Gels as Promising Delivery Systems: Physicochemical Property Characterization and Recent Applications
Abstract
:1. Introduction
2. Physical Properties of Gels
2.1. pH
2.2. Structure
2.3. Mechanical Properties
2.4. Rheological Property
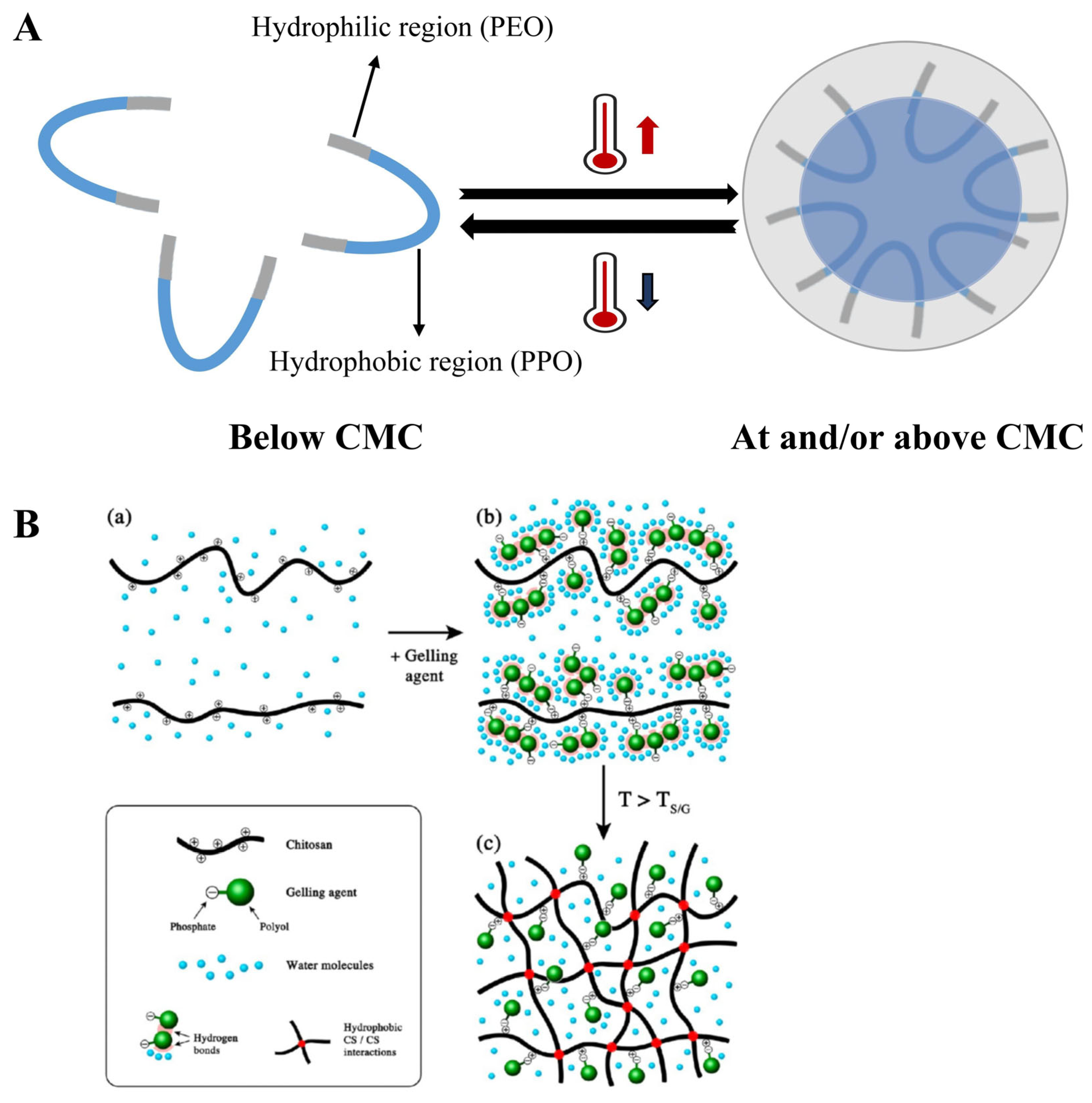
2.5. Phase Transition
3. The Applied Analysis of the Gel
3.1. Analysis of Gels’ pH
3.2. Analysis of Gels’ Structure
3.3. Analysis of Gels’ Mechanical Properties
3.4. Application of Rheometers in Gel
3.5. Analysis of Phase Transition Temperature
| Poly (N-Isopropylacrylamide) (PNIPAAm) with Transition Temperature of 32 °C [115]. | ||||||
|---|---|---|---|---|---|---|
| Thermoresponsive Excipients | Other Major Excipients | Purpose of Addition | Application | Tsol-Gel | Reference | Mechanism |
| PNIPAAm | Hyaluronic acid | Transition temperature of PNIPAAm gels is close to body temperature, so additions used for adjusting Tsol-gel are not necessary. But IPN/semi-IPN network can be formed by adding various hydrophilic polymers to increase mechanical strength, adhesiveness, and biocompatibility and achieve uniform and delayed drug release. | Injection | 30~33 °C | [268] | Figure 10B |
| Poly (vinyl alcohol) | / | 33 °C | [260] | |||
| Hyaluronic acid | Ocular | 34.4~35.5 °C | [276] | |||
| Hyaluronic acid | Ocular | 33 °C | [276] | |||
| Gelatin | / | 37 °C | [277] | |||
| Salecan | / | 32 °C | [116] | |||
| Cellulose nanocrystals | Wound dressing | 36~39 °C | [278] | |||
| Poloxamer with transition temperature of 15~32 °C [279]. | ||||||
| Thermoresponsive excipients | Concentration | Purpose of addition | Application | Tsol-gel | Reference | Mechanism |
| Poloxamer 407 | 16.5% | Adjusting concentration of P407 and P188 (P407 usually 15–30% [106]) for the fine-tuning of PPO and PEO ratios to achieve a formulation with an optimal phase transition temperature [280] | Ocular | 27.1 °C | [281] | Figure 11A |
| 15% | Injection | 35.3 °C | [282] | |||
| 18% | Ocular | 34.3 °C | [283] | |||
| 20.45% | Nasal | 31.99 °C | [284] | |||
| 18% | Nasal | 32 °C | [285] | |||
| Poloxamer 407/188 | 20%: 5% | Ocular | 28.4 °C | [281] | ||
| 17.3%: 1.2% | Buccal | 31.5~ 33.5 °C | [118] | |||
| 24.07%: 1.22% | Rectal | 32.8 °C | [265] | |||
| Chitosan with transition temperature of 30 °C [119]. | ||||||
| Thermoresponsive excipients | Other major excipients | Purpose of addition | Application | Tsol-gel | Reference | Mechanism |
| Chitosan | β-glycerophosphate | Encourage the creation of the gel, enhancing its robustness and the ideal duration for gelation | Injection | 37 °C | [286] | Figure 11B |
| β-glycerophosphate | Injection | 32.6 °C | [269] | |||
| β-glycerophosphate | Injection | 28.36 °C | [270] | |||
| Gelatin/β-glycerophosphate | Ocular | 37 °C | [271] | |||
| Gelatin/β-glycerol phosphate | / | 32.17 °C | [272] | |||
| Hydroxypropyl methylcellulose/glycerol | Hydroxypropyl methylcellulose aids in thermogelation, while glycerol reduces the temperature of phase transition. | Injection | 32 °C | [274] | ||
| Mixed usage of multiple thermoresponsive excipients. | ||||||
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suharyani, I.; Fouad Abdelwahab Mohammed, A.; Muchtaridi, M.; Wathoni, N.; Abdassah, M. Evolution of Drug Delivery Systems for Recurrent Aphthous Stomatitis. Drug Des. Devel Ther. 2021, 15, 4071–4089. [Google Scholar] [CrossRef] [PubMed]
- Tokita, M. Transport Phenomena in Gel. Gels 2016, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef]
- Wychowaniec, J.K.; Saini, H.; Scheibe, B.; Dubal, D.P.; Schneemann, A.; Jayaramulu, K. Hierarchical porous metal-organic gels and derived materials: From fundamentals to potential applications. Chem. Soc. Rev. 2022, 51, 9068–9126. [Google Scholar] [CrossRef]
- Mayr, J.; Saldías, C.; Díaz Díaz, D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Ullah, F.; Othman, M.B.; Javed, F.; Ahmad, Z.; Md Akil, H. Classification, processing and application of hydrogels: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 414–433. [Google Scholar] [CrossRef]
- Kashyap, N.; Kumar, N.; Kumar, M.N. Hydrogels for pharmaceutical and biomedical applications. Crit. Rev. Ther. Drug Carr. Syst. 2005, 22, 107–149. [Google Scholar] [CrossRef]
- Dehshahri, A.; Kumar, A.; Madamsetty, V.S.; Uzieliene, I.; Tavakol, S.; Azedi, F.; Fekri, H.S.; Zarrabi, A.; Mohammadinejad, R.; Thakur, V.K. New Horizons in Hydrogels for Methotrexate Delivery. Gels 2020, 7, 2. [Google Scholar] [CrossRef]
- Gacanin, J.; Synatschke, C.V.; Weil, T. Biomedical Applications of DNA-Based Hydrogels. Adv. Funct. Mater. 2020, 30, 1906253. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Lee, D.S. Injectable biodegradable hydrogels. Macromol. Biosci. 2010, 10, 563–579. [Google Scholar] [CrossRef]
- Mastropietro, D.J.; Omidian, H.; Park, K. Drug delivery applications for superporous hydrogels. Expert. Opin. Drug Deliv. 2012, 9, 71–89. [Google Scholar] [CrossRef] [PubMed]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid Nanoparticles and Their Hydrogel Composites for Drug Delivery: A Review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Kumar, N.; Bhattacharya, C.; Sagiri, S.S.; Jain, K.; Pal, K.; Ray, S.S.; Nayak, B. Organogels: Properties and Applications in Drug Delivery. Des. Monomers Polym. 2011, 14, 95–108. [Google Scholar] [CrossRef]
- Kumar, R.; Katare, O.P. Lecithin organogels as a potential phospholipid-structured system for topical drug delivery: A review. AAPS PharmSciTech 2005, 6, E298–E310. [Google Scholar] [CrossRef] [PubMed]
- Pisal, S.; Shelke, V.; Mahadik, K.; Kadam, S. Effect of organogel components on in vitro nasal delivery of propranolol hydrochloride. AAPS PharmSciTech 2004, 5, e63. [Google Scholar] [CrossRef] [PubMed]
- Plourde, F.; Motulsky, A.; Couffin-Hoarau, A.C.; Hoarau, D.; Ong, H.; Leroux, J.C. First report on the efficacy of l-alanine-based in situ-forming implants for the long-term parenteral delivery of drugs. J. Control Release 2005, 108, 433–441. [Google Scholar] [CrossRef]
- Pan, A.; Roy, S.G.; Haldar, U.; Mahapatra, R.D.; Harper, G.R.; Low, W.L.; De, P.; Hardy, J.G. Uptake and Release of Species from Carbohydrate Containing Organogels and Hydrogels. Gels 2019, 5, 43. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Zhang, Y.; Luo, B.; Liu, X.; Cao, Y.; Pei, R. Construction of adhesive and bioactive silk fibroin hydrogel for treatment of spinal cord injury. Acta Biomater. 2023, 158, 178–189. [Google Scholar] [CrossRef]
- Luo, C.; Guo, A.; Li, J.; Tang, Z.; Luo, F. Janus Hydrogel to Mimic the Structure and Property of Articular Cartilage. ACS Appl. Mater. Interfaces 2022, 14, 35434–35443. [Google Scholar] [CrossRef]
- Xiao, Y.; Gu, Y.; Qin, L.; Chen, L.; Chen, X.; Cui, W.; Li, F.; Xiang, N.; He, X. Injectable thermosensitive hydrogel-based drug delivery system for local cancer therapy. Colloids Surf. B Biointerfaces 2021, 200, 111581. [Google Scholar] [CrossRef]
- Sutthasupa, S.; Padungkit, C.; Suriyong, S. Colorimetric ammonia (NH3) sensor based on an alginate-methylcellulose blend hydrogel and the potential opportunity for the development of a minced pork spoilage indicator. Food Chem. 2021, 362, 130151. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, K.; Li, X.; Zhang, X.; Zhang, D.; Wang, L.N.; Lee, C.S. A double-crosslinked self-healing antibacterial hydrogel with enhanced mechanical performance for wound treatment. Acta Biomater. 2021, 124, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Liu, Y.; Chen, S.; Lin, Y.; Yue, Y. A Schiff base hydrogel dressing loading extracts from Periplaneta Americana for diabetic wound healing. Int. J. Biol. Macromol. 2023, 230, 123256. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Sun, H.; Yue, Z.; Yu, C.; Jiang, L.; Dong, X.; Yao, M.; Shi, M.; Liang, L.; Wan, Y.; et al. Zwitterionic Polysaccharide-Based Hydrogel Dressing as a Stem Cell Carrier to Accelerate Burn Wound Healing. Adv. Healthc. Mater. 2023, 12, e2202309. [Google Scholar] [CrossRef]
- Alsaab, H.; Bonam, S.P.; Bahl, D.; Chowdhury, P.; Alexander, K.; Boddu, S.H. Organogels in Drug Delivery: A Special Emphasis on Pluronic Lecithin Organogels. J. Pharm. Pharm. Sci. 2016, 19, 252–273. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Gündoğdu, E.A.; Cağlar, E.S.; Özgenç, E.; Gonzalez-Alvarez, M.; Gonzalez-Alvarez, I.; Okur, N. Polymer based Gels: Recent and Future Applications in Drug Delivery Field. Curr. Drug Deliv. 2023, 20, 1288–1313. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Wang, Y.; Yang, Y.; Xu, L.; Li, S. Degradation of glutamate-based organogels for biodegradable implants: In vitro study and in vivo observation. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 80–90. [Google Scholar] [CrossRef]
- Dai, M.; Bai, L.; Zhang, H.; Ma, Q.; Luo, R.; Lei, F.; Fei, Q.; He, N. A novel flunarizine hydrochloride-loaded organogel for intraocular drug delivery in situ: Design, physicochemical characteristics and inspection. Int. J. Pharm. 2020, 576, 119027. [Google Scholar] [CrossRef]
- Bourdon, F.; Lecoeur, M.; Leconte, L.; Ultré, V.; Kouach, M.; Odou, P.; Vaccher, C.; Foulon, C. Evaluation of Pentravan(®), Pentravan(®) Plus, Phytobase(®), Lipovan(®) and Pluronic Lecithin Organogel for the transdermal administration of antiemetic drugs to treat chemotherapy-induced nausea and vomiting at the hospital. Int. J. Pharm. 2016, 515, 774–787. [Google Scholar] [CrossRef]
- Querobino, S.M.; de Faria, N.C.; Vigato, A.A.; da Silva, B.G.M.; Machado, I.P.; Costa, M.S.; Costa, F.N.; de Araujo, D.R.; Alberto-Silva, C. Sodium alginate in oil-poloxamer organogels for intravaginal drug delivery: Influence on structural parameters, drug release mechanisms, cytotoxicity and in vitro antifungal activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 1350–1361. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, D.; Chen, J.; Xi, K.; Da, X.; Guo, H.; Zhang, D.; Gao, T.; Lu, T.; Gao, G.; et al. Photoelastic Organogel with Multiple Stimuli Responses. Small 2022, 18, e2204140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Pan, Y.; Dong, Q.; Tang, X.; Xin, Y.; Yin, B.; Zhu, J.; Kou, X.; Ho, C.T.; Huang, Q. Development of organogel-based emulsions to enhance the loading and bioaccessibility of 5-demethylnobiletin. Food Res. Int. 2021, 148, 110592. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Diaz-Dussan, D.; Wu, M.; Peng, Y.Y.; Wang, J.; Zeng, H.; Duan, W.; Kong, L.; Hao, X.; Narain, R. Dual-Cross-Linked Network Hydrogels with Multiresponsive, Self-Healing, and Shear Strengthening Properties. Biomacromolecules 2021, 22, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.L.; Kirilov, P.; Roullin, V.G. Organogels, promising drug delivery systems: An update of state-of-the-art and recent applications. J. Control Release 2018, 271, 1–20. [Google Scholar] [CrossRef]
- Qin, M.; Yuan, W.; Zhang, X.; Cheng, Y.; Xu, M.; Wei, Y.; Chen, W.; Huang, D. Preparation of PAA/PAM/MXene/TA hydrogel with antioxidant, healable ability as strain sensor. Colloids Surf. B Biointerfaces 2022, 214, 112482. [Google Scholar] [CrossRef]
- Bollu, A.; Giri, P.; Dalabehera, N.R.; Asmi, A.R.; Sharma, N.K. Unnatural Amino Acid: 4-Aminopyrazolonyl Amino Acid Comprising Tri-Peptides Forms Organogel with Co-Solvent (EtOAc:Hexane). Front. Chem. 2022, 10, 821971. [Google Scholar] [CrossRef]
- Liu, H.; Bi, X.; Wu, Y.; Pan, M.; Ma, X.; Mo, L.; Wang, J.; Li, X. Cationic self-assembled peptide-based molecular hydrogels for extended ocular drug delivery. Acta Biomater. 2021, 131, 162–171. [Google Scholar] [CrossRef]
- Toro-Vazquez, J.F.; Morales-Rueda, J.; Torres-Martínez, A.; Charó-Alonso, M.A.; Mallia, V.A.; Weiss, R.G. Cooling rate effects on the microstructure, solid content, and rheological properties of organogels of amides derived from stearic and (R)-12-hydroxystearic acid in vegetable oil. Langmuir 2013, 29, 7642–7654. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Wu, D.; Xue, J.; Qiu, Y.; Liao, M.; Pei, Q.; Goorsky, M.S.; He, X. Homogeneous Freestanding Luminescent Perovskite Organogel with Superior Water Stability. Adv. Mater. 2019, 31, e1902928. [Google Scholar] [CrossRef]
- Cai, Z.; Tang, Y.; Wei, Y.; Wang, P.; Zhang, H. Double—Network hydrogel based on exopolysaccharides as a biomimetic extracellular matrix to augment articular cartilage regeneration. Acta Biomater. 2022, 152, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.P.; John, J.; Holmqvist, P.; Olsson, U.; Chandran, S.; Joseph, B. Adsorption of Anionic Dyes Using a Poly(styrene-block-4-vinylpyridine) Block Copolymer Organogel. Langmuir 2021, 37, 3996–4006. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Florio, D.; Panzetta, V.; Roviello, V.; Netti, P.A.; Di Natale, C.; Marasco, D. Hydrogelation tunability of bioinspired short peptides. Soft Matter 2022, 18, 8418–8426. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, S.; Zhang, Y.; Zhang, H.; Ma, Y.; Xu, M.; An, P.; Zhou, Y.; Halila, S.; Wei, Y.; et al. Injectable chitosan/xyloglucan composite hydrogel with mechanical adaptivity and endogenous bioactivity for skin repair. Carbohydr. Polym. 2023, 313, 120904. [Google Scholar] [CrossRef]
- Zheng, W.; Hao, Y.; Wang, D.; Huang, H.; Guo, F.; Sun, Z.; Shen, P.; Sui, K.; Yuan, C.; Zhou, Q. Preparation of triamcinolone acetonide-loaded chitosan/fucoidan hydrogel and its potential application as an oral mucosa patch. Carbohydr. Polym. 2021, 272, 118493. [Google Scholar] [CrossRef]
- Guo, J.; Yao, H.; Li, X.; Chang, L.; Wang, Z.; Zhu, W.; Su, Y.; Qin, L.; Xu, J. Advanced Hydrogel systems for mandibular reconstruction. Bioact. Mater. 2023, 21, 175–193. [Google Scholar] [CrossRef]
- Zafar, A.; Alsaidan, O.A.; Imam, S.S.; Yasir, M.; Alharbi, K.S.; Khalid, M. Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation. Gels 2022, 8, 418. [Google Scholar] [CrossRef]
- Drozdov, A.D.; deClaville Christiansen, J. Modeling the effects of pH and ionic strength on swelling of polyelectrolyte gels. J. Chem. Phys. 2015, 142, 114904. [Google Scholar] [CrossRef]
- Drozdov, A.D.; Christiansen, J.D. The Effects of pH and Ionic Strength of Swelling of Cationic Gels. Int. J. Appl. Mech. 2016, 08, 1650059. [Google Scholar] [CrossRef]
- Andrade, F.; Roca-Melendres, M.M.; Durán-Lara, E.F.; Rafael, D.; Schwartz, S., Jr. Stimuli-Responsive Hydrogels for Cancer Treatment: The Role of pH, Light, Ionic Strength and Magnetic Field. Cancers 2021, 13, 1164. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Hydrogels: Experimental characterization and mathematical modelling of their mechanical and diffusive behaviour. Chem. Soc. Rev. 2018, 47, 2357–2373. [Google Scholar] [CrossRef] [PubMed]
- Basu, N.; Chakraborty, A.; Ghosh, R. Carbohydrate Derived Organogelators and the Corresponding Functional Gels Developed in Recent Time. Gels 2018, 4, 52. [Google Scholar] [CrossRef]
- Onaciu, A.; Munteanu, R.A.; Moldovan, A.I.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.; Rastogi, S.K.; Dominguez, J.; Cantu, T.; Brittain, W.; Irvin, J.; Betancourt, T. Biodegradable DNA-enabled poly(ethylene glycol) hydrogels prepared by copper-free click chemistry. J. Biomater. Sci. Polym. Ed. 2016, 27, 22–39. [Google Scholar] [CrossRef]
- Soares, P.A.; de Seixas, J.R.; Albuquerque, P.B.; Santos, G.R.; Mourão, P.A.; Barros, W., Jr.; Correia, M.T.; Carneiro-da-Cunha, M.G. Development and characterization of a new hydrogel based on galactomannan and κ-carrageenan. Carbohydr. Polym. 2015, 134, 673–679. [Google Scholar] [CrossRef]
- Treesuppharat, W.; Rojanapanthu, P.; Siangsanoh, C.; Manuspiya, H.; Ummartyotin, S. Synthesis and characterization of bacterial cellulose and gelatin-based hydrogel composites for drug-delivery systems. Biotechnol. Rep. 2017, 15, 84–91. [Google Scholar] [CrossRef]
- Vigato, A.A.; Querobino, S.M.; de Faria, N.C.; de Freitas, A.C.P.; Leonardi, G.R.; de Paula, E.; Cereda, C.M.S.; Tófoli, G.R.; de Araujo, D.R. Synthesis and characterization of nanostructured lipid-poloxamer organogels for enhanced skin local anesthesia. Eur. J. Pharm. Sci. 2019, 128, 270–278. [Google Scholar] [CrossRef]
- Xing, P.; Chen, H.; Bai, L.; Hao, A.; Zhao, Y. Superstructure Formation and Topological Evolution Achieved by Self-Organization of a Highly Adaptive Dynamer. ACS Nano 2016, 10, 2716–2727. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, self-assembled hydrogel composed of a modified aromatic dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Meibom, A.; Plane, F.; Cheng, T.; Grandjean, G.; Haldimann, O.; Escrig, S.; Jensen, L.; Daraspe, J.; Mucciolo, A.; De Bellis, D.; et al. Correlated cryo-SEM and CryoNanoSIMS imaging of biological tissue. BMC Biol. 2023, 21, 126. [Google Scholar] [CrossRef]
- Liberman, L.; Kleinerman, O.; Davidovich, I.; Talmon, Y. Micrograph contrast in low-voltage SEM and cryo-SEM. Ultramicroscopy 2020, 218, 113085. [Google Scholar] [CrossRef] [PubMed]
- Skilling, K.J.; Citossi, F.; Bradshaw, T.D.; Ashford, M.; Kellam, B.; Marlow, M. Insights into low molecular mass organic gelators: A focus on drug delivery and tissue engineering applications. Soft Matter 2014, 10, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.R.; Coates, I.A.; Boucheteau, T.R.; Miravet, J.F.; Escuder, B.; Castelletto, V.; Hamley, I.W.; Smith, D.K. Low-molecular-weight gelators: Elucidating the principles of gelation based on gelator solubility and a cooperative self-assembly model. J. Am. Chem. Soc. 2008, 130, 9113–9121. [Google Scholar] [CrossRef] [PubMed]
- Bielejewski, M.; Tritt-Goc, J. Evidence of solvent-gelator interaction in sugar-based organogel studied by field-cycling NMR relaxometry. Langmuir 2010, 26, 17459–17464. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, Y.E. Structure and dynamics of hydrogels and organogels: An NMR spectroscopy approach. Progress. Polym. Sci. 2011, 36, 1184–1253. [Google Scholar] [CrossRef]
- Dandapat, M.; Mandal, D. Local viscosity and solvent relaxation experienced by rod-like fluorophores in AOT/4-chlorophenol/m-xylene organogels. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 170, 150–156. [Google Scholar] [CrossRef]
- Shalaby, E.S.; Shalaby, S.I. Optimization of folic acid Span 60-organogel to enhance its in vitro and in vivo photoprotection: A comparative study. Ther. Deliv. 2022, 13, 517–530. [Google Scholar] [CrossRef]
- Gattani, V.; Dawre, S. Development of favipiravir loaded PLGA nanoparticles entrapped in in-situ gel for treatment of Covid-19 via nasal route. J. Drug Deliv. Sci. Technol. 2023, 79, 104082. [Google Scholar] [CrossRef]
- Ullah, K.H.; Rasheed, F.; Naz, I.; Ul Haq, N.; Fatima, H.; Kanwal, N.; Ur-Rehman, T. Chitosan Nanoparticles Loaded Poloxamer 407 Gel for Transungual Delivery of Terbinafine HCl. Pharmaceutics 2022, 14, 2353. [Google Scholar] [CrossRef]
- Jamshaid, H.; Din, F.U.; Malik, M.; Mukhtiar, M.; Choi, H.G.; Ur-Rehman, T.; Khan, G.M. A cutback in Imiquimod cutaneous toxicity; comparative cutaneous toxicity analysis of Imiquimod nanotransethosomal gel with 5% marketed cream on the BALB/c mice. Sci. Rep. 2022, 12, 14244. [Google Scholar] [CrossRef]
- Chettupalli, A.K.; Ananthula, M.; Amarachinta, P.R.; Bakshi, V.; Yata, V.K. Design, formulation, in-vitro and ex-vivo evaluation of atazanavir loaded cubosomal gel. Biointerface Res. Appl. Chem. 2021, 11, 12037–12054. [Google Scholar]
- Neupane, R.; Boddu, S.H.S.; Al-Tabakha, M.M.; Jacob, S.; Babu, R.J.; Tiwari, A.K. Percutaneous absorption and Skin accumulation of Lorazepam-Diphenhydramine- Haloperidol Carbopol gel in Porcine Ear Skin. AAPS PharmSciTech 2023, 24, 183. [Google Scholar] [CrossRef] [PubMed]
- Arpornmaeklong, P.; Jaiman, N.; Apinyauppatham, K.; Fuongfuchat, A.; Boonyuen, S. Effects of Calcium Carbonate Microcapsules and Nanohydroxyapatite on Properties of Thermosensitive Chitosan/Collagen Hydrogels. Polymers 2023, 15, 416. [Google Scholar] [CrossRef]
- Ma, W.; Chen, H.; Cheng, S.; Wu, C.; Wang, L.; Du, M. Gelatin hydrogel reinforced with mussel-inspired polydopamine-functionalized nanohydroxyapatite for bone regeneration. Int. J. Biol. Macromol. 2023, 240, 124287. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xu, H.; Yang, Y.; Wang, X.; An, W.; Hu, Y.; Xu, S. A nonswellable gradient hydrogel with tunable mechanical properties. J. Mater. Chem. B 2020, 8, 2702–2708. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Wang, P.; Yang, R.; Tan, X.; Shi, T.; Ma, J.; Xue, W.; Chi, B. Bio-fabricated nanocomposite hydrogel with ROS scavenging and local oxygenation accelerates diabetic wound healing. J. Mater. Chem. B 2022, 10, 4083–4095. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Zhang, Z.; Jia, F.; Gao, G. Acetylated Distarch Phosphate-Mediated Tough and Conductive Hydrogel for Antibacterial Wearable Sensors. ACS Appl. Mater. Interfaces 2022, 14, 51420–51428. [Google Scholar] [CrossRef]
- Si, C.; Tian, X.; Wang, Y.; Wang, Z.; Wang, X.; Lv, D.; Wang, A.; Wang, F.; Geng, L.; Zhao, J.; et al. A Polyvinyl Alcohol-Tannic Acid Gel with Exceptional Mechanical Properties and Ultraviolet Resistance. Gels 2022, 8, 751. [Google Scholar] [CrossRef]
- Xie, M.; Zheng, Z.; Pu, S.; Jia, Y.G.; Wang, L.; Chen, Y. Macroporous Adhesive Nano-Enabled Hydrogels Generated from Air-in-Water Emulsions. Macromol. Biosci. 2022, 22, e2100491. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, M.; Zhao, Z. Facile fabrication of self-healing, injectable and antimicrobial cationic guar gum hydrogel dressings driven by hydrogen bonds. Carbohydr. Polym. 2023, 310, 120723. [Google Scholar] [CrossRef]
- Li, D.; Fei, X.; Xu, L.; Wang, Y.; Tian, J.; Li, Y. Pressure-sensitive antibacterial hydrogel dressing for wound monitoring in bed ridden patients. J. Colloid. Interface Sci. 2022, 627, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Bassi da Silva, J.; Ferreira, S.B.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing Mucoadhesion in Polymer Gels: The Effect of Method Type and Instrument Variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed]
- Senyiğit, Z.A.; Karavana, S.Y.; Eraç, B.; Gürsel, O.; Limoncu, M.H.; Baloğlu, E. Evaluation of chitosan based vaginal bioadhesive gel formulations for antifungal drugs. Acta Pharm. 2014, 64, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Kunstman, P.; Roszak, R.; Białas, W. Rheological and textural properties of microemulsion-based polymer gels with indomethacin. Drug Dev. Ind. Pharm. 2016, 42, 854–861. [Google Scholar] [CrossRef]
- Jain, S.K.; Kaur, M.; Kalyani, P.; Mehra, A.; Kaur, N.; Panchal, N. Microsponges enriched gel for enhanced topical delivery of 5-fluorouracil. J. Microencapsul. 2019, 36, 677–691. [Google Scholar] [CrossRef]
- Tuğcu-Demiröz, F.; Acartürk, F.; Özkul, A. Preparation and characterization of bioadhesive controlled-release gels of cidofovir for vaginal delivery. J. Biomater. Sci. Polym. Ed. 2015, 26, 1237–1255. [Google Scholar] [CrossRef]
- Rencber, S.; Cheaburu-Yilmaz, C.N.; Kose, F.A.; Karavana, S.Y.; Yilmaz, O. Preparation and characterization of alginate and chitosan IPC based gel formulation for mucosal application. Cellul. Chem. Technol. 2019, 53, 655–665. [Google Scholar] [CrossRef]
- Liang, Y.; Chitrakar, B.; Liu, Z.; Ming, X.; Xu, D.; Mo, H.; Shi, C.; Zhu, X.; Hu, L.; Li, H. Preparation and characterization of 3D-printed antibacterial hydrogel with benzyl isothiocyanate. Int. J. Bioprint 2023, 9, 671. [Google Scholar] [CrossRef]
- Das, D.; Cho, H.; Kim, N.; Pham, T.T.H.; Kim, I.G.; Chung, E.J.; Noh, I. A terpolymeric hydrogel of hyaluronate-hydroxyethyl acrylate-gelatin methacryloyl with tunable properties as biomaterial. Carbohydr. Polym. 2019, 207, 628–639. [Google Scholar] [CrossRef]
- Stojkov, G.; Niyazov, Z.; Picchioni, F.; Bose, R.K. Relationship between Structure and Rheology of Hydrogels for Various Applications. Gels 2021, 7, 255. [Google Scholar] [CrossRef]
- Huang, N. Rheological Characterization of Pharmaceutical and Cosmetic Formulations for Cutaneous Applications. Curr. Pharm. Des. 2019, 25, 2349–2363. [Google Scholar] [CrossRef] [PubMed]
- Kirilov, P.; Gauffre, F.; Franceschi-Messant, S.; Perez, E.; Rico-Lattes, I. Rheological characterization of a new type of colloidal dispersion based on nanoparticles of gelled oil. J. Phys. Chem. B 2009, 113, 11101–11108. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yin, H.; Chen, X.; Chen, T.H.; Liu, H.M.; Rao, S.S.; Tan, Y.J.; Qian, Y.X.; Liu, Y.W.; Hu, X.K.; et al. Ångstrom-scale silver particle-embedded carbomer gel promotes wound healing by inhibiting bacterial colonization and inflammation. Sci. Adv. 2020, 6, eaba0942. [Google Scholar] [CrossRef] [PubMed]
- Simsolo, E.E.; Eroğlu, İ.; Tanrıverdi, S.T.; Özer, Ö. Formulation and Evaluation of Organogels Containing Hyaluronan Microparticles for Topical Delivery of Caffeine. AAPS PharmSciTech 2018, 19, 1367–1376. [Google Scholar] [CrossRef]
- Antoniuk, I.; Kaczmarek, D.; Kardos, A.; Varga, I.; Amiel, C. Supramolecular Hydrogel Based on pNIPAm Microgels Connected via Host⁻Guest Interactions. Polymers 2018, 10, 566. [Google Scholar] [CrossRef]
- Garcia-Hernandez, A.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Sosa-Hernandez, E.; Alvarez-Ramirez, J. Effects of clay concentration on the morphology and rheological properties of xanthan gum-based hydrogels reinforced with montmorillonite particles. J. Appl. Polym. Sci. 2017, 134, 44517. [Google Scholar] [CrossRef]
- Fariña, M.; Torres, M.D.; Moreira, R. Starch hydrogels from discarded chestnuts produced under different temperature-time gelatinisation conditions. Int. J. Food Sci. Technol. 2019, 54, 1179–1186. [Google Scholar] [CrossRef]
- Farrell, K.; Joshi, J.; Kothapalli, C.R. Injectable uncrosslinked biomimetic hydrogels as candidate scaffolds for neural stem cell delivery. J. Biomed. Mater. Res. A 2017, 105, 790–805. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Derkach, S.R.; Kulichikhin, V.G. Rheology of Gels and Yielding Liquids. Gels 2023, 9, 715. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Greco, F.; Busilacchi, A.; Sollazzo, V.; Gigante, A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: A review. Carbohydr. Polym. 2012, 89, 723–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, C.; Zhao, D.; Zhang, S.; Ma, G.; Su, Z.; Li, X. On-line monitoring of the sol-gel transition temperature of thermosensitive chitosan/β-glycerophosphate hydrogels by low field NMR. Carbohydr. Polym. 2020, 238, 116196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Jin, X.; Li, H.; Zhang, R.R.; Wu, C.W. Injectable and body temperature sensitive hydrogels based on chitosan and hyaluronic acid for pH sensitive drug release. Carbohydr. Polym. 2018, 186, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhao, X.; Guo, B.; Han, Y. Preparation, thermal response mechanisms and biomedical applications of thermosensitive hydrogels for drug delivery. Expert. Opin. Drug Deliv. 2023, 20, 641–672. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef]
- Chen, Y.; Lee, J.H.; Meng, M.; Cui, N.; Dai, C.Y.; Jia, Q.; Lee, E.S.; Jiang, H.B. An Overview on Thermosensitive Oral Gel Based on Poloxamer 407. Materials 2021, 14, 4522. [Google Scholar] [CrossRef]
- Huang, Z.; Feng, Q.L.; Yu, B.; Li, S.J. Biomimetic properties of an injectable chitosan/nano-hydroxyapatite/collagen composite. Mater. Sci. Eng. C-Mater. Biol. Appl. 2011, 31, 683–687. [Google Scholar] [CrossRef]
- Budhori, A.; Tiwari, A.; Tiwari, V.; Sharma, A.; Kumar, M.; Gautam, G.; Virmani, T.; Kumar, G.; Alhalmi, A.; Noman, O.M.; et al. QbD Design, Formulation, Optimization and Evaluation of Trans-Tympanic Reverse Gelatination Gel of Norfloxacin: Investigating Gene-Gene Interactions to Enhance Therapeutic Efficacy. Gels 2023, 9, 657. [Google Scholar] [CrossRef]
- Chung, Y.M.; Simmons, K.L.; Gutowska, A.; Jeong, B. Sol-gel transition temperature of PLGA-g-PEG aqueous solutions. Biomacromolecules 2002, 3, 511–516. [Google Scholar] [CrossRef]
- Garripelli, V.K.; Kim, J.K.; Namgung, R.; Kim, W.J.; Repka, M.A.; Jo, S. A novel thermosensitive polymer with pH-dependent degradation for drug delivery. Acta Biomater. 2010, 6, 477–485. [Google Scholar] [CrossRef]
- Pandit, N.K.; McIntyre, H.J. Cosolvent effects on the gel formation and gel melting transitions of Pluronic F127 gels. Pharm. Dev. Technol. 1997, 2, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Winnik, F.M. Fluorescence studies of aqueous solutions of poly (N-isopropylacrylamide) below and above their LCST. Macromolecules 1990, 23, 233–242. [Google Scholar] [CrossRef]
- Inal, S.; Kölsch, J.D.; Chiappisi, L.; Janietz, D.; Gradzielski, M.; Laschewsky, A.; Neher, D. Structure-related differences in the temperature-regulated fluorescence response of LCST type polymers. J. Mater. Chem. C 2013, 1, 6603–6612. [Google Scholar] [CrossRef]
- Fan, R.; Cheng, Y.; Wang, R.; Zhang, T.; Zhang, H.; Li, J.; Song, S.; Zheng, A. Thermosensitive Hydrogels and Advances in Their Application in Disease Therapy. Polymers 2022, 14, 2379. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, C.; Shen, W.; Cheng, Z.; Yu, L.L.; Ping, Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J. Control Release 2007, 120, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Hu, X.; Qi, X.; Yu, H.; Liu, Y.; Li, J.; Zhang, J.; Dong, W. A novel thermo-responsive hydrogel based on salecan and poly(N-isopropylacrylamide): Synthesis and characterization. Colloids Surf. B Biointerfaces 2015, 125, 1–11. [Google Scholar] [CrossRef]
- Tuomela, A.; Hirvonen, J.; Peltonen, L. Stabilizing Agents for Drug Nanocrystals: Effect on Bioavailability. Pharmaceutics 2016, 8, 16. [Google Scholar] [CrossRef]
- Kapourani, A.; Kirimkiroglou, K.; Chachlioutaki, K.; Koromili, M.; Ritzoulis, C.; Assimopoulou, A.N.; Andreadis, D.A.; Fatouros, D.G.; Barmpalexis, P. Evaluation of pilocarpine in situ forming buccal gels as potential formulations for the management of xerostomia. J. Drug Deliv. Sci. Technol. 2023, 86, 104698. [Google Scholar] [CrossRef]
- Goycoolea, F.; Argüelles-Monal, W.; Lizardi, J.; Peniche, C.; Heras, A.; Galed, G.; Díaz, E. Temperature and pH-sensitive chitosan hydrogels: DSC, rheological and swelling evidence of a volume phase transition. Polym. Bull. 2007, 58, 225–234. [Google Scholar] [CrossRef]
- Liu, L.; Ma, H.; Yu, J.; Fan, Y. Fabrication of glycerophosphate-based nanochitin hydrogels for prolonged release under in vitro physiological conditions. Cellulose 2021, 28, 4887–4897. [Google Scholar] [CrossRef]
- Kurniawansyah, I.S.; Rusdiana, T.; Sopyan, I.; Ramoko, H.; Wahab, H.A.; Subarnas, A. In situ ophthalmic gel forming systems of poloxamer 407 and hydroxypropyl methyl cellulose mixtures for sustained ocular delivery of chloramphenicole: Optimization study by factorial design. Heliyon 2020, 6, e05365. [Google Scholar] [CrossRef] [PubMed]
- Fouda, N.H.; Abdelrehim, R.T.; Hegazy, D.A.; Habib, B.A. Sustained ocular delivery of Dorzolamide-HCl via proniosomal gel formulation: In-vitro characterization, statistical optimization, and in-vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2018, 25, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.S.; Ravi, P.R.; Mir, S.I.; Khan, M.S.; Kathuria, H.; Katnapally, P.; Bhatnagar, U. Design, Characterization and Pharmacokinetic-Pharmacodynamic Evaluation of Poloxamer and Kappa-Carrageenan-Based Dual-Responsive In Situ Gel of Nebivolol for Treatment of Open-Angle Glaucoma. Pharmaceutics 2023, 15, 405. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Shah, J.; Jacob, S.; Al-Dhubiab, B.E.; Sreeharsha, N.; Morsy, M.A.; Gupta, S.; Attimarad, M.; Shinu, P.; Venugopala, K.N. Experimental design, formulation and in vivo evaluation of a novel topical in situ gel system to treat ocular infections. PLoS ONE 2021, 16, e0248857. [Google Scholar] [CrossRef]
- Cavet, M.E.; Glogowski, S.; Lowe, E.R.; Phillips, E. Rheological Properties, Dissolution Kinetics, and Ocular Pharmacokinetics of Loteprednol Etabonate (Submicron) Ophthalmic Gel 0.38. J. Ocul. Pharmacol. Ther. 2019, 35, 291–300. [Google Scholar] [CrossRef]
- Pires, P.C.; Rodrigues, M.; Alves, G.; Santos, A.O. Strategies to Improve Drug Strength in Nasal Preparations for Brain Delivery of Low Aqueous Solubility Drugs. Pharmaceutics 2022, 14, 588. [Google Scholar] [CrossRef]
- Ramesh, Y.; Abhirami, B.; Gnana, S.K.; Kaveri, S.; Sulthana, S.N.; Sravya, A.; Sujatha, K. Formulation and evaluation of oxymetazoline hydrochloride nasal gels. J. Drug Deliv. Ther. 2018, 8, 49–57. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Osman, D.A.; Abd El-Fattah, A.I. Formulation and in vitro evaluation of a thermoreversible mucoadhesive nasal gel of itopride hydrochloride. Drug Dev. Ind. Pharm. 2018, 44, 1857–1867. [Google Scholar] [CrossRef]
- Gu, F.; Fan, H.; Cong, Z.; Li, S.; Wang, Y.; Wu, C. Preparation, characterization, and in vivo pharmacokinetics of thermosensitive in situ nasal gel of donepezil hydrochloride. Acta Pharm. 2020, 70, 411–422. [Google Scholar] [CrossRef]
- Abdulla, N.A.; Balata, G.F.; El-Ghamry, H.A.; Gomaa, E. Intranasal delivery of Clozapine using nanoemulsion-based in-situ gels: An approach for bioavailability enhancement. Saudi Pharm. J. 2021, 29, 1466–1485. [Google Scholar] [CrossRef]
- Malviya, V.; Ladhake, V.; Gajbiye, K.; Satao, J.; Tawar, M. Design and characterization of phase transition system of zolmitriptan hydrochloride for nasal drug delivery system. Int. J. Pharm. Sci. Nanotechnol. 2020, 13, 4942–4951. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Bhasin, B. Transdermal lipid vesicular delivery of iloperidone: Formulation, in vitro and in vivo evaluation. Colloids Surf. B Biointerfaces 2019, 183, 110409. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Koteswara Rao, S.N.; Panda, S.P.; Umasankar, K.; Narender, M.; Suryakumari, C.H. Formulation and Evaluation of Tacrolimus Transdermal Gel. J. Drug Deliv. Ther. 2019, 9, 110–118. [Google Scholar] [CrossRef]
- Gao, X.; Brogden, N.K. Development of Hydrogels for Microneedle-Assisted Transdermal Delivery of Naloxone for Opioid-Induced Pruritus. J. Pharm. Sci. 2019, 108, 3695–3703. [Google Scholar] [CrossRef]
- El-Housiny, S.; Shams Eldeen, M.A.; El-Attar, Y.A.; Salem, H.A.; Attia, D.; Bendas, E.R.; El-Nabarawi, M.A. Fluconazole-loaded solid lipid nanoparticles topical gel for treatment of pityriasis versicolor: Formulation and clinical study. Drug Deliv. 2018, 25, 78–90. [Google Scholar] [CrossRef]
- Khan, A.S.; Shah, K.U.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.A.; Alhashem, Y.N.; Ghazanfar, S.; Khan, K.A.; Niazi, Z.R.; Farid, A. Tacrolimus-Loaded Solid Lipid Nanoparticle Gel: Formulation Development and In Vitro Assessment for Topical Applications. Gels 2022, 8, 129. [Google Scholar] [CrossRef]
- Dragicevic, N.; Krajisnik, D.; Milic, J.; Fahr, A.; Maibach, H. Development of hydrophilic gels containing coenzyme Q(10)-loaded liposomes: Characterization, stability and rheology measurements. Drug Dev. Ind. Pharm. 2019, 45, 43–54. [Google Scholar] [CrossRef]
- Dudhipala, N.; Phasha Mohammed, R.; Adel Ali Youssef, A.; Banala, N. Effect of lipid and edge activator concentration on development of aceclofenac-loaded transfersomes gel for transdermal application: In vitro and ex vivo skin permeation. Drug Dev. Ind. Pharm. 2020, 46, 1334–1344. [Google Scholar] [CrossRef]
- Chandra, A.; Aggarwal, G.; Manchanda, S.; Narula, A. Development of Topical Gel of Methotrexate Incorporated Ethosomes and Salicylic Acid for the Treatment of Psoriasis. Pharm. Nanotechnol. 2019, 7, 362–374. [Google Scholar] [CrossRef]
- Ramadan, A.A.; Elbakry, A.M.; Esmaeil, A.H.; Khaleel, S.A. Pharmaceutical and pharmacokinetic evaluation of novel rectal mucoadhesive hydrogels containing tolmetin sodium. J. Pharm. Investig. 2018, 48, 673–683. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Rabea, Y.K.; El-Ela, F.I.A.; Khallaf, R.A. Glycerosomal thermosensitive in situ gel of duloxetine HCl as a novel nanoplatform for rectal delivery: In vitro optimization and in vivo appraisal. Drug Deliv. Transl. Res. 2022, 12, 3083–3103. [Google Scholar] [CrossRef]
- Arpa, M.D.; Yoltaş, A.; Onay Tarlan, E.; Şenyüz, C.; Sipahi, H.; Aydın, A.; Üstündağ Okur, N. New therapeutic system based on hydrogels for vaginal candidiasis management: Formulation-characterization and in vitro evaluation based on vaginal irritation and direct contact test. Pharm. Dev. Technol. 2020, 25, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.U.; Rajput, A.P.; Belgamwar, V.S.; Chalikwar, S.S. Development and characterization of amphotericin B nanoemulsion-loaded mucoadhesive gel for treatment of vulvovaginal candidiasis. Heliyon 2022, 8, e11489. [Google Scholar] [CrossRef] [PubMed]
- Sakran, W.; Abdel-Rashid, R.S.; Saleh, F.; Abdel-Monem, R. Ethosomal gel for rectal transmucosal delivery of domperidone: Design of experiment, in vitro, and in vivo evaluation. Drug Deliv. 2022, 29, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Dobard, C.W.; Makarova, N.; West-Deadwyler, R.; Taylor, A.; Dinh, C.; Martin, A.; Lipscomb, J.; Mitchell, J.; Khalil, G.; Garcia-Lerma, G.; et al. Efficacy of Vaginally Administered Gel Containing Emtricitabine and Tenofovir Against Repeated Rectal Simian Human Immunodeficiency Virus Exposures in Macaques. J. Infect. Dis. 2018, 218, 1284–1290. [Google Scholar] [CrossRef]
- Salatin, S.; Tarzamani, M.; Farjami, A.; Jelvehgari, M. Development and characterization of a novel mucoadhesive sol-gel suppository of sumatriptan: Design, optimization, in vitro and ex vivo evaluation for rectal drug delivery. Ther. Deliv. 2022, 13, 95–108. [Google Scholar] [CrossRef]
- Belo, S.; Touchard, J.; Secretan, P.H.; Vidal, F.; Boudy, V.; Cisternino, S.; Schlatter, J. Stability of Pentobarbital Hydrogel for Rectal Administration in Pediatric Procedural Sedation. Hosp. Pharm. 2021, 56, 332–337. [Google Scholar] [CrossRef]
- Aframian, D.J.; Davidowitz, T.; Benoliel, R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral. Dis. 2006, 12, 420–423. [Google Scholar] [CrossRef]
- Chuenbarn, T.; Sirirak, J.; Tuntarawongsa, S.; Okonogi, S.; Phaechamud, T. Design and Comparative Evaluation of Vancomycin HCl-Loaded Rosin-Based In Situ Forming Gel and Microparticles. Gels 2022, 8, 231. [Google Scholar] [CrossRef]
- Priprem, A.; Damrongrungruang, T.; Limsitthichaikoon, S.; Khampaenjiraroch, B.; Nukulkit, C.; Thapphasaraphong, S.; Limphirat, W. Topical Niosome Gel Containing an Anthocyanin Complex: A Potential Oral Wound Healing in Rats. AAPS PharmSciTech 2018, 19, 1681–1692. [Google Scholar] [CrossRef]
- Sanjana, A.; Ahmed, M.G.; Gowda Bh, J. Preparation and evaluation of in-situ gels containing hydrocortisone for the treatment of aphthous ulcer. J. Oral. Biol. Craniofac Res. 2021, 11, 269–276. [Google Scholar] [CrossRef]
- Raheema, D.A.; Kassab, H.J. Preparation and in-vitro Evaluation of Secnidazole as Periodontal In-situ Gel for Treatment of Periodontal Disease. Iraqi J. Pharm. Sci. 2022, 31, 50–61. [Google Scholar] [CrossRef]
- Banna, A.H.E.; Youssef, F.S.; Elzorba, H.Y.; Soliman, A.M.; Mohamed, G.G.; Ismail, S.H.; Mousa, M.R.; Elbanna, H.A.; Osman, A.S. Evaluation of the wound healing effect of neomycin-silver nano-composite gel in rats. Int. J. Immunopathol. Pharmacol. 2022, 36, 3946320221113486. [Google Scholar] [CrossRef] [PubMed]
- Shah, H.; Nair, A.B.; Shah, J.; Bharadia, P.; Al-Dhubiab, B.E. Proniosomal gel for transdermal delivery of lornoxicam: Optimization using factorial design and in vivo evaluation in rats. Daru 2019, 27, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, F.; Wang, L.; Yang, Y.; Khan, B.M.; Cheong, K.L.; Liu, Y. Preparation and evaluation of Bletilla striata polysaccharide/carboxymethyl chitosan/Carbomer 940 hydrogel for wound healing. Int. J. Biol. Macromol. 2019, 132, 729–737. [Google Scholar] [CrossRef]
- Wen, X.; Bao, D.; Chen, M.; Zhang, A.; Liu, C.; Sun, R. Preparation of CMC/HEC crosslinked hydrogels for drug delivery. BioResources 2015, 10, 8339–8351. [Google Scholar] [CrossRef]
- Akbari, J.; Saeedi, M.; Morteza-Semnani, K.; Hashemi, S.M.H.; Babaei, A.; Eghbali, M.; Mohammadi, M.; Rostamkalaei, S.S.; Asare-Addo, K.; Nokhodchi, A. Innovative topical niosomal gel formulation containing diclofenac sodium (niofenac). J. Drug Target. 2022, 30, 108–117. [Google Scholar] [CrossRef]
- Madni, A.; Rahim, M.A.; Mahmood, M.A.; Jabar, A.; Rehman, M.; Shah, H.; Khan, A.; Tahir, N.; Shah, A. Enhancement of Dissolution and Skin Permeability of Pentazocine by Proniosomes and Niosomal Gel. AAPS PharmSciTech 2018, 19, 1544–1553. [Google Scholar] [CrossRef]
- Akar, E.; Altınışık, A.; Seki, Y. Preparation of pH- and ionic-strength responsive biodegradable fumaric acid crosslinked carboxymethyl cellulose. Carbohydr. Polym. 2012, 90, 1634–1641. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Cheng, H. Impact of κ-Carrageenan on the Cold-Set Pea Protein Isolate Emulsion-Filled Gels: Mechanical Property, Microstructure, and In Vitro Digestive Behavior. Foods 2024, 13, 483. [Google Scholar] [CrossRef]
- Alkufi, H.K.; Kassab, H.J. Formulation and Evaluation of Sustained Release Sumatriptan Mucoadhesive Intranasal in-Situ Gel. Iraqi J. Pharm. Sci. 2019, 28, 95–104. [Google Scholar] [CrossRef]
- Al-Joufi, F.; Elmowafy, M.; Alruwaili, N.K.; Alharbi, K.S.; Shalaby, K.; Alsharari, S.D.; Ali, H.M. Mucoadhesive In Situ Rectal Gel Loaded with Rifampicin: Strategy to Improve Bioavailability and Alleviate Liver Toxicity. Pharmaceutics 2021, 13, 336. [Google Scholar] [CrossRef]
- Sadeq, Z.A.; Sabri, L.A.; Al-Kinani, K.K. Natural polymer Effect on gelation and rheology of ketotifen-loaded pH-sensitive in situ ocular gel (Carbapol). J. Of. Adv. Pharm. Educ. And. Res. 2022, 12, 45–50. [Google Scholar] [CrossRef]
- Thombre, K.P.; Sharma, D.; Lanjewar, A.M. Formulation and evaluation pharmaceutical aqueous gel of powdered Cordia dichotoma leaves with guava leaves. Am. J. PharmTech Res. 2018, 8, 268–277. [Google Scholar] [CrossRef]
- Alipour, S.; Azari, H.; Ahmadi, F. In situ thermosensitive gel of levodopa: Potential formulation for nose to brain delivery in Parkinson disease. Trends Pharm. Sci. 2020, 6, 97–104. [Google Scholar]
- Arafa, M.G.; El-Kased, R.F.; Elmazar, M.M. Thermoresponsive gels containing gold nanoparticles as smart antibacterial and wound healing agents. Sci. Rep. 2018, 8, 13674. [Google Scholar] [CrossRef]
- Pathak, K.; Kumar, A.; Yadav, E. Development and correlation between in vitro drug release and in vitro permeation of thermally triggered mucoadhesive in situ nasal gel of repaglinide PVP K30 complex. Int. J. Pharm. Sci. Drug Res. 2019, 11, 22–30. [Google Scholar] [CrossRef]
- Obayes, K.K.; Thomas, L.M. Development and Characterization of Hyaluronic Acid-Incorporated Thermosensitive Nasal in situ Gel of Meclizine Hydrochloride. Al-Rafidain J. Med. Sci. 2024, 6, 97–104. [Google Scholar] [CrossRef]
- Gondaliya, R.P.; Shah, P.D.; Nerkar, P.P.; Mahajan, H.S.; Ige, P.P. Buccal Gel Of Verapamil HCl Based On Fenugreek Mucilage And Xanthan Gum: In-Vitro Evaluation. Am. J. PharmTech Res. 2013, 3, 410–419. [Google Scholar]
- Park, Y.J.; Yong, C.S.; Kim, H.M.; Rhee, J.D.; Oh, Y.K.; Kim, C.K.; Choi, H.G. Effect of sodium chloride on the release, absorption and safety of diclofenac sodium delivered by poloxamer gel. Int. J. Pharm. 2003, 263, 105–111. [Google Scholar] [CrossRef]
- Al-Wiswasi, N.N.; Al-Khedairy, E.B. Formulation and in vitro evaluation of in-situ gelling liquid suppositories for naproxen. Iraqi J. Pharm. Sci. 2008, 17, 31–38. [Google Scholar] [CrossRef]
- Gupta, V.; Dwivedi, A.; Trivedi, N.; Jain, N.; Garud, N.; Jain, D. Formulation and evaluation of naproxen gel containing tulsi oil as penetration enhancer. Int. J. Pharm. Clin. Res. 2009, 1, 153–155. [Google Scholar]
- El-Leithy, E.S.; Shaker, D.S.; Ghorab, M.K.; Abdel-Rashid, R.S. Evaluation of mucoadhesive hydrogels loaded with diclofenac sodium-chitosan microspheres for rectal administration. AAPS PharmSciTech 2010, 11, 1695–1702. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, H.S.; Shah, S.K.; Surana, S.J. Nasal in situ gel containing hydroxy propyl β-cyclodextrin inclusion complex of artemether: Development and in vitro evaluation. J. Incl. Phenom. Macrocycl. Chem. 2011, 70, 49–58. [Google Scholar] [CrossRef]
- Khan, S.; Gajbhiye, C.; Singhavi, D.J.; Yeole, P. In situ Gel of Metoprolol Tartrate: Physicochemical Characterization, In vitro Diffusion and Histological Studies. Indian. J. Pharm. Sci. 2012, 74, 564–570. [Google Scholar] [CrossRef]
- Nerkar, P.P.; Gattani, S.G. Cress seed mucilage based buccal mucoadhesive gel of venlafaxine: In vivo, in vitro evaluation. J. Mater. Sci. Mater. Med. 2012, 23, 771–779. [Google Scholar] [CrossRef]
- Bhandwalkar, M.J.; Avachat, A.M. Thermoreversible nasal in situ gel of venlafaxine hydrochloride: Formulation, characterization, and pharmacodynamic evaluation. AAPS PharmSciTech 2013, 14, 101–110. [Google Scholar] [CrossRef]
- Nerkar, P.P.; Gattani, S.G. Oromucosal delivery of venlafaxine by linseed mucilage based gel: In vitro and in vivo evaluation in rabbits. Arch. Pharm. Res. 2013, 36, 846–853. [Google Scholar] [CrossRef]
- Singh, R.M.; Kumar, A.; Pathak, K. Thermally triggered mucoadhesive in situ gel of loratadine: β-cyclodextrin complex for nasal delivery. AAPS PharmSciTech 2013, 14, 412–424. [Google Scholar] [CrossRef]
- Mali, K.; Dhawale, S.; Dias, R.; Havaldar, V.; Ghorpade, V.; Salunkhe, N. Nasal Mucoadhesive In Situ Gel of Granisetron Hydrochloride using Natural Polymers. J. Appl. Pharm. Sci. 2015, 5, 084–093. [Google Scholar] [CrossRef]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D.; Shengule, S. Formulation and evaluation of thermoreversible mucoadhesive in-situ gel for intranasal delivery of naratriptan hydrochloride. J. Drug Deliv. Sci. Technol. 2015, 29, 238–244. [Google Scholar] [CrossRef]
- Sherafudeen, S.P.; Vasantha, P.V. Development and evaluation of in situ nasal gel formulations of loratadine. Res. Pharm. Sci. 2015, 10, 466–476. [Google Scholar] [PubMed]
- Salunke, S.R.; Patil, S.B. Ion activated in situ gel of gellan gum containing salbutamol sulphate for nasal administration. Int. J. Biol. Macromol. 2016, 87, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Shelke, S.; Shahi, S.; Jalalpure, S.; Dhamecha, D. Poloxamer 407-based intranasal thermoreversible gel of zolmitriptan-loaded nanoethosomes: Formulation, optimization, evaluation and permeation studies. J. Liposome Res. 2016, 26, 313–323. [Google Scholar] [CrossRef]
- Verma, P.; Prashar, N.; Kumar, V.; Chaudhary, H. Nasal (In-situ) gel (Phenylepherine HCl) for allergic rhinitis congestion treatment: Development and characterization. Am. J. PharmTech Res. 2016, 6, 1–17. [Google Scholar]
- Rajeshwari, H.R.; Dhamecha, D.; Jagwani, S.; Patil, D.; Hegde, S.; Potdar, R.; Metgud, R.; Jalalpure, S.; Roy, S.; Jadhav, K.; et al. Formulation of thermoreversible gel of cranberry juice concentrate: Evaluation, biocompatibility studies and its antimicrobial activity against periodontal pathogens. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1506–1514. [Google Scholar] [CrossRef]
- Karpagavalli, L.; Gopalasrsatheeskumar, K.; Narayanan, N.; Maheswaran, A.; Raj, A.I.; Priya, J.H. Formulation and evaluation of zolpidem nasal in situ gel. World J. Pharm. Res. 2017, 6, 940–951. [Google Scholar]
- Pathan Bashir, I.; Mene, H.; Bairagi, S. Quality by design (QbD) approach to formulate in situ gelling system for nose to brain delivery of Fluoxetine hydrochloride: Ex-vivo and In-vivo study. Ars. Pharm. 2017, 58, 107–114. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, S.; Wang, H.; Bie, H. A mucoadhesive, thermoreversible in situ nasal gel of geniposide for neurodegenerative disease’s. PLoS ONE 2017, 12, e0189478. [Google Scholar] [CrossRef]
- Danao, K.; Gangane, P.; Mahajan, N.; Mahajan, U. Formation and Development of Ondansetron Hydrochloride Nasal in situ gel. Int. J. Res. Dev. Pharm. Life Sci. 2018, 7, 3100–3103. [Google Scholar] [CrossRef]
- Thombre, N.A.; Pawar, K.N.; Patil, Y.A.; Kshirsagar, S.J. Novel thiolated Ceasalpinia Pulcherrima: Synthesis, characterization and evaluation as mucoadhesive in situ gelling agent. Pharm. J. Sri Lanka 2018, 8, 1–19. [Google Scholar] [CrossRef]
- Ravikrishna, V.; Krishnaveni, J. Development and evaluation of clozapine intranasal mucoadhesive in situ gels for brain targeting. J. Drug Deliv. Ther. 2019, 9, 198–207. [Google Scholar] [CrossRef]
- Naresh, W.R.; Dilip, D.V.; Sunil, K.P. Xyloglucan based nasal in situ gel formulation of mirtazapine for treatment of depression. Indian. J. Pharm. Educ. Res. 2020, 54, S210–S219. [Google Scholar] [CrossRef]
- Parashar, P.; Diwaker, N.; Kanoujia, J.; Singh, M.; Yadav, A.; Singh, I.; Saraf, S.A. In situ gel of lamotrigine for augmented brain delivery: Development characterization and pharmacokinetic evaluation. J. Pharm. Investig. 2020, 50, 95–105. [Google Scholar] [CrossRef]
- Patil, M.V.; Jadhav, R.L.; Shaikh, S.N.; Belhekar, S.N. Formulation and evaluation thermoreversible gel of antifungal agent for treatment of vaginal infection. J. Pharm. Res. Int. 2020, 32, 58–66. [Google Scholar] [CrossRef]
- Gambhire, M.S.; Gambhire, V.M.; Bachhav, J.R. Formulation development and optimization of mucoadhesive in situ gelling system for nasal administration of piracetam. J. Maharaja Sayajirao Univ. Baroda 2021, 55, 21–31. [Google Scholar]
- Patel, M.S.; Patel, A.D.; Doshi, N.R.; Vyas, G. Design and development of novel floating in situ gel of amoxicillin for the treatment of peptic ulcer disease caused by Helicobacter pylori. Acta Sci. Pharm. Sci. 2021, 5, 81–93. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Koli, A.R.; Desai, D.T.; Parikh, R.K.; Shah, D.O. Tailored Doxycycline Hyclate Loaded In Situ Gel for the Treatment of Periodontitis: Optimization, In Vitro Characterization, and Antimicrobial Studies. AAPS PharmSciTech 2021, 22, 77. [Google Scholar] [CrossRef]
- Richu, E.; Sneha, W.; Mundhe, B.S.; Rayate, S.A.; Sahebrao, A.; Swati, T.; Anil, J. Formulate and evaluate gel containing nanostructured lipids of Pongamia pinnata. Plant Arch. 2021, 21, 515–525. [Google Scholar] [CrossRef]
- Viswanadhan Vasantha, P.; Sherafudeen, S.P.; Rahamathulla, M.; Mathew, S.T.; Murali, S.; Alshehri, S.; Shakeel, F.; Alam, P.; Sirhan, A.Y.; Narayana Iyer, B.A. Combination of Cellulose Derivatives and Chitosan-Based Polymers to Investigate the Effect of Permeation Enhancers Added to In Situ Nasal Gels for the Controlled Release of Loratadine and Chlorpheniramine. Polymers 2023, 15, 1206. [Google Scholar] [CrossRef]
- Pandit, A.P.; Pol, V.V.; Kulkarni, V.S. Xyloglucan Based In Situ Gel of Lidocaine HCl for the Treatment of Periodontosis. J. Pharm. 2016, 2016, 3054321. [Google Scholar] [CrossRef] [PubMed]
- Baloğlu, E.; Karavana, S.Y.; Hyusein, I.Y.; Köse, T. Design and formulation of mebeverine HCl semisolid formulations for intraorally administration. AAPS PharmSciTech 2010, 11, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sguizzato, M.; Bories, C.; Nastruzzi, C.; Cortesi, R. Production and Characterization of a Clotrimazole Liposphere Gel for Candidiasis Treatment. Polymers 2018, 10, 160. [Google Scholar] [CrossRef]
- Shiehzadeh, F.; Mohebi, D.; Chavoshian, O.; Daneshmand, S. Formulation, Characterization, and Optimization of a Topical Gel Containing Tranexamic Acid to Prevent Superficial Bleeding: In Vivo and In Vitro Evaluations. Turk. J. Pharm. Sci. 2023, 20, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Pepe, A.; Baldisserotto, A.; Barbari, R.; Montesi, L.; Drechsler, M.; Mariani, P.; Cortesi, R. Niosomes for Topical application of antioxidant molecules: Design and in vitro behavior. Gels 2023, 9, 107. [Google Scholar] [CrossRef]
- Kumari, P.; Bais, S. Formulation and Pharmacological Evaluation of Herbal Gel Containing Curcuma longa. INNOSC Theranostics Pharmacol. Sci. 2023, 5, 1–6. [Google Scholar] [CrossRef]
- Alam, P.; Shakeel, F.; Foudah, A.I.; Alshehri, S.; Salfi, R.; Alqarni, M.H.; Aljarba, T.M. Central Composite Design (CCD) for the Optimisation of Ethosomal Gel Formulation of Punica granatum Extract: In Vitro and In Vivo Evaluations. Gels 2022, 8, 511. [Google Scholar] [CrossRef]
- Shoaeba, S.; Sharav, D.; Jain, H.; Sahu, A.; Meshram, D. Formulation and evaluation of in situ ophthalmic gel of loteprednol etabonate. J. Appl. Pharm. Res. 2021, 9, 25–29. [Google Scholar]
- Jadhao Umesh, T.; Rathod Sayali, P.; Dhembre Gunesh, N.; Sable Shital, D.; Lokhande Sneha, S. Formulation and critical evaluation of piroxicam gel. Pharma Innov. 2021, 10, 89–94. [Google Scholar]
- Ahmed, M.M.; Fatima, F.; Anwer, M.K.; Ibnouf, E.O.; Kalam, M.A.; Alshamsan, A.; Aldawsari, M.F.; Alalaiwe, A.; Ansari, M.J. Formulation and in vitro evaluation of topical nanosponge-based gel containing butenafine for the treatment of fungal skin infection. Saudi Pharm. J. 2021, 29, 467–477. [Google Scholar] [CrossRef]
- Singh, N.; Goyal, K.; Sondhi, S.; Jindal, S. Development and characterization of Barbaloin Gel for the safe and effective treatment of Psoriasis. J. Drug Deliv. Ther. 2020, 10, 188–197. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Ferrara, F.; Drechsler, M.; Hallan, S.S.; Huang, N.; Simelière, F.; Khunti, N.; Cortesi, R.; Marchetti, N.; et al. Nanoparticulate Gels for Cutaneous Administration of Caffeic Acid. Nanomaterials 2020, 10, 961. [Google Scholar] [CrossRef] [PubMed]
- Samuel, A.J.; Mulla, N. Formulation and evaluation of herbal topical gel containing leaves extract of Andrographis paniculata. J. Drug Deliv. Ther. 2020, 10, 48–51. [Google Scholar] [CrossRef]
- Kashyap, A.; Das, A.; Ahmed, A.B. Formulation and evaluation of transdermal topical gel of ibuprofen. J. Drug Deliv. Ther. 2020, 10, 20–25. [Google Scholar] [CrossRef]
- Gupta, S.; Wairkar, S.; Bhatt, L.K. Isotretinoin and α-tocopherol acetate-loaded solid lipid nanoparticle topical gel for the treatment of acne. J. Microencapsul. 2020, 37, 557–565. [Google Scholar] [CrossRef]
- Dange, V.; Dinde, S.; Doiphode, A.; Dhavane, S.; Dudhal, B.; Shid, S.; Yadav, A. Formulation and evaluation of herbal gel containing Lantana camara for management of Acne vulgaris. J. Univ. Shanghai Sci. Technol. 2020, 22, 799–809. [Google Scholar]
- Dandagi, P.M.; Pandey, P.; Gadad, A.P.; Mastiholimath, V.S. Formulation and evaluation of microemulsion based luliconazole gel for topical delivery. Indian J. Pharm. Educ. Res. 2020, 54, 293–301. [Google Scholar] [CrossRef]
- Al-Nima, A.M.; Qasim, Z.S.; Al-Kotaji, M. Formulation, evaluation and anti-microbial potential of topical Licorice root extract gel. Iraqi J. Pharm. 2020, 17, 37–56. [Google Scholar] [CrossRef]
- Priyanka, N.V.S.; Neeraja, P.; Mangilal, T.; Kumar, M.R. Formulation and Evaluation of Gel Loaded with Microspheres of Apremilast for Transdermal Delivery System. Asian J. Pharm. Clin. Res. 2019, 12, 411–417. [Google Scholar] [CrossRef]
- Khedekar, Y.B.; Mojad, A.; Malsane, P. Formulation, development, and evaluation of Silymarin loaded topical gel for fungal infection. J. Adv. Pharm. 2019, 8, 6–9. [Google Scholar]
- Cytotoxic effect of transdermal invasomal anastrozole gel on MCF-7 breast cancer cell line. J. Appl. Pharm. Sci. 2019, 9, 50–58. [CrossRef]
- Parkhe, G.; Jain, P.; Patel, S. Formulation and Evaluation of Acyclovir Loaded Novel Nano-Emulsion Gel for Topical Treatment of Herpes Simplex Viral Infections. J. Drug Deliv. Ther. 2018, 8, 265–270. [Google Scholar] [CrossRef]
- Jain, V.; Jain, P.K.; Shende, S.; Mishra, R. Formulation and Evaluation of Gel Containing Ethosomes Entrapped with Tretinoin. J. Drug Deliv. Ther. 2018, 8, 315–321. [Google Scholar] [CrossRef]
- Sridevi, P.; Vijayanand, P.; Raju, M.B. Formulation and evaluation of anti-inflammatory herbal gel containing isolated solanesol. Ann. Phytomed 2017, 6, 127–131. [Google Scholar] [CrossRef]
- Jyothi, D.; Koland, M. Formulation and evaluation of an he rbal anti-inflammatory gel containing Trigonella foenum greacum seed extract. Int. J. Pharm. Pharm. Sci. 2016, 8, 41–44. [Google Scholar]
- Avinash, S.; Gowda, D.; Suresh, J.; Ram, A.; Srivastava, A.; Osmani, R. Formulation and evaluation of topical gel using Eupatorium glandulosum michx. for wound healing activity. Sch. Res. Libr. 2016, 8, 255–266. [Google Scholar]
- Gujjar, S.; Madhavi, B.; Karki, R. Formulation and evaluation of topical gel containing nanostructured lipid carriers dispersion of an antifungal drug. Acta Pharm. Sci. 2019, 57, 57–75. [Google Scholar] [CrossRef]
- Patlolla, V.G.R.; Peter Holbrook, W.; Gizurarson, S.; Kristmundsdottir, T. Doxycycline and Monocaprin In Situ Hydrogel: Effect on Stability, Mucoadhesion and Texture Analysis and In Vitro Release. Gels 2019, 5, 47. [Google Scholar] [CrossRef]
- Al-Kassas, R.S.; El-Khatib, M.M. Ophthalmic controlled release in situ gelling systems for ciprofloxacin based on polymeric carriers. Drug Deliv. 2009, 16, 145–152. [Google Scholar] [CrossRef]
- Karavana, S.Y.; Güneri, P.; Ertan, G. Benzydamine hydrochloride buccal bioadhesive gels designed for oral ulcers: Preparation, rheological, textural, mucoadhesive and release properties. Pharm. Dev. Technol. 2009, 14, 623–631. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Figueiras, A.; Freire, C.; Santos, D.; Veiga, F. Combining strategies to optimize a gel formulation containing miconazole: The influence of modified cyclodextrin on textural properties and drug release. Drug Dev. Ind. Pharm. 2010, 36, 705–714. [Google Scholar] [CrossRef]
- Baloglu, E.; Karavana, S.Y.; Senyigit, Z.A.; Guneri, T. Rheological and mechanical properties of poloxamer mixtures as a mucoadhesive gel base. Pharm. Dev. Technol. 2011, 16, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Korkm, E.; Gokce, E.H.; Ozer, O. Development and evaluation of coenzyme Q10 loaded solid lipid nanoparticle hydrogel for enhanced dermal delivery. Acta Pharm. 2013, 63, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Pawar, J.; Narkhede, R.; Amin, P.; Tawde, V. Design and Evaluation of Topical Diclofenac Sodium Gel Using Hot Melt Extrusion Technology as a Continuous Manufacturing Process with Kolliphor® P407. AAPS PharmSciTech 2017, 18, 2303–2315. [Google Scholar] [CrossRef] [PubMed]
- Barati, M.; Mohammadi Samani, S.; Pourtalebi Jahromi, L.; Ashrafi, H.; Azadi, A. Controlled-release in-situ gel forming formulation of tramadol containing chitosan-based pro-nanogels. Int. J. Biol. Macromol. 2018, 118, 1449–1454. [Google Scholar] [CrossRef]
- Cunha, S.; Forbes, B.; Lobo, J.M.S.; Silva, A.C. Thermosensitive nasal in situ gels of lipid-based Nanosystems to improve the treatment of Alzheimer’s disease. Proceedings 2020, 78, 37. [Google Scholar] [CrossRef]
- Patlolla, V.G.R.; Holbrook, W.P.; Gizurarson, S.; Kristmundsdottir, P. Evaluation of in vitro mucoadhesiveness and texture profile analysis of doxycycline in situ hydrogels. Pharmazie 2020, 75, 7–12. [Google Scholar] [CrossRef]
- Tomás, M.; Rolo, J.; Gaspar, C.; Palmeira-de-Oliveira, A.; Simões, S.; Katz, D.F.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Sodium bicarbonate gels: A new promising strategy for the treatment of vulvovaginal candidosis. Eur. J. Pharm. Sci. 2021, 157, 105621. [Google Scholar] [CrossRef]
- Gökçe, G.; Karavana, S.Y.; Bağriyanik, A.; Pekçetin, Ç.; Algin Yapar, E.; Aybar Tural, G.; Gökçe, E.H. Design and in vitro, in vivo evaluation of antioxidant bioadhesive gels for burn treatment. Turk. J. Biol. 2022, 46, 251–262. [Google Scholar] [CrossRef]
- Toma, I.; Tefas, L.R.; Bogdan, C.; Tomuță, I. Development and Characterization of Loratadine Liposomal Gel Using QbD Approach. Farmacia 2022, 70, 204–213. [Google Scholar] [CrossRef]
- Faris Taufeq, F.Y.; Habideen, N.H.; Rao, L.N.; Podder, P.K.; Katas, H. Potential Hemostatic and Wound Healing Effects of Thermoresponsive Wound Dressing Gel Loaded with Lignosus rhinocerotis and Punica granatum Extracts. Gels 2023, 9, 48. [Google Scholar] [CrossRef]
- Gilani, S.J.; Jumah, M.N.B.; Zafar, A.; Imam, S.S.; Yasir, M.; Khalid, M.; Alshehri, S.; Ghuneim, M.M.; Albohairy, F.M. Formulation and Evaluation of Nano Lipid Carrier-Based Ocular Gel System: Optimization to Antibacterial Activity. Gels 2022, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Campanholi, K.; da Silva Junior, R.C.; Gonçalves, R.S.; de Oliveira, M.C.; Pozza, M.; Leite, A.T.; da Silva, L.H.; Malacarne, L.C.; Bruschi, M.L.; Castilha, L.D.; et al. Photo-Phytotherapeutic Gel Composed of Copaifera reticulata, Chlorophylls, and k-Carrageenan: A New Perspective for Topical Healing. Pharmaceutics 2022, 14, 2580. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Peh, K.K.; Al-Hanbali, O. Effect of Carbopol and polyvinylpyrrolidone on the mechanical, rheological, and release properties of bioadhesive polyethylene glycol gels. AAPS PharmSciTech 2000, 1, E24. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Haldar, P.K.; Chhetri, A. Experimentation on Hibiscus rosa Flower Mucilage as a Potential Nasal Mucoadhesive its Characterization as an In-situ Gel for some Anxiolytics. J. Young Pharm. 2016, 8, 82. [Google Scholar] [CrossRef]
- Altuntaş, E.; Yener, G. Formulation and Evaluation of Thermoreversible In Situ Nasal Gels Containing Mometasone Furoate for Allergic Rhinitis. AAPS PharmSciTech 2017, 18, 2673–2682. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y.; Şenyiğit, Z.A.; Eraç, B.; Limoncu, M.H.; Baloğlu, E. Mucoadhesive in situ gel formulation for vaginal delivery of clotrimazole: Formulation, preparation, and in vitro/in vivo evaluation. Pharm. Dev. Technol. 2017, 22, 551–561. [Google Scholar] [CrossRef]
- Rençber, S.; Karavana, S.Y. Formulation and optimization of gellan gum-poloxamer based dexamethasone mucoadhesive in situ gel. J. Res. Pharm. 2020, 201, 529–538. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, Z.; Wu, M.; Shen, Y.; Li, G.; Ma, T. Fabrication of emulsion gel based on polymer sanxan and its potential as a sustained-release delivery system for β-carotene. Int. J. Biol. Macromol. 2020, 164, 597–605. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Alruwaili, N.K.; Alhakamy, N.A.; Abualsunun, W.A.; Bakhaidar, R.B.; Bahmdan, R.H.; Rizg, W.Y.; et al. Oral gel loaded with penciclovir-lavender oil nanoemulsion to enhance bioavailability and alleviate pain associated with herpes labialis. Drug Deliv. 2021, 28, 1043–1054. [Google Scholar] [CrossRef]
- Alkhalidi, H.M.; Hosny, K.M.; Rizg, W.Y. Oral Gel Loaded by Fluconazole—Sesame Oil Nanotransfersomes: Development, Optimization, and Assessment of Antifungal Activity. Pharmaceutics 2020, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bao, Q.; Shen, J.; Lalla, R.V.; Burgess, D.J. Mucoadhesive in situ forming gel for oral mucositis pain control. Int. J. Pharm. 2020, 580, 116138. [Google Scholar] [CrossRef] [PubMed]
- Barreto-Henriksson, H.; Llorente, M.; Larsson, A.; Brisby, H.; Gold, J.; Schuster, E.; Ström, A. Determination of mechanical and rheological properties of a cell-loaded peptide gel during ECM production. Int. J. Pharm. 2019, 563, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, J.; Shi, Y.; Wan, Y. Rheological, mechanical and degradable properties of injectable chitosan/silk fibroin/hydroxyapatite/glycerophosphate hydrogels. J. Mech. Behav. Biomed. Mater. 2016, 64, 161–172. [Google Scholar] [CrossRef]
- Deng, L.; Li, Y.; Zhong, G.; Lei, W.; Peng, Y.; Zhu, Z. Effects of Starch Addition on KGM Sol’s Pasting, Rheological Properties, and Gel Texture. ACS Omega 2023, 8, 33299–33309. [Google Scholar] [CrossRef]
- Goel, H.; Gupta, N.; Santhiya, D.; Dey, N.; Bohidar, H.B.; Bhattacharya, A. Bioactivity reinforced surface patch bound collagen-pectin hydrogel. Int. J. Biol. Macromol. 2021, 174, 240–253. [Google Scholar] [CrossRef]
- Rubinstein, B.J.; Ranney, J.D.; Khoshakhlagh, P.; Strasnick, B.; Horn-Ranney, E.L. A novel gel patch for minimally invasive repair of tympanic membrane perforations. Int. J. Pediatr. Otorhinolaryngol. 2018, 115, 27–32. [Google Scholar] [CrossRef]
- Luo, G.F.; Chen, W.H.; Zhang, X.Z. 100th Anniversary of Macromolecular Science Viewpoint: Poly(N-isopropylacrylamide)-Based Thermally Responsive Micelles. ACS Macro Lett. 2020, 9, 872–881. [Google Scholar] [CrossRef]
- Yang, L.; Fan, X.; Zhang, J.; Ju, J. Preparation and Characterization of Thermoresponsive Poly(N-Isopropylacrylamide) for Cell Culture Applications. Polymers 2020, 12, 389. [Google Scholar] [CrossRef]
- Zhang, J.T.; Bhat, R.; Jandt, K.D. Temperature-sensitive PVA/PNIPAAm semi-IPN hydrogels with enhanced responsive properties. Acta Biomater. 2009, 5, 488–497. [Google Scholar] [CrossRef]
- Kim, E.Y.; Gao, Z.G.; Park, J.S.; Li, H.; Han, K. rhEGF/HP-beta-CD complex in poloxamer gel for ophthalmic delivery. Int. J. Pharm. 2002, 233, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Li, L.; Huang, C.; Li, W.; Wu, C. Optimization and physicochemical characterization of thermosensitive poloxamer gel containing puerarin for ophthalmic use. Chem. Pharm. Bull. 2006, 54, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-Y. Optimization for formulation of Zhi Xiong San thermo-sensitive gel by central composite design-response surface method and study on characterization of its nasal mucosal permeability. Chin. Tradit. Herbal. Drugs 2014, 45, 1845–1849. [Google Scholar] [CrossRef]
- Nasir, F.; Iqbal, Z.; Khan, J.A.; Khan, A.; Khuda, F.; Ahmad, L.; Khan, A.; Khan, A.; Dayoo, A.; Roohullah. Development and evaluation of diclofenac sodium thermorevesible subcutaneous drug delivery system. Int. J. Pharm. 2012, 439, 120–126. [Google Scholar] [CrossRef]
- Arkin, A.; Elham, A.; Anwar, A.; Kalimanjiang, G.; Iminjan, M. Optimization and Evaluation of the Quercus infectoria Galls Thermosensitive In Situ Gel for Rectal Delivery. Evid. Based Complement. Altern. Med. 2022, 2022, 8451055. [Google Scholar] [CrossRef]
- Sahoo, D.; Sahoo, S.; Mohanty, P.; Sasmal, S.; Nayak, P. Chitosan: A new versatile bio-polymer for various applications. Des. Monomers Polym. 2009, 12, 377–404. [Google Scholar] [CrossRef]
- Nithya, S.; Nimal, T.R.; Baranwal, G.; Suresh, M.K.; Anju, C.P.; Anil Kumar, V.; Gopi Mohan, C.; Jayakumar, R.; Biswas, R. Preparation, characterization and efficacy of lysostaphin-chitosan gel against Staphylococcus aureus. Int. J. Biol. Macromol. 2018, 110, 157–166. [Google Scholar] [CrossRef]
- Ha, D.I.; Lee, S.B.; Chong, M.S.; Lee, Y.M.; Kim, S.Y.; Park, Y.H. Preparation of thermo-responsive and injectable hydrogels based on hyaluronic acid and poly (N-isopropylacrylamide) and their drug release behaviors. Macromol. Res. 2006, 14, 87–93. [Google Scholar] [CrossRef]
- Furlani, F.; Rossi, A.; Grimaudo, M.A.; Bassi, G.; Giusto, E.; Molinari, F.; Lista, F.; Montesi, M.; Panseri, S. Controlled Liposome Delivery from Chitosan-Based Thermosensitive Hydrogel for Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 894. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Dou, K.; Li, K.; Zhou, Q.; Fu, Q. Injectable thermo-sensitive hydrogel containing ADSC-derived exosomes for the treatment of cavernous nerve injury. Carbohydr. Polym. 2023, 300, 120226. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Hung, K.H.; Tsai, T.H.; Lee, C.J.; Ku, R.Y.; Chiu, A.W.; Chiou, S.H.; Liu, C.J. Sustained delivery of latanoprost by thermosensitive chitosan-gelatin-based hydrogel for controlling ocular hypertension. Acta Biomater. 2014, 10, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Yang, S.H.; Liu, C.C.; Gefen, A.; Lin, F.H. Thermosensitive hydrogel made of ferulic acid-gelatin and chitosan glycerophosphate. Carbohydr. Polym. 2013, 92, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Bonde, S.; Chandarana, C.; Prajapati, P.; Vashi, V. A comprehensive review on recent progress in chitosan composite gels for biomedical uses. Int. J. Biol. Macromol. 2024, 272, 132723. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef]
- Supper, S.; Anton, N.; Seidel, N.; Riemenschnitter, M.; Schoch, C.; Vandamme, T. Rheological study of chitosan/polyol-phosphate systems: Influence of the polyol part on the thermo-induced gelation mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Li, N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1282–1287. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive nanocomposite hydrogels based on Gelatin/poly (N–isopropylacrylamide)(PNIPAM) for controlled drug delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-Responsive Poly(N-Isopropylacrylamide)-Cellulose Nanocrystals Hybrid Hydrogels for Wound Dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef]
- Laniesse, D.; Smith, D.A.; Knych, H.K.; Mosley, C.; Guzman, D.S.; Beaufrère, H. In vitro characterization of a formulation of butorphanol tartrate in a poloxamer 407 base intended for use as a parenterally administered slow-release analgesic agent. Am. J. Vet. Res. 2017, 78, 677–687. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, J.; Li, Y.; Huang, J.; Huang, Z.; Huang, Y.; Pan, X.; Wu, C. Thermo-sensitive gel in glaucoma therapy for enhanced bioavailability: In vitro characterization, in vivo pharmacokinetics and pharmacodynamics study. Life Sci. 2018, 212, 80–86. [Google Scholar] [CrossRef]
- Dumortier, G.; El Kateb, N.; Sahli, M.; Kedjar, S.; Boulliat, A.; Chaumeil, J.C. Development of a thermogelling ophthalmic formulation of cysteine. Drug Dev. Ind. Pharm. 2006, 32, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nasir, F.; Iqbal, Z.; Khan, A.; Khan, J.A.; Khan, A.; Khuda, F.; Zakir, S.; Yousaf, N.; Khan, I.; Shah, Y.; et al. Development and evaluation of pluronic- and methylcellulose-based thermoreversible drug delivery system for insulin. Drug Dev. Ind. Pharm. 2014, 40, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.; Marzok, S.; Ibrahim, M. Development of optimized mucoadhesive thermosensitive pluronic based in situ gel for controlled delivery of Latanoprost: Antiglaucoma efficacy and stability approaches. J. Drug Deliv. Sci. Technol. 2019, 53, 101134. [Google Scholar] [CrossRef]
- Rao, M.; Agrawal, D.K.; Shirsath, C. Thermoreversible mucoadhesive in situ nasal gel for treatment of Parkinson’s disease. Drug Dev. Ind. Pharm. 2017, 43, 142–150. [Google Scholar] [CrossRef]
- Zaki, N.M.; Awad, G.A.; Mortada, N.D.; Abd Elhady, S.S. Enhanced bioavailability of metoclopramide HCl by intranasal administration of a mucoadhesive in situ gel with modulated rheological and mucociliary transport properties. Eur. J. Pharm. Sci. 2007, 32, 296–307. [Google Scholar] [CrossRef]
- Ohmes, J.; Saure, L.M.; Schütt, F.; Trenkel, M.; Seekamp, A.; Scherließ, R.; Adelung, R.; Fuchs, S. Injectable Thermosensitive Chitosan-Collagen Hydrogel as A Delivery System for Marine Polysaccharide Fucoidan. Mar. Drugs 2022, 20, 402. [Google Scholar] [CrossRef]
- Gan, L.; Wang, L.; Chen, J.; Tang, L. Complications of XEN gel stent implantation for the treatment of glaucoma: A systematic review. Front. Med. 2024, 11, 1360051. [Google Scholar] [CrossRef]
- Sivalingam, S.; Tamm-Daniels, I.; Nakada, S.Y. Office-based ureteral stent placement under local anesthesia for obstructing stones is safe and efficacious. Urology 2013, 81, 498–502. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef]
- Soliman, I.I.; Soliman, N.A.; Abdou, E.M. Formulation and stability study of chlorpheniramine maleate nasal gel. Pharm. Dev. Technol. 2010, 15, 484–491. [Google Scholar] [CrossRef]
- Mohan, E.C.; Kandukuri, J.M.; Allenki, V. Preparation and evaluation of in-situ-gels for ocular drug delivery. J. Pharm. Res. 2009, 2, 1089–1094. [Google Scholar]
- Prasanth, V.; Ranjan, S. Formulation and evaluation of in situ ocular gel of levofloxacin. J. Drug Deliv. Ther. 2017, 7, 68–73. [Google Scholar] [CrossRef]
- Majeed, A.; Khan, N.A. Ocular in situ gel: An overview. J. Drug Deliv. Ther. 2019, 9, 337–347. [Google Scholar] [CrossRef]
- Simões, S.M.; Veiga, F.; Torres-Labandeira, J.J.; Ribeiro, A.C.; Sandez-Macho, M.I.; Concheiro, A.; Alvarez-Lorenzo, C. Syringeable Pluronic-α-cyclodextrin supramolecular gels for sustained delivery of vancomycin. Eur. J. Pharm. Biopharm. 2012, 80, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Nayak, S.K.; Maji, S.; Anis, A.; Kim, D.; Pal, K. Environment sensitive hydrogels for drug delivery applications. Eur. Polym. J. 2019, 120, 109220. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.J.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B Biointerfaces 2020, 188, 110757. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; You, T.; Zhao, B.; Dong, L.; Huang, C.; Zhou, X.; Xing, M.; Qian, W.; Luo, G. 3D Printing-Based Hydrogel Dressings for Wound Healing. Adv. Sci. 2024, 11, e2404580. [Google Scholar] [CrossRef]
- Tamo, A.K.; Djouonkep, L.D.W.; Selabi, N.B.S. 3D Printing of Polysaccharide-Based Hydrogel Scaffolds for Tissue Engineering Applications: A Review. Int. J. Biol. Macromol. 2024, 270, 132123. [Google Scholar] [CrossRef]
- Motealleh, A.; Schäfer, A.H.; Fromm, O.; Kehr, N.S. 3D-Printed Oxygen-Carrying Nanocomposite Hydrogels for Enhanced Cell Viability under Hypoxic and Normoxic Conditions. Biomacromolecules 2021, 22, 4758–4769. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Liu, C.; Wei, X.; Guo, J.; Luo, Y. 3D-Printed hydrogel scaffolds with drug- and stem cell-laden core/shell filaments for cancer therapy and soft tissue repair. J. Mater. Chem. B 2024, 12, 11491–11501. [Google Scholar] [CrossRef] [PubMed]
- Kass, L.; Thang, M.; Zhang, Y.; DeVane, C.; Logan, J.; Tessema, A.; Perry, J.; Hingtgen, S. Development of a biocompatible 3D hydrogel scaffold using continuous liquid interface production for the delivery of cell therapies to treat recurrent glioblastoma. Bioeng. Transl. Med. 2024, 9, e10676. [Google Scholar] [CrossRef] [PubMed]
- Zaszczyńska, A.; Niemczyk-Soczynska, B.; Sajkiewicz, P. A Comprehensive Review of Electrospun Fibers, 3D-Printed Scaffolds, and Hydrogels for Cancer Therapies. Polymers 2022, 14, 5278. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef]
- Gantenbein, S.; Colucci, E.; Käch, J.; Trachsel, E.; Coulter, F.B.; Rühs, P.A.; Masania, K.; Studart, A.R. Three-dimensional printing of mycelium hydrogels into living complex materials. Nat. Mater. 2023, 22, 128–134. [Google Scholar] [CrossRef]
- Söhling, N.; Neijhoft, J.; Nienhaus, V.; Acker, V.; Harbig, J.; Menz, F.; Ochs, J.; Verboket, R.D.; Ritz, U.; Blaeser, A.; et al. 3D-Printing of Hierarchically Designed and Osteoconductive Bone Tissue Engineering Scaffolds. Materials 2020, 13, 1836. [Google Scholar] [CrossRef]
- Wang, L.; Ye, C.; Xue, X.; Xie, M.; Zhi, Y.; Feng, X.; Zhao, P.; Zhou, J.; Mi, M.; Li, J.; et al. 3D-Printed Breast Prosthesis that Smartly Senses and Targets Breast Cancer Relapse. Adv. Sci. 2024, 11, e2402345. [Google Scholar] [CrossRef]
- Phan, V.H.G.; Murugesan, M.; Huong, H.; Le, T.T.; Phan, T.H.; Manivasagan, P.; Mathiyalagan, R.; Jang, E.S.; Yang, D.C.; Li, Y.; et al. Cellulose Nanocrystals-Incorporated Thermosensitive Hydrogel for Controlled Release, 3D Printing, and Breast Cancer Treatment Applications. ACS Appl. Mater. Interfaces 2022, 14, 42812–42826. [Google Scholar] [CrossRef]
- Kiseleva, M.; Lescot, T.; Selivanova, S.V.; Fortin, M.A. Gold-Enhanced Brachytherapy by a Nanoparticle-Releasing Hydrogel and 3D-Printed Subcutaneous Radioactive Implant Approach. Adv. Healthc. Mater. 2023, 12, e2300305. [Google Scholar] [CrossRef]
- Kiseleva, M.; Omar, M.M.; Boisselier, É.; Selivanova, S.V.; Fortin, M.A. A Three-Dimensional Printable Hydrogel Formulation for the Local Delivery of Therapeutic Nanoparticles to Cervical Cancer. ACS Biomater. Sci. Eng. 2022, 8, 1200–1214. [Google Scholar] [CrossRef]
- Herreros-Pomares, A.; Zhou, X.; Calabuig-Fariñas, S.; Lee, S.J.; Torres, S.; Esworthy, T.; Hann, S.Y.; Jantus-Lewintre, E.; Camps, C.; Zhang, L.G. 3D printing novel in vitro cancer cell culture model systems for lung cancer stem cell study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111914. [Google Scholar] [CrossRef]
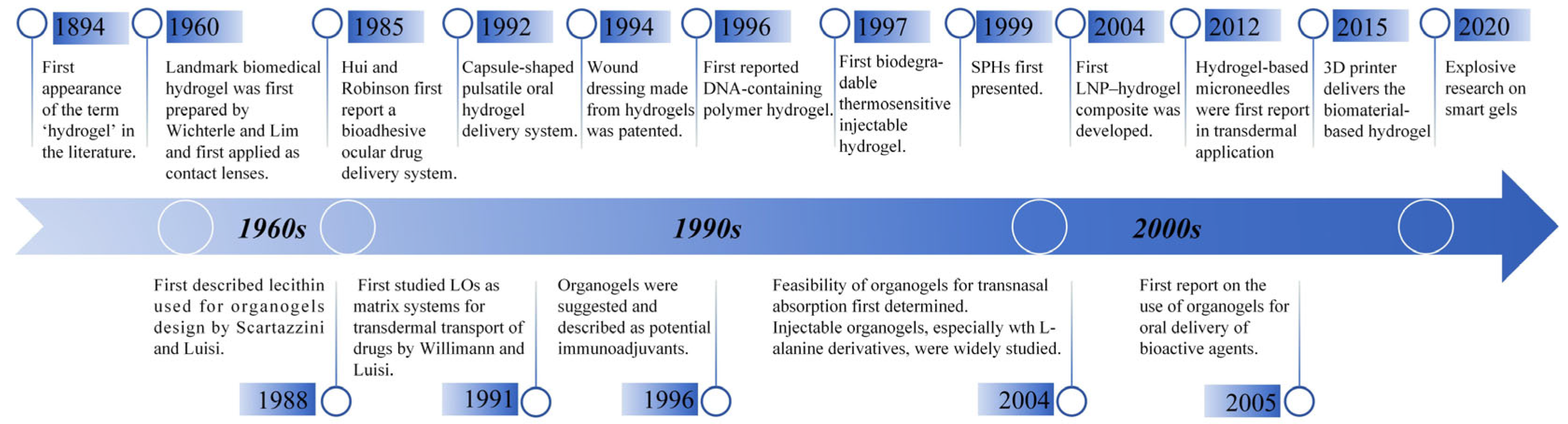
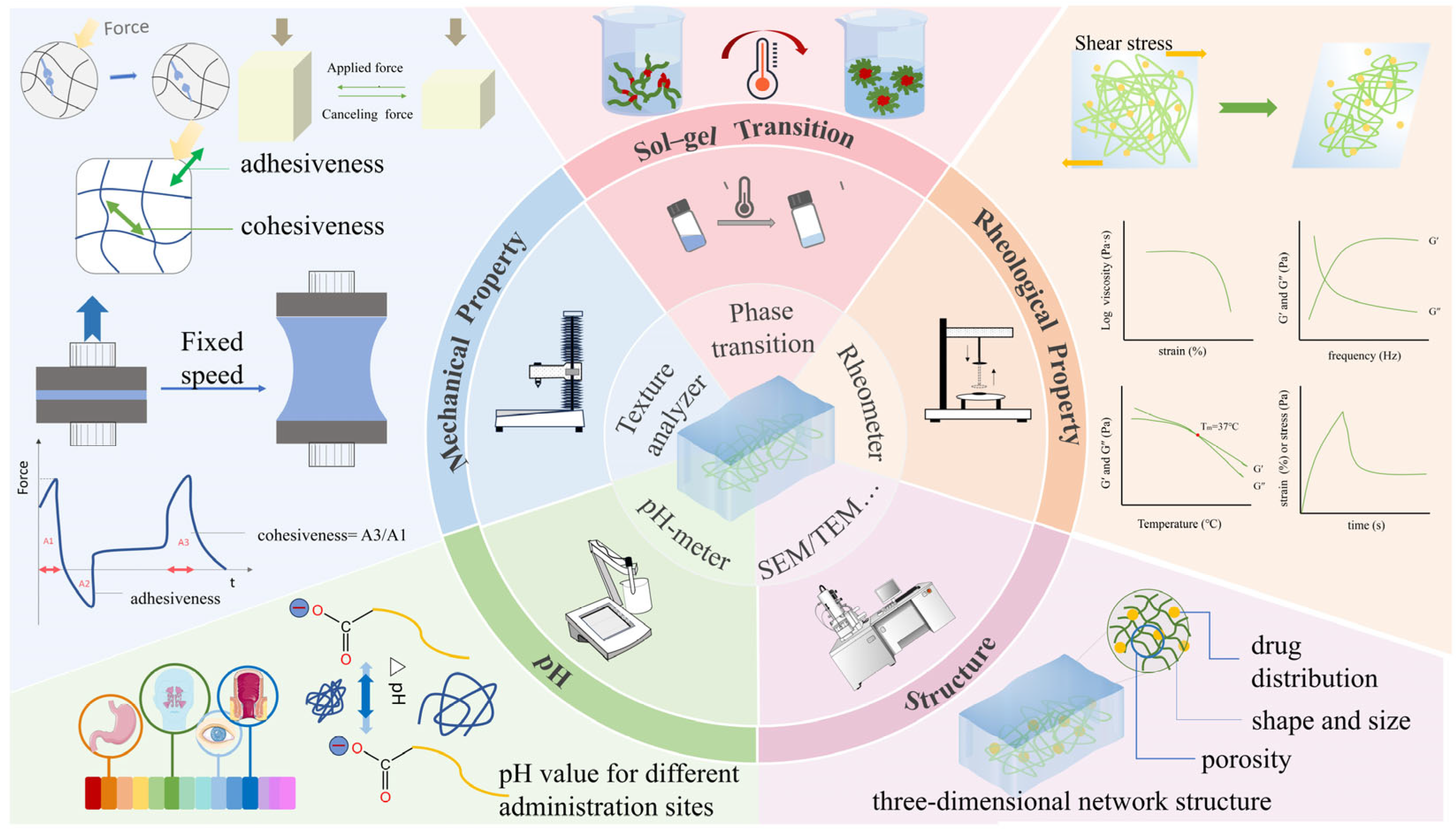
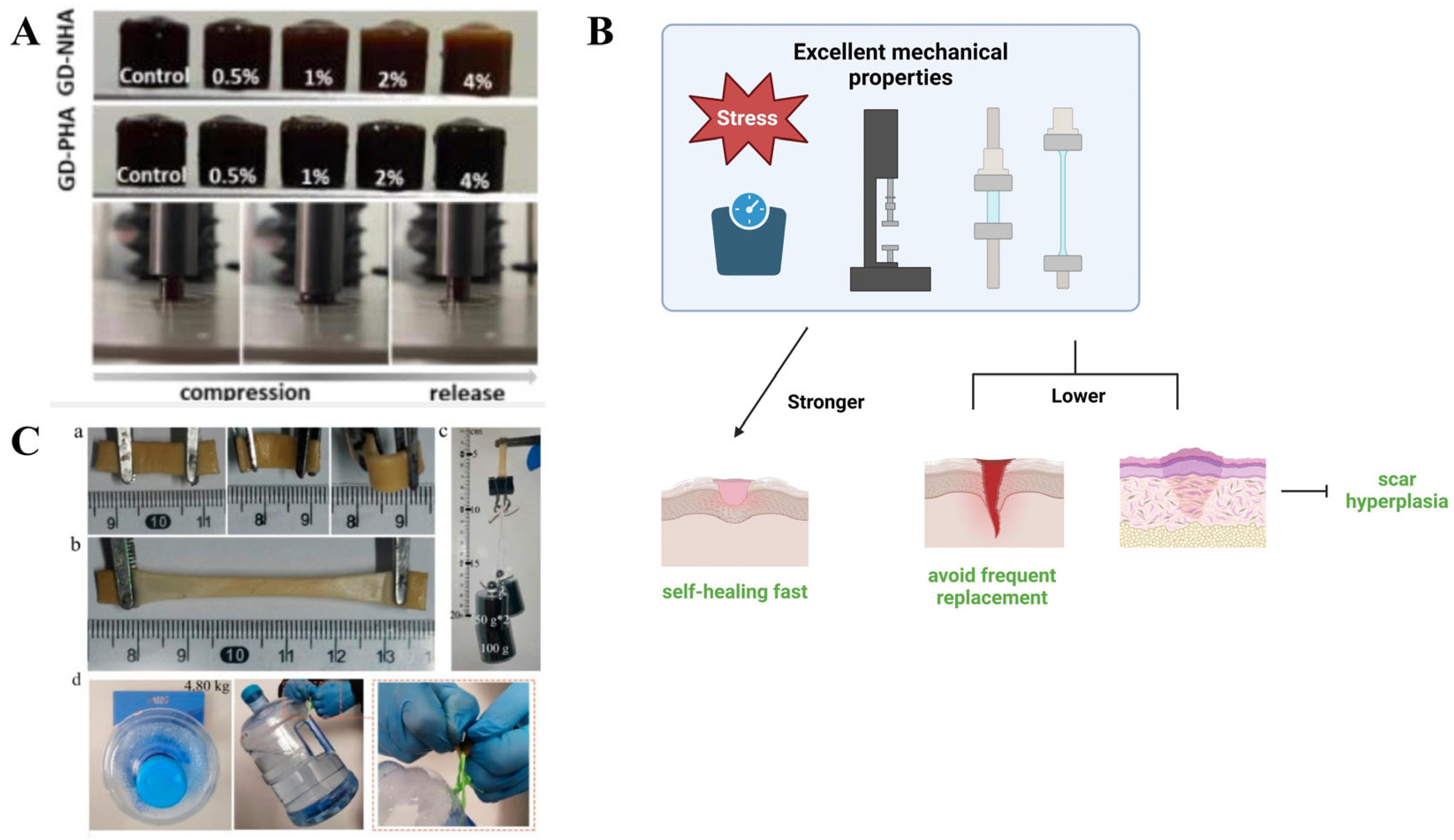
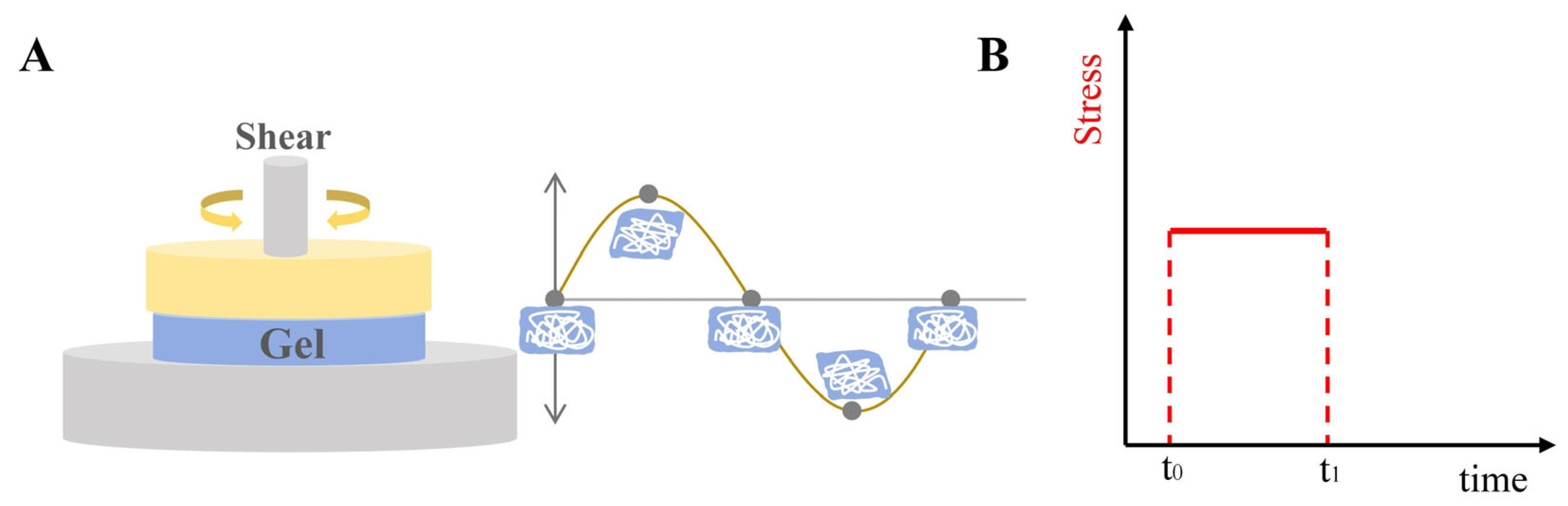
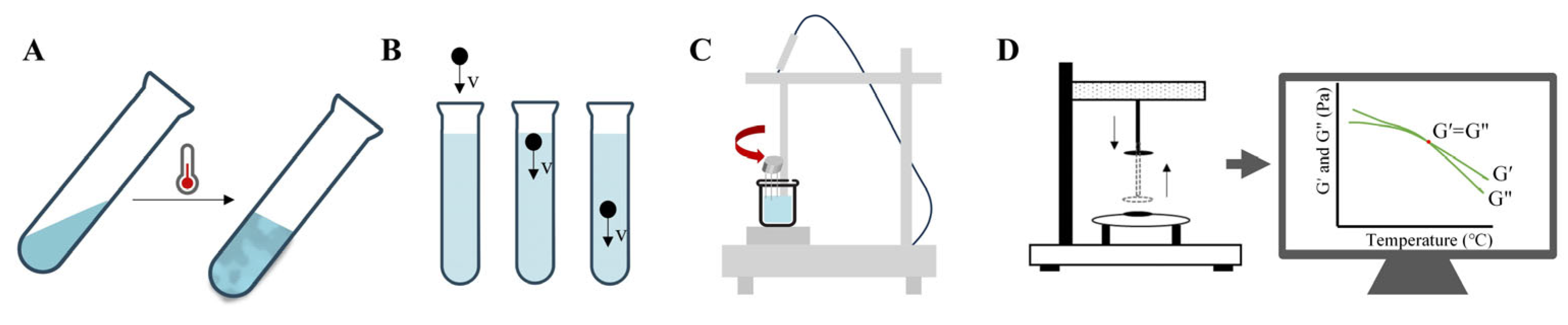
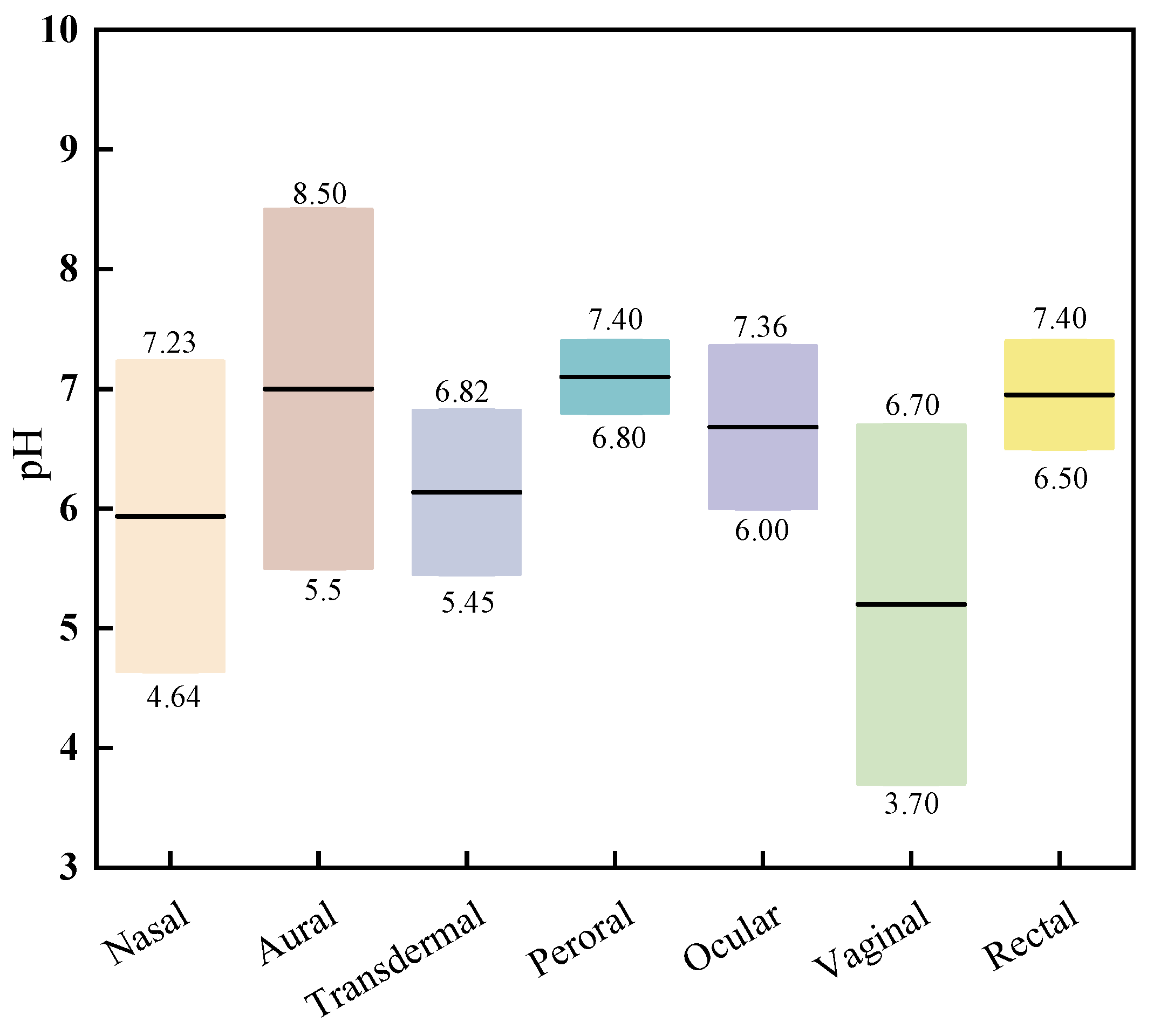
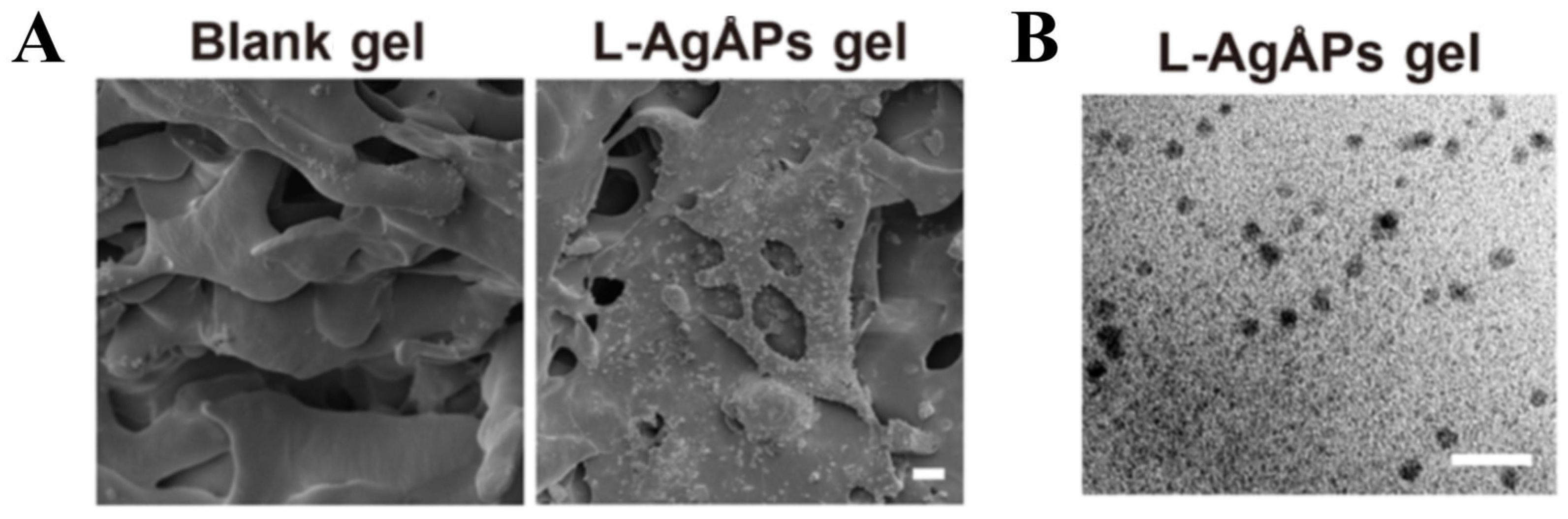



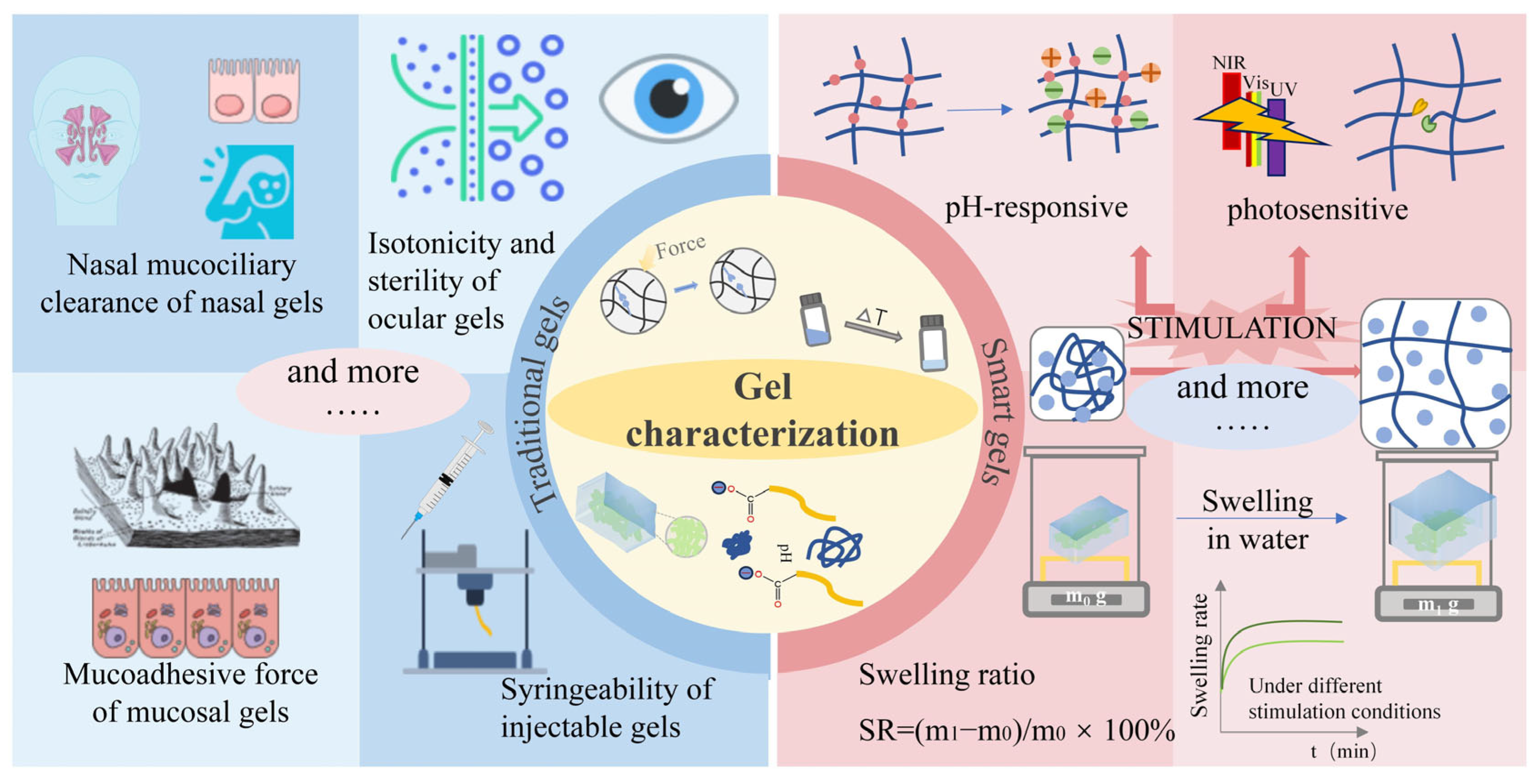
| Test | Measurement Methods | Image | Common Rules | Significance |
|---|---|---|---|---|
| Strain sweep | Characterize gels through the application of escalating oscillatory strain at a steady frequency |  | In a specific area of gels experiencing low shear stress, the moduli remain unaffected by the escalating stress. | To determine LVR region of gels |
| Frequency sweep | Assess the viscoelastic characteristics of gels through a comparison of G′ and G″ values across various frequencies | 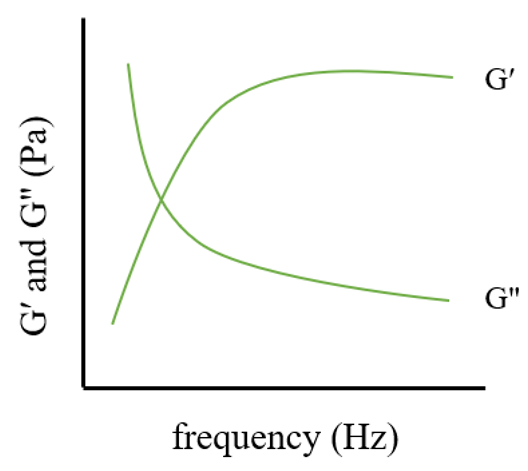 | Every gel exhibits characteristics of a viscoelastic fluid, characterized by upward gradients on the G′ and G″ axes, leading to a more rapid rise in the loss modulus. | To determine the G′ and G″ crossover points |
| Temperature sweep | Measure transition temperature of gels by increasing temperature and record when G′ value equals with G″ value. | 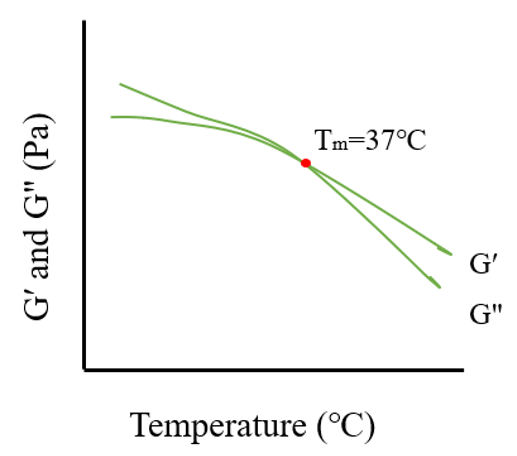 | As temperature increases, G′ and G″ show a downward trend, and there will exist a crossover point at a certain temperature, known as the phase transition temperature. | To determine phase transition temperature; especially used in thermosensitive gels |
| Creep test | Record compliance over time when applying stress and after stress relief | 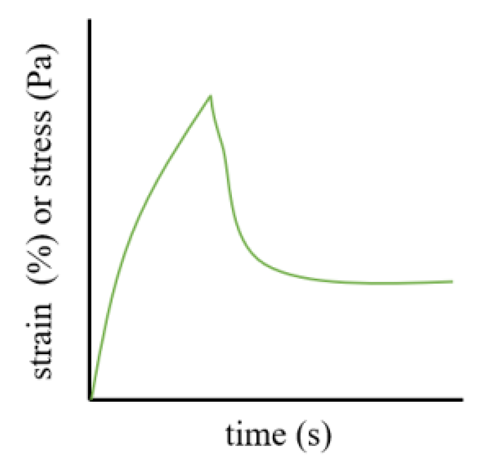 | During the loading step, the J value is inversely proportional to the stiffness and recovery time, and the slope and creep compliance during the recovery step can reflect the elastic behavior and stiffness of the gel. | To evaluate the stiffness and elasticity of gels |
| Structural Characterization | Methods | Aim | Data Analysis | Reference |
|---|---|---|---|---|
| Microstructure Characterization | SEM/TEM/AFM, etc. | To observe micromorphology of gel | Shape or size | [71,94,135,150,153,154] |
| FT-IR | The aim is to determine the potential interplay between excipients and active drugs in gel formulations. | Span60 (-CH) 2956 and 2849 cm−1 (-OH in carboxyl group) 2916 cm−1 (-C=O in ester) 1736 cm−1 (-CH) 2930, 2899, 2867, and 2834 cm−1 (-C=C in alkenes) 1671 cm−1 | [158] | |
| Span20 (-OH) 3392 cm−1 (-CH2- asymmetric stretching) 2923 cm−1 (-CH2-symmetric stretching) 2834 cm−1 (-C=O stretching) 1738 cm−1 | [157] | |||
| Tween20 (-OH stretching) 3488 cm−1 (-CH2-asymmetric stretching) 2920 cm−1 (-CH2-symmetric stretching) 2860 cm−1 (-C=O stretching) 1734 cm−1 | [157] | |||
| Carbomer (-NH and -C=O) 3357 cm−1 and 1639 cm−1 | [94] | |||
| Carbomer with the existence of triethanolamine (-N-H) around 3316 cm−1 (-C-H) 2931 cm−1 (-C-O-C) 1200 ~1250 cm−1 (=C-H) 800~850 cm−1 1643 cm−1 peaks signify the presence of hydrogen bonds in the carbonyl group of carbopol when hydrated. | [72] | |||
| Poloxamer 407 (-C-O-C) 1240 cm−1 (-O-H) 3647.2 cm−1 (-N-H) 3347 cm−1 | [68] | |||
| Chitosan (-O-H and -N-H) 3348 cm−1 (-C-H) 2867 cm−1 (-CONH2) 1643 cm−1 (-C-O) 1072 cm−1 | [69] | |||
| Sodium alginate (mannuronic acid) 880 cm−1 (uronic acid) 1056 cm−1 (-OH) 2283 cm−1 (-CH2) 2928 cm−1 | [47] |
| Types | Pattern | Composition | Peculiarity | Range of Application | Notes |
|---|---|---|---|---|---|
| Coaxial cylinder fixture | 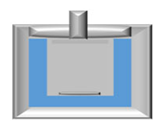 | It consists of an inner cylinder and an outer cylinder. The inner cylinder can rotate, while the outer cylinder remains stationary. The sample fills the annular space between the inner and outer cylinders. | The diameter of the inner cylinder is lesser, whereas the outer cylinder exhibits a greater diameter, and both are approximately equal in height. | 1. Low-to-medium viscosity fluids 2. Some organic solvents or easily oxidized liquid samples | Ensure the inner and outer cylinders are properly aligned during their setup and operation. |
| Taper plate fixture | 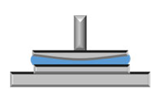 | It consists of a cone and a flat plate. The apex of the cone is in close contact with the plate, and the sample fills the small space between the cone and the plate. | The angle of the cone is usually small, typically between 1° and 4°, to ensure relatively uniform shear stresses and shear rates during the measurement. | 1. High-viscosity fluids, as well as pasty and semi-solid samples 2. Precious samples | 1. The cone and plate need to be carefully cleaned to avoid the impact of residual samples on subsequent measurements. 2. Pay attention to temperature control. |
| Parallel plate fixture | 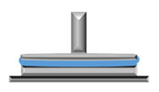 | It consists of two parallel disks. The sample is placed between the two disks, the diameter of which can be selected according to the amount of sample and the measurement requirements. | The spacing between the two disks can be precisely adjusted by the instrument to accommodate samples of different viscosities and thicknesses. | The fluids of various viscosities, as well as thin film-like and sheet-like samples | 1. Ensure the sample is evenly distributed between the parallel plates. 2. Prevent sample edge extrusion. 3. Pay attention to the temperature uniformity between the parallel plates. |
| Gel Type | Rheometer Model | Fixture | Temperature/°C | Shear Rates/s−1 | Frequency | Strain/% | Reference |
|---|---|---|---|---|---|---|---|
| Oral gel | / | / | 25 ± 1 | 2, 10, 20, 30, 40, 50, 60 | / | / | [250] |
| / | / | 25 ± 5 | 2, 10, 20, 30, 40, 50, 60 | / | / | [251] | |
| ARES-G2, TA | steel cone | 15~45 | shear stress (0.1~500 Pa) | / | 0.1 | [252] | |
| Gel injection | Anton Paar Physica MCR 300 | cone plate | 25 | 0.7 Hz | 0.7 Hz | 0.1 | [253] |
| Anton Paar Physica MCR 300 | cone plate | 20 | 6.28 rad | 0.0628 Hz~62.8 rad/s | 0.01~10 | [253] | |
| Kinexus Pro, Malvern | parallel plate | 25~45 | / | 0.1~100 Hz | 1 | [254] | |
| Topical gels | TA | parallel plate | 25 | 0.1~200 | 0.1~100 rad/s | 5 | [255] |
| TA, AR-500 | cone plate | / | / | 1~100 rad/s | / | [256] | |
| MCR502, Anton Paar | parallel plate | / | / | 1 Hz | 0.001~10 | [257] | |
| Ophthalmic gel | / | parallel plate | 25 ± 0.1 | / | angular velocity (0.5~100 rpm) | / | [122] |
| Anton Paar, MCR 302 | parallel plate | 20~37 | / | / | / | [123] | |
| / | / | 34 ± 1 | / | angular velocity (0.5~100 rpm) | / | [124] | |
| / | vaned rotor | 25 | 1000~10−5 | / | / | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.; Qi, Z.; Cao, Y.; Li, R.; Wu, J.; Tang, R.; Gao, Y.; Du, R.; Liu, M. Gels as Promising Delivery Systems: Physicochemical Property Characterization and Recent Applications. Pharmaceutics 2025, 17, 249. https://doi.org/10.3390/pharmaceutics17020249
Wang E, Qi Z, Cao Y, Li R, Wu J, Tang R, Gao Y, Du R, Liu M. Gels as Promising Delivery Systems: Physicochemical Property Characterization and Recent Applications. Pharmaceutics. 2025; 17(2):249. https://doi.org/10.3390/pharmaceutics17020249
Chicago/Turabian StyleWang, Enzhao, Zhaoying Qi, Yuzhou Cao, Ruixiang Li, Jing Wu, Rongshuang Tang, Yi Gao, Ruofei Du, and Minchen Liu. 2025. "Gels as Promising Delivery Systems: Physicochemical Property Characterization and Recent Applications" Pharmaceutics 17, no. 2: 249. https://doi.org/10.3390/pharmaceutics17020249
APA StyleWang, E., Qi, Z., Cao, Y., Li, R., Wu, J., Tang, R., Gao, Y., Du, R., & Liu, M. (2025). Gels as Promising Delivery Systems: Physicochemical Property Characterization and Recent Applications. Pharmaceutics, 17(2), 249. https://doi.org/10.3390/pharmaceutics17020249







