PLGA-Based Strategies for Intranasal and Pulmonary Applications
Abstract
:1. The Role of PLGA in Enhancing Nasal and Pulmonary Drug Delivery Systems
2. Therapeutic Applications of PLGA in Nasal and Pulmonary Route
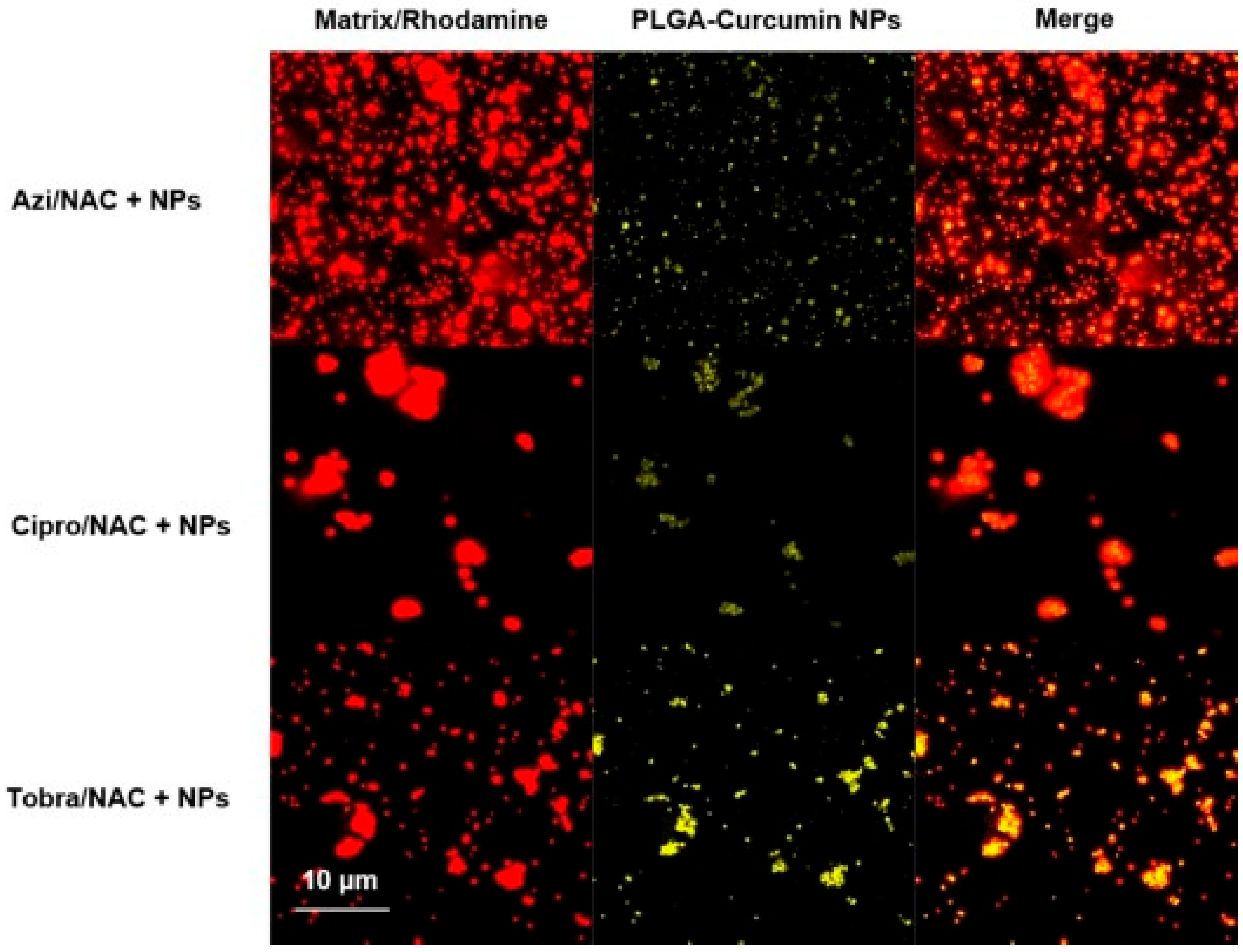
2.1. Enhancing Antibiotic Therapies for Respiratory Infections
2.2. Anti-Inflammatory and Anti-Fibrotic Applications
2.3. Combination Therapies for Complex Respiratory Conditions
2.4. PLGA-Based Pulmonary Delivery of Peptides and Proteins
2.5. Innovations in Nanoparticle Design for Biologics
2.6. Intranasal PLGA-Based Formulations for CNS Drug Delivery
2.7. Oncology Applications of PLGA in Lung and Brain Cancers
3. Key Materials Supporting PLGA Formulations in Intranasal and Pulmonary Delivery
3.1. Improving Stability and Circulation with PEG and PVA
3.2. Optimizing Aerosolization with Lactose, Sorbitol, and Surfactants
3.3. Specialized Ligands for CNS Targeting
3.4. Porogens and Agents for Controlled Release and Cellular Uptake
3.5. Immune-Boosting Adjuvants for Vaccines
3.6. Structural and Functional Enhancers in PLGA-Based Formulations
4. Factors Affecting Delivery Performance of PLGA-Based Carriers
4.1. Impact of Particle Size
4.1.1. Optimal Particle Size for Pulmonary Delivery
4.1.2. Nanoparticles for Pulmonary Applications
4.1.3. Particle Size for Intranasal Delivery and Brain Targeting
4.1.4. Disease-Specific Particle Size Optimization
4.2. Impact of Size Distribution
4.2.1. Uniform Size Distribution for Pulmonary Delivery
4.2.2. Controlled Size Distribution in Porous Particles
4.2.3. Narrow Size Distribution in Nanoparticles
4.2.4. Effect of Size Distribution on Intranasal Delivery
4.2.5. Technological Approaches for Size Control
4.2.6. Polydispersity and Stability
4.3. Impact of Particle Aerodynamic Performance
4.3.1. Optimal Aerodynamic Diameter for Lung Deposition
4.3.2. Enhanced Aerodynamics Through Porosity
4.3.3. Efficient Aerosolization for Pulmonary Applications
4.3.4. Targeted Lung Retention and Cellular Uptake
4.3.5. Role of Fine Particle Fraction (FPF) in Delivery Efficiency
4.3.6. Improved Aerodynamics via Particle Engineering
4.4. Impact of Surface Charge
4.4.1. Colloidal Stability and Surface Charge
4.4.2. Surface Charge and Cellular Uptake
4.4.3. Mucoadhesion and Nasal Retention
4.4.4. Impact of Charge on Immune Response and Drug Targeting
4.4.5. Charge Modulation for Enhanced Delivery
4.5. Impact of Particle Porosity
4.5.1. Aerodynamic Performance and Pulmonary Deposition
4.5.2. Prolonged Drug Retention and Sustained Release
4.5.3. Drug Loading and Tissue Targeting
4.5.4. Porogen-Assisted Porosity Engineering
4.5.5. Enhanced Mucosal and Cellular Uptake
4.6. Impact of Particle Morphology
4.6.1. Spherical and Smooth Morphology for Stability and Delivery Efficiency
4.6.2. Rough and Textured Morphologies for Aerodynamic Efficiency
4.6.3. Uniformity and Homogeneity in Morphology
4.6.4. Surface Features for Targeted Delivery
4.7. Impact of Drug Loading and Encapsulation Efficiency
4.7.1. High Encapsulation Efficiency for Enhanced Drug Retention
4.7.2. Optimized Drug Loading for Sustained Release
4.7.3. Role of Surface Modifications in Enhancing Encapsulation
4.7.4. High Encapsulation Efficiency for Combination Therapies
4.7.5. Encapsulation for Enhanced Stability and Biological Activity
4.7.6. Impact of Formulation Techniques on Encapsulation
4.8. Impact of Drug Release
4.8.1. Sustained Release for Prolonged Therapeutic Effects
4.8.2. Prolonged Release for Targeted Pulmonary Delivery
4.8.3. Controlled Drug Release for Brain-Targeted Delivery
4.8.4. Dual-Drug and Multi-Drug Release Profiles
4.8.5. Biphasic and Controlled Release Profiles
4.8.6. Effect of Surface Modifications on Drug Release
4.8.7. Sustained Release for Enhanced Mucosal and Systemic Responses
4.8.8. Prolonged Drug Release Enhancing Pulmonary Retention
5. Techniques for Designing Tailored PLGA Drug Delivery Systems
5.1. Emulsion Solvent Evaporation for High Encapsulation Efficiency
5.2. Nanoprecipitation for Targeted CNS Delivery
5.3. Spray-Drying for Pulmonary Drug Delivery
5.4. Freeze-Drying for Stability and Long-Term Storage
5.5. Advanced Techniques: Electrospraying and Microfluidics
5.6. Surface Modifications for Enhanced Bioavailability
5.7. Emerging Techniques: Flow Focusing® and Supercritical Fluid Processing
5.8. Optimization Tools for Tailored PLGA Formulations
6. Assessing PLGA-Based Carriers in Pulmonary and Intranasal Delivery
6.1. Physicochemical Characterization and Morphology Analysis
6.2. Biodegradability, Biocompatibility, and Cytotoxicity Studies
6.3. Drug Loading, Release Kinetics, and Sustained Delivery
6.4. Aerodynamic Properties and Pulmonary Delivery
6.5. Mucoadhesion and Nasal Permeation Studies
6.6. Cellular Uptake, Targeting, and Biodistribution
6.7. Immunogenicity and Immune Response Studies
6.8. Therapeutic Efficacy in Disease Models
6.9. Pharmacokinetics and Toxicity Studies
6.10. Imaging and Visualization Techniques
6.11. Advanced Fabrication Techniques and Optimization
7. Proven Benefits of Using PLGA in Intranasal and Pulmonary Drug Delivery
7.1. Sustained and Controlled Drug Release for Chronic Diseases
7.2. Advancing Pulmonary Fibrosis Treatments
7.3. Enhanced Lung Deposition and Retention
7.4. Biocompatibility and Long-Term Safety
7.5. Expanding Systemic Applications Through Pulmonary Delivery
7.6. Non-Invasive CNS Delivery via Intranasal Route
7.7. Reduced Side Effects and Enhanced Therapeutic Efficacy
7.8. Targeted Oncology Therapies
7.9. Enhanced Stability and Bioavailability
7.10. Expanding Delivery Applications with Surface Modifications
7.11. Combination Therapies and Multifactorial Disease Management
7.12. Advances in Cancer and Vaccine Applications
7.13. Gene Therapies and RNA-Based Therapeutics
7.14. Prolonged Efficacy in Chronic Conditions
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahar, R.; Chakraborty, A.; Nainwal, N.; Bahuguna, R.; Sajwan, M.; Jakhmola, V. Application of PLGA as a Biodegradable and Biocompatible Polymer for Pulmonary Delivery of Drugs. AAPS PharmSciTech 2023, 24, 39. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, M.C.; Pais, A.; Sousa, J.J.S.; Brillaut, J.; Olivier, J.C. Development of levofloxacin-loaded PLGA microspheres of suitable properties for sustained pulmonary release. Int. J. Pharm. 2019, 556, 117–124. [Google Scholar] [CrossRef]
- Alghareeb, S.; Asare-Addo, K.; Conway, B.R.; Adebisi, A.O. PLGA nanoparticles for nasal drug delivery. J. Drug Deliv. Sci. Technol. 2024, 95, 105564. [Google Scholar] [CrossRef]
- Areny-Balaguero, A.; Mekseriwattana, W.; Camprubi-Rimblas, M.; Stephany, A.; Roldan, A.; Sole-Porta, A.; Artigas, A.; Closa, D.; Roig, A. Fluorescent PLGA Nanocarriers for Pulmonary Administration: Influence of the Surface Charge. Pharmaceutics 2022, 14, 1447. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Takami, T.; Murakami, Y. Porous PLGA microparticles formed by “one-step” emulsification for pulmonary drug delivery: The surface morphology and the aerodynamic properties. Colloids Surf. B Biointerfaces 2017, 159, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Sodha, S.; Gupta, P. PLGA and PEG based porous microparticles as vehicles for pulmonary somatropin delivery. Eur. J. Pharm. Biopharm. 2023, 191, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Motawea, A.; Ahmed, D.A.M.; El-Mansy, A.A.; Saleh, N.M. Crucial Role of PLGA Nanoparticles in Mitigating the Amiodarone-Induced Pulmonary Toxicity. Int. J. Nanomed. 2021, 16, 4713–4737. [Google Scholar]
- Dhanda, D.S.; Tyagi, P.; Mirvish, S.S.; Kompella, U.B. Supercritical fluid technology based large porous celecoxib-PLGA microparticles do not induce pulmonary fibrosis and sustain drug delivery and efficacy for several weeks following a single dose. J. Control. Release 2013, 168, 239–250. [Google Scholar] [CrossRef]
- Martin-Banderas, L.; Holgado, M.A.; Alvarez-Fuentes, J.; Fernandez-Arevalo, M. Use of Flow Focusing(R) technology to produce tobramycin-loaded PLGA microparticles for pulmonary drug delivery. Med. Chem. 2012, 8, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, M.; Liu, X.; Jin, Y. Drug-Loaded PLGA Electrospraying Porous Microspheres for the Local Therapy of Primary Lung Cancer via Pulmonary Delivery. ACS Omega 2017, 2, 2273–2279. [Google Scholar] [CrossRef]
- Albarki, M.A.; Donovan, M.D. Uptake of Cationic PAMAM-PLGA Nanoparticles by the Nasal Mucosa. Sci. Pharm. 2022, 90, 72. [Google Scholar] [CrossRef]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson’s disease treatment. Int. J. Nanomedicine 2016, 11, 6547–6559. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 12. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Engineered PLGA nano- and micro-carriers for pulmonary delivery: Challenges and promises. J. Pharm. Pharmacol. 2012, 64, 1217–1235. [Google Scholar] [CrossRef]
- Lababidi, N.; Montefusco-Pereira, C.V.; de Souza Carvalho-Wodarz, C.; Lehr, C.M.; Schneider, M. Spray-dried multidrug particles for pulmonary co-delivery of antibiotics with N-acetylcysteine and curcumin-loaded PLGA-nanoparticles. Eur. J. Pharm. Biopharm. 2020, 157, 200–210. [Google Scholar] [CrossRef]
- Yang, Z.L.; Wang, L.M.; Tian, L.; Zhang, X.P.; Huang, G.H. Tadalafil-loaded PLGA microspheres for pulmonary administration: Preparation and evaluation. Braz. J. Pharm. Sci. 2019, 55, e17536. [Google Scholar] [CrossRef]
- Ali, A.A.E.; Taher, M.; Mohamed, F. Microencapsulation of alpha-mangostin into PLGA microspheres and optimization using response surface methodology intended for pulmonary delivery. J. Microencapsul. 2013, 30, 728–740. [Google Scholar] [CrossRef]
- Almutairi, M.; Hefnawy, A.; Almotairy, A.; Alobaida, A.; Alyahya, M.; Althobaiti, A.; Adel Ali Youssef, A.; Elkanayati, R.M.; Ashour, E.A.; Smyth, H.D.C.; et al. Formulation and evaluation of inhaled Sildenafil-loaded PLGA microparticles for treatment of pulmonary arterial hypertension (PAH): A novel high drug loaded formulation and scalable process via hot melt extrusion technology (Part Ⅰ). Int. J. Pharm. 2024, 655, 124044. [Google Scholar] [CrossRef]
- Koushik, K.; Dhanda, D.S.; Cheruvu, N.P.; Kompella, U.B. Pulmonary delivery of deslorelin: Large-porous PLGA particles and HPbetaCD complexes. Pharm. Res. 2004, 21, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Chalikwar, S.S.; Mene, B.S.; Pardeshi, C.V.; Belgamwar, V.S.; Surana, S.J. Self-Assembled, Chitosan Grafted PLGA Nanoparticles for Intranasal Delivery: Design, Development and Ex Vivo Characterization. Polym.-Plast. Technol. Eng. 2013, 52, 368–380. [Google Scholar] [CrossRef]
- Yamamoto, H.; Kuno, Y.; Sugimoto, S.; Takeuchi, H.; Kawashima, Y. Surface-modified PLGA nanosphere with chitosan improved pulmonary delivery of calcitonin by mucoadhesion and opening of the intercellular tight junctions. J. Control. Release 2005, 102, 373–381. [Google Scholar] [CrossRef]
- Pawar, D.; Mangal, S.; Goswami, R.; Jaganathan, K.S. Development and characterization of surface modified PLGA nanoparticles for nasal vaccine delivery: Effect of mucoadhesive coating on antigen uptake and immune adjuvant activity. Eur. J. Pharm. Biopharm. 2013, 85, 550–559. [Google Scholar] [CrossRef]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele di Villa Bianca, R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef]
- Shah, S.R.; Prajapati, H.R.; Sheth, D.B.; Gondaliya, E.M.; Vyas, A.J.; Soniwala, M.M.; Chavda, J.R. Pharmacokinetics and in vivo distribution of optimized PLGA nanoparticles for pulmonary delivery of levofloxacin. J. Pharm. Pharmacol. 2020, 72, 1026–1037. [Google Scholar] [CrossRef] [PubMed]
- Dawre, S.; Waghela, S.; Saraogi, G. Statistically designed vitamin D3 Encapsulated PLGA microspheres dispersed in thermoresponsive in-situ gel for nasal delivery. J. Drug Deliv. Sci. Technol. 2022, 75, 103688. [Google Scholar] [CrossRef]
- Feng, T.; Tian, H.; Xu, C.; Lin, L.; Xie, Z.; Lam, M.H.; Liang, H.; Chen, X. Synergistic co-delivery of doxorubicin and paclitaxel by porous PLGA microspheres for pulmonary inhalation treatment. Eur. J. Pharm. Biopharm. 2014, 88, 1086–1093. [Google Scholar] [CrossRef]
- Ziaei, E.; Emami, J.; Rezazadeh, M.; Kazemi, M. Pulmonary Delivery of Docetaxel and Celecoxib by PLGA Porous Microparticles for Their Synergistic Effects Against Lung Cancer. Anticancer Agents Med. Chem. 2022, 22, 951–967. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Gregoire, N.; Sousa, J.J.; Pais, A.A.; Lamarche, I.; Gobin, P.; Olivier, J.C.; Marchand, S.; Couet, W. Pulmonary pharmacokinetics of levofloxacin in rats after aerosolization of immediate-release chitosan or sustained-release PLGA microspheres. Eur. J. Pharm. Sci. 2016, 93, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alshraiedeh, N.H.; Zayed, A.L.; Altaani, B.M. Low Molecular Weight Chitosan-Coated PLGA Nanoparticles for Pulmonary Delivery of Tobramycin for Cystic Fibrosis. Pharmaceuticals 2018, 11, 28. [Google Scholar] [CrossRef]
- Debnath, S.K.; Saisivam, S.; Omri, A. PLGA Ethionamide Nanoparticles for Pulmonary Delivery: Development and in vivo evaluation of dry powder inhaler. J. Pharm. Biomed. Anal. 2017, 145, 854–859. [Google Scholar] [CrossRef]
- Gunday Tureli, N.; Tureli, A.E.; Schneider, M. Optimization of ciprofloxacin complex loaded PLGA nanoparticles for pulmonary treatment of cystic fibrosis infections: Design of experiments approach. Int. J. Pharm. 2016, 515, 343–351. [Google Scholar] [CrossRef]
- Bonaccorso, A.; Musumeci, T.; Carbone, C.; Vicari, L.; Lauro, M.R.; Puglisi, G. Revisiting the role of sucrose in PLGA-PEG nanocarrier for potential intranasal delivery. Pharm. Dev. Technol. 2018, 23, 265–274. [Google Scholar] [CrossRef]
- Lee, W.T.; Lee, H.; Kim, J.; Jung, Y.; Choi, E.; Jeong, J.H.; Jeong, J.H.; Lee, J.H.; Youn, Y.S. Alveolar macrophage phagocytosis-evading inhaled microgels incorporating nintedanib-PLGA nanoparticles and pirfenidone-liposomes for improved treatment of pulmonary fibrosis. Bioact. Mater. 2024, 33, 262–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bajaj, N.; Xu, P.; Ohn, K.; Tsifansky, M.D.; Yeo, Y. Development of highly porous large PLGA microparticles for pulmonary drug delivery. Biomaterials 2009, 30, 1947–1953. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Emami, J.; Najafabadi, A.R.; Gilani, K.; Minaiyan, M.; Mahdavi, H.; Nokhodchi, A. Effect of carrier morphology and surface characteristics on the development of respirable PLGA microcapsules for sustained-release pulmonary delivery of insulin. Int. J. Pharm. 2010, 389, 74–85. [Google Scholar] [CrossRef]
- Aguiar, M.M.; Rodrigues, J.M.; Silva Cunha, A. Encapsulation of insulin-cyclodextrin complex in PLGA microspheres: A new approach for prolonged pulmonary insulin delivery. J. Microencapsul. 2004, 21, 553–564. [Google Scholar] [CrossRef]
- Osman, R.; Kan, P.L.; Awad, G.; Mortada, N.; El-Shamy, A.E.; Alpar, O. Enhanced properties of discrete pulmonary deoxyribonuclease I (DNaseI) loaded PLGA nanoparticles during encapsulation and activity determination. Int. J. Pharm. 2011, 408, 257–265. [Google Scholar] [CrossRef]
- Pirooznia, N.; Hasannia, S.; Lotfi, A.S.; Ghanei, M. Encapsulation of Alpha-1 antitrypsin in PLGA nanoparticles: In Vitro characterization as an effective aerosol formulation in pulmonary diseases. J. Nanobiotechnol. 2012, 10, 20. [Google Scholar] [CrossRef]
- Devrim, B.; Bozkir, A.; Canefe, K. Preparation and evaluation of PLGA microparticles as carrier for the pulmonary delivery of rhIL-2: I. Effects of some formulation parameters on microparticle characteristics. J. Microencapsul. 2011, 28, 582–594. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef]
- El-Zaafarany, G.M.; Soliman, M.E.; Mansour, S.; Cespi, M.; Palmieri, G.F.; Illum, L.; Casettari, L.; Awad, G.A.S. A Tailored Thermosensitive PLGA-PEG-PLGA/Emulsomes Composite for Enhanced Oxcarbazepine Brain Delivery via the Nasal Route. Pharmaceutics 2018, 10, 217. [Google Scholar] [CrossRef]
- Kaur, S.; Manhas, P.; Swami, A.; Bhandari, R.; Sharma, K.K.; Jain, R.; Kumar, R.; Pandey, S.K.; Kuhad, A.; Sharma, R.K.; et al. Bioengineered PLGA-chitosan nanoparticles for brain targeted intranasal delivery of antiepileptic TRH analogues. Chem. Eng. J. 2018, 346, 630–639. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, Y.; Liu, Y.; Hu, X.; Han, H.; Li, Q. Artesunate-loaded porous PLGA microsphere as a pulmonary delivery system for the treatment of non-small cell lung cancer. Colloids Surf. B Biointerfaces 2021, 206, 111937. [Google Scholar] [CrossRef]
- Chung, K.; Ullah, I.; Kim, N.; Lim, J.; Shin, J.; Lee, S.C.; Jeon, S.; Kim, S.H.; Kumar, P.; Lee, S.K. Intranasal delivery of cancer-targeting doxorubicin-loaded PLGA nanoparticles arrests glioblastoma growth. J. Drug Target. 2020, 28, 617–626. [Google Scholar] [CrossRef]
- Imamoglu, S.; Devrim Gökberk, B.; Eryilmaz, W.; Bozkir, A. Effect of surfactant types and concentrations on levofloxacin-loaded PLGA microparticles for pulmonary delivery—An in vitro study. J. Res. Pharm. 2022, 26, 1156–1176. [Google Scholar] [CrossRef]
- Kawashima, Y.; Yamamoto, H.; Takeuchi, H.; Fujioka, S.; Hino, T. Pulmonary delivery of insulin with nebulized DL-lactide/glycolide copolymer (PLGA) nanospheres to prolong hypoglycemic effect. J. Control. Release 1999, 62, 279–287. [Google Scholar] [CrossRef]
- Feng, T.S.; Tian, H.Y.; Xu, C.N.; Lin, L.; Lam, M.H.W.; Liang, H.J.; Chen, X.S. Doxorubicin-loaded PLGA microparticles with internal pores for long-acting release in pulmonary tumor inhalation treatment. Chin. J. Polym. Sci. 2015, 33, 947–954. [Google Scholar] [CrossRef]
- Bitencourt, C.D.; Gelfuso, G.M.; Pereira, P.A.T.; de Assis, P.A.; Tefé-Silva, C.; Ramos, S.G.; Arantes, E.C.; Faccioli, L.H. Hyaluronidase-Loaded PLGA Microparticles as a New Strategy for the Treatment of Pulmonary Fibrosis. Tissue Eng. Part A 2015, 21, 246–256. [Google Scholar] [CrossRef]
- Chen, Y.L.; Li, Y.; He, Y.J.; Shen, L.; Qiao, L.; Li, J.; Zhao, Y.Z.; Gao, J.N.; Hao, Y.H. Targeted lung therapy with rosmarinic acid encapsulated in PLGA microspheres for radiation-induced pulmonary fibrosis. J. Drug Deliv. Sci. Technol. 2024, 96, 105710. [Google Scholar] [CrossRef]
- Saghir, S.A.M.; Al-Gabri, N.A.; Khafaga, A.F.; El-Shaer, N.H.; Alhumaidh, K.A.; Elsadek, M.F.; Ahmed, B.M.; Alkhawtani, D.M.; Abd El-Hack, M.E. Thymoquinone-PLGA-PVA Nanoparticles Ameliorate Bleomycin-Induced Pulmonary Fibrosis in Rats via Regulation of Inflammatory Cytokines and iNOS Signaling. Animals 2019, 9, 951. [Google Scholar] [CrossRef]
- Shahabadi, N.; Moshiri, M.; Roohbakhsh, A.; Imenshahidi, M.; Hashemi, M.; Amin, F.; Yazdian-Robati, R.; Salmasi, Z.; Etemad, L. A dose-related positive effect of inhaled simvastatin-loaded PLGA nanoparticles on paraquat-induced pulmonary fibrosis in rats. Basic Clin. Pharmacol. Toxicol. 2022, 131, 251–261. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Khallaf, R.A.; Mahmoud, M.O.; Hussein, R.R.S.; El-Kalaawy, A.M.; Abdel-Razik, A.H.; Aboud, H.M. Intratracheally Inhalable Nifedipine-Loaded Chitosan-PLGA Nanocomposites as a Promising Nanoplatform for Lung Targeting: Snowballed Protection via Regulation of TGF-beta/beta-Catenin Pathway in Bleomycin-Induced Pulmonary Fibrosis. Pharmaceuticals 2021, 14, 225. [Google Scholar] [CrossRef]
- Huang, J.; Chen, Z.; Li, Y.; Li, L.; Zhang, G. Rifapentine-linezolid-loaded PLGA microspheres for interventional therapy of cavitary pulmonary tuberculosis: Preparation and in vitro characterization. Drug Des. Devel Ther. 2017, 11, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, I.; Taniguchi, Y.; Tamura, Y.; Ochiai, K.; Makino, K. Effects of L-leucine on PLGA microparticles for pulmonary administration prepared using spray drying: Fine particle fraction and phagocytotic ratio of alveolar macrophages. Colloid. Surf. A-Physicochem. Eng. Asp. 2018, 537, 411–417. [Google Scholar] [CrossRef]
- Tomoda, K.; Kojima, S.; Kajimoto, M.; Watanabe, D.; Nakajima, T.; Makino, K. Effects of pulmonary surfactant system on rifampicin release from rifampicin-loaded PLGA microspheres. Colloids Surf. B Biointerfaces 2005, 45, 1–6. [Google Scholar] [CrossRef]
- Tafaghodi, M.; Tabassi, S.A.S.; Jaafari, M.R. Nasal immunization by (PLGA) nanospheres encapsulated with tetanus toxoid and (CpG-ODN). Iran. J. Pharm. Res. 2007, 6, 151–158. [Google Scholar]
- Park, S.; Park, J.Y.; Nahm, J.H.; Kim, G.; Cho, Y.; Kang, W.; Key, J. Systemic delivery of nintedanib using PLGA-based discoidal polymeric particles for idiopathic pulmonary fibrosis treatment. Mater. Today Chem. 2022, 26, 101181. [Google Scholar] [CrossRef]
- Gupta, V.; Ahsan, F. Influence of PEI as a core modifying agent on PLGA microspheres of PGE(1), a pulmonary selective vasodilator. Int. J. Pharm. 2011, 413, 51–62. [Google Scholar] [CrossRef]
- Lazo, R.E.L.; Oliveira, B.D.; Cobre, A.D.; Ferreira, L.M.; Felipe, K.B.; de Oliveira, P.R.; Murakami, F.S. Engineering porous PLGA microparticles for pulmonary delivery of sildenafil citrate. Powder Technol. 2023, 430, 118999. [Google Scholar] [CrossRef]
- Gupta, V.; Davis, M.; Hope-Weeks, L.J.; Ahsan, F. PLGA microparticles encapsulating prostaglandin E1-hydroxypropyl-beta-cyclodextrin (PGE1-HPbetaCD) complex for the treatment of pulmonary arterial hypertension (PAH). Pharm. Res. 2011, 28, 1733–1749. [Google Scholar] [CrossRef]
- Zhao, X.; Ni, S.; Song, Y.; Hu, K. Intranasal delivery of Borneol/R8dGR peptide modified PLGA nanoparticles co-loaded with curcumin and cisplatin alleviate hypoxia in pediatric brainstem glioma which improves the synergistic therapy. J. Control. Release 2023, 362, 121–137. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.; Mehta, T. Intranasal delivery of chitosan decorated PLGA core /shell nanoparticles containing flavonoid to reduce oxidative stress in the treatment of Alzheimer’s disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102242. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Y.; Wang, A.; Duan, X.; Sun, Y.; Wang, L.; Chu, L.; Lv, Y.; Cui, N.; Fan, X.; et al. Temozolomide hexadecyl ester targeted plga nanoparticles for drug-resistant glioblastoma therapy via intranasal administration. Front. Pharmacol. 2022, 13, 965789. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Gauba, P.; Tyagi, A.; Dang, S.T. Surface modified PLGA nanoparticles of gabapentin: Biodistribution and pharmacodynamic investigation via intranasal route. J. Drug Deliv. Sci. Technol. 2024, 97, 105786. [Google Scholar] [CrossRef]
- Tanna, V.; Vora, A.; Shah, P.; Nair, A.B.; Shah, J.; Sawarkar, S.P. PLGA Nanoparticles Based Mucoadhesive Nasal In Situ Gel for Enhanced Brain Delivery of Topiramate. AAPS PharmSciTech 2024, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Handa, M.; Sanap, S.N.; Bhatta, R.S.; Patil, G.P.; Ghose, S.; Singh, D.P.; Shukla, R. Combining donepezil and memantine via mannosylated PLGA nanoparticles for intranasal delivery: Characterization and preclinical studies. Biomater. Adv. 2023, 154, 213663. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, T.; Di Benedetto, G.; Carbone, C.; Bonaccorso, A.; Amato, G.; Lo Faro, M.J.; Burgaletto, C.; Puglisi, G.; Bernardini, R.; Cantarella, G. Intranasal Administration of a TRAIL Neutralizing Monoclonal Antibody Adsorbed in PLGA Nanoparticles and NLC Nanosystems: An In Vivo Study on a Mouse Model of Alzheimer’s Disease. Biomedicines 2022, 10, 985. [Google Scholar] [CrossRef]
- Gattani, V.; Dawre, S. Development of favipiravir loaded PLGA nanoparticles entrapped in in-situ gel for treatment of Covid-19 via nasal route. J. Drug Deliv. Sci. Technol. 2023, 79, 104082. [Google Scholar] [CrossRef]
- Jaganathan, K.S.; Vyas, S.P. Strong systemic and mucosal immune responses to surface-modified PLGA microspheres containing recombinant hepatitis B antigen administered intranasally. Vaccine 2006, 24, 4201–4211. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, F.; Earley, B.; Cassidy, J.P.; Markey, B.; Doherty, S.; Welsh, M.D. Comparing the immune response to a novel intranasal nanoparticle PLGA vaccine and a commercial BPI3V vaccine in dairy calves. BMC Vet. Res. 2015, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Genta, I.; Colonna, C.; Conti, B.; Caliceti, P.; Salmaso, S.; Speziale, P.; Pietrocola, G.; Chiesa, E.; Modena, T.; Dorati, R. CNA-loaded PLGA nanoparticles improve humoral response againstS. aureus-mediated infections in a mouse model: Subcutaneous vs. nasal administration strategy. J. Microencapsul. 2016, 33, 750–762. [Google Scholar] [CrossRef]
- Souci, L.; Jaunet, H.; Le Diguerher, G.; Guionnet, J.M.; Beven, V.; Paboeuf, F.; Montier, T.; Dory, D. Intranasal inoculations of naked or PLGA-PEI nanovectored DNA vaccine induce systemic and mucosal antibodies in pigs: A feasibility study. Res. Vet. Sci. 2020, 132, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Bailey, B.A.; Desai, K.-G.H.; Ochyl, L.J.; Ciotti, S.M.; Moon, J.J.; Schwendeman, S.P. Self-encapsulating Poly(lactic-co-glycolic acid) (PLGA) Microspheres for Intranasal Vaccine Delivery. Mol. Pharm. 2017, 14, 3228–3237. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Lin, M.Y.; Bal, S.M.; van Meijgaarden, K.E.; Franken, K.L.; Friggen, A.H.; Junginger, H.E.; Borchard, G.; Klein, M.R.; Ottenhoff, T.H. Pulmonary delivery of DNA encoding Mycobacterium tuberculosis latency antigen Rv1733c associated to PLGA-PEI nanoparticles enhances T cell responses in a DNA prime/protein boost vaccination regimen in mice. Vaccine 2009, 27, 4010–4017. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Qin, L.; Yue, M.; Xue, J.; Cui, Z.; Zhan, X.; Gai, J.; Zhang, X.; Guan, J.; et al. Tunable rigidity of PLGA shell-lipid core nanoparticles for enhanced pulmonary siRNA delivery in 2D and 3D lung cancer cell models. J. Control. Release 2024, 366, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Slutter, B.; Bal, S.; Keijzer, C.; Mallants, R.; Hagenaars, N.; Que, I.; Kaijzel, E.; van Eden, W.; Augustijns, P.; Lowik, C.; et al. Nasal vaccination with N-trimethyl chitosan and PLGA based nanoparticles: Nanoparticle characteristics determine quality and strength of the antibody response in mice against the encapsulated antigen. Vaccine 2010, 28, 6282–6291. [Google Scholar] [CrossRef]
- Mohamed, F.; van der Walle, C.F. PLGA microcapsules with novel dimpled surfaces for pulmonary delivery of DNA. Int. J. Pharm. 2006, 311, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Bivas-Benita, M.; Romeijn, S.; Junginger, H.E.; Borchard, G. PLGA-PEI nanoparticles for gene delivery to pulmonary epithelium. Eur. J. Pharm. Biopharm. 2004, 58, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Csaba, N.; Sánchez, A.; Alonso, M.J. PLGA:: Poloxamer and PLGA:: Poloxamine blend nanostructures as carriers for nasal gene delivery. J. Control. Release 2006, 113, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Gupta, V.; Ahsan, F. PEG-PLGA based large porous particles for pulmonary delivery of a highly soluble drug, low molecular weight heparin. J. Control. Release 2012, 162, 310–320. [Google Scholar] [CrossRef]
- Niwa, T.; Takeuchi, H.; Hino, T.; Kawashima, Y. [Aerosolization of lactide/glycolide copolymer (PLGA) nanospheres for pulmonary delivery of peptide-drugs]. Yakugaku Zasshi 1995, 115, 732–741. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Hamishehkar, H.; Najafabadi, A.R.; Gilani, K.; Minaiyan, M.; Mahdavi, H.; Mirzadeh, H.; Fakhari, A.; Nokhodchi, A. Particle size design of PLGA microspheres for potential pulmonary drug delivery using response surface methodology. J. Microencapsul. 2009, 26, 1–8. [Google Scholar] [CrossRef]
- Chung, E.P.; Cotter, J.D.; Prakapenka, A.V.; Cook, R.L.; DiPerna, D.M.; Sirianni, R.W. Targeting Small Molecule Delivery to the Brain and Spinal Cord via Intranasal Administration of Rabies Virus Glycoprotein (RVG29)-Modified PLGA Nanoparticles. Pharmaceutics 2020, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, J.; Kamal, Z.; Guo, P.; Wu, X.; Lu, L.; Wu, H.; Qiu, M. Odorranalectin modified PEG-PLGA/PEG-PBLG curcumin-loaded nanoparticle for intranasal administration. Drug Dev. Ind. Pharm. 2020, 46, 899–909. [Google Scholar] [CrossRef]
- Mohaghegh, M.; Tafaghodi, M. Dextran microspheres could enhance immune responses against PLGA nanospheres encapsulated with tetanus toxoid and Quillaja saponins after nasal immunization in rabbit. Pharm. Dev. Technol. 2011, 16, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Isabel, C.Z.; Luis, A.D.; Samuel, E.S.; Elizabeth, P.S.; Dea, H.R.; Sergio, A.A. “Novel mucoadhesive PLGA-PVM/MA micro-nanocomposites loaded with felodipine intended for pulmonary administration by nebulization”. Int. J. Pharm. 2022, 628, 122295. [Google Scholar] [CrossRef]
- Pawar, D.; Goyal, A.K.; Mangal, S.; Mishra, N.; Vaidya, B.; Tiwari, S.; Jain, A.K.; Vyas, S.P. Evaluation of mucoadhesive PLGA microparticles for nasal immunization. AAPS J. 2010, 12, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Carcaboso, A.M.; Hernández, R.M.; Igartua, M.; Rosas, J.E.; Patarroyo, M.E.; Pedraz, J.L. Potent, long lasting systemic antibody levels and mixed Th1/Th2 immune response after nasal immunization with malaria antigen loaded PLGA microparticles. Vaccine 2004, 22, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Puras, G.; Salvador, A.; Igartua, M.; Hernández, R.M.; Pedraz, J.L. Encapsulation of Aβ1–15 in PLGA microparticles enhances serum antibody response in mice immunized by subcutaneous and intranasal routes. Eur. J. Pharm. Sci. 2011, 44, 200–206. [Google Scholar] [CrossRef]
- Maaz, A.; Blagbrough, I.S.; De Bank, P.A. A Cell-Based Nasal Model for Screening the Deposition, Biocompatibility, and Transport of Aerosolized PLGA Nanoparticles. Mol. Pharm. 2024, 21, 1108–1124. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Murakami, Y. Development of PEG-PLA/PLGA microparticles for pulmonary drug delivery prepared by a novel emulsification technique assisted with amphiphilic block copolymers. Colloids Surf. B Biointerfaces 2011, 87, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Zhang, Z.; Li, Z.; Huang, G. Preparation and in vitro evaluation of etoposide-loaded PLGA microspheres for pulmonary drug delivery. Drug Deliv. 2014, 21, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Nanaki, S.G.; Spyrou, K.; Bekiari, C.; Veneti, P.; Baroud, T.N.; Karouta, N.; Grivas, I.; Papadopoulos, G.C.; Gournis, D.; Bikiaris, D.N. Hierarchical Porous Carbon-PLLA and PLGA Hybrid Nanoparticles for Intranasal Delivery of Galantamine for Alzheimer’s Disease Therapy. Pharmaceutics 2020, 12, 227. [Google Scholar] [CrossRef]
- Karavasili, C.; Bouropoulos, N.; Sygellou, L.; Amanatiadou, E.P.; Vizirianakis, I.S.; Fatouros, D.G. PLGA/DPPC/trimethylchitosan spray-dried microparticles for the nasal delivery of ropinirole hydrochloride: In vitro, ex vivo and cytocompatibility assessment. Mater. Sci. Eng. C 2016, 59, 1053–1062. [Google Scholar] [CrossRef]
- Ghaderinia, P.; Shapouri, R.; Rostamizadeh, K.; Khodavandi, A.; Mahdavi, M. Immunogenic Evaluation of MPEG-PCL & PLGA Nanoparticles Containing Klebsiella pneumoniae K2O1 Capsular Antigen in Pulmonary Infection Model of Mice. IEEE Trans. Nanobioscience 2023, 22, 393–400. [Google Scholar] [CrossRef]
- Musumeci, T.; Serapide, M.F.; Pellitteri, R.; Dalpiaz, A.; Ferraro, L.; Dal Magro, R.; Bonaccorso, A.; Carbone, C.; Veiga, F.; Sancini, G.; et al. Oxcarbazepine free or loaded PLGA nanoparticles as effective intranasal approach to control epileptic seizures in rodents. Eur. J. Pharm. Biopharm. 2018, 133, 309–320. [Google Scholar] [CrossRef]
- Al-Saikhan, F.I.; Abd-Elaziz, M.A.; Al-Shdefat, R.; Anwar, M.K.; Iqbal, M.S. Preparation and Evaluation of Aviptadil Acetate Loaded Plga Microparticles: A Preliminary Study to Treat Pulmonary Hypertension. Lat. Am. J. Pharm. 2019, 38, 545–552. [Google Scholar]
- Chintapula, U.; Yang, S.; Nguyen, T.; Li, Y.; Jaworski, J.; Dong, H.; Nguyen, K.T. Supramolecular Peptide Nanofiber/PLGA Nanocomposites for Enhancing Pulmonary Drug Delivery. ACS Appl. Mater. Interfaces 2022, 14, 56498–56509. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; Nozik-Grayck, E.; McMurtry, I.F.; Oka, M.; Komatsu, M.; Ahsan, F. Inhaled PLGA particles of prostaglandin E(1) ameliorate symptoms and progression of pulmonary hypertension at a reduced dosing frequency. Mol. Pharm. 2013, 10, 1655–1667. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Pan, L.; Zhang, Y.; Wang, Y.; Zhang, Z.; Lu, J.; Zhou, P.; Fang, Y.; Jiang, S. Intranasal delivery of cationic PLGA nano/microparticles-loaded FMDV DNA vaccine encoding IL-6 elicited protective immunity against FMDV challenge. PLoS ONE 2011, 6, e27605. [Google Scholar] [CrossRef]
- Haque, S.; Pouton, C.W.; McIntosh, M.P.; Ascher, D.B.; Keizer, D.W.; Whittaker, M.R.; Kaminskas, L.M. The impact of size and charge on the pulmonary phatmacokinetics and immunological response of the lungs to PLGA nanoparticles after intratracheal administration to rats. Nanomed.-Nanotechnol. Biol. Med. 2020, 30, 102291. [Google Scholar] [CrossRef]
- Aghaei Delche, N.; Kheiri, R.; Ghorbani Nejad, B.; Sheikhi, M.; Razavi, M.S.; Rahimzadegan, M.; Salmasi, Z. Recent progress in the intranasal PLGA-based drug delivery for neurodegenerative diseases treatment. Iran. J. Basic. Med. Sci. 2023, 26, 1107–1119. [Google Scholar] [CrossRef]
- Piazza, J.; Hoare, T.; Molinaro, L.; Terpstra, K.; Bhandari, J.; Selvaganapathy, P.R.; Gupta, B.; Mishra, R.K. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)-block-poly(D,L)-lactic-co-glycolic acid (PEG-PLGA) nanoparticles for the treatment of schizophrenia. Eur. J. Pharm. Biopharm. 2014, 87, 30–39. [Google Scholar] [CrossRef]


| Drug(s) | Therapeutic Purpose | References |
|---|---|---|
| Levofloxacin, Tobramycin, Ciprofloxacin, Azithromycin | Treatment of bacterial infections, including pulmonary infections, biofilm penetration, cystic fibrosis, and chronic lung diseases. | [9,15,23,24,28,29,31,45] |
| Insulin | Prolonged pulmonary delivery for diabetes management with enhanced hypoglycemic effects and optimized formulations. | [35,36,46] |
| Celecoxib, Docetaxel, Paclitaxel | Lung cancer treatment with enhanced tumor targeting, sustained delivery, synergistic effects, and reduced side effects. | [8,10,26,27,47] |
| Alpha-mangostin, Rosmarinic acid, Simvastatin, Thymoquinone, Nifedipine | Treatment of pulmonary fibrosis and inflammation, reducing oxidative stress, and improving lung function. | [17,48,49,50,51,52] |
| Ethionamide, Rifampicin, Rifapentine, Linezolid | Pulmonary tuberculosis therapy with targeted drug release, prolonged lung retention, and macrophage-targeted delivery. | [30,53,54,55,56] |
| Recombinant human interleukin-2 (rhIL-2) | Pulmonary delivery of cytokines for immune modulation, retained bioactivity, and therapeutic protein release. | [33,39] |
| Nintedanib, Pirfenidone | Anti-fibrotic agents for treating idiopathic pulmonary fibrosis with prolonged lung retention and reduced fibrosis progression. | [33,49,57] |
| Sildenafil citrate, Tadalafil, Prostaglandin E1 | Pulmonary arterial hypertension management with improved bioavailability, reduced toxicity, and extended drug release. | [16,18,19,58,59,60] |
| Doxorubicin, Artesunate, Oridonin, Curcumin, Temozolomide | Pulmonary and CNS cancer therapies with enhanced tumor targeting, reduced tumor hypoxia, and prolonged survival rates. | [10,43,44,61,62,63] |
| Vitamin D3, Amiodarone, Gabapentin, Topiramate | Neurological and pulmonary therapies with improved systemic delivery, brain targeting, and reduced toxicity. | [7,25,41,64,65] |
| Rotigotine, Donepezil, Memantine, Huperzine A, Gabapentin, Ropinirole Hydrochloride (RH) | CNS disorders such as Parkinson’s and Alzheimer’s disease with improved brain targeting, therapeutic efficacy, and bioavailability. | [12,13,40,62,66,67] |
| Favipiravir | Treatment of viral infections such as COVID-19 via enhanced nasal delivery systems and sustained release. | [32,68] |
| Deslorelin, Alpha-1 antitrypsin, Calcitonin, Somatropin | Sustained release for systemic and pulmonary hormone replacement, protein therapies, or calcium regulation. | [6,19,21,38] |
| Vaccines (e.g., SPf66 malaria, BPI3V, CNA19, HBsAg) | Nasal and pulmonary vaccine delivery with robust mucosal and systemic immune responses and optimized antigen stability. | [22,69,70,71,72,73] |
| DNA/RNA Therapeutics (e.g., FMDV DNA, siRNA) | Gene therapy for respiratory diseases, with high transfection efficiency, targeted delivery, and enhanced immune responses. | [74,75,76,77,78,79] |
| Polymers and Excipients | Key Patterns and Applications | References |
|---|---|---|
| PLGA, PEG-PLGA, Chitosan, PVA, Mannitol, Sorbitol, Lactose | Biodegradable and biocompatible systems for sustained drug release, enhanced bioavailability, and reduced cytotoxicity. | [1,2,5,16,28,36,39] |
| PLGA, Chitosan, DPPC, Leucine, Cyclodextrins | Enhanced mucoadhesion and nasal or pulmonary delivery with controlled particle size and drug release properties. | [11,20,34,68,81,87] |
| PLGA, Chitosan, TMC, Glycol Chitosan, Lactoferrin | Improved systemic and CNS delivery, enhanced brain targeting for neurodegenerative and CNS disorders. | [3,12,13,25,40,62] |
| PLGA, PEG, PVA, Poloxamer, Poloxamine | Tunable aerodynamic and physical properties for pulmonary drug delivery. | [4,6,16,60,75,82] |
| PLGA, PEI, Poloxamer, Tween 20 | Efficient gene and siRNA delivery systems with high transfection efficiency and targeted gene therapy potential. | [74,75,78,79] |
| PLGA, QS-21, CpG-ODN, Chitosan | Effective vaccine delivery with robust systemic and mucosal immune responses. | [56,73,85,87,88] |
| PLGA, Amphiphilic Block Copolymers, Borneol | Multifunctional drug carriers for localized therapy and enhanced therapeutic efficacy. | [10,26,27,77] |
| PLGA, PVA, Chitosan, Kolliphor | Anti-inflammatory and antimicrobial applications in pulmonary therapies. | [7,9,15,45] |
| PLGA, DPPC, Sorbitol, Leucine, Cyclodextrins | Pulmonary fibrosis treatment with enhanced therapeutic effects and reduced inflammation. | [48,49,50,52] |
| PLGA, Chitosan, PVA, Lactose | Mucoadhesive formulations for effective nasal vaccine delivery. | [22,72,76,89] |
| PLGA, PEI, PVA, Lactose | DNA and RNA delivery platforms with potential applications in pulmonary and nasal gene therapy. | [75,77,78] |
| Physicochemical Properties | Description | References |
|---|---|---|
| Sustained Drug Release | Controlled and prolonged release ranging from hours to weeks for localized or systemic delivery. | [1,2,10,16,19,25,36,39,59,92] |
| High Encapsulation Efficiency | Achieved >70% efficiency for diverse drugs, maintaining stability and preventing degradation. | [10,17,25,31,36,37,39,53,59,85,92] |
| Particle Size Control | Tunable sizes from nanoscale (<200 nm) to microscale (1–20 µm) for specific delivery needs. | [4,22,25,34,35,54,57,65,81] |
| Surface Charge Modifications | Cationic and anionic modifications to enhance mucoadhesion, uptake, or lung retention. | [3,4,11,20,74,76] |
| Aerodynamic Properties for Pulmonary Delivery | Optimized aerodynamic diameters (1–5 µm) and low density for deep lung deposition. | [5,14,16,25,37,58,82] |
| Porosity and Surface Morphology | Porous/dimpled particles enhance drug release, retention, and avoid macrophage uptake. | [5,8,19,27,34,58,69] |
| Biodegradability and Biocompatibility | Safe degradation into lactic and glycolic acid; no cytotoxic effects; suitable for long-term use. | [1,2,5,6,7,22] |
| Colloidal Stability | Formulations resist aggregation, ensuring reliable delivery and extended shelf life. | [4,29,30,31,49,70] |
| Targeting and Mucoadhesive Properties | Improved mucoadhesion and tissue targeting through polymers like chitosan and ligands. | [3,12,20,21,40,66,87] |
| Hydrophilic and Hydrophobic Drug Compatibility | Versatile drug encapsulation enabling both hydrophilic and hydrophobic drug formulations. | [9,19,28,43,54,65] |
| Enhanced Intracellular Uptake and Endosomal Escape | Nanoparticles designed for intracellular delivery with superior escape mechanisms. | [12,62,67,75,83,98] |
| Fine Particle Fraction (FPF) | High FPF for inhalation formulations, enabling effective deep lung drug delivery. | [17,35,45,54,68,81] |
| Drug Loading Capacity | High loading efficiency (up to 30%) for achieving therapeutic drug concentrations. | [19,34,39,52,89] |
| Dual Drug Delivery Systems | Co-delivery of drugs for synergistic therapeutic effects, especially for cancer and infections. | [15,26,27,71] |
| Controlled Degradation Profiles | PLGA formulations tailored for specific degradation rates based on polymer composition. | [1,10,19,57] |
| Immune Response Modulation | Surface-modified PLGA particles to enhance antigen presentation and sIgA production. | [22,69,72,73] |
| Preparation/Processing Technique | Key Features and Applications | References |
|---|---|---|
| Emulsion Solvent Evaporation | Widely used for hydrophilic and hydrophobic drugs, providing high encapsulation efficiency and size control. | [2,19,21,24,31,36,37,39,45,46,47,53,63,88] |
| Double Emulsion Solvent Evaporation (w/o/w) | Effective for encapsulating proteins, peptides, and hydrophilic drugs, retaining bioactivity and minimizing burst release. | [2,19,34,36,39,40,58,60,87,89] |
| Spray Drying | Produces low-density, fine particles for pulmonary applications with excellent aerodynamic properties. | [5,15,23,35,54,86,91,94] |
| Nanoprecipitation | Ideal for stable, small nanoparticles with high drug loading for nasal, pulmonary, and CNS delivery. | [7,10,11,12,13,31,32,41,62] |
| Freeze Drying (Lyophilization) | Stabilizes formulations, prevents aggregation, and improves storage for long-term use. | [23,24,25,30,96] |
| Supercritical Fluid Technology | Environmentally friendly method for preparing porous microparticles with controlled porosity and size. | [8] |
| Flow Focusing® Technology | Produces highly uniform particles, particularly for chronic disease treatments via pulmonary delivery. | [9] |
| Hot-Melt Extrusion | Combined with porogens for controlled-release microparticles for pulmonary hypertension therapy. | [18] |
| Membrane Emulsification | Ensures uniform particle sizes with high encapsulation efficiency, particularly for hydrophobic drugs. | [49] |
| One-step Emulsification | Simplified process for producing porous microparticles with enhanced lung retention properties. | [5,99] |
| Top-down Particle Engineering | Produces discoidal particles tailored for improved lung deposition and controlled aerodynamic performance. | [57] |
| Surface Modification (e.g., Ligand/Chitosan Coating) | Enhances targeting, mucoadhesion, and immune response; applied in nasal, pulmonary, and vaccine delivery. | [3,20,21,22,29,40,66,78,100] |
| Cryoprotectant-Assisted Formulations | Stabilizes nanoparticles during freeze drying while maintaining bioactivity and redispersibility. | [32,96] |
| Electrospraying | Produces porous microparticles for cancer and fibrosis therapy with high drug loading and fine particle fractions. | [10] |
| Box–Behnken Design and Optimization | Optimizes particle size, drug release, and encapsulation efficiency for therapeutic tailoring. | [12,31,40,68,82] |
| Microfluidics | Advanced technique for precise shell-core nanoparticles and siRNA delivery. | [75] |
| Combination Technologies (e.g., Spray Drying + Nanoprecipitation) | Hybrid approaches for co-delivery systems and synergistic drug combinations. | [15,26,27,91] |
| Porogens for Porous Structures (e.g., Ammonium Bicarbonate) | Enhances porosity, lung retention, and controlled drug release for pulmonary applications. | [18,19,34] |
| Design of Experiments (DoEs) | Applied for optimizing formulations to achieve desired drug release profiles and physicochemical properties. | [31,49,57,60,68,82] |
| Testing/Evaluation Method | Description | References |
|---|---|---|
| In Vitro Drug Release Studies | Evaluates release kinetics, focusing on sustained, controlled, or burst release profiles, often paired with modeling approaches. | [24,26,28,30,31,60,71,92] |
| Particle Size and Morphology Analysis | Uses SEM, TEM, and laser diffraction to assess uniformity, aerodynamic performance, and surface characteristics. | [5,22,35,43,54,57,97] |
| Encapsulation Efficiency and Drug Loading | Measures drug entrapment efficiency and loading capacity to ensure optimized therapeutic payloads. | [17,19,39,78,80] |
| In Vivo Pharmacokinetics and Bioavailability | Analyzes plasma drug concentration, lung retention, and bioavailability for systemic and localized therapies. | [19,28,33,51,59,65] |
| Mucoadhesion and Nasal Permeation Tests | Evaluates formulations’ adhesion to mucosal surfaces and their ability to permeate nasal epithelium for enhanced delivery. | [20,21,22,25,68,71] |
| Aerodynamic Property Testing | FPF, MMAD, and respirable fractions are measured to ensure efficient pulmonary deposition and retention. | [5,8,10,18,24,34,52,54,86] |
| Cytotoxicity and Biocompatibility Assays | Includes MTT assays, ROS production tests, and histopathological studies to confirm safe use in cells and tissues. | [4,7,17,62,64,75] |
| Cellular Uptake and Endosomal Escape | Analyzes nanoparticle internalization by cells and their escape from endosomal pathways to enhance therapeutic effects. | [11,12,67,75,83,98] |
| Immunological Testing for Vaccine Formulations | Measures IgA/IgG titers, cytokine responses, and mucosal/systemic immunity for evaluating vaccine efficacy. | [69,72,73,74,87,88] |
| Inflammatory and Oxidative Stress Marker Analysis | Reduces markers related to fibrosis, cancer, and inflammation, particularly for pulmonary and systemic diseases. | [33,49,50,51,52] |
| Histopathology and Tissue Analysis | Examines tissue-level toxicity and therapeutic impact using staining, imaging, and immunohistochemistry. | [7,41,43,57,65,75] |
| Pharmacodynamics and Therapeutic Efficacy Studies | Focuses on therapeutic outcomes such as blood glucose control, cancer inhibition, and fibrosis reduction. | [26,44,46,51,57,61] |
| Antibacterial and Antitumor Activity Testing | Tests bacterial killing efficiency, apoptosis induction, and tumor growth inhibition in vitro and in vivo. | [9,15,27,44,61] |
| Safety, Stability, and Shelf-Life Testing | Evaluates formulation stability during storage, including freeze drying and aggregation prevention. | [10,23,25,37,96] |
| Gene Delivery and Transfection Efficiency | Includes siRNA/DNA internalization, immune response monitoring, and cellular transfection efficacy. | [74,75,76,77,78] |
| Metabolic Stability and Degradation Studies | Monitors polymer degradation, metabolic stability, and retention of bioactivity during release. | [24,40,49,60,89] |
| Advanced Imaging and Biodistribution | Uses fluorescence imaging, CT scans, and molecular tomography to track biodistribution and targeting efficiency. | [49,62,79,83,96] |
| Design of Experiments (DoEs) for Optimization | Utilized to optimize particle size, encapsulation efficiency, drug release profiles, and aerodynamic properties. | [25,31,57,60,68,82] |
| Benefit | Description | References |
|---|---|---|
| Sustained and Controlled Drug Release | Enables prolonged therapeutic effects, reduces dosing frequency, and improves patient compliance. | [1,2,19,24,36,39,92] |
| High Encapsulation Efficiency and Drug Loading | Ensures effective delivery of hydrophilic and hydrophobic drugs with minimal waste. | [10,17,18,51,53,85] |
| Tailored Particle Size for Specific Applications | Allows precision in pulmonary, nasal, and systemic drug delivery by optimizing aerodynamic and absorption properties. | [5,25,35,40,54,57,82] |
| Enhanced Bioavailability and Targeting | Improves drug delivery to specific tissues or organs, including lungs, CNS, and mucosal surfaces. | [3,12,13,21,33,62] |
| Biocompatibility and Safety | Biodegradable and biocompatible, with degradation into lactic and glycolic acids; non-toxic even for long-term use. | [1,5,6,7,22] |
| Adaptability to Complex Formulations | Compatible with co-delivery of multiple drugs, enabling synergistic effects in therapies. | [15,26,27,87] |
| Versatility in Preparation Methods | Can be prepared using diverse techniques such as emulsification, nanoprecipitation, spray drying, and supercritical fluids. | [2,8,9,10,49] |
| Immune Modulation and Vaccine Efficiency | Enhances mucosal and systemic immune responses for effective vaccination via nasal and pulmonary routes. | [22,69,72,73,88] |
| Improved Stability and Shelf-Life | Freeze drying and cryoprotectant-assisted techniques ensure long-term stability of formulations. | [23,24,25,96] |
| Enhanced Intracellular Uptake | Surface-modified nanoparticles increase cellular uptake, ensuring effective drug delivery to target cells. | [11,12,67,75,83,98] |
| Reduction of Side Effects | Localized drug delivery minimizes systemic toxicity, particularly in cancer, hypertension, and pulmonary therapies. | [7,18,27,59] |
| Effective Pulmonary Delivery | Optimized aerodynamic properties and low-density formulations enhance lung deposition and retention. | [5,14,15,16,60] |
| Support for Gene Therapy and siRNA Delivery | High transfection efficiency and stability support the development of genetic therapies. | [74,75,77,78] |
| Antioxidant and Anti-Inflammatory Benefits | Encapsulation of bioactive compounds like curcumin and simvastatin reduces oxidative stress and inflammation. | [17,49,50,51,62] |
| Potential for Disease-Specific Customization | Customizable formulations address diseases such as tuberculosis, diabetes, cancer, fibrosis, and CNS disorders. | [28,33,36,47,57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidian, H.; Wilson, R.L. PLGA-Based Strategies for Intranasal and Pulmonary Applications. Pharmaceutics 2025, 17, 207. https://doi.org/10.3390/pharmaceutics17020207
Omidian H, Wilson RL. PLGA-Based Strategies for Intranasal and Pulmonary Applications. Pharmaceutics. 2025; 17(2):207. https://doi.org/10.3390/pharmaceutics17020207
Chicago/Turabian StyleOmidian, Hossein, and Renae L. Wilson. 2025. "PLGA-Based Strategies for Intranasal and Pulmonary Applications" Pharmaceutics 17, no. 2: 207. https://doi.org/10.3390/pharmaceutics17020207
APA StyleOmidian, H., & Wilson, R. L. (2025). PLGA-Based Strategies for Intranasal and Pulmonary Applications. Pharmaceutics, 17(2), 207. https://doi.org/10.3390/pharmaceutics17020207






