Rasmussen, J.; Bodea, G.O.; Amarilla, A.A.; Gerdes, P.; Sanchez-Luque, F.J.; Ajjikuttira, P.; Modhiran, N.; Liang, B.; Faivre, J.; et al. No Evidence of Human Genome Integration of SARS-CoV-2 Found by Long-Read DNA Sequencing. Cell Rep. 2021, 36, 109530. [Google Scholar] [CrossRef] [PubMed]
- Merchant, H.A. Comment on Alden et al. Intracellular Reverse Transcription of Pfizer Biontech COVID-19 mRNA Vaccine BNT-162b2 in Vitro in Human Liver Cell Line. Curr. Issues Mol. Biol. 2022, 44, 1115–1126. Curr. Issues Mol. Biol. 2022, 44, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Cristoni, S.; Marino, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Lettieri, G.; Piscopo, M. Detection of Recombinant Spike Protein in the Blood of Individuals Vaccinated against SARS-CoV-2: Possible Molecular Mechanisms. Proteom. Clin. Appl. 2023, 17, e2300048. [Google Scholar] [CrossRef]
- Krauson, A.J.; Casimero, F.V.C.; Siddiquee, Z.; Stone, J.R. Duration of SARS-CoV-2 mRNA Vaccine Persistence and Factors Associated with Cardiac Involvement in Recently Vaccinated Patients. NPJ Vaccines 2023, 8, 141. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyarto, B.Z. The mRNA-LNP Platform’s Lipid Nanoparticle Component Used in Preclinical Vaccine Studies Is Highly Inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Stanworth, S.J.; New, H.V.; Apelseth, T.O.; Brunskill, S.; Cardigan, R.; Doree, C.; Germain, M.; Goldman, M.; Massey, E.; Prati, D.; et al. Effects of the COVID-19 Pandemic on Supply and Use of Blood for Transfusion. Lancet Haematol. 2020, 7, e756–e764. [Google Scholar] [CrossRef]
- Chang, L.; Yan, Y.; Wang, L. Coronavirus Disease 2019: Coronaviruses and Blood Safety. Transfus. Med. Rev. 2020, 34, 75–80. [Google Scholar] [CrossRef]
- Bouhou, S.; Lahjouji, K.; Masrar, A. Blood Donor Eligibility after COVID-19 Vaccination: The Current State of Recommendations. Pan Afr. Med. J. 2021, 40, 207. [Google Scholar] [CrossRef]
- Jacobs, J.W.; Bibb, L.A.; Savani, B.N.; Booth, G.S. Refusing Blood Transfusions from COVID-19-Vaccinated Donors: Are We Repeating History? Br. J. Haematol. 2022, 196, 585–588. [Google Scholar] [CrossRef]
- Hunain, R.; Uday, U.; Rackimuthu, S.; Nawaz, F.A.; Narain, K.; Essar, M.Y.; Rehman, M.U.; Ahmad, S.; Butt, A. Effects of SARS-CoV-2 Vaccination on Blood Donation and Blood Banks in India. Ann. Med. Surg. 2022, 78, 103772. [Google Scholar] [CrossRef]
- Parry, P.I.; Lefringhausen, A.; Turni, C.; Neil, C.J.; Cosford, R.; Hudson, N.J.; Gillespie, J. ‘Spikeopathy’: COVID-19 Spike Protein Is Pathogenic, from Both Virus and Vaccine mRNA. Biomedicines 2023, 11, 2287. [Google Scholar] [CrossRef]
- Castruita, J.A.S.; Schneider, U.V.; Mollerup, S.; Leineweber, T.D.; Weis, N.; Bukh, J.; Pedersen, M.S.; Westh, H. SARS-CoV-2 Spike mRNA Vaccine Sequences Circulate in Blood up to 28 Days after COVID-19 Vaccination. APMIS 2023, 131, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Roubinian, N.H.; Greene, J.; Liu, V.X.; Lee, C.; Mark, D.G.; Vinson, D.R.; Spencer, B.R.; Bruhn, R.; Bravo, M.; Stone, M.; et al. Clinical Outcomes in Hospitalized Plasma and Platelet Transfusion Recipients Prior to and Following Widespread Blood Donor SARS-CoV-2 Infection and Vaccination. Transfusion 2024, 64, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; De Mejia, C.M.; Heffes-Doon, A.; Lin, X.; Botros, B.; Gurzenda, E.; Clauss-Pascarelli, C.; Nayak, A. Biodistribution of mRNA COVID-19 Vaccines in Human Breast Milk. EBioMedicine 2023, 96, 104800. [Google Scholar] [CrossRef] [PubMed]
- Huynh, T.V.; Rethi, L.; Lee, T.W.; Higa, S.; Kao, Y.H.; Chen, Y.J. Spike Protein Impairs Mitochondrial Function in Human Cardiomyocytes: Mechanisms Underlying Cardiac Injury in COVID-19. Cells 2023, 12, 877. [Google Scholar] [CrossRef]
- Schwartz, L.; Aparicio-Alonso, M.; Henry, M.; Radman, M.; Attal, R.; Bakkar, A. Toxicity of the Spike Protein of COVID-19 Is a Redox Shift Phenomenon: A Novel Therapeutic Approach. Free Radic. Biol. Med. 2023, 206, 106–110. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 Spike Protein Promotes Mapk and Nf-Kb Activation in Human Lung Cells and Inflammatory Cytokine Production in Human Lung and Intestinal Epithelial Cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef]
- Niu, C.; Liang, T.; Chen, Y.; Zhu, S.; Zhou, L.; Chen, N.; Qian, L.; Wang, Y.; Li, M.; Zhou, X.; et al. SARS-CoV-2 Spike Protein Induces the Cytokine Release Syndrome by Stimulating T Cells to Produce More Il-2. Front. Immunol. 2024, 15, 1444643. [Google Scholar] [CrossRef]
- Robles, J.P.; Zamora, M.; Adan-Castro, E.; Siqueiros-Marquez, L.; de la Escalera, G.M.; Clapp, C. The Spike Protein of SARS-CoV-2 Induces Endothelial Inflammation through Integrin Alpha5beta1 and Nf-Kappab Signaling. J. Biol. Chem. 2022, 298, 101695. [Google Scholar] [CrossRef]
- Avolio, E.; Carrabba, M.; Milligan, R.; Williamson, M.K.; Beltrami, A.P.; Gupta, K.; Elvers, K.T.; Gamez, M.; Foster, R.R.; Gillespie, K.; et al. The SARS-CoV-2 Spike Protein Disrupts Human Cardiac Pericytes Function through Cd147 Receptor-Mediated Signalling: A Potential Non-Infective Mechanism of COVID-19 Microvascular Disease. Clin. Sci. 2021, 135, 2667–2689. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Galbusera, M.; Pezzotta, A.; Gastoldi, S.; Imberti, B.; Perna, A.; Ruggenenti, P.; Donadelli, R.; Benigni, A.; et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front. Immunol. 2022, 13, 827146. [Google Scholar] [CrossRef] [PubMed]
- Perico, L.; Morigi, M.; Pezzotta, A.; Locatelli, M.; Imberti, B.; Corna, D.; Cerullo, D.; Benigni, A.; Remuzzi, G. SARS-CoV-2 Spike Protein Induces Lung Endothelial Cell Dysfunction and Thrombo-Inflammation Depending on the C3a/C3a Receptor Signalling. Sci. Rep. 2023, 13, 11392. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.M.; Ferrari, M.; Lynch, N.J.; Yaseen, S.; Dudler, T.; Gragerov, S.; Demopulos, G.; Heeney, J.L.; Schwaeble, W.J. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front. Immunol. 2021, 12, 714511. [Google Scholar] [CrossRef]
- Cao, X.; Nguyen, V.; Tsai, J.; Gao, C.; Tian, Y.; Zhang, Y.; Carver, W.; Kiaris, H.; Cui, T.; Tan, W. The SARS-CoV-2 Spike Protein Induces Long-Term Transcriptional Perturbations of Mitochondrial Metabolic Genes, Causes Cardiac Fibrosis, and Reduces Myocardial Contractile in Obese Mice. Mol. Metab. 2023, 74, 101756. [Google Scholar] [CrossRef]
- Rao, E.; Grover, P.; Zhang, H. Thrombosis after SARS-CoV-2 Infection or COVID-19 Vaccination: Will a Nonpathologic Anti-Pf4 Antibody Be a Solution?-a Narrative Review. J. BioX Res. 2022, 5, 97–103. [Google Scholar]
- Fujimura, Y.; Holland, L.Z. COVID-19 Microthrombosis: Unusually Large Vwf Multimers Are a Platform for Activation of the Alternative Complement Pathway under Cytokine Storm. Int. J. Hematol. 2022, 115, 457–469. [Google Scholar] [CrossRef]
- Indes, J.E.; Koleilat, I.; Hatch, A.N.; Choinski, K.; Jones, D.B.; Aldailami, H.; Billett, H.; Denesopolis, J.M.; Lipsitz, E. Early Experience with Arterial Thromboembolic Complications in Patients with COVID-19. J. Vasc. Surg. 2021, 73, 381–389.e1. [Google Scholar] [CrossRef]
- Vettori, M.; Dima, F.; Henry, B.M.; Carpene, G.; Gelati, M.; Celegon, G.; Salvagno, G.L.; Lippi, G. Effects of Different Types of Recombinant SARS-CoV-2 Spike Protein on Circulating Monocytes’ Structure. Int. J. Mol. Sci. 2023, 24, 9373. [Google Scholar] [CrossRef]
- Vettori, M.; Carpene, G.; Salvagno, G.L.; Gelati, M.; Dima, F.; Celegon, G.; Favaloro, E.J.; Lippi, G. Effects of Recombinant SARS-CoV-2 Spike Protein Variants on Platelet Morphology and Activation. Semin. Thromb. Hemost. 2023, 50, 275–283. [Google Scholar] [CrossRef]
- Zhou, Y.; Nishikawa, M.; Kanno, H.; Yang, R.; Ibayashi, Y.; Xiao, T.H.; Peterson, W.; Herbig, M.; Nitta, N.; Miyata, S.; et al. Long-Term Effects of Pfizer-Biontech COVID-19 Vaccinations on Platelets. Cytometry A 2023, 103, 162–167. [Google Scholar] [CrossRef]
- Thachil, J.; Favaloro, E.J.; Lippi, G. D-Dimers-Normal Levels Versus Elevated Levels Due to a Range of Conditions, Including D-Dimeritis, Inflammation, Thromboembolism, Disseminated Intravascular Coagulation, and COVID-19. Semin. Thromb. Hemost. 2022, 48, 672–679. [Google Scholar] [CrossRef]
- Grobbelaar, L.M.; Venter, C.; Vlok, M.; Ngoepe, M.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. SARS-CoV-2 Spike Protein S1 Induces Fibrin(Ogen) Resistant to Fibrinolysis: Implications for Microclot Formation in COVID-19. Biosci. Rep. 2021, 41, BSR20210611. [Google Scholar] [CrossRef]
- DeOre, B.J.; Tran, K.A.; Andrews, A.M.; Ramirez, S.H.; Galie, P.A. SARS-CoV-2 Spike Protein Disrupts Blood-Brain Barrier Integrity Via Rhoa Activation. J. Neuroimmune Pharmacol. 2021, 16, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Montezano, A.C.; Camargo, L.L.; Mary, S.; Neves, K.B.; Rios, F.J.; Stein, R.; Lopes, R.A.; Beattie, W.; Thomson, J.; Herder, V.; et al. SARS-CoV-2 Spike Protein Induces Endothelial Inflammation Via Ace2 Independently of Viral Replication. Sci. Rep. 2023, 13, 14086. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, J.; Koller, A. Systemic Endothelial Inflammation: A Key Driver of Adverse Reactions to mRNA-Containing COVID-19 Vaccines. Vaccines 2025, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Dézsi, L.; Kökény, G.; Szénási, G.; Révész, C.; Mészáros, T.; Barta, B.A.; Facsko, R.; Szilasi, A.; Bakos, T.; Kozma, G.T. Acute Anaphylactic and Multiorgan Inflammatory Effects of Comirnaty in Pigs: Evidence of Spike Protein mRNA Transfection and Paralleling Inflammatory Cytokine Upregulation. bioRxiv 2025, 1–28. [Google Scholar] [CrossRef]
- Platt, L.R.; Estivariz, C.F.; Sutter, R.W. Vaccine-Associated Paralytic Poliomyelitis: A Review of the Epidemiology and Estimation of the Global Burden. J. Infect. Dis. 2014, 210 (Suppl. 1), S380–S389. [Google Scholar] [CrossRef]
- Baraff, L.J.; Manclark, C.R.; Cherry, J.D.; Christenson, P.; Marcy, S.M. Analyses of Adverse Reactions to Diphtheria and Tetanus Toxoids and Pertussis Vaccine by Vaccine Lot, Endotoxin Content, Pertussis Vaccine Potency and Percentage of Mouse Weight Gain. Pediatr. Infect. Dis. J. 1989, 8, 502–507. [Google Scholar] [CrossRef]
- Ipp, M.; Goldbach, M.; Greenberg, S.; Gold, R. Effect of Needle Change and Air Bubble in Syringe on Minor Adverse Reactions Associated with Diphtheria-Tetanus Toxoids-Pertussis-Polio Vaccination in Infants. Pediatr. Infect. Dis. J. 1990, 9, 291–293. [Google Scholar]
- Golaz, A.; Hardy, I.R.; Glushkevich, T.G.; Areytchiuk, E.K.; Deforest, A.; Strebel, P.; Wharton, M.; Sutter, R.W. Evaluation of a Single Dose of Diphtheria-Tetanus Toxoids among Adults in Odessa, Ukraine, 1995: Immunogenicity and Adverse Reactions. J. Infect. Dis. 2000, 181 (Suppl. 1), S203–S207. [Google Scholar] [CrossRef] [PubMed]
- Relyveld, E.H.; Bizzini, B.; Gupta, R.K. Rational Approaches to Reduce Adverse Reactions in Man to Vaccines Containing Tetanus and Diphtheria Toxoids. Vaccine 1998, 16, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.S.; Kalman, D.D. Successful Vaccination with Tetanus and Diphtheria and Acelluar Pertussis Vaccine after Adverse Reaction to Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine or Diphtheria and Tetanus Vaccine in Pediatric Patients. Ann. Allergy Asthma Immunol. 2019, 123, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Dall, C. Pediatric RSV Vaccine Trials on Hold, Fda Says. CIDRAP. Available online: https://www.cidrap.umn.edu/respiratory-syncytial-virus-rsv/pediatric-rsv-vaccine-trials-hold-fda-says?utm_source=chatgpt.com (accessed on 22 September 2025).
- FDA. Vaccines and Related Biological Products Advisory Committee Meeting: Considerations for Respiratory Syncytial Virus (RSV) Vaccine Safetyin Pediatric Populations. In FDA Briefing Document December 12, 2024; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/media/184301/download?utm_source=chatgpt.com (accessed on 22 September 2025).
- Andries, O.; Cafferty, S.M.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-Methylpseudouridine-Incorporated mRNA Outperforms Pseudouridine-Incorporated mRNA by Providing Enhanced Protein Expression and Reduced Immunogenicity in Mammalian Cell Lines and Mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.J.; Kim, D.; Kang, S.; Li, K.; Cha, I.; Sasaki, A.; Porras, J.; Xia, T.; Jung, J.U. Viral Codon Optimization on SARS-CoV-2 Spike Boosts Immunity in the Development of COVID-19 mRNA Vaccines. J. Med. Virol. 2023, 95, e29183. [Google Scholar] [CrossRef]
- Kachaev, Z.M.; Lebedeva, L.A.; Kozlov, E.N.; Shidlovskii, Y.V. Interplay of mRNA Capping and Transcription Machineries. Biosci. Rep. 2020, 40, BSR20192825. [Google Scholar] [CrossRef]
- Warminski, M.; Depaix, A.; Ziemkiewicz, K.; Spiewla, T.; Zuberek, J.; Drazkowska, K.; Kedzierska, H.; Popielec, A.; Baranowski, M.R.; Sklucka, M.; et al. Trinucleotide Cap Analogs with Triphosphate Chain Modifications: Synthesis, Properties, and Evaluation as mRNA Capping Reagents. Nucleic Acids Res. 2024, 52, 10788–10809. [Google Scholar] [CrossRef]
- Muttach, F.; Muthmann, N.; Rentmeister, A. Synthetic mRNA Capping. Beilstein J. Org. Chem. 2017, 13, 2819–2832. [Google Scholar] [CrossRef]
- Imprachim, N.; Yosaatmadja, Y.; Newman, J.A. Crystal Structures and Fragment Screening of SARS-CoV-2 Nsp14 Reveal Details of Exoribonuclease Activation and mRNA Capping and Provide Starting Points for Antiviral Drug Development. Nucleic Acids Res. 2023, 51, 475–487. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, Y.; Zhang, S.; Chen, S.J. mRNA Vaccine Sequence and Structure Design and Optimization: Advances and Challenges. J. Biol. Chem. 2024, 301, 108015. [Google Scholar] [CrossRef]
- Wang, Y.S.; Kumari, M.; Chen, G.H.; Hong, M.H.; Yuan, J.P.; Tsai, J.L.; Wu, H.C. mRNA-Based Vaccines and Therapeutics: An in-Depth Survey of Current and Upcoming Clinical Applications. J. Biomed. Sci. 2023, 30, 84. [Google Scholar] [CrossRef] [PubMed]
- Grandi, C.; Emmaneel, M.; Nelissen, F.H.T.; Roosenboom, L.W.M.; Petrova, Y.; Elzokla, O.; Hansen, M.M.K. Decoupled Degradation and Translation Enables Noise Modulation by Poly(a) Tails. Cell Syst. 2024, 15, 526–543.e7. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Lutz, J.; Kowalczyk, A.; Schlake, T.; Heidenreich, R. Rnactive(R) Technology: Generation and Testing of Stable and Immunogenic mRNA Vaccines. Methods Mol. Biol. 2017, 1499, 89–107. [Google Scholar] [PubMed]
- Rauch, S.; Lutz, J.; Muhe, J.; Kowalczyk, A.; Schlake, T.; Heidenreich, R. Sequence-Optimized mRNA Vaccines against Infectious Disease. Methods Mol. Biol. 2024, 2786, 183–203. [Google Scholar] [PubMed]
- Kramps, T. Introduction to RNA Vaccines Post COVID-19. Methods Mol. Biol. 2024, 2786, 1–22. [Google Scholar]
- Fu, Q.; Zhao, X.; Hu, J.; Jiao, Y.; Yan, Y.; Pan, X.; Wang, X.; Jiao, F. mRNA Vaccines in the Context of Cancer Treatment: From Concept to Application. J. Transl. Med. 2025, 23, 12. [Google Scholar] [CrossRef]
- Nallagatla, S.R.; Bevilacqua, P.C. Nucleoside Modifications Modulate Activation of the Protein Kinase Pkr in an RNA Structure-Specific Manner. RNA 2008, 14, 1201–1213. [Google Scholar] [CrossRef]
- Mueller, S.; Papamichail, D.; Coleman, J.R.; Skiena, S.; Wimmer, E. Reduction of the Rate of Poliovirus Protein Synthesis through Large-Scale Codon Deoptimization Causes Attenuation of Viral Virulence by Lowering Specific Infectivity. J. Virol. 2006, 80, 9687–9696. [Google Scholar] [CrossRef]
- Bentele, K.; Saffert, P.; Rauscher, R.; Ignatova, Z.; Bluthgen, N. Efficient Translation Initiation Dictates Codon Usage at Gene Start. Mol. Syst. Biol. 2013, 9, 675. [Google Scholar] [CrossRef]
- Scharff, L.B.; Childs, L.; Walther, D.; Bock, R. Local Absence of Secondary Structure Permits Translation of mRNAs That Lack Ribosome-Binding Sites. PLoS Genet. 2011, 7, e1002155. [Google Scholar] [CrossRef]
- Georgakopoulos-Soares, I.; Parada, G.E.; Hemberg, M. Secondary Structures in Rna Synthesis, Splicing and Translation. Comput. Struct. Biotechnol. J. 2022, 20, 2871–2884. [Google Scholar] [CrossRef] [PubMed]
- Kozak, M. Pushing the Limits of the Scanning Mechanism for Initiation of Translation. Gene 2002, 299, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Ota, N.; Itani, M.; Aoki, T.; Sakurai, A.; Fujisawa, T.; Okada, Y.; Noda, K.; Arakawa, Y.; Tokuda, S.; Tanikawa, R. Expression of SARS-CoV-2 Spike Protein in Cerebral Arteries: Implications for Hemorrhagic Stroke Post-mRNA Vaccination. J. Clin. Neurosci. 2025, 136, 111223. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Goldsmith, J.A.; Schaub, J.M.; DiVenere, A.M.; Kuo, H.C.; Javanmardi, K.; Le, K.C.; Wrapp, D.; Lee, A.G.; Liu, Y.; et al. Structure-Based Design of Prefusion-Stabilized SARS-CoV-2 Spikes. Science 2020, 369, 1501–1505. [Google Scholar] [CrossRef]
- Tan, T.J.C.; Mou, Z.; Lei, R.; Ouyang, W.O.; Yuan, M.; Song, G.; Andrabi, R.; Wilson, I.A.; Kieffer, C.; Dai, X.; et al. High-Throughput Identification of Prefusion-Stabilizing Mutations in SARS-CoV-2 Spike. Nat. Commun. 2023, 14, 2003. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Cottrell, C.A.; Wang, N.; Pallesen, J.; Yassine, H.M.; Turner, H.L.; Corbett, K.S.; Graham, B.S.; McLellan, J.S.; Ward, A.B. Pre-Fusion Structure of a Human Coronavirus Spike Protein. Nature 2016, 531, 118–121. [Google Scholar] [CrossRef]
- Pallesen, J.; Wang, N.; Corbett, K.S.; Wrapp, D.; Kirchdoerfer, R.N.; Turner, H.L.; Cottrell, C.A.; Becker, M.M.; Wang, L.; Shi, W.; et al. Immunogenicity and Structures of a Rationally Designed Prefusion Mers-Cov Spike Antigen. Proc. Natl. Acad. Sci. USA 2017, 114, E7348–E7357. [Google Scholar] [CrossRef]
- Juraszek, J.; Rutten, L.; Blokland, S.; Bouchier, P.; Voorzaat, R.; Ritschel, T.; Bakkers, M.J.G.; Renault, L.L.R.; Langedijk, J.P.M. Stabilizing the Closed SARS-CoV-2 Spike Trimer. Nat. Commun. 2021, 12, 244. [Google Scholar] [CrossRef]
- Korchinsky, N.; Davis, A.M.; Boros, L.G. Nutritional Deuterium Depletion and Health: A Scoping Review. Metabolomics 2024, 20, 117. [Google Scholar] [CrossRef]
- Parhiz, H.; Brenner, J.S.; Patel, P.N.; Papp, T.E.; Shahnawaz, H.; Li, Q.; Shi, R.; Zamora, M.E.; Yadegari, A.; Marcos-Contreras, O.A.; et al. Added to Pre-Existing Inflammation, mRNA-Lipid Nanoparticles Induce Inflammation Exacerbation (Ie). J. Control. Release 2022, 344, 50–61. [Google Scholar] [CrossRef]
- Szoke, D.; Kovacs, G.; Kemecsei, E.; Balint, L.; Szotak-Ajtay, K.; Aradi, P.; Dinnyes, A.S.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; et al. Nucleoside-Modified VEGF mRNA Induces Organ-Specific Lymphatic Growth and Reverses Experimental Lymphedema. Nat. Commun. 2021, 12, 3460. [Google Scholar] [CrossRef]
- Szebeni, J.; Storm, G.; Ljubimova, J.Y.; Castells, M.; Phillips, E.J.; Turjeman, K.; Barenholz, Y.; Crommelin, D.J.A.; Dobrovolskaia, M.A. Applying Lessons Learned from Nanomedicines to Understand Rare Hypersensitivity Reactions to mRNA-Based SARS-CoV-2 Vaccines. Nat. Nanotechnol. 2022, 17, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pfizer Australia Pty Ltd. Nonclinical Evaluation Report: BNT162b2 [mRNA] COVID-19 Vaccine (Comirnaty). Available online: https://www.tga.gov.au/sites/default/files/foi-2389-06.pdf (accessed on 22 September 2025).
- Anchordoquy, T.; Artzi, N.; Balyasnikova, I.V.; Barenholz, Y.; La-Beck, N.M.; Brenner, J.S.; Chan, W.C.W.; Decuzzi, P.; Exner, A.A.; Gabizon, A.; et al. Mechanisms and Barriers in Nanomedicine: Progress in the Field and Future Directions. ACS Nano 2024, 18, 13983–13999. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hoorn, D.; Aitha, A.; Breier, D.; Peer, D. The Immunostimulatory Nature of mRNA Lipid Nanoparticles. Adv. Drug Deliv. Rev. 2024, 205, 115175. [Google Scholar] [CrossRef] [PubMed]
- Vogt, W. Anaphylatoxins: Possible Roles in Disease. Complement 1986, 3, 177–188. [Google Scholar] [CrossRef]
- Schanzenbacher, J.; Kohl, J.; Karsten, C.M. Anaphylatoxins Spark the Flame in Early Autoimmunity. Front. Immunol. 2022, 13, 958392. [Google Scholar] [CrossRef]
- Alving, C.R.; Kinsky, S.C.; Haxby, J.A.; Kinsky, C.B. Antibody Binding and Complement Fixation by a Liposomal Model Membrane. Biochemistry 1969, 8, 1582–1587. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy Caused by Liposomes, Micellar Carriers of Intravenous Drugs, and Radiocontrast Agents. Crit. Rev.™ Ther. Drug Carr. Syst. 2001, 18, 567–606. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A New Class of Drug-Induced Acute Immune Toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Szebeni, J. Complement Activation-Related Pseudoallergy: A Stress Reaction in Blood Triggered by Nanomedicines and Biologicals. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef]
- Dezsi, L.; Meszaros, T.; Kozma, G.; Mária, H.-V.; Olah, C.Z.; Szabo, M.; Patko, Z.; Fulop, T.; Hennies, M.; Szebeni, M.; et al. A Naturally Hypersensitive Porcine Model May Help Understand the Mechanism of COVID-19 mRNA Vaccine-Induced Rare (Pseudo) Allergic Reactions: Complement Activation as a Possible Contributing Factor. Geroscience 2022, 44, 597–618. [Google Scholar] [CrossRef]
- de Vrieze, J. Suspicions Grow That Nanoparticles in Pfizer’s COVID-19 Vaccine Trigger Rare Allergic Reactions. ScienceInsider/News Piece, Published Online December 21, 2020. Available online: https://doi.org/10.1126/science.abg2359 (accessed on 30 September 2025).
- Szebeni, J.; Fontana, J.L.; Wassef, N.; Mongan, P.D.; Morse, D.S.; Dobbins, D.E.; Stahl, G.L.; Bunger, R.; Alving, C.R. Hemodynamic Changes Induced by Liposomes and Liposome-Encapsulated Hemoglobin in Pigs: A Model for Pseudoallergic Cardiopulmonary Reactions to Liposomes: Role of Complement and Inhibition by Soluble Cr1 and Anti-C5a Antibody. Circulation 1999, 99, 2302–2309. [Google Scholar] [CrossRef]
- Barth, I.; Weisser, K.; Gaston-Tischberger, D.; Mahler, V.; Keller-Stanislawski, B. Anaphylactic Reactions after COVID-19 Vaccination in Germany. Allergol. Sel. 2023, 7, 90–100. [Google Scholar] [CrossRef]
- Barta, B.A.; Radovits, T.; Dobos, A.B.; Kozma, G.T.; Mészáros, T.; Berényi, P.; Facskó, R.; Fülöp, T.; Merkely, B.; Szebeni, J. Comirnaty-Induced Cardiopulmonary Distress and Other Symptoms of Complement-Mediated Pseudo-Anaphylaxis in a Hyperimmune Pig Model: Causal Role of Anti-Peg Antibodies. Vaccine X 2024, 19, 100497. [Google Scholar] [CrossRef]
- Yang, Q.; Lai, S.K. Anti-Peg Immunity: Emergence, Characteristics, and Unaddressed Questions. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 655–677. [Google Scholar] [CrossRef]
- Chen, E.; Chen, B.M.; Su, Y.C.; Chang, Y.C.; Cheng, T.L.; Barenholz, Y.; Roffler, S.R. Premature Drug Release from Polyethylene Glycol (PEG)-Coated Liposomal Doxorubicin Via Formation of the Membrane Attack Complex. ACS Nano 2020, 14, 7808–7822. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-Peg Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to Pegylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Szebeni, J. Complement Activation: A Potential Threat on the Safety of Poly(Ethylene Glycol)-Coated Nanomedicines. ACS Nano 2020, 14, 7682–7688. [Google Scholar] [CrossRef]
- USFDA. Guidance for Industry: Immunogenicity Assessment for Therapeutic Protein Products. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-assessment-therapeutic-protein-products (accessed on 22 September 2025).
- Lederer, K.; Castano, D.; Atria, D.G.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid Nanoparticles Enhance the Efficacy of mRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid Nanoparticles Enhance the Efficacy of mRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2022, 55, 1136–1138. [Google Scholar] [CrossRef]
- Hemmrich, E.; McNeil, S. Active Ingredient Vs Excipient Debate for Nanomedicines. Nat. Nanotechnol. 2023, 18, 692–695. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified mRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Ferraresso, F.; Badior, K.; Seadler, M.; Zhang, Y.; Wietrzny, A.; Cau, M.F.; Haugen, A.; Rodriguez, G.G.; Dyer, M.R.; Cullis, P.R.; et al. Protein Is Expressed in All Major Organs after Intravenous Infusion of mRNA -Lipid Nanoparticles in Swine. Mol. Ther. Methods Clin. Dev. 2024, 32, 101314. [Google Scholar] [CrossRef] [PubMed]
- Lachmann, P.J. The Amplification Loop of the Complement Pathways. Adv. Immunol. 2009, 104, 115–149. [Google Scholar] [PubMed]
- Khalid, M.B.; Frischmeyer-Guerrerio, P.A. The Conundrum of COVID-19 mRNA Vaccine-Induced Anaphylaxis. J. Allergy Clin. Immunol. Glob. 2023, 2, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, P.W.; Allison, M.E.; Akkaraju, S.; Goodnow, C.C.; Fearon, D.T. C3d of Complement as a Molecular Adjuvant: Bridging Innate and Acquired Immunity. Science 1996, 271, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Wills-Karp, M. Complement Activation Pathways: A Bridge between Innate and Adaptive Immune Responses in Asthma. Proc. Am. Thorac. Soc. 2007, 4, 247–251. [Google Scholar] [CrossRef]
- Kozma, G.T.; Meszaros, T.; Berenyi, P.; Facsko, R.; Patko, Z.; Olah, C.Z.; Nagy, A.; Fulop, T.G.; Glatter, K.A.; Radovits, T.; et al. Role of Anti-Polyethylene Glycol (Peg) Antibodies in the Allergic Reactions to PEG-Containing COVID-19 Vaccines: Evidence for Immunogenicity of Peg. Vaccine 2023, 41, 4561–4570. [Google Scholar] [CrossRef]
- Carreno, J.M.; Singh, G.; Tcheou, J.; Srivastava, K.; Gleason, C.; Muramatsu, H.; Desai, P.; Aberg, J.A.; Miller, R.L.; PARIS Study Group; et al. mRNA-1273 but Not Bnt162b2 Induces Antibodies against Polyethylene Glycol (Peg) Contained in mRNA-Based Vaccine Formulations. Vaccine 2022, 40, 6114–6124. [Google Scholar] [CrossRef]
- Ju, Y.; Lee, W.S.; Pilkington, E.H.; Kelly, H.G.; Li, S.; Selva, K.J.; Wragg, K.M.; Subbarao, K.; Nguyen, T.H.O.; Rowntree, L.C.; et al. Anti-Peg Antibodies Boosted in Humans by SARS-CoV-2 Lipid Nanoparticle mRNA Vaccine. ACS Nano 2022, 16, 11769–11780. [Google Scholar] [CrossRef]
- Bavli, Y.; Chen, B.M.; Gross, G.; Hershko, A.; Turjeman, K.; Roffler, S.; Barenholz, Y. Anti-PEG Antibodies before and after a First Dose of ComirnatyR (mRNA-LNP-Based SARS-CoV-2 Vaccine). J. Control. Release 2023, 354, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.H.Y.; Leung, J.; Zhang, Y.; Strong, C.; Basha, G.; Momeni, A.; Chen, Y.; Jan, E.; Abdolahzadeh, A.; Wang, X.; et al. Induction of Bleb Structures in Lipid Nanoparticle Formulations of mRNA Leads to Improved Transfection Potency. Adv. Mater. 2023, 35, e2303370. [Google Scholar] [CrossRef] [PubMed]
- Unruh, T.; Gotz, K.; Vogel, C.; Frohlich, E.; Scheurer, A.; Porcar, L.; Steiniger, F. Mesoscopic Structure of Lipid Nanoparticle Formulations for mRNA Drug Delivery: Comirnaty and Drug-Free Dispersions. ACS Nano 2024, 18, 9746–9764. [Google Scholar] [CrossRef] [PubMed]
- Pfizer; BioNtech. Comirnaty Original/Omicron Ba.4-5 Dispersion for Injection. Available online: https://labeling.pfizer.com/ShowLabeling.aspx?id=19823 (accessed on 22 September 2025).
- Raoult, D. Confirmation of the Presence of Vaccine DNA in the Pfizer Anti-COVID-19 Vaccine. Hal Open Sci. 2024, 04778576. [Google Scholar]
- Lee, M.; Ki-Yeob, J. Foreign Materials in Blood Samples of Recipients of COVID19 Vaccines. Int. J. Vaccine Theory Pract. Res. 2022, 2, 249–265. [Google Scholar] [CrossRef]
- Diblasi, L.; Monteverde, M.; Nonis, D.; Sangorrín, M. At Least 55 Undeclared Chemical Elements Found in COVID-19 Vaccines from Astrazeneca, Cansino, Moderna, Pfizer, Sinopharm and Sputnik V, with Precise Icp-Ms. Int. J. Vaccine Theory Pract. Res. 2024, 3, 1367–1393. [Google Scholar] [CrossRef]
- Kämmerer, U.; Schulz, V.; Steger, K. Biontech Rna-Based COVID-19 Injections Contain Large Amounts of Residual DNA Including an Sv40 Promoter/Enhancer Sequence. Sci. Public. Health Policy Law 2024, 5, 2019–2024. [Google Scholar]
- CGC. Case Briefing Document. Available online: http://ukcitizen2021.org/Case_Briefing_Document_and_lab_report_Ref_AUC_101_Report%20.pdf (accessed on 22 September 2025).
- Doherty, M.; Whicher, J.T.; Dieppe, P.A. Activation of the Alternative Pathway of Complement by Monosodium Urate Monohydrate Crystals and Other Inflammatory Particles. Ann. Rheum. Dis. 1983, 42, 285–291. [Google Scholar] [CrossRef]
- Russell, I.J.; Mansen, C.; Kolb, L.M.; Kolb, W.P. Activation of the Fifth Component of Human Complement (C5) Induced by Monosodium Urate Crystals: C5 Convertase Assembly on the Crystal Surface. Clin. Immunol. Immunopathol. 1982, 24, 239–250. [Google Scholar] [CrossRef]
- Fields, T.R.; Abramson, S.B.; Weissmann, G.; Kaplan, A.P.; Ghebrehiwet, B. Activation of the Alternative Pathway of Complement by Monosodium Urate Crystals. Clin. Immunol. Immunopathol. 1983, 26, 249–257. [Google Scholar] [CrossRef]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of Complement-Activating Particles in Aqueous Solutions of Taxol: Possible Role in Hypersensitivity Reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar]
- Baranyi, L.; Szebeni, J.; Savay, S.; Bodo, M.; Basta, M.; Bentley, T.B.; Bunger, R.; Alving, C.R. Complement-Dependent Shock and Tissue Damage Induced by Intravenous Injection of Cholesterol-Enriched Liposomes in Rats. J. Appl. Res. 2003, 3, 221–231. Available online: https://jarcet.com/articles/Vol3Iss3/Baranyi.htm?utm_source=chatgpt.com (accessed on 30 September 2025).
- Ratajczak, M.Z.; Lee, H.; Wysoczynski, M.; Wan, W.; Marlicz, W.; Laughlin, M.J.; Kucia, M.; Janowska-Wieczorek, A.; Ratajczak, J. Novel Insight into Stem Cell Mobilization-Plasma Sphingosine-1-Phosphate Is a Major Chemoattractant That Directs the Egress of Hematopoietic Stem Progenitor Cells from the Bone Marrow and Its Level in Peripheral Blood Increases During Mobilization Due to Activation of Complement Cascade/Membrane Attack Complex. Leukemia 2010, 24, 976–985. [Google Scholar] [PubMed]
- Klein, C.P.; de Groot, K.; van Kamp, G. Activation of Complement C3 by Different Calcium Phosphate Powders. Biomaterials 1983, 4, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Hasselbacher, P. Binding of Immunoglobulin and Activation of Complement by Asbestos Fibers. J. Allergy Clin. Immunol. 1979, 64, 294–298. [Google Scholar] [CrossRef]
- Ramos da Silva, J.; Rodrigues, K.B.; Pelegrin, G.F.; Sales, N.S.; Muramatsu, H.; de Oliveira Silva, M.; Porchia, B.; Moreno, A.C.R.; Aps, L.; Venceslau-Carvalho, A.A.; et al. Single Immunizations of Self-Amplifying or Non-Replicating mRNA-LNP Vaccines Control HPV-Associated Tumors in Mice. Sci. Transl. Med. 2023, 15, eabn3464. [Google Scholar] [CrossRef]
- Valdes Angues, R.; Perea Bustos, Y. SARS-CoV-2 Vaccination and the Multi-Hit Hypothesis of Oncogenesis. Cureus 2023, 15, e50703. [Google Scholar] [CrossRef]
- Kruger, U. COVID Vaccination and Turbo-Cancer. Multiple Tumors in Multiple Organs. Available online: https://www.globalresearch.ca/turbo-cancer-we-have-problem/5789172 (accessed on 22 September 2025).
- Wiedmeier-Nutor, J.E.; Iqbal, M.; Rosenthal, A.C.; Bezerra, E.D.; Garcia-Robledo, J.E.; Bansal, R.; Johnston, P.B.; Hathcock, M.; Larsen, J.T.; Bergsagel, P.L.; et al. Response to COVID-19 Vaccination Post-Car T Therapy in Patients with Non-Hodgkin Lymphoma and Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2023, 23, 456–462. [Google Scholar] [CrossRef]
- Sekizawa, A.; Hashimoto, K.; Kobayashi, S.; Kozono, S.; Kobayashi, T.; Kawamura, Y.; Kimata, M.; Fujita, N.; Ono, Y.; Obuchi, Y.; et al. Rapid Progression of Marginal Zone B-Cell Lymphoma after COVID-19 Vaccination (BNT-162b2): A Case Report. Front. Med. 2022, 9, 963393. [Google Scholar] [CrossRef]
- Gibo, M.; Kojima, S.; Fujisawa, A.; Kikuchi, T.; Fukushima, M. Increased Age-Adjusted Cancer Mortality after the Third mRNA-Lipid Nanoparticle Vaccine Dose During the COVID-19 Pandemic in Japan. Cureus 2024, 16, e57860. [Google Scholar]
- Nacionales, D.C.; Weinstein, J.S.; Yan, X.J.; Albesiano, E.; Lee, P.Y.; Kelly-Scumpia, K.M.; Lyons, R.; Satoh, M.; Chiorazzi, N.; Reeves, W.H. B Cell Proliferation, Somatic Hypermutation, Class Switch Recombination, and Autoantibody Production in Ectopic Lymphoid Tissue in Murine Lupus. J. Immunol. 2009, 182, 4226–4236. [Google Scholar] [CrossRef]
- Gentilini, P.; Lindsay, J.C.; Konishi, N.; Fukushima, M.; Polykretis, P. A Case Report of Acute Lymphoblastic Leukaemia (ALL)/Lymphoblastic Lymphoma (LBL) Following the Second Dose of Comirnaty®: An Analysis of the Potential Pathogenic Mechanism Based on of the Existing Literature. Preprints 2024, 2024031661. [Google Scholar] [CrossRef]
- Hill, A.B. The Environment and Disease: Association or Causation? Proc. R. Soc. Med. 1965, 58, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Menetrier-Caux, C.; Ray-Coquard, I.; Blay, J.Y.; Caux, C. Lymphopenia in Cancer Patients and Its Effects on Response to Immunotherapy: An Opportunity for Combination with Cytokines? J. Immunother. Cancer 2019, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; El-Deiry, W.S. Transfected SARS-CoV-2 Spike DNA for Mammalian Cell Expression Inhibits P53 Activation of P21(Waf1), Trail Death Receptor Dr5 and Mdm2 Proteins in Cancer Cells and Increases Cancer Cell Viability after Chemotherapy Exposure. Oncotarget 2024, 15, 275–284. [Google Scholar] [CrossRef]
- Stockert, J.A.; Gupta, A.; Herzog, B.; Yadav, S.S.; Tewari, A.K.; Yadav, K.K. Predictive Value of Pseudouridine in Prostate Cancer. Am. J. Clin. Exp. Urol. 2019, 7, 262–272. [Google Scholar]
- Stockert, J.A.; Weil, R.; Yadav, K.K.; Kyprianou, N.; Tewari, A.K. Pseudouridine as a Novel Biomarker in Prostate Cancer. Urol. Oncol. 2021, 39, 63–71. [Google Scholar] [CrossRef]
- Han, X.; Wang, M.; Zhao, Y.L.; Yang, Y.; Yang, Y.G. RNA Methylations in Human Cancers. Semin. Cancer Biol. 2021, 75, 97–115. [Google Scholar] [CrossRef]
- Raszek, M.; Cowley, D.; Redwan, E.M.; Vladimir, N.; Uversky, V.N.; Rubio-Casillas, A. Exploring the Possible Link between the Spike Protein Immunoglobulin G4 Antibodies and Cancer Progression. Explor. Immunol. 2024, 4, 267–284. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Shirjang, S.; Baradaran, B. Micro-Rnas: The New Potential Biomarkers in Cancer Diagnosis, Prognosis and Cancer Therapy. Cell. Mol. Biol. 2015, 61, 1–10. [Google Scholar] [PubMed]
- Chang, W.; Chang, Q.; Lu, H.; Liu, S.; Li, Y.; Chen, C. Microrna-873-5p Suppresses Cell Malignant Behaviors of Thyroid Cancer Via Targeting Cxcl5 and Regulating P53 Pathway. Hum. Vaccines Immunother. 2022, 18, 2065837. [Google Scholar] [CrossRef] [PubMed]
- Trifylli, E.M.; Kriebardis, A.G.; Koustas, E.; Papadopoulos, N.; Fortis, S.P.; Tzounakas, V.L.; Anastasiadi, A.T.; Sarantis, P.; Vasileiadi, S.; Tsagarakis, A.; et al. A Current Synopsis of the Emerging Role of Extracellular Vesicles and Micro-mRNAs in Pancreatic Cancer: A Forward-Looking Plan for Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 3406. [Google Scholar] [CrossRef]
- Maiese, A.; Baronti, A.; Manetti, A.C.; Di Paolo, M.; Turillazzi, E.; Frati, P.; Fineschi, V. Death after the Administration of COVID-19 Vaccines Approved by Ema: Has a Causal Relationship Been Demonstrated? Vaccines 2022, 10, 308. [Google Scholar] [CrossRef]
- Watson, O.J.; Barnsley, G.; Toor, J.; Hogan, A.B.; Winskill, P.; Ghani, A.C. Global Impact of the First Year of COVID-19 Vaccination: A Mathematical Modelling Study. Lancet Infect. Dis. 2022, 22, 1293–1302. [Google Scholar] [CrossRef]
- Mesle, M.M.; Brown, J.; Mook, P.; Hagan, J.; Pastore, R.; Bundle, N.; Spiteri, G.; Ravasi, G.; Nicolay, N.; Andrews, N.; et al. Estimated Number of Deaths Directly Averted in People 60 Years and Older as a Result of COVID-19 Vaccination in the Who European Region, December 2020 to November 2021. Eurosurveillance 2021, 26, 2101021. [Google Scholar] [CrossRef]
- Sorli, A.S. The Discrepancy Between the Number of Saved Lives with COVID-19 Vaccination and Statistics of Our World Data. J. Clin. Trials 2025, S32, 001. [Google Scholar]
- Beattie, K.A. Worldwide Bayesian Causal Impact Analysis of Vaccine Administration on Deaths and Cases Associated with COVID-19: A Bigdata Analysis of 145 Countries. Preprint 2021. [Google Scholar] [CrossRef]
- Kakeya, H.; Nitta, T.; Kamijima, Y.; Miyazawa, T. Significant Increase in Excess Deaths after Repeated COVID-19 Vaccination in Japan. JMA J. 2025, 8, 584–586. [Google Scholar] [CrossRef]
- Yaraghi, P.; Kheyri, A.; Mikaeili, N.; Boroumand, A.; Abbasifard, M.; Farhangnia, P.; Rezagholizadeh, F.; Khorramdelazad, H. Nanoparticle-Mediated Enhancement of DNA Vaccines: Revolutionizing Immunization Strategies. Int. J. Biol. Macromol. 2025, 302, 140558. [Google Scholar] [CrossRef]
- Saleh, M.; El-Moghazy, A.; Elgohary, A.H.; Saber, W.I.A.; Helmy, Y.A. Revolutionizing Nanovaccines: A New Era of Immunization. Vaccines 2025, 13, 126. [Google Scholar] [CrossRef]
- Leong, K.Y.; Tham, S.K.; Poh, C.L. Revolutionizing Immunization: A Comprehensive Review of mRNA Vaccine Technology and Applications. Virol. J. 2025, 22, 71. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Ramet, M.; et al. Efficacy and Safety of an mRNA-Based Rsv Pref Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef] [PubMed]
- ModernaTX, Inc. Prescribing Information: (Respiratory Syncytial Virus Vaccine) Injectable Suspension, for Intramuscular Use. Available online: https://www.fda.gov/media/179005/download?attachment=&utm_source=chatgpt.com (accessed on 30 September 2025).
- FDA. FDA Approves and Authorizes Updated mRNA COVID-19 Vaccines to Better Protect against Currently Circulating Variants. In FDA New Release; FDA: Silver Spring, MD, USA, 2024. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-and-authorizes-updated-mrna-covid-19-vaccines-better-protect-against-currently (accessed on 22 September 2025).
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA Vaccines in Disease Prevention and Treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Ladak, R.J.; He, A.J.; Huang, Y.H.; Ding, Y. The Current Landscape of mRNA Vaccines against Viruses and Cancer-a Mini Review. Front. Immunol. 2022, 13, 885371. [Google Scholar] [CrossRef]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA Vaccines: Clinical Advances and Future Opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [CrossRef]
- Xie, W.; Chen, B.; Wong, J. Evolution of the Market for mRNA Technology. Nat. Rev. Drug Discov. 2021, 20, 735–736. [Google Scholar] [CrossRef]
- Insights, Fortune Business. Lipid Nanoparticles Market Size, Share & Industry Analysis, by Type (Solid Lipid Nanoparticles (SLNP), Nanostructured Lipid Carriers (NSLC), and Others), by Application (Therapeutics, Research), by End-User (Pharmaceutical & Biotechnology Companies, Academic & Research Institutes, and Others), and Regional Forecast, 2024–2032. Available online: https://www.fortunebusinessinsights.com/lipid-nanoparticles-market-106960 (accessed on 30 September 2025).
- Intelligence, Mordor. mRNA Vaccine and Therapeutics Market Size & Share Analysis—Growth Trends & Forecasts (2024–2029). Available online: https://www.mordorintelligence.com/industry-reports/mrna-vaccines-and-therapeutics-market (accessed on 22 September 2025).
- Nathan-Kazis, J.; Kennedy’s Edict Results in New, Narrowed Vaccine Guidance from CDC. Barron’s Online 30 May 2025. Available online: https://www.barrons.com/articles/vaccines-cdc-covid-19-pregnant-women-children-kennedy-6f19ab5f (accessed on 30 September 2025).
- Food and Drug Administration: FDA Approves Required Updated Warning in Labeling of mRNA COVID-19 Vaccines Regarding Myocarditis and Pericarditis Following Vaccination 25 June 2025. Available online: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-approves-required-updated-warning-labeling-mrna-covid-19-vaccines-regarding-myocarditis-and (accessed on 22 September 2025).
- Scribner, H.; Rubin, A. A Guide to the New COVID Vaccine Recommendations. AXIOS. Available online: https://www.axios.com/2025/08/28/cdc-covid-vaccine-rules-recommendations-high-risk-fda (accessed on 22 September 2025).
- Lim, D. 2025. FDA Revokes Emergency Use of COVID-19 Vaccines. Politico, 27 August. Available online: https://www.politico.com/news/2025/08/27/fda-revokes-emergency-use-of-covid-19-vaccines-00530626 (accessed on 22 September 2025).
- Daily Beast. 2025 RFK Jr. Celebrates as FDA Ends COVID Vaccine Mandate 27 August 2025. Available online: https://www.thedailybeast.com/rfk-jr-celebrates-as-fda-ends-covid-vaccine-mandate (accessed on 22 September 2025).
- El-Deiry, W.; Kupperwasser, C. Workup Safety Uncertainties of mRNA COVID Vaccines. Meeting of the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention, Atlanta, GA, 18–19 September 2025. Available online: https://www.cdc.gov/acip/downloads/agendas/final-posted-2025-09-19-508.pdf (accessed on 22 September 2025).
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR-Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef]
- Hu, M.; Shoaibi, A.; Feng, Y.; Lloyd, P.C.; Wong, H.L.; Smith, E.R.; Amend, K.L.; Kline, A.; Beachler, D.C.; Gruber, J.F.; et al. Safety of Ancestral Monovalent BNT162b2, mRNA-1273, and NVX-CoV2373 COVID-19 Vaccines in US Children Aged 6 Months to 17 Years. JAMA Netw. Open 2024, 7, e248192. [Google Scholar] [CrossRef]
- Eens, S.; Van Hecke, M.; Van den Bogaert, S.; Favere, K.; Cools, N.; Fransen, E.; Roskams, T.; Heidbuchel, H.; Guns, P.J. A Murine Model of mRNA COVID-19 Vaccine-Induced Myocarditis: A Shot in the Dark? JACC Basic Transl. Sci. 2024, 9, 1026–1028. [Google Scholar] [CrossRef]
- Zuin, M.; Zimelli, E.; Valle, C.D.; Cavedon, S.; Rigatelli, G.; Bilato, C. Diagnosis of Acute Myocarditis Following mRNA Vaccines against SARS-CoV-2: A Methodological Review. Viruses 2023, 15, 929. [Google Scholar] [CrossRef] [PubMed]
- Zirkenbach, V.A.; Ignatz, R.M.; Öttl, R.; Cehreli, Z.; Stroikova, V.; Kaya, M.; Lehmann, L.H.; Preusch, M.R.; Frey, N.; Kaya, Z. Effect of SARS-CoV-2 mRNA-Vaccine on the Induction of Myocarditis in Different Murine Animal Models. Int. J. Mol. Sci. 2023, 24, 5011. [Google Scholar] [CrossRef] [PubMed]
- Yeni, M. COVID-19 BNT162b2 mRNA vaccine induced myocarditis with left ventricular thrombus in a young male. Acta Cardiol. 2023, 78, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T. Acute Myocarditis after the Third Dose of COVID-19 mRNA-1273 Vaccine. J. Gen. Fam. Med. 2023, 24, 188–189. [Google Scholar] [CrossRef]
- Weerts, V.; Lempereur, M.; Léonard, P.; Lancellotti, P. Facing COVID-19: Myocarditis following vaccination with mRNA SARS-CoV-2. Rev. Medicale Liege 2023, 78, 141–146. [Google Scholar]
- Wassif, M.; Lo, P.; Satouris, P.; Swan, L.; Tardo, D.; Kovacic, J.C.; Muller, D.; Muthiah, K.; Kotlyar, E.; Bart, N.K. Acute Myocarditis and Pericarditis after mRNA COVID-19 Vaccinations-a Single-Centre Retrospective Analysis. Heart Lung Circ. 2023, 32, 467–479. [Google Scholar] [CrossRef]
- Vila-Olives, R.; Uribarri, A.; Martínez-Martínez, M.; Argudo, E.; Bonilla, C.; Chiscano, L.; Herrador, L.; Gabaldón, A.; Buera, I.; Vidal, M.; et al. Fulminant myocarditis following SARS-CoV-2 mRNA vaccination rescued with venoarterial ECMO: A report of two cases. Perfusion 2023, 39, 655–659. [Google Scholar] [CrossRef]
- Ulucay, A.S.; Singh, G.; Kanuri, S.H. Do COVID-19 viral infection and its mRNA vaccine carry an equivalent risk of myocarditis? Review of the current evidence, insights, and future directions. Indian Heart J. 2023, 75, 217–223. [Google Scholar] [CrossRef]
- Tome, J.; Cowan, L.T.; Fung, I.C.-H. A Pharmacoepidemiological Study of Myocarditis and Pericarditis Following the First Dose of mRNA COVID-19 Vaccine in Europe. Microorganisms 2023, 11, 1099. [Google Scholar] [CrossRef]
- Straus, W.; Urdaneta, V.; Esposito, D.B.; Mansi, J.A.; Rodriguez, C.S.; Burton, P.; Vega, J.M. Analysis of Myocarditis Among 252 Million mRNA-1273 Recipients Worldwide. Clin. Infect. Dis. 2023, 76, e544–e552. [Google Scholar] [CrossRef]
- Stowe, J.; Miller, E.; Andrews, N.; Whitaker, H.J. Risk of myocarditis and pericarditis after a COVID-19 mRNA vaccine booster and after COVID-19 in those with and without prior SARS-CoV-2 infection: A self-controlled case series analysis in England. PLoS Med. 2023, 20, e1004245. [Google Scholar] [CrossRef] [PubMed]
- Sim, J.-Y.; Kim, S.-Y.; Kim, E.-K. The incidence and clinical characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination in Republic of Korea adolescents from July 2021 to September 2022. Osong Public Health Res. Perspect. 2023, 14, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Shime, M.; Nozaki, Y.; Morita, A.; Ishiodori, T.; Murakami, T.; Yamasaki, H.; Yamamoto, M.; Takada, H. Life-Threatening Severe Acute Respiratory Syndrome Coronavirus-2 mRNA Vaccine-Associated Myocarditis after COVID-19 Myocarditis. J. Paediatr. Child Health 2023, 59, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Shenton, P.; Cheng, D.; Simm, P.; Jones, B.; Crawford, N. Myocarditis following COVID-19 mRNA vaccinations: Twin and sibling case series. Vaccine X 2023, 14, 100350. [Google Scholar] [CrossRef]
- Altman, N.L.; Berning, A.A.; Mann, S.C.; Quaife, R.A.; Gill, E.A.; Auerbach, S.R.; Campbell, T.B.; Bristow, M.R. Vaccination-Associated Myocarditis and Myocardial Injury. Circ. Res. 2023, 132, 1338–1357. [Google Scholar] [CrossRef]
- Jabagi, M.J.; Botton, J.; Bertrand, M.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Myocardial Infarction, Stroke, and Pulmonary Embolism After BNT162b2 mRNA COVID-19 Vaccine in People Aged 75 Years or Older. JAMA 2022, 327, 80–82. [Google Scholar] [CrossRef]
- Botton, J.; Jabagi, M.J.; Bertrand, M.; Baricault, B.; Drouin, J.; Le Vu, S.; Weill, A.; Farrington, P.; Zureik, M.; Dray-Spira, R. Risk for Myocardial Infarction, Stroke, and Pulmonary Embolism Following COVID-19 Vaccines in Adults Younger Than 75 Years in France. Ann. Intern. Med. 2022, 175, 1250–1257. [Google Scholar] [CrossRef]
- Liko, J.; Cieslak, P.R. Assessment of Risk for Sudden Cardiac Death Among Adolescents and Young Adults After Receipt of COVID-19 Vaccine—Oregon, June 2021–December 2022. MMWR-Morb. Mortal. Wkly. Rep. 2024, 73, 317–320. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: SARS-CoV-2 mRNA Vaccines and the Possible Mechanism of Vaccine-Induced Immune Thrombotic Thrombocytopenia (VITT). Med. Sci. Monit. 2021, 27, e932899-e2. [Google Scholar] [CrossRef]
- Rzymski, P.; Perek, B.; Flisiak, R. Thrombotic Thrombocytopenia after COVID-19 Vaccination: In Search of the Underlying Mechanism. Vaccines 2021, 9, 559. [Google Scholar] [CrossRef]
- Ostrowski, S.R.; Søgaard, O.S.; Tolstrup, M.; Stærke, N.B.; Lundgren, J.; Østergaard, L.; Hvas, A.-M. Inflammation and Platelet Activation After COVID-19 Vaccines—Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis. Front. Immunol. 2021, 12, 779453. [Google Scholar] [CrossRef]
- Yoshida, K.; Tanaka, K.; Suto, Y.; Fukuda, H. Repeated Cardioembolic Stroke after COVID-19 mRNA Vaccination: A Case Report. J. Stroke Cerebrovasc. Dis. 2022, 31, 106233. [Google Scholar] [CrossRef] [PubMed]
- Chui, C.S.L.; Fan, M.; Wan, E.Y.F.; Leung, M.T.Y.; Cheung, E.; Yan, V.K.C.; Gao, L.; Ghebremichael-Weldeselassie, Y.; Man, K.K.; Lau, K.K.; et al. Thromboembolic events and hemorrhagic stroke after mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccination: A self-controlled case series study. eClinicalMedicine 2022, 50, 101504. [Google Scholar] [CrossRef] [PubMed]
- Gadi, S.R.V.; Brunker, P.A.R.; Al-Samkari, H.; Sykes, D.B.; Saff, R.R.; Lo, J.; Bendapudi, P.; Leaf, D.E.; Leaf, R.K. Severe autoimmune hemolytic anemia following receipt of SARS-CoV-2 mRNA vaccine. Transfusion 2021, 61, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- D’agostino, V.; Caranci, F.; Negro, A.; Piscitelli, V.; Tuccillo, B.; Fasano, F.; Sirabella, G.; Marano, I.; Granata, V.; Grassi, R.; et al. A Rare Case of Cerebral Venous Thrombosis and Disseminated Intravascular Coagulation Temporally Associated to the COVID-19 Vaccine Administration. J. Pers. Med. 2021, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, M.; Moslemi, M.; Pakmehr, A.; Vahed, S.Z.; Khalaji, A.; Moslemi, H.; Vahedi, A. TTP-like syndrome and its relationship with complement activation in critically ill patients with COVID-19: A cross-sectional study. Heliyon 2023, 9, e17370. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, T.; De Wit, Y.; Schärz, N.; van Mierlo, G.; Angelillo-Scherrer, A.; Brodard, J.; Schefold, J.C.; Hirzel, C.; Jongerius, I.; Zeerleder, S. Immunothrombosis and Complement Activation Contribute to Disease Severity and Adverse Outcome in COVID-19. J. Innate Immun. 2023, 15, 850–864. [Google Scholar] [CrossRef]
- Joob, B.; Wiwanitkit, V. COVID-19, Vaccination, Multisystem Inflammatory Syndrome, Aneurysm, Screening and Post Vaccination Death. Int. J. Prev. Med. 2023, 14, 74. [Google Scholar] [CrossRef]
- Afshar, Z.M.; Pirzaman, A.T.; Liang, J.J.; Sharma, A.; Pirzadeh, M.; Babazadeh, A.; Hashemi, E.; Deravi, N.; Abdi, S.; Allahgholipour, A.; et al. Do we miss rare adverse events induced by COVID-19 vaccination? Front. Med. 2022, 9, 933914. [Google Scholar] [CrossRef]
- Monadhel, H.; Abbas, A.; Mohammed, A. COVID-19 Vaccinations and Their Side Effects: A Scoping Systematic Review. F1000Research 2023, 12, 604. [Google Scholar] [CrossRef]
- Dzau, V.J.; Hodgkinson, C.P. RNA Therapeutics for the Cardiovascular System. Circulation 2024, 149, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.K.; Paliwal, V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2021, 43, 3–40. [Google Scholar] [CrossRef] [PubMed]
- Ogata, S.; Ishii, Y.; Asano, K.; Kobayashi, E.; Kubota, S.; Takahashi, K.; Miyaji, Y.; Higashiyama, Y.; Joki, H.; Doi, H.; et al. Sensory Ataxic Guillain-Barré Syndrome with Dysgeusia after mRNA COVID-19 Vaccination. Intern. Med. 2022, 61, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.E.; Goddard, K.; Lewis, N.; Fireman, B.; Myers, T.R.; Bakshi, N.; Weintraub, E.; Donahue, J.G.; Nelson, J.C.; Xu, S.; et al. Incidence of Guillain-Barré Syndrome After COVID-19 Vaccination in the Vaccine Safety Datalink. JAMA Netw. Open 2022, 5, e228879. [Google Scholar] [CrossRef] [PubMed]
- Keh, R.Y.S.; Scanlon, S.; Datta-Nemdharry, P.; Donegan, K.; Cavanagh, S.; Foster, M.; Skelland, D.; Palmer, J.; Machado, P.M.; Keddie, S.; et al. COVID-19 vaccination and Guillain-Barré syndrome: Analyses using the National Immunoglobulin Database. Brain 2023, 146, 739–748. [Google Scholar] [CrossRef]
- Meo, S.A.; Shaikh, N.; Abukhalaf, F.A.; Meo, A.S. Exploring the adverse events of Oxford–AstraZeneca, Pfizer-BioNTech, Moderna, and Johnson and Johnson COVID-19 vaccination on Guillain–Barré Syndrome. Sci. Rep. 2024, 14, 18767. [Google Scholar] [CrossRef]
- de Sa, K.S.G.; Silva, J.; Bayarri-Olmos, R.; Brinda, R.; Constable, R.A.R.; Diaz, P.A.C.; Kwon, D.-I.; Rodrigues, G.; Wenxue, L.; Baker, C.; et al. A Causal Link between Autoantibodies and Neurological Symptoms in Long COVID. medRxiv 2024. [Google Scholar] [CrossRef]
- Ogunjimi, O.B.; Tsalamandris, G.; Paladini, A.; Varrassi, G.; Zis, P. Guillain-Barré Syndrome Induced by Vaccination Against COVID-19: A Systematic Review and Meta-Analysis. Cureus 2023, 15, 1–9. [Google Scholar] [CrossRef]
- Ha, J.; Park, S.; Kang, H.; Kyung, T.; Kim, N.; Kim, D.K.; Kim, H.; Bae, K.; Song, M.C.; Lee, K.J.; et al. Real-world data on the incidence and risk of Guillain–Barré syndrome following SARS-CoV-2 vaccination: A prospective surveillance study. Sci. Rep. 2023, 13, 3773. [Google Scholar] [CrossRef]
- Abara, W.E.; Gee, J.; Marquez, P.; Woo, J.; Myers, T.R.; DeSantis, A.; Baumblatt, J.A.G.; Woo, E.J.; Thompson, D.; Nair, N.; et al. Reports of Guillain-Barré Syndrome After COVID-19 Vaccination in the United States. JAMA Netw. Open 2023, 6, e2253845. [Google Scholar] [CrossRef]
- Hosseini, R.; Askari, N. A review of neurological side effects of COVID-19 vaccination. Eur. J. Med. Res. 2023, 28, 102. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Schultze, A.; Tazare, J.; Tamborska, A.; Singh, B.; Donegan, K.; Stowe, J.; Morton, C.E.; Hulme, W.J.; Curtis, H.J.; et al. Safety of COVID-19 vaccination and acute neurological events: A self-controlled case series in England using the OpenSAFELY platform. Vaccine 2022, 40, 4479–4487. [Google Scholar] [CrossRef] [PubMed]
- Soeiro, T.; Salvo, F.; Pariente, A.; Grandvuillemin, A.; Jonville-Béra, A.-P.; Micallef, J. Type I interferons as the potential mechanism linking mRNA COVID-19 vaccines to Bell’s palsy. Therapies 2021, 76, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.J.; Clothier, H.J.; Kattan, G.S.; Boyd, J.H.; Buttery, J.P. Acute disseminated encephalomyelitis and transverse myelitis following COVID-19 vaccination—A self-controlled case series analysis. Vaccine 2024, 42, 2212–2219. [Google Scholar] [CrossRef] [PubMed]
- Poli, K.; Poli, S.; Ziemann, U. Multiple Autoimmune Syndromes Including Acute Disseminated Encephalomyelitis, Myasthenia Gravis, and Thyroiditis Following Messenger Ribonucleic Acid-Based COVID-19 Vaccination: A Case Report. Front. Neurol. 2022, 13, 913515. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Kraemer, M. A neurologist’s rhombencephalitis after comirnaty vaccination. A change of perspective. Neurol. Res. Pract. 2021, 3, 56. [Google Scholar] [CrossRef]
- Núñez, I.; García-Grimshaw, M.; Valencia, C.Y.C.; Callejas, D.E.A.; Alfaro, M.L.M.; Saniger-Alba, M.d.M.; Gutiérrez-Romero, A.; Carrillo-Mezo, R.; Ceballos-Liceaga, S.E.; Baptista-Rosas, R.C.; et al. Seizures following COVID-19 vaccination in Mexico: A nationwide observational study. Epilepsia 2022, 63, e144–e149. [Google Scholar] [CrossRef]
- Doron, A.; Eviatar-Ribak, T.; Vituri, A.; Shahar, S.; Fahoum, F.; Goldstein, L. The COVID-19 pfizer BioNTech mRNA vaccine and the frequency of seizures. Clin. Neurol. Neurosurg. 2023, 233, 107952. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Akhtar, N.; Al Jerdi, S.; Kamran, S.; Joseph, S.; Morgan, D.; Uy, R.; Abid, F.B.; Al-Khal, A.; Bertollini, R.; et al. Association between COVID-19 vaccination and stroke: A nationwide case-control study in Qatar. Int. J. Infect. Dis. 2024, 145, 107095. [Google Scholar] [CrossRef]
- Lu, Y.; Matuska, K.; Nadimpalli, G.; Ma, Y.; Duma, N.; Zhang, H.T.; Chiang, Y.; Lyu, H.; Chillarige, Y.; Kelman, J.A.; et al. Stroke Risk After COVID-19 Bivalent Vaccination Among US Older Adults. JAMA 2024, 331, 938–950. [Google Scholar] [CrossRef]
- Stefanou, M.I.; Palaiodimou, L.; de Sousa, D.A.; Theodorou, A.; Bakola, E.; Katsaros, D.E.; Halvatsiotis, P.; Tzavellas, E.; Naska, A.; Coutinho, J.M.; et al. Acute Arterial Ischemic Stroke Following COVID-19 Vaccination: A Systematic Review and Meta-Analysis. Neurology 2022, 99, e1465–e1474. [Google Scholar] [CrossRef]
- Filippatos, F.; Tatsi, E.-B.; Michos, A. Immunology of Multisystem Inflammatory Syndrome after COVID-19 in Children: A Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 5711. [Google Scholar] [CrossRef] [PubMed]
- Efe, C.; Uzun, S.; Matter, M.S.; Beretta-Piccoli, B.T. Autoimmune-Like Hepatitis Related to SARS-CoV-2 Vaccination: Towards a Clearer Definition. Liver Int. 2025, 45, e16209. [Google Scholar] [CrossRef] [PubMed]
- Widhani, A.; Hasibuan, A.S.; Rismawati, R.; Maria, S.; Koesnoe, S.; Hermanadi, M.I.; Ophinni, Y.; Yamada, C.; Harimurti, K.; Sari, A.N.L.; et al. Efficacy, Immunogenicity, and Safety of COVID-19 Vaccines in Patients with Autoimmune Diseases: A Systematic Review and Meta-Analysis. Vaccines 2023, 11, 1456. [Google Scholar] [CrossRef] [PubMed]
- Hanberg, J.S.; Fu, X.; Wang, X.; Patel, N.J.; Kawano, Y.; Schiff, A.; Kowalski, E.N.; Cook, C.E.; Vanni, K.M.M.; Guzzo, K.; et al. Effectiveness of a fourth dose of COVID-19 mRNA vaccine in patients with systemic autoimmune rheumatic diseases using disease-modifying antirheumatic drugs: An emulated target trial. Lancet Rheumatol. 2024, 6, e21–e30. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, M.H.; Park, S.J.; Choi, M.G.; Chun, E.M. Autoimmune Adverse Event Following COVID-19 Vaccination in Seoul, South Korea. J. Allergy Clin. Immunol. 2024, 153, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-C.; Huang, I.-H.; Wang, C.-Y.; Chi, C.-C. New Onset and Exacerbation of Autoimmune Bullous Dermatosis Following COVID-19 Vaccination: A Systematic Review. Vaccines 2024, 12, 465. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Yasumi, M.; Shiraishi, K.; Kamijo, K.; Kamae, T.; Karasuno, T. Multiple microthromboses with autoimmune hemolytic anemia after BNT162b2 mRNA vaccination. [Rinsho Ketsueki] Jpn. J. Clin. Hematol. 2023, 64, 1421–1425. [Google Scholar] [CrossRef]
- Nnawuba, K.C.; Boral, B.M.; Donnell, R.W. Probable warm autoimmune hemolytic anemia proceeding the administration of the Pfizer mRNA COVID-19 vaccine. Immunohematology 2022, 38, 106–107. [Google Scholar]
- De Bruyne, S.; Van Landeghem, S.; Schauwvlieghe, A.; Noens, L. Life-Threatening Autoimmune Hemolytic Anemia Fol-lowing mRNA COVID-19 Vaccination: Don’t Be Too Prudent with the Red Gold. Clin. Chem. Lab. Med. (CCLM) 2022, 60, e125–e128. [Google Scholar] [CrossRef]
- Atoui, A.; Jarrah, K.; Al Mahmasani, L.; Bou-Fakhredin, R.; Taher, A.T. Deep venous thrombosis and pulmonary embolism after COVID-19 mRNA vaccination. Ann. Hematol. 2022, 101, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Ciftel, S.; Tuzun, Z. Subacute Thyroiditis Following SARS-CoV-2 Vaccination: An Autoimmune/Inflammatory Syndrome Induced by Adjuvants (Asia Syndrome). Acta Endocrinol. 2023, 19, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Alsudais, A.S.; Alkanani, R.S.; Fathi, A.B.; Almuntashiri, S.S.; Jamjoom, J.N.; Alzhrani, M.A.; Althubaiti, A.; Radi, S. Autoimmune diabetes mellitus after COVID-19 vaccination in adult population: A systematic review of case reports. BMC Endocr. Disord. 2023, 23, 164. [Google Scholar] [CrossRef] [PubMed]
- Abe, N.; Bohgaki, M.; Kasahara, H. SARS-CoV-2 mRNA Vaccination-induced Autoimmune Polyarthritis-like Rheumatoid Arthritis. Mayo Clin. Proc. 2022, 97, 1574–1575. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Layhadi, J.A.; Hourcade, D.E.; Fulton, W.T.; Tan, T.J.; Dunham, D.; Chang, I.; Vel, M.S.; Fernandes, A.; Lee, A.S.; et al. Elucidating allergic reaction mechanisms in response to SARS-CoV-2 mRNA vaccination in adults. Allergy 2024, 79, 2502–2523. [Google Scholar] [CrossRef] [PubMed]
- Takata, H.; Shimizu, T.; Yamade, R.; Elsadek, N.E.; Emam, S.E.; Ando, H.; Ishima, Y.; Ishida, T. Anti-PEG IgM production induced by PEGylated liposomes as a function of administration route. J. Control. Release 2023, 360, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Snow, T.T.; Lee, A.S.; Shah, M.M.; Heider, A.; Blomkalns, A.; Betts, B.; Buzzanco, A.S.; Gonzalez, J.; Chinthrajah, R.S.; et al. Assessment of Allergic and Anaphylactic Reactions to mRNA COVID-19 Vaccines with Confirmatory Testing in a US Regional Health System. JAMA Netw. Open 2021, 4, e2125524. [Google Scholar] [CrossRef]
- Jaggers, J.; Wolfson, A.R. mRNA COVID-19 Vaccine Anaphylaxis: Epidemiology, Risk Factors, and Evaluation. Curr. Allergy Asthma Rep. 2023, 23, 195–200. [Google Scholar] [CrossRef]
- Meroni, P.L.; Croci, S.; Lonati, P.A.; Pregnolato, F.; Spaggiari, L.; Besutti, G.; Bonacini, M.; Ferrigno, I.; Rossi, A.; Hetland, G.; et al. Complement activation predicts negative outcomes in COVID-19: The experience from Northen Italian patients. Autoimmun. Rev. 2022, 22, 103232. [Google Scholar] [CrossRef]
- Aochi, S.; Uehara, M.; Yamamoto, M. IgG4-related Disease Emerging after COVID-19 mRNA Vaccination. Intern. Med. 2023, 62, 1547–1551. [Google Scholar] [CrossRef]
- Hirano, H.; Asada, H. Exponential decline, ceiling effect, downregulation, and T-cell response in immunoglobulin G antibody levels after messenger RNA vaccine boosters: A case report. J. Med. Case Rep. 2024, 18, 631. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, A.; Chammartin, F.; Audigé, A.; Eichenberger, A.L.; Braun, D.L.; Amico, P.; Stoeckle, M.P.; Hasse, B.; Papadimitriou-Olivgeris, M.; Manuel, O.; et al. Antibody and T-Cell Response to Bivalent Booster SARS-CoV-2 Vaccines in People With Compromised Immune Function: COVERALL-3 Study. J. Infect. Dis. 2024, 230, e847–e859. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Bouteau, A.; Herbst, C.; Igyártó, B.Z. Pre-exposure to mRNA-LNP inhibits adaptive immune responses and alters innate immune fitness in an inheritable fashion. PLoS Pathog. 2022, 18, e1010830. [Google Scholar] [CrossRef] [PubMed]
- Chau, C.Y.C.; Chow, L.L.W.; Sridhar, S.; Shih, K.C. Ophthalmological Considerations for COVID-19 Vaccination in Patients with Inflammatory Eye Diseases and Autoimmune Disorders. Ophthalmol. Ther. 2021, 10, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Kim, S.; Han, J.; Kim, S.S.; Han, S.H.; Lee, S.W.; Kim, Y.J. Neuro-Ophthalmic Adverse Events of COVID-19 Infection and Vaccines: A Nationwide Cohort Study. Investig. Ophthalmol. Vis. Sci. 2023, 64, 37. [Google Scholar] [CrossRef]
- Tiede, A.; Sachs, U.J.; Czwalinna, A.; Werwitzke, S.; Bikker, R.; Krauss, J.K.; Donnerstag, F.G.; Weißenborn, K.; Höglinger, G.U.; Maasoumy, B.; et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccination. Blood 2021, 138, 350–353. [Google Scholar] [CrossRef]
- Pietri, T.; Micallef, J.; Gervoise-Boyer, M.; Boyer, P. P-308 Spontaneous reports of menstrual cycle disorders after mRNA COVID-19 vaccine. Hum. Reprod. 2022, 37 (Suppl. S1), deac107.294. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, S.; Dai, Z.; Hao, L.; Luan, C.; Guo, Q.; Meng, C.; Zhang, Y. Effects of COVID-19 and mRNA vaccines on human fertility. Hum. Reprod. 2022, 37, 5–13. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Drover, S.S.M.; Fell, D.B.; Austin, P.C.; D’SOuza, R.; Guttmann, A.; Buchan, S.A.; Wilson, S.E.; Nasreen, S.; Brown, K.A.; et al. Association between maternal mRNA COVID-19 vaccination in early pregnancy and major congenital anomalies in offspring: Population based cohort study with sibling matched analysis. BMJ Med. 2024, 3, e000743. [Google Scholar] [CrossRef]
- Fell, D.B.; Dhinsa, T.; Alton, G.D.; Török, E.; Dimanlig-Cruz, S.; Regan, A.K.; Sprague, A.E.; Buchan, S.A.; Kwong, J.C.; Wilson, S.E.; et al. Association of COVID-19 Vaccination in Pregnancy With Adverse Peripartum Outcomes. JAMA 2022, 327, 1478–1487. [Google Scholar] [CrossRef]
- Ruderman, R.S.; Mormol, J.; Trawick, E.; Perry, M.F.; Allen, E.C.; Millan, D.; Miller, E.S. Association of COVID-19 Vaccination During Early Pregnancy With Risk of Congenital Fetal Anomalies. JAMA Pediatr. 2022, 176, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, M.; Xue, X.; Li, N.; Chen, L.; Shi, J. Association Between Time Interval from COVID-19 Vaccination to In Vitro Fertilization and Pregnancy Rate After Fresh Embryo Transfer. JAMA Netw. Open 2022, 5, e2236609. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, Y.; Zhang, Y.; Li, M.; Su, X.; Zhou, Y.; Zhang, Z.; Jin, L. Association of COVID-19 vaccination before conception with maternal thyroid function during early pregnancy: A single-center study in China. J. Med. Virol. 2023, 95, e28245. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Elwood, C.; Money, D.M.; Dunne, C. Myths Versus Facts: COVID-19 Vaccine Effects on Pregnancy, Fertility, and Menstruation. Manag. Menopause New Guidel. 2022, 64, 354–358. [Google Scholar]
- Barros, F.C.; Gunier, R.B.; Rego, A.; Sentilhes, L.; Rauch, S.; Gandino, S.; Teji, J.S.; Thornton, J.G.; Kachikis, A.B.; Nieto, R.; et al. Maternal vaccination against COVID-19 and neonatal outcomes during Omicron: INTERCOVID-2022 study. Am. J. Obstet. Gynecol. 2024, 231, 460.e1–460.e17. [Google Scholar] [CrossRef] [PubMed]
- Peretz-Machluf, R.; Gilboa, M.; Bookstein-Peretz, S.; Segal, O.; Regev, N.; Meyer, R.; Regev-Yochay, G.; Yinon, Y.; Toussia-Cohen, S. Obstetric and Early Neonatal Outcomes Following Second and Third COVID-19 Vaccination in Pregnancy. Isr. Med. Assoc. J. IMAJ 2024, 26, 12–17. [Google Scholar]
- Santimano, A.J.; Al-Zoubi, R.M.; Al-Qudimat, A.R.; Al Darwish, M.B.; Ojha, L.K.; Rejeb, M.A.; Hamad, Y.; Elrashid, M.A.; Ruxshan, N.M.; El Omri, A.; et al. Efficacy and Clinical Outcomes of mRNA COVID-19 Vaccine in Pregnancy: A Systematic Review and Meta-Analysis. Intervirology 2024, 67, 40–54. [Google Scholar] [CrossRef]
- Moro, P.L.; Carlock, G.; Fifadara, N.; Habenicht, T.; Zhang, B.; Strid, P.; Marquez, P. Safety Monitoring of Bivalent mRNA COVID-19 Vaccine among Pregnant Persons in the Vaccine Adverse Event Reporting System—United States, September 1, 2022–March 31, 2023. Vaccine 2024, 42, 2380–2384. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jung, J.; Kang, M.; Choi, M.J.; Choi, W.S.; Bin Seo, Y.; Hyun, H.-J.; Yoon, Y.; Choe, Y.J.; Cho, G.J.; et al. The risk of pregnancy-related adverse outcomes after COVID-19 vaccination: Propensity score-matched analysis with influenza vaccination. Vaccine 2025, 44, 126506. [Google Scholar] [CrossRef]
- Ghanbari, E.P.; Jakobs, K.; Puccini, M.; Reinshagen, L.; Friebel, J.; Haghikia, A.; Kränkel, N.; Landmesser, U.; Rauch-Kröhnert, U. The Role of NETosis and Complement Activation in COVID-19-Associated Coagulopathies. Biomedicines 2023, 11, 1371. [Google Scholar] [CrossRef]
- Brito, S.; Ferreira, N.; Mateus, S.; Bernardo, M.; Pinto, B.; Lourenço, A.; Grenho, F. A Case of Autoimmune Hemolytic Anemia Following COVID-19 Messenger Ribonucleic Acid Vaccination. Cureus 2021, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.; Tay, E.; D’Souza, A. Localised swelling at sites of dermal filler injections following administration of COVID-19 vaccines: A systematic review. Singap. Med. J. 2024, 65, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Jue, M.-S.; Joh, H.C.; Kim, S.H.; Ko, J.Y. Stevens–Johnson Syndrome/Toxic Epidermal Necrolysis Overlap After the Third Dose of BNT162b2 mRNA COVID-19 Vaccination and Literature Review. Dermatitis® 2023, 34, 158–159. [Google Scholar] [CrossRef] [PubMed]
- Franzblau, L.E.; Mauskar, M.; Wysocki, C.A. Macrophage Activation Syndrome Complicated by Toxic Epidermal Necrolysis Following SARS-CoV-2 mRNA Vaccination. J. Clin. Immunol. 2023, 43, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Beamish, I.V.; Bogoch, I.I.; Carr, D. Delayed Inflammatory Reaction to Dermal Fillers after COVID-19 Vaccination: A Case Report. Can. J. Emerg. Med. 2022, 24, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, Y.; Sasaki, H.; Miyata, N.; Miyazaki, K.; Okudela, K.; Tateishi, Y.; Hayashi, H.; Kawana-Tachikawa, A.; Iwashita, H.; Maeda, K.; et al. An autopsy case of COVID-19-like acute respiratory distress syndrome after mRNA-1273 SARS-CoV-2 vaccination. Int. J. Infect. Dis. 2022, 121, 98–101. [Google Scholar] [CrossRef]
- Lopatynsky-Reyes, E.Z.; Acosta-Lazo, H.; Ulloa-Gutierrez, R.; Ávila-Aguero, M.L.; Chacon-Cruz, E. BCG Scar Local Skin Inflammation as a Novel Reaction Following mRNA COVID-19 Vaccines in Two International Healthcare Workers. Cureus 2021, 13, 1–6. [Google Scholar] [CrossRef]
- Ben-Fredj, N.; Chahed, F.; Ben-Fadhel, N.; Mansour, K.; Ben-Romdhane, H.; El Mabrouk, R.S.; Chadli, Z.; Ghedira, D.; Belhadjali, H.; Chaabane, A.; et al. Case series of chronic spontaneous urticaria following COVID-19 vaccines: An unusual skin manifestation. Eur. J. Clin. Pharmacol. 2022, 78, 1959–1964. [Google Scholar] [CrossRef]
- Grieco, T.; Ambrosio, L.; Trovato, F.; Vitiello, M.; Demofonte, I.; Fanto, M.; Paolino, G.; Pellacani, G. Effects of Vaccination against COVID-19 in Chronic Spontaneous and Inducible Urticaria (CSU/CIU) Patients: A Monocentric Study. J. Clin. Med. 2022, 11, 1822. [Google Scholar] [CrossRef]
- Iwamura, N.; Eguchi, K.; Takatani, A.; Tsutsumi, K.; Koga, T.; Araki, T.; Aramaki, T.; Terada, K.; Ueki, Y. A Case Series of Rheumatoid Arthritis Flare Including Extra-Articular Manifestations Following SARS-CoV-2 mRNA Vaccination: A Compre-hensive Cytokine Assay. Cureus 2024, 16, e58740. [Google Scholar] [CrossRef]
- Fong, W.; Woon, T.H.; Chew, L.-C.; Low, A.; Law, A.; Poh, Y.J.; Yeo, S.I.; Leung, Y.Y.; Ma, M.; Santosa, A.; et al. Prevalence and factors associated with flares following COVID-19 mRNA vaccination in patients with rheumatoid arthritis, psoriatic arthritis and spondyloarthritis: A national cohort study. Hortic. Bras. 2023, 63, 38. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Park, J.; Jeong, Y.D.; Jo, H.; Kim, S.; Woo, S.; Son, Y.; Kim, H.J.; Lee, K.; et al. Global burden of vaccine-associated hepatobiliary and gastrointestinal adverse drug reactions, 1967–2023: A comprehensive analysis of the international pharmacovigilance database. J. Med. Virol. 2024, 96, e29792. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Oh, E.H.; Han, D.S. Polyposis of gastrointestinal tract after COVID-19 mRNA vaccination: A report of two cases. Clin. Endosc. 2024, 57, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Mujukian, A.; Kumar, R.; Li, D.; Debbas, P.; Botwin, G.J.; Cheng, S.; Ebinger, J.; Braun, J.; McGovern, D.; Melmed, G.Y.; et al. Postvaccination Symptoms After SARS-CoV-2 mRNA Vaccination Among Patients With Inflammatory Bowel Disease: A Prospective, Comparative Study. Inflamm. Bowel Dis. 2024, 30, 602–616. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Coscia, G.; Fishbein, J.S.; Innamorato, A.; Ali, A.; Farzan, S. Gastrointestinal reflux contributes to laryngopharyngeal symptoms that mimic anaphylaxis: COVID-19 vaccination experience. J. Allergy Clin. Immunol. Glob. 2024, 3, 100176. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; Pose, E.; Wittner, M.; Londoño, M.-C.; Schaub, G.; Cook, J.; Dimitriadis, S.; Meacham, G.; Irwin, S.; Lim, Z.; et al. Immune responses and clinical outcomes after COVID-19 vaccination in patients with liver disease and liver transplant recipients. J. Hepatol. 2024, 80, 109–123. [Google Scholar] [CrossRef]
- Taida, T.; Kato, J.; Ishikawa, K.; Akizue, N.; Ohta, Y.; Okimoto, K.; Saito, K.; Matsusaka, K.; Matsumura, T.; Kato, N. Severe ulcerative colitis induced by COVID-19 vaccination. Clin. J. Gastroenterol. 2024, 17, 447–450. [Google Scholar] [CrossRef]
- Cannatelli, R.; Ferretti, F.; Carmagnola, S.; Bergna, I.M.B.; Monico, M.C.; Maconi, G.; Ardizzone, S. Risk of adverse events and reported clinical relapse after COVID-19 vaccination in patients with IBD. Gut 2022, 71, 1926–1928. [Google Scholar] [CrossRef]
- Eguchi, G.; Murakoshi, M.; Miyaoka, F.; Shimbo, A.; Irabu, H.; Kanamori, T.; Udagawa, T.; Morio, T.; Shimizu, M. Acute tubulointerstitial nephritis following coronavirus disease 2019 mRNA vaccination: A pediatric case report. CEN Case Rep. 2024, 14, 261–265. [Google Scholar] [CrossRef]
- Abramson, M.; Yu, S.M.-W.; Campbell, K.N.; Chung, M.; Salem, F. IgA Nephropathy After SARS-CoV-2 Vaccination. Kidney Med. 2021, 3, 860–863. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, K.T.; Park, E.; Pyun, J.H.; Kim, J.H.; Chung, B.I. Urological Complications after COVID 19 Vaccine Ac-cording to Age, Sex and Manufacturer. World J. Urol. 2023, 41, 2255–2263. [Google Scholar] [CrossRef] [PubMed]
- Imhof, C.; Messchendorp, A.L.; Bungener, L.B.; Hepkema, B.G.; Kho, M.M.L.; Reinders, M.E.J.; Bemelman, F.J.; Hilbrands, L.B.; Gansevoort, R.T.; Sanders, J.S.F.; et al. The effect of COVID-19 vaccination on kidney function and HLA antibody formation in patients with end-stage kidney disease and on kidney replacement treatment. Clin. Kidney J. 2024, 17, sfae122. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Reddy, N.T.S.; Reddy, J.; Krishna, D.V.V.; Dehade, A.; Agrawal, N.K. Patterns and outcomes of late onset thyroid disturbances after COVID-19 vaccination: A report of 75 cases. Trop. Med. Int. Health 2024, 29, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jafarzadeh, A.; Nemati, M.; Jafarzadeh, S.; Nozari, P.; Mortazavi, S.M.J. Thyroid dysfunction following vaccination with COVID-19 vaccines: A basic review of the preliminary evidence. J. Endocrinol. Investig. 2022, 45, 1835–1863. [Google Scholar] [CrossRef] [PubMed]
- Aburisheh, K.H.; Enabi, H.M.; Alodah, N.A.; Alotary, B.H.; Algheryafi, L.A.; Almairi, A.M.; Aldhewaila, A.A. New Onset of Type 1 Diabetes Mellitus Post-COVID-19 Vaccine. J. Med. Cases 2024, 15, 367–370. [Google Scholar] [CrossRef]
- Mungmunpuntipantip, R.; Wiwanitkit, V. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine 2022, 78, 641. [Google Scholar] [CrossRef]
- Aydoğan, B.İ.; Ünlütürk, U.; Cesur, M. Type 1 diabetes mellitus following SARS-CoV-2 mRNA vaccination. Endocrine 2022, 78, 42–46. [Google Scholar] [CrossRef]
- Xiong, X.; Lui, D.T.W.; Chung, M.S.H.; Au, I.C.H.; Lai, F.T.T.; Wan, E.Y.F.; Chui, C.S.L.; Li, X.; Cheng, F.W.T.; Cheung, C.-L.; et al. Incidence of diabetes following COVID-19 vaccination and SARS-CoV-2 infection in Hong Kong: A population-based cohort study. PLoS Med. 2023, 20, e1004274. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, D.; Pan, Y.; Li, H.; Zhao, W.; Lu, T.; Kong, W.; Ding, M.; Wang, X.; Zhang, G. Serological response and immune-related adverse events following COVID-19 vaccination in cancer patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Rev. Med. Virol. 2024, 34, e2495. [Google Scholar] [CrossRef]
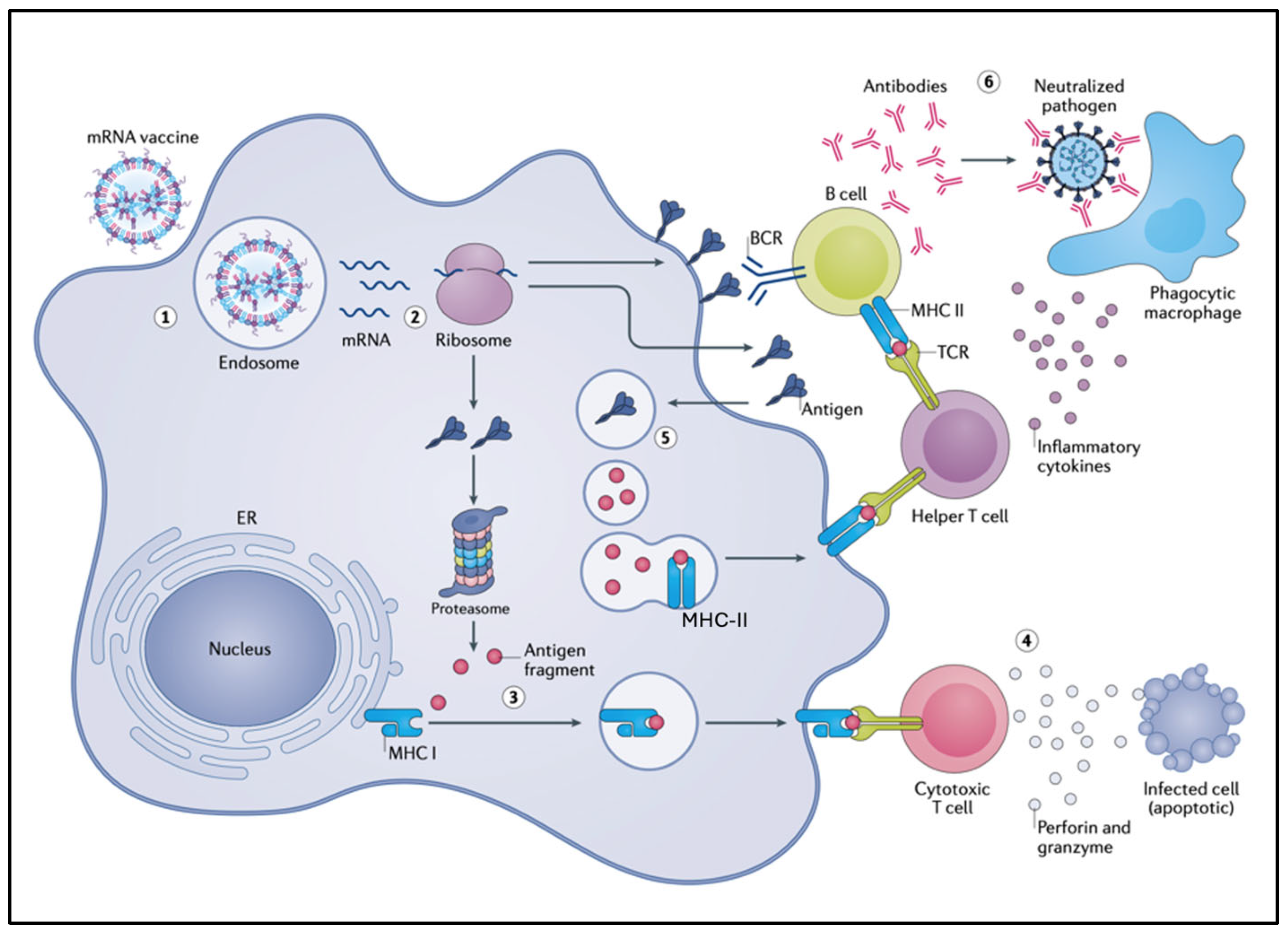
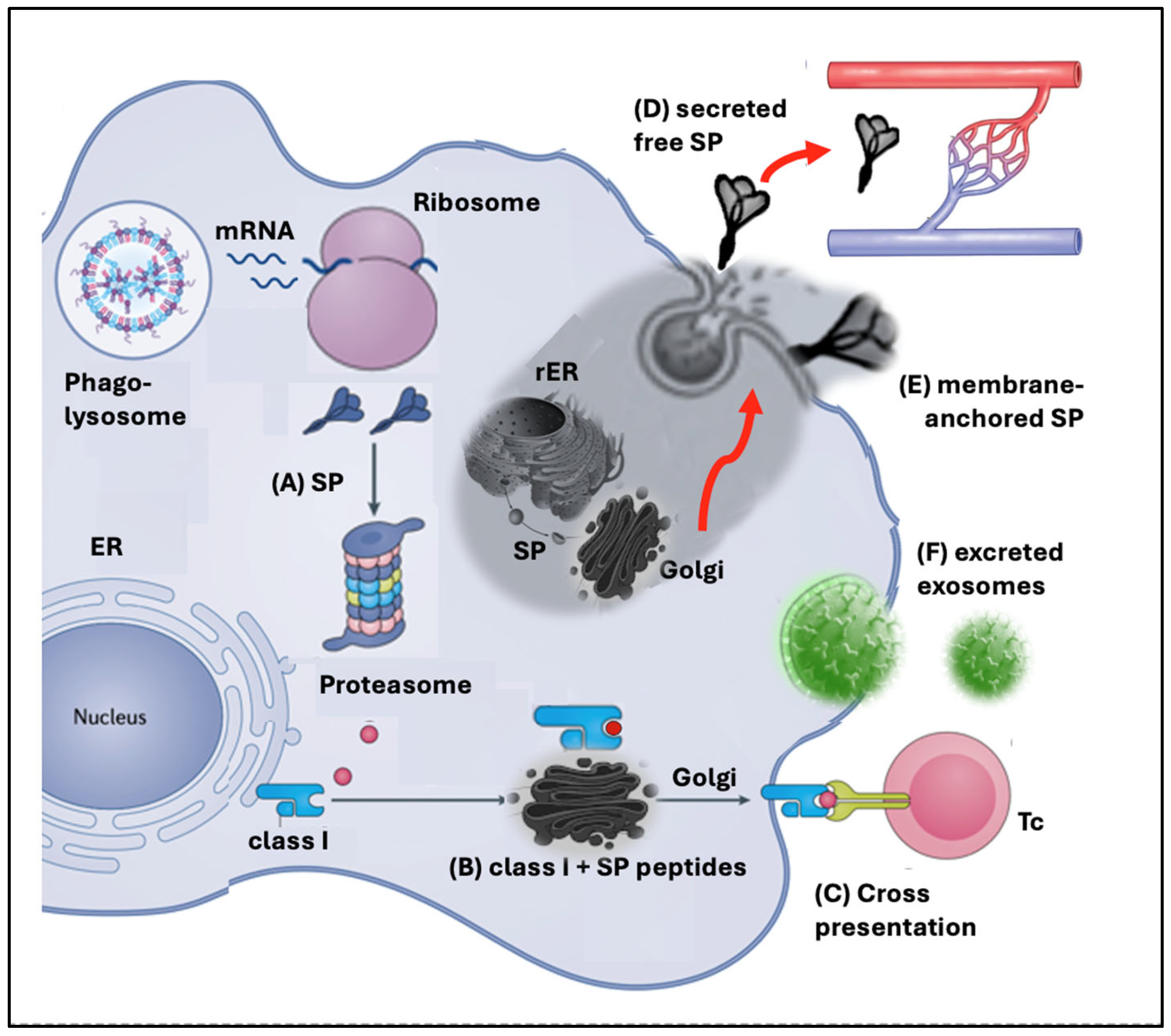
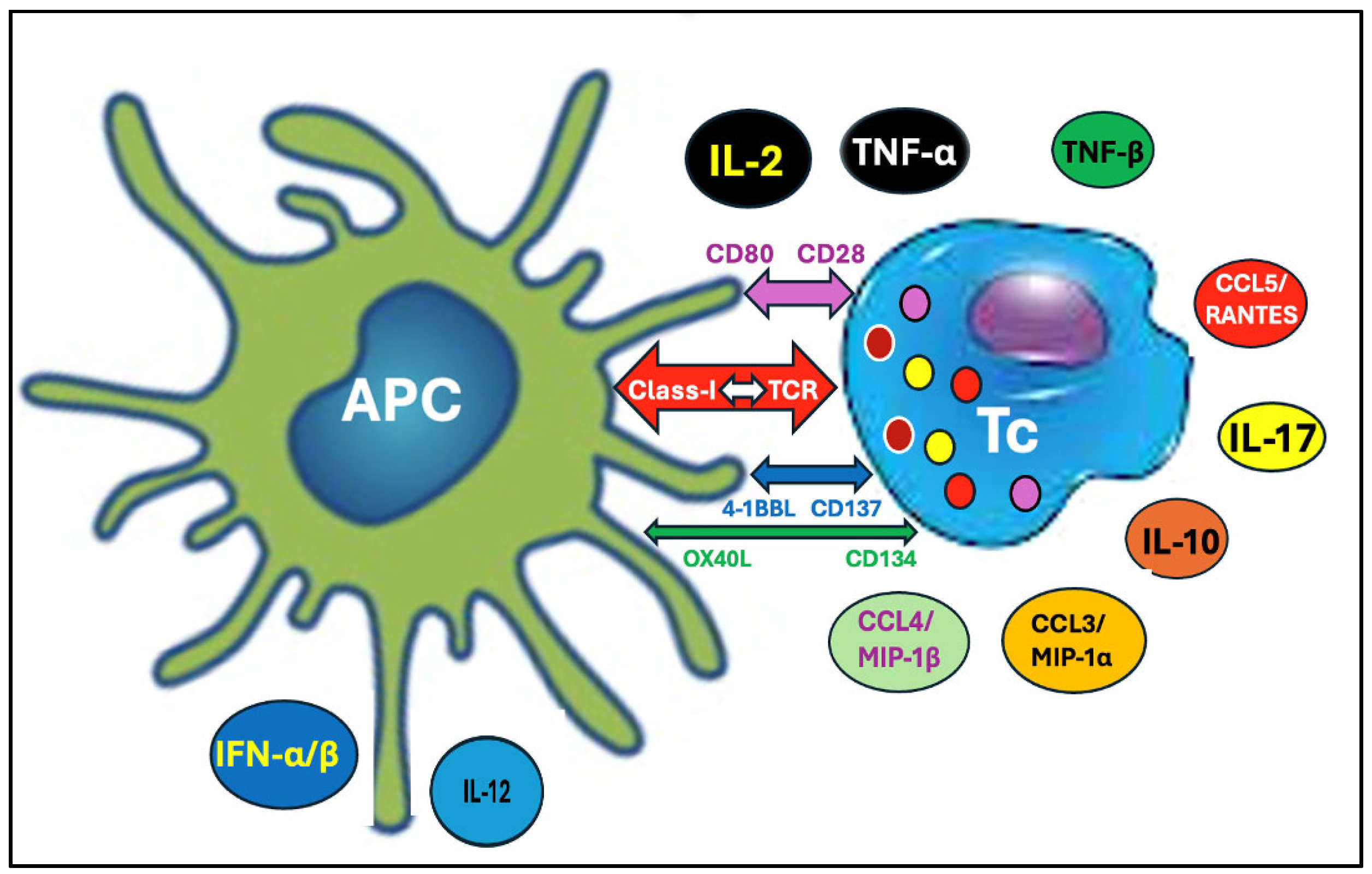
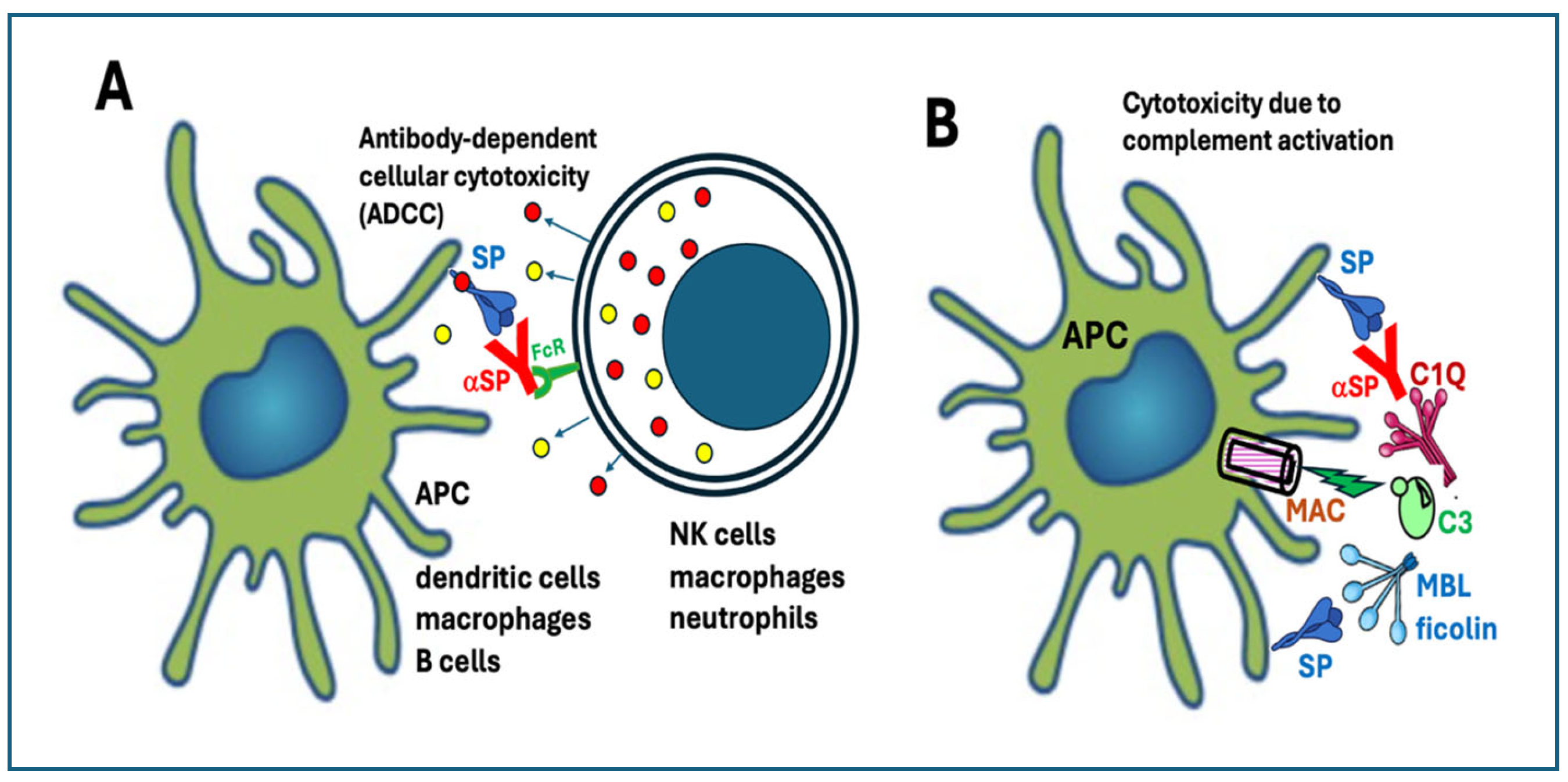

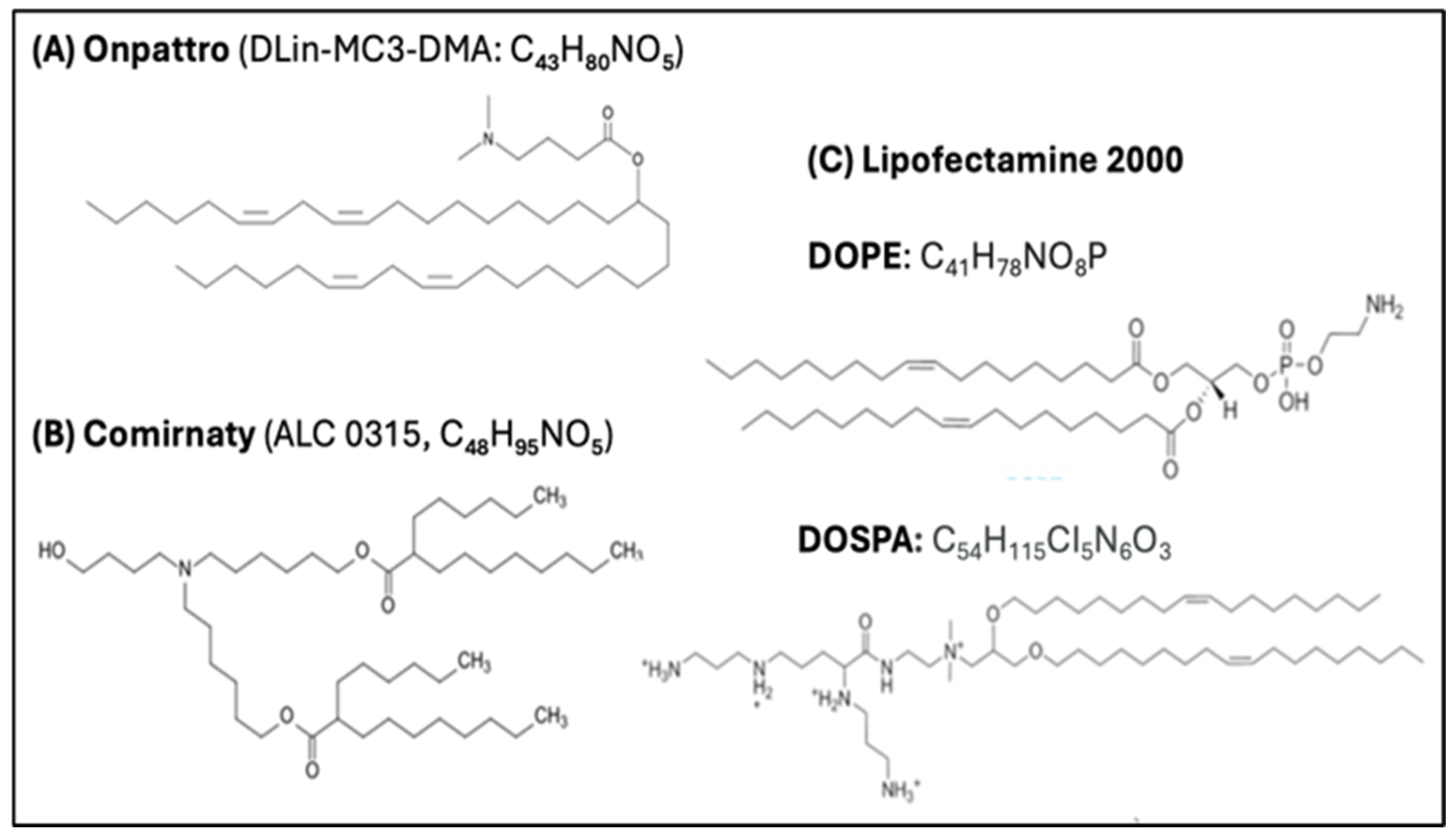
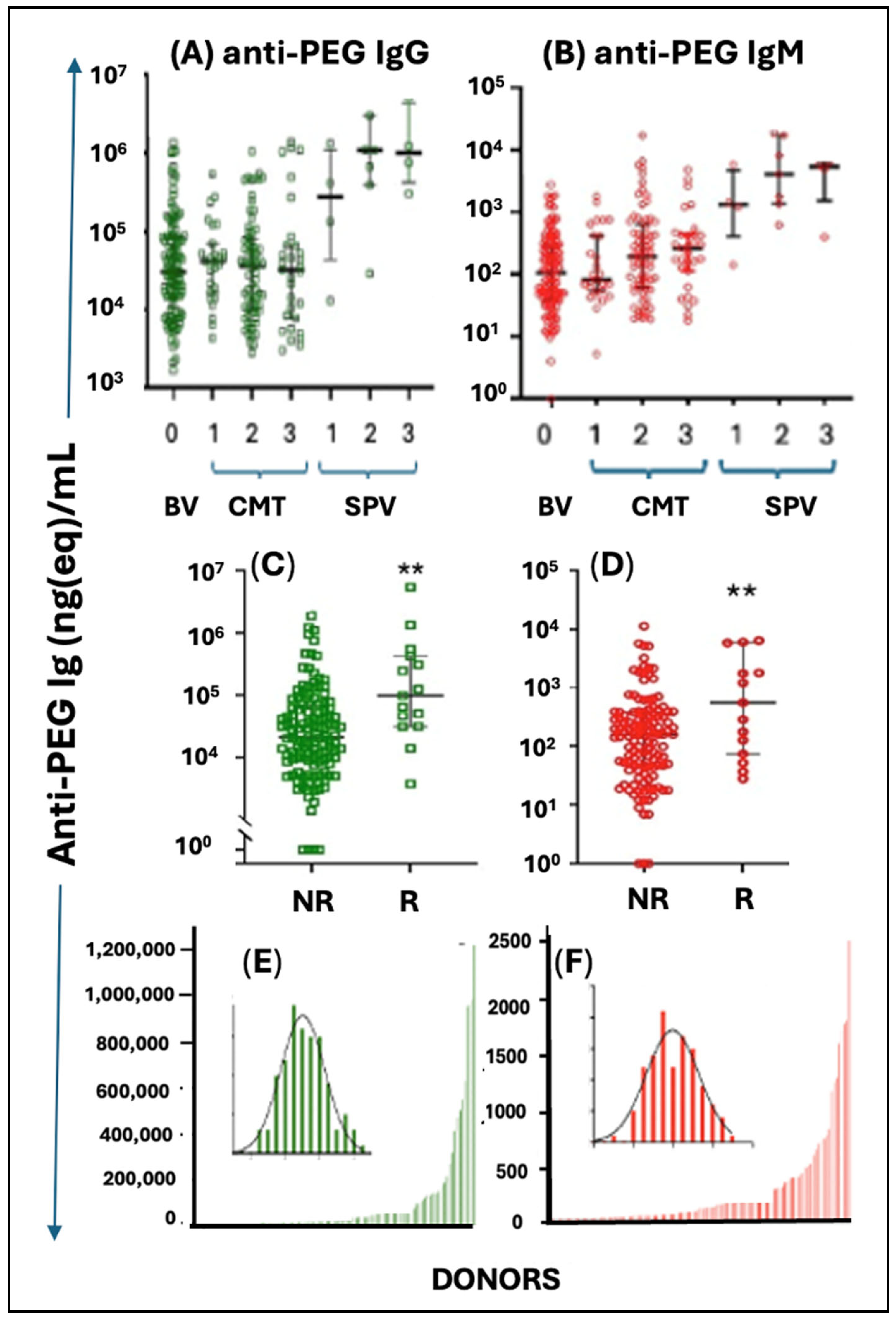
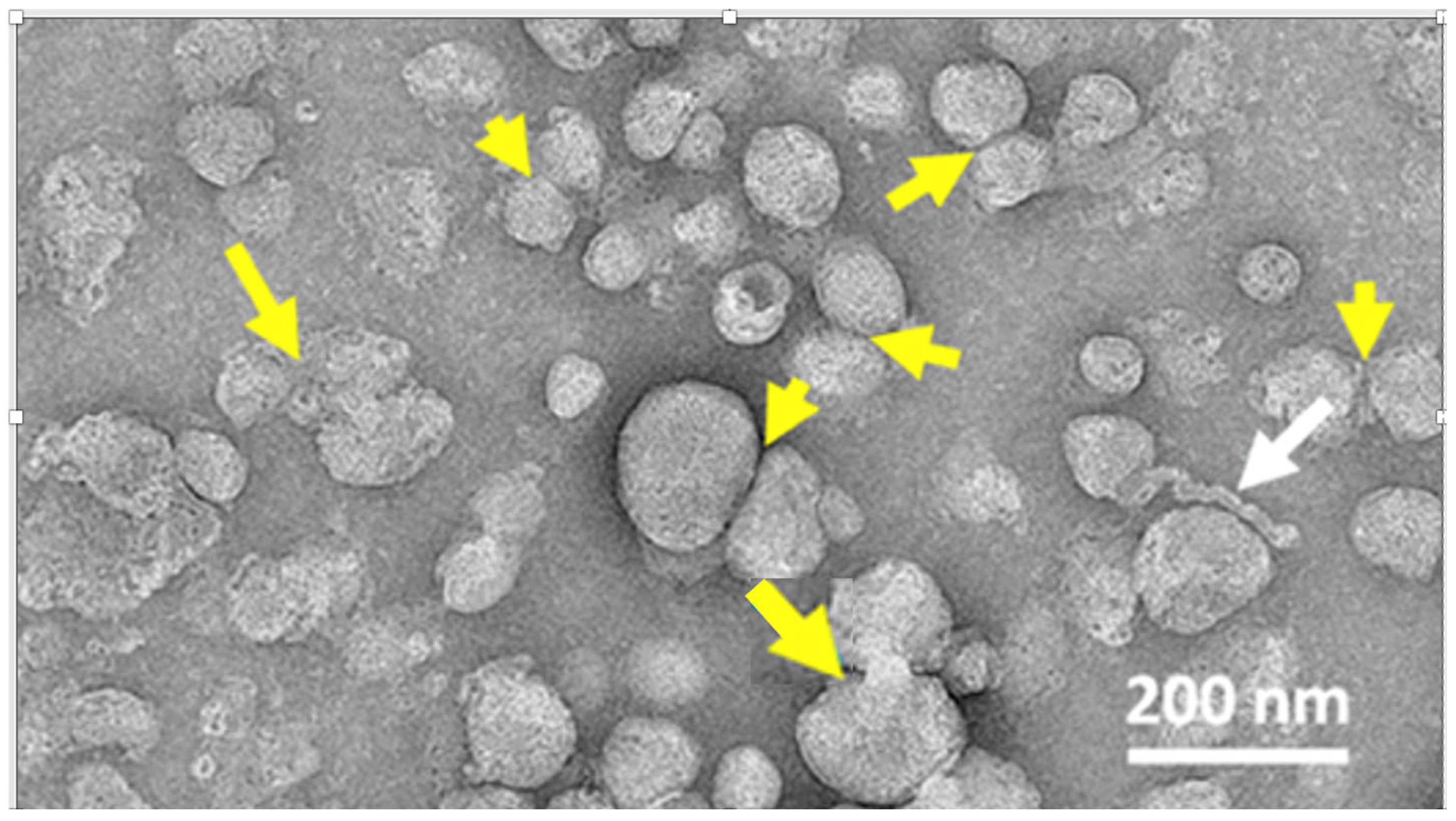
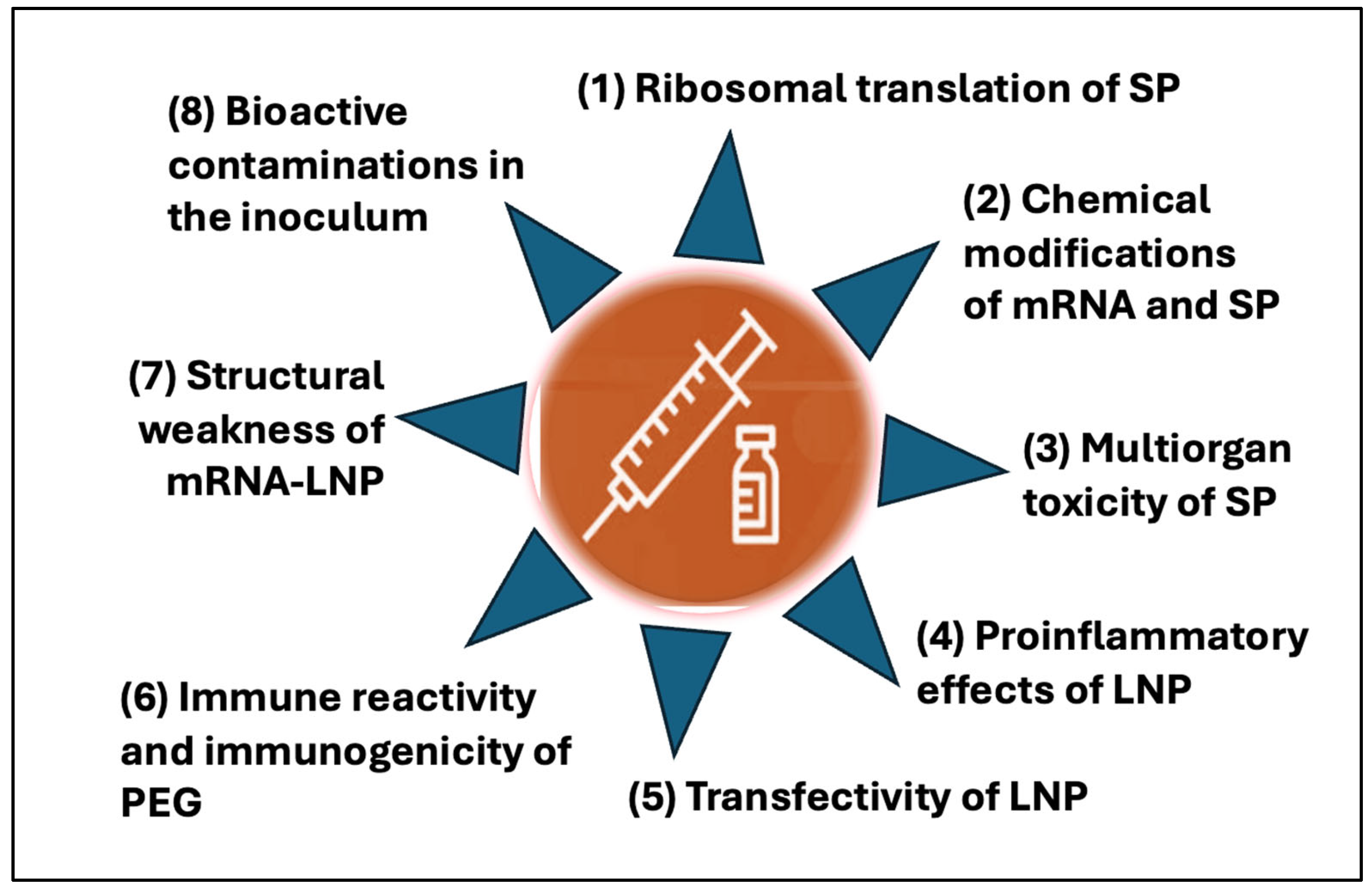
| Unique Vaccine Properties | Collateral Immune Effects/ Unintended Processes | Clinical Manifestations |
|---|---|---|
|
|
|
|
| |
|
|
|
|
| |
|
| |
|
|
|
|
|
|
|
|
|
| Systemic Toxicities | References |
|---|---|
| Complement activation | [147,148,149] |
| Endothelial inflammation, microvascular damage | [80,145,146,147,148] |
| Oxidative (mitochondrial) damage | [36,141,142,150] |
| Myocardium contractility decrease | [150] |
| Cardiac fibrosis | |
| Cytokine release | [143,144] |
| (Micro)thrombosis | [131,148,151,152,153] |
| Red blood cell aggregation | [84] |
| White cell activation and/or cytotoxicity | [93,154,155] |
| Platelet activation and/or coagulation abnormalities | [156,157] |
| Fibrin thrombus formation | [158] |
| Blood–brain barrier damage | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szebeni, J. Unique Features and Collateral Immune Effects of mRNA-LNP COVID-19 Vaccines: Plausible Mechanisms of Adverse Events and Complications. Pharmaceutics 2025, 17, 1327. https://doi.org/10.3390/pharmaceutics17101327
Szebeni J. Unique Features and Collateral Immune Effects of mRNA-LNP COVID-19 Vaccines: Plausible Mechanisms of Adverse Events and Complications. Pharmaceutics. 2025; 17(10):1327. https://doi.org/10.3390/pharmaceutics17101327
Chicago/Turabian StyleSzebeni, János. 2025. "Unique Features and Collateral Immune Effects of mRNA-LNP COVID-19 Vaccines: Plausible Mechanisms of Adverse Events and Complications" Pharmaceutics 17, no. 10: 1327. https://doi.org/10.3390/pharmaceutics17101327
APA StyleSzebeni, J. (2025). Unique Features and Collateral Immune Effects of mRNA-LNP COVID-19 Vaccines: Plausible Mechanisms of Adverse Events and Complications. Pharmaceutics, 17(10), 1327. https://doi.org/10.3390/pharmaceutics17101327







