Investigations of In Vitro Anti-Acetylcholinesterase, Anti-Diabetic, Anti-Inflammatory, and Anti-Cancer Efficacy of Garden Cress (Lepidium sativum Linn.) Seed Extracts, as Well as In Vivo Biochemical and Hematological Assays
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Seeds and Preparation of Extracts
2.2. Phyto-Chemical Evaluation of L. sativum Seed Extracts
2.3. In Vitro Studies of L. sativum Seed Extracts
2.4. In Vivo Studies of L. sativum Seed Extracts
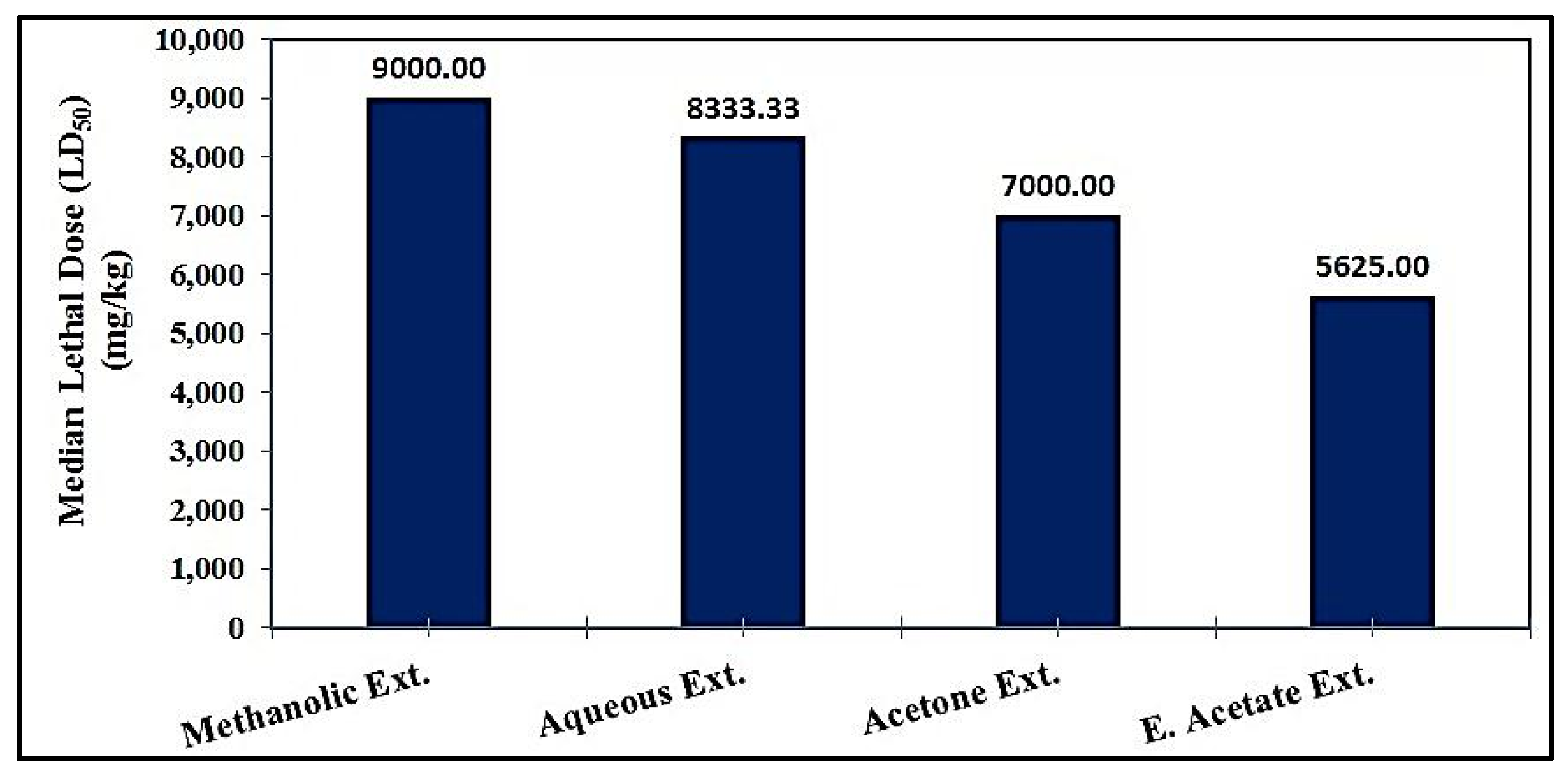
2.4.1. Median Lethal Dose (LD50)
2.4.2. Experimental Design
2.4.3. Sample Collections
2.4.4. Biochemical Assays
Hematological and Biochemical Measurements
Biochemical Assays in Supernatants of Tissues Homogenates
2.5. Statistical Analysis
3. Results
3.1. Phyto-Chemical Evaluation of L. sativum Seed Extracts
3.2. In Vitro Studies on L. sativum Seed Extracts
3.2.1. Antioxidant Activity
3.2.2. Scavenging Activity
3.2.3. Anti-Alzheimer’s and Anti-Diabetic Activities
3.2.4. Anti-Arthritic and Anti-Inflammatory Activities
3.2.5. Cytotoxic Activity and Enzymatic Assays
3.3. In Vivo Study on L. sativum Seed Extracts
3.3.1. Median Lethal Dose (LD50)
3.3.2. Hematological and Biochemical Measurements
3.3.3. Biochemical Assays in Supernatants of Tissue Homogenates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase enzyme |
| ALP | Alkaline phosphatase |
| AST | Aspartate transaminase |
| ABTS | 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) |
| BUN | Blood urea nitrogen |
| CAT | Catalase |
| CK | Creatin kinase |
| COX-1 | Cyclooxygenase-1 enzyme |
| COX-2 | Cyclooxygenase-2 enzyme |
| MTT | 3-(4,5-Dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| DPPH | 1,1-Diphenyl-2-picryl-hydrazyl |
| GGT | Gamma-glutamyl transferase |
| GPx | Glutathione peroxidase enzyme |
| HCT | Hematocrit |
| HB | Hemoglobin |
| HDL-c | High-density lipoprotein-cholesterol |
| Caco-2 | Human colon cancer |
| HepG-2 | Human hepatocellular carcinoma |
| A549 | Human lung cancer |
| IL-6 | Interleukin-6 |
| IRP | Iron-reducing power |
| LDH | Lactate dehydrogenase |
| L. sativum | Lepidium sativum Linn. |
| LPO | Lipid peroxidation product |
| 5-LOX | 5-Lipoxygenase enzyme |
| LDL-c | Low-density lipoprotein-cholesterol |
| NO | Nitric oxide |
| PLT | Platelet count |
| RBCs | Red blood cells |
| GSH | Reduced glutathione |
| ALT | Serum alanine transaminase |
| SOD | Superoxide dismutase |
| IC50 | The median inhibitory concentration |
| LD50 | The median lethal doses |
| TAC | Total antioxidant capacity |
| TAC | Total antioxidant capacity |
| TC | Total cholesterol |
| TPC | Total protein carbonyl |
| TG | Triglycerides |
| TNF-α | Tumor necrosis factor-α |
| WBCs | White blood cells |
References
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W.; et al. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.W.; Li, R.; Geetha, T.; Tao, Y.X.; Babu, J.R. Nerve growth factor in metabolic complications and Alzheimer’s disease: Physiology and therapeutic potential. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165858. [Google Scholar] [CrossRef] [PubMed]
- Zilliox, L.A. Diabetes and peripheral nerve disease. Clin. Geriatr. Med. 2021, 37, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E.; Mouton, A.J.; da Silva, A.A.; Omoto, A.C.; Wang, Z.; Li, X.; do Carmo, J.M. Obesity, kidney dysfunction, and inflammation: Interactions in hypertension. Cardiovasc. Res. 2021, 117, 1859–1876. [Google Scholar] [CrossRef]
- Benzaquen, D.; Lawrence, Y.R.; Taussky, D.; Zwahlen, D.; Oehler, C.; Champion, A. The Crosstalk between Nerves and Cancer—A Poorly Understood Phenomenon and New Possibilities. Cancers 2024, 16, 1875. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Feng, J.; Zheng, Y.; Guo, M.; Ares, I.; Martínez, M.; Lopez-Torres, B.; Martínez-Larrañaga, M.R.; Wang, X.; Anadón, A.; Martínez, M.A. Oxidative stress, the blood–brain barrier and neurodegenerative diseases: The critical beneficial role of dietary antioxidants. Acta Pharm. Sin. B 2023, 13, 3988–4024. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes mellitus: Classification, mediators, and complications; A gate to identify potential targets for the development of new effective treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef]
- Lucena, P.B.; Heneka, M.T. Inflammatory aspects of Alzheimer’s disease. Acta Neuropathol. 2024, 148, 31. [Google Scholar] [CrossRef]
- Moawad, M.H.E.D.; Serag, I.; Alkhawaldeh, I.M.; Abbas, A.; Sharaf, A.; Alsalah, S.; Sadeq, M.A.; Shalaby, M.M.M.; Hefnawy, M.T.; Abouzid, M.; et al. Exploring the mechanisms and therapeutic approaches of mitochondrial dysfunction in Alzheimer’s disease: An educational literature review. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, H.; Qu, S. Therapeutic potential of fucoidan in central nervous system disorders: A systematic review. Int. J. Biol. Macromol. 2024, 277, 134397. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Simó, M.; Navarro, X.; Yuste, V.J.; Bruna, J. Autonomic nervous system and cancer. Clin. Auton. Res. 2018, 28, 301–314. [Google Scholar] [CrossRef]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Aqafarini, A.; Lotfi, M.; Norouzi, M.; Karimzadeh, G. Induction of tetraploidy in garden cress: Morphological and cytological changes. Plant Cell Tissue Organ Cult. 2019, 137, 627–635. [Google Scholar] [CrossRef]
- Vaishnavi; Gupta, R.; Choudhary, P. Botanical description of garden cress (Lepidium sativum L.) plant and physical characteristics of its seeds. J. Pharmacogn. Phytochem. 2020, 9, 2424–2428. [Google Scholar]
- Kumar, N.; Singh, S. Nutritional composition, nutraceutical aspects, and medicinal benefits of garden cress (Lepidium sativum) seeds—A geographical and processing perspective. Trends Food Sci. Technol. 2024, 154, 104791. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Oraby, H.F. Chapter 20—Lepidium sativum Seeds: Therapeutic significance and health-promoting potential. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 273–289. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Gupta, N. Chemical composition, antibacterial and antioxidant activities of oils obtained by different extraction methods from Lepidium sativum L. seeds. Ind. Crops Prod. 2020, 156, 112876. [Google Scholar] [CrossRef]
- Tounsi, N.; Djerdjouri, B.; Yahia, O.A.; Belkebir, A. Pro-oxidant versus anti-oxidant effects of seeds aglycone extracts of Lepidium sativum and Eruca vesicaria Linn., in vitro, and on neutrophil nitro-oxidative functions. J. Food Sci. Technol. 2019, 56, 5492–5499. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, M.; Kılıç, S.; Pekmezci, E.; Kartal, M. Evaluating antiinflammatory and antiandrogenic effects of garden cress (Lepidium sativum L.) in HaCaT cells. Rec. Nat. Prod. 2018, 12, 595–601. [Google Scholar] [CrossRef]
- Sharma, R.K.; Vyas, K.; Manda, H. Evaluation on antifungal effect of ethanolic extract of Lepidium sativum seed. Int. J. Phytopharrnacol. 2012, 3, 117–120. [Google Scholar]
- Desai, S.S.; Walvekar, M.V.; Khairmode, S.P. Free radical scavenging activity of Lepidium sativum seed extract in hfd/stz induced diabetes. Int. J. Pharma. Bio. Sci. 2018, 9, 127–132. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; Omar, N.I.; Hasan, E.A.; Ahmed, K.A.; Youssef, A.M. Assessment of the biological activities of Egyptian purslane (Portulaca oleracea) extract after incorporating metal nanoparticles, in vitro and in vivo study. Asian Pac. J. Cancer Prev. 2022, 23, 287–310. [Google Scholar] [CrossRef]
- Odeh, D.M.; Odeh, M.M.; Hafez, T.S.; Hassan, A.S. Bioactive Fused Pyrazoles Inspired by the Adaptability of 5-Aminopyrazole Derivatives: Recent Review. Molecules 2025, 30, 366. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Hassan, A.S.; Morsy, N.M.; Hafez, T.S.; Saleh, F.M.; Hassaneen, H.M. Design, synthesis, molecular prediction and biological evaluation of pyrazole-azomethine conjugates as antimicrobial agents. Synth. Commun. 2021, 51, 1564–1580. [Google Scholar]
- Almehizia, A.A.; Naglah, A.M.; Aljafen, S.S.; Hassan, A.S.; Aboulthana, W.M. Assessment of the In Vitro Biological Activities of Schiff Base-Synthesized Copper Oxide Nanoparticles as an Anti-Diabetic, Anti-Alzheimer, and Anti-Cancer Agent. Pharmaceutics 2025, 17, 180. [Google Scholar] [CrossRef]
- Mukhtar, S.S.; Hassan, A.S.; Morsy, N.M.; Hafez, T.S.; Hassaneen, H.M.; Saleh, F.M. Overview on synthesis, reactions, applications, and biological activities of Schiff bases. Egypt. J. Chem. 2021, 64, 6541–6554. [Google Scholar]
- Hassan, A.S.; Hafez, T.S.; Ali, M.M.; Khatab, T.K. Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 417–429. [Google Scholar]
- Naglah, A.M.; Almehizia, A.A.; Ghazwani, M.; Al-Wasidi, A.S.; Naglah, A.A.; Aboulthana, W.M.; Hassan, A.S. In Vitro Enzymatic and Computational Assessments of Pyrazole–Isatin and Pyrazole–Indole Conjugates as Anti-Diabetic, Anti-Arthritic, and Anti-Inflammatory Agents. Pharmaceutics 2025, 17, 293. [Google Scholar] [CrossRef] [PubMed]
- Iii, V.D.J.; Kumar, I.; Palandurkar, K.; Giri, R.; Giri, K. Lepidium sativum: Bone healer in traditional medicine, an experimental validation study in rats. J. Fam. Med. Prim. Care 2020, 9, 812–818. [Google Scholar] [CrossRef]
- George, V.C.; Kumar, D.R.; Rajkumar, V.; Suresh, P.K.; Kumar, R.A. Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of Annona muricata Linn. in normal and immortalized human cell lines. Asian Pac. J. Cancer Prev. 2012, 13, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. Pharm. Belg. 1994, 49, 462–468. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Alam, A.K. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Chakraborthy, G.S. Free radical scavenging activity of Costus speciosus leaves. Indian J. Pharm. Educ. Res. 2009, 43, 96–98. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wickramaratne, M.N.; Punchihewa, J.; Wickramaratne, D. In-vitro alpha amylase inhibitory activity of the leaf extracts of Adenanthera pavonina. BMC Complement. Altern. Med. 2016, 16, 466. [Google Scholar] [CrossRef]
- Pistia-Brueggeman, G.; Hollingsworth, R.I. A preparation and screening strategy for glycosidase inhibitors. Tetrahedron 2001, 57, 8773–8778. [Google Scholar] [CrossRef]
- Das, S.; Sureshkumar, P. Effect of methanolic root extract of Blepharispermum subsesssile DC in controlling arthritic activity. Res. J. Biotechnol. 2016, 11, 65–74. [Google Scholar]
- Oyedapo, O.O.; Famurewa, A.J. Antiprotease and Membrane Stabilizing Activities of Extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetraptera. Int. J. Pharmacogn. 1995, 33, 65–69. [Google Scholar] [CrossRef]
- Meera, S.; Ramaiah, N.; Kalidindi, N. Illustration of anti-rheumatic mechanism of rheumavedic capsule. Saudi Pharm. J. 2011, 19, 279–284. [Google Scholar] [CrossRef]
- Alaa, A.M.; El-Azab, A.S.; Abou-Zeid, L.A.; ElTahir, K.E.; Abdel-Aziz, N.I.; Ayyad, R.R.; Al-Obaid, A.M. Synthesis, anti-inflammatory, analgesic and COX-1/2 inhibition activities of anilides based on 5, 5-diphenylimidazolidine-2, 4-dione scaffold: Molecular docking studies. Eur. J. Med. Chem. 2016, 115, 121–131. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Li, J.; Liu, H.; Zhang, Y.; Yang, Z.; Liu, W. Design, synthesis, biological evaluation and docking study of novel indole-2-amide as anti-inflammatory agents with dual inhibition of COX and 5-LOX. Eur. J. Med. Chem. 2019, 180, 41–50. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Hassan, A.S.; Mady, M.F.; Awad, H.M.; Hafez, T.S. Synthesis and antitumor activity of some new pyrazolo [1,5-a] pyrimidines. Chin. Chem. Lett. 2017, 28, 388–393. [Google Scholar]
- Hassan, A.S.; Awad, H.M.; Magd-El-Din, A.A.; Hafez, T.S. Synthesis and in vitro antitumor evaluation of novel Schiff bases. Med. Chem. Res. 2018, 27, 915–927. [Google Scholar] [CrossRef]
- Pandey, P.; Khan, F.; Alzahrani, F.A.; Qari, H.A.; Oves, M. A Novel Approach to Unraveling the Apoptotic Potential of Rutin (Bioflavonoid) via Targeting Jab1 in Cervical Cancer Cells. Molecules 2021, 26, 5529. [Google Scholar] [CrossRef] [PubMed]
- Paget, G.E.; Barnes, J.M. Chapter 6—Toxicity tests. In Evaluation of Drug Activities: Pharmacometrics; Laurance, D.R., Bacharach, A.L., Eds.; Academic Press: Cambridge, MA, USA, 1964; Volume 1, pp. 135–166. [Google Scholar] [CrossRef]
- Sorg, D.A.; Buckner, B. A simple method of obtaining venous blood from small laboratory animals. Proc. Soc. Exp. Biol. Med. 1964, 115, 1131–1132. [Google Scholar] [CrossRef]
- Schumann, G.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef]
- Beutler, E.; Duron, O.; Kelly, B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Nishikimi, M.; Appaji, N.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem. Biophys. Res. Comm. 1972, 46, 849–864. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the Quantitive and Qualitative Charecterization of Erythrocyte Glutathione Peroxidase. J. Lab. Clin. Med. 1967, 70, 158–163. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, H.; Novick, D.; Wallach, D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J. Biol. Chem. 1990, 265, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- March, C.J.; Mosley, B.; Larsen, A.; Cerretti, D.P.; Braedt, G.; Price, V.; Gillis, S.; Henney, C.S.; Kronheim, S.R.; Grabstein, K.; et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature 1985, 315, 641–647. [Google Scholar] [CrossRef]
- Babbush, K.M.; Babbush, R.A.; Khachemoune, A. The therapeutic use of antioxidants for melasma. J. Drugs Dermatol. 2020, 19, 788–792. [Google Scholar] [CrossRef]
- Painuli, S.; Quispe, C.; Herrera-Bravo, J.; Semwal, P.; Martorell, M.; Almarhoon, Z.M.; Seilkhan, A.; Ydyrys, A.; Rad, J.S.; Alshehri, M.M.; et al. Nutraceutical Profiling, Bioactive Composition, and Biological Applications of Lepidium sativum L. Oxid. Med. Cell Longev. 2022, 2022, 2910411. [Google Scholar] [CrossRef]
- Chatoui, K.; Harhar, H.; El Kamli, T.; Tabyaoui, M. Chemical composition and antioxidant capacity of Lepidium sativum seeds from four regions of Morocco. Evid. Based Complement. Alternat. Med. 2020, 2020, 7302727. [Google Scholar] [CrossRef]
- Kumar, V.; Tomar, V.; Ranade, S.A.; Yadav, H.; Srivastava, M. Phytochemical, antioxidant investigations and fatty acid composition of Lepidium sativum seeds. J. Environ. Biol. 2020, 41, 59–65. [Google Scholar] [CrossRef]
- Kassem, I.A.; Farghaly, A.A.; Ghaly, N.S.; Hassan, Z.M.; Nabil, M. Composition and genoprotective effect of the flavonoidal content of Lepidium sativum L. methanolic seed extract against cyclophosphamide-induced DNA damage in mice. Pharmacog. J. 2020, 12, 1728–1734. [Google Scholar] [CrossRef]
- Baregama, C.; Shringi, M.; Goyal, A.; Gupta, K. TLC Solvent System Development, HPTLC and GC-MS Analysis of Methanolic Extract of Lepidium Sativum Linn Seeds. J. Pharm. Negat. Results 2022, 13, 3473–3485. [Google Scholar]
- Hassan, A.S.; Aboulthana, W.M. Synthesis, In Vitro Biological Investigation, and In Silico Analysis of Pyrazole-Based Derivatives as Multi-target Agents. Egypt. J. Chem. 2023, 66, 441–455. [Google Scholar] [CrossRef]
- Solaiman, M.M. Agglutination effect of selected medicinal plant leaf crude extracts on ABO blood group. Am. J. Plant Biol. 2021, 6, 11–18. [Google Scholar] [CrossRef]
- Attia, E.S.; Amer, A.H.; Hasanein, M.A. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat. Prod. Res. 2019, 33, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Ouattar, H.; Zouirech, O.; Kara, M.; Assouguem, A.; Almutairi, S.M.; Al-Hemaid, F.M.; Rasheed, R.A.; Ullah, R.; Abbasi, A.M.; Aouane, M.; et al. In Vitro Study of the Phytochemical Composition and Antioxidant, Immunostimulant, and Hemolytic Activities of Nigella sativa (Ranunculaceae) and Lepidium sativum Seeds. Molecules 2022, 27, 5946. [Google Scholar] [CrossRef]
- Goyzueta-Mamani, L.D.; Ccahuana, H.L.B.; Chávez-Fumagalli, M.A.; Alvarez, K.L.; Aguilar-Pineda, J.A.; Vera-Lopez, K.J.; Cardenas, C.L.L. In Silico Analysis of Metabolites from Peruvian Native Plants as Potential Therapeutics against Alzheimer’s Disease. Molecules 2022, 27, 918. [Google Scholar] [CrossRef]
- López, A.F.F.; Martínez, O.M.M.; Hernández, H.F.C. Evaluation of Amaryllidaceae alkaloids as inhibitors of human acetylcholinesterase by QSAR analysis and molecular docking. J. Mol. Struct. 2021, 1225, 129142. [Google Scholar] [CrossRef]
- Abbas-Mohammadi, M.; Farimani, M.M.; Salehia, P.; Ebrahimi, S.N.; Sonboli, A.; Kelso, C.; Skropeta, D. Acetylcholinesterase-inhibitory activity of Iranian plants: Combined HPLC/bioassay-guided fractionation, molecular networking and docking strategies for the dereplication of active compounds. J. Pharm. Biomed. Anal. 2018, 158, 471–479. [Google Scholar]
- El-Shamarka, M.E.A.; Aboulthana, W.M.; Omar, N.I.; Mahfouz, M.M. Evaluation of the biological efficiency of Terminalia chebula fruit extract against neurochemical changes induced in brain of diabetic rats: An epigenetic study. Inflammopharmacology 2024, 32, 1439–1460. [Google Scholar] [CrossRef]
- Niño, J.; Hernández, J.A.; Correa, Y.M.; Mosquera, O.M. In vitro inhibition of acetylcholinesterase by crude plant extracts from Colombian flora. Mem. Inst. Oswaldo Cruz 2006, 101, 783–785. [Google Scholar] [CrossRef]
- Balkis, A.; Tran, K.; Lee, Y.Z.; Ng, K. Screening flavonoids for inhibition of acetylcholinesterase identified baicalein as the most potent inhibitor. J. Agric. Sci. 2015, 7, 26–35. [Google Scholar] [CrossRef]
- Kozieł, K.; Urbanska, E.M. Kynurenine Pathway in Diabetes Mellitus-Novel Pharmacological Target? Cells 2023, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Naglah, A.M.; Almehizia, A.A.; Al-Wasidi, A.S.; Alharbi, A.S.; Alqarni, M.H.; Hassan, A.S.; Aboulthana, W.M. Exploring the Potential Biological Activities of Pyrazole-Based Schiff Bases as Anti-Diabetic, Anti-Alzheimer’s, Anti-Inflammatory, and Cytotoxic Agents: In Vitro Studies with Computational Predictions. Pharmaceuticals 2024, 17, 655. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.F.; Aboulthana, W.M.; Sherief, M.A.; El-Bassyouni, G.T.; Mousa, S.M. Synthesis, structural, molecular docking, and in vitro biological activities of Cu-doped ZnO nanomaterials. Sci. Rep. 2024, 14, 9027. [Google Scholar]
- Aboulthana, W.M.K.; Refaat, E.; Khaled, S.E.; Ibrahim, N.E.; Youssef, A.M. Metabolite Profiling and Biological Activity Assessment of Casuarina equisetifolia Bark after Incorporating Gold Nanoparticles. Asian Pac. J. Cancer Prev. 2022, 23, 3457–3471. [Google Scholar] [CrossRef] [PubMed]
- Abdelazeem, N.M.; Aboulthana, W.M.; Hassan, A.S.; Almehizia, A.A.; Naglah, A.M.; Alkahtani, H.M. Synthesis, in silico ADMET prediction analysis, and pharmacological evaluation of sulfonamide derivatives tethered with pyrazole or pyridine as anti-diabetic and anti-Alzheimer’s agents. Saudi Pharm. J. 2024, 32, 102025. [Google Scholar] [CrossRef]
- Raj, R.; Thomas, S.; Gorantla, V. Accelerated atherosclerosis in rheumatoid arthritis: A systematic review. F1000Research 2023, 11, 466. [Google Scholar] [CrossRef]
- Alkahtani, H.M.; Almehizia, A.A.; Al-Omar, M.A.; Obaidullah, A.J.; Zen, A.A.; Hassan, A.S.; Aboulthana, W.M. In Vitro Evaluation and Bioinformatics Analysis of Schiff Bases Bearing Pyrazole Scaffold as Bioactive Agents: Antioxidant, Anti-Diabetic, Anti-Alzheimer, and Anti-Arthritic. Molecules 2023, 28, 7125. [Google Scholar] [CrossRef]
- Almehizia, A.A.; Aboulthana, W.M.; Naglah, A.M.; Hassan, A.S. In vitro biological studies and computational prediction-based analyses of pyrazolo [1,5-a]pyrimidine derivatives. RSC Adv. 2024, 14, 8397–8408. [Google Scholar]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein-polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Chu, Q.; Bao, B.; Wu, W. Mechanism of interaction between phenolic compounds and proteins based on non-covalent and covalent interactions. Med. Res. 2018, 2, 180014. [Google Scholar]
- Sinha, S.K.; Prasad, S.K.; Islam, M.A.; Gurav, S.S.; Patil, R.B.; AlFaris, N.A.; Aldayel, T.S.; AlKehayez, N.M.; Wabaidur, S.M.; Shakya, A. Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and non-structural protein-15: A pharmacoinformatics study. J. Biomol. Struct. Dyn. 2021, 39, 4686–4700. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, N.; Shukla, A.; Makhal, P.N.; Kaki, V.R. Natural product-driven dual COX-LOX inhibitors: Overview of recent studies on the development of novel anti-inflammatory agents. Heliyon 2023, 9, e14569. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, A.A.; Tadros, M.I.; El-Refaie, W.M. Improving the efficacy of Cyclooxegenase-2 inhibitors in the management of oral cancer: Insights into the implementation of nanotechnology and mucoadhesion. J. Drug Deliv. Sci. Technol. 2021, 61, 102240. [Google Scholar] [CrossRef]
- Ali, D.E.; Gedaily, R.A.E.; Ezzat, S.M.; El Sawy, M.A.; Meselhy, M.R.; Abdel-Sattar, E. In silico and in vitro anti-inflammatory study of phenolic compounds isolated from Eucalyptus maculata resin. Sci. Rep. 2023, 13, 2093. [Google Scholar] [CrossRef]
- El-Feky, A.M.; El-Rashedy, A.A. Sterols and flavonoids in strawberry calyx with free radical scavenging, anti-inflammatory, and molecular dynamic study. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 108. [Google Scholar] [CrossRef]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef]
- Cruz, S.; Gomes, S.E.; Borralho, P.M.; Rodrigues, C.M.P.; Gaudêncio, S.P.; Pereira, F. In Silico HCT116 Human Colon Cancer Cell-Based Models En Route to the Discovery of Lead-Like Anticancer Drugs. Biomolecules 2018, 8, 56. [Google Scholar] [CrossRef]
- ElNaker, N.A.; Yousef, A.F.; Yousef, L.F. A review of Arthrocnemum (Arthrocaulon) macrostachyum chemical content and bioactivity. Phytochem. Rev. 2020, 19, 1427–1448. [Google Scholar] [CrossRef]
- Huang, C.; Lu, C.K.; Tu, M.C.; Chang, J.H.; Chen, Y.J.; Tu, Y.H.; Huang, H.C. Polyphenol-rich Avicennia marina leaf extracts induce apoptosis in human breast and liver cancer cells and in a nude mouse xenograft model. Oncotarget 2016, 7, 35874–35893. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, H.; Ma, L.; Liu, A. Polysaccharides from the peels of Citrus aurantifolia induces apoptosis in transplanted H22 cells in mice. Int. J. Biol. Macromol. 2017, 101, 680–689. [Google Scholar] [CrossRef]
- Roy, A.; Bharadvaja, N. Effect of different culture media on shoot multiplication and stigmasterol content in accessions of Centella asiatica. Int. J. Ayurvedic Herb. Med. 2017, 7, 2643–2650. [Google Scholar] [CrossRef]
- Tong, Q.Y.; He, Y.; Zhao, Q.B.; Qing, Y.; Huang, W.; Wu, X.H. Cytotoxicity and apoptosis-inducing effect of steroidal saponins from Dioscorea zingiberensis wright against cancer cells. Steroids 2012, 77, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bai, H.; Zhang, X.; Liu, J.; Cao, P.; Liao, N. Inhibitory effect of oleanolic acid on hepatocellular carcinoma via ERK-p53-mediated cell cycle arrest and mitochondrial-dependent apoptosis. Carcinogenesis 2013, 34, 1323–1330. [Google Scholar] [CrossRef]
- Tion, M.T.; Fotina, H.; Saganuwan, S.A. Phytochemical screening, proximate analysis, median lethal dose (LD50), hematological and biochemical effects of various extracts of Abrus precatorius seeds in Mus musculus. J. Adv. Vet. Anim. Res. 2018, 5, 354–360. [Google Scholar]
- Chalasani, N.; Aljadhey, H.; Kesterson, J.; Murray, M.D.; Hall, S.D. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004, 126, 1287–1292. [Google Scholar] [CrossRef]
- Luka, J.; Mbaya, A.W.; Biu, A.A.; Nwosu, C.O. The effect of crude ethanolic extract of Diospyros mespiliformis (Ebenaceae) on the clinicopathological parameters of Yankasa sheep experimentally infected with Haemonchus contortus. Comp. Clin. Pathol. 2015, 24, 445–452. [Google Scholar] [CrossRef]
- Hazarika, I.; Geetha, K.M.; Sundari, P.S.; Madhu, D. Acute oral toxicity evaluation of extracts of Hydrocotyle sibthorpioides in wister albino rats as per OECD 425 TG. Toxicol. Rep. 2019, 6, 321–328. [Google Scholar] [CrossRef]
- Ebbo, A.A.; Sani, D.; Suleiman, M.M.; Ahmad, A.; Hassan, A.Z. Acute and sub-chronic toxicity evaluation of the crude methanolic extract of Diospyros mespiliformis hochst ex a. Dc (ebenaceae) and its fractions. Toxicol. Rep. 2020, 7, 1138–1144. [Google Scholar] [CrossRef]
- Nafiu, M.; Akanji, M.A.; Yakubu, M.T. Effect of aqueous extract of Cochlospermum planchonii rhizome on some kidney and liver functional indicies of albino rats. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 22–26. [Google Scholar] [CrossRef]
- Singh, C.; Prakash, C.; Tiwari, K.N.; Mishra, S.K.; Kumar, V. Premna integrifolia ameliorates cyclophosphamide-induced hepatotoxicity by modulation of oxidative stress and apoptosis. Biomed. Pharmacother. 2018, 107, 634–643. [Google Scholar] [CrossRef]
- Yang, W.; Shao, F.; Wang, J.; Shen, T.; Zhao, Y.; Fu, X.; Zhang, L.; Li, H. Ethyl Acetate Extract from Artemisia argyi Prevents Liver Damage in ConA-Induced Immunological Liver Injury Mice via Bax/Bcl-2 and TLR4/MyD88/NF-κB Signaling Pathways. Molecules 2022, 27, 7883. [Google Scholar] [CrossRef] [PubMed]
- Seif, M.M.; Madboli, A.; Marrez, D.A.; Aboulthana, W.M.K. Hepato-Renal protective Effects of Egyptian Purslane Extract against Experimental Cadmium Toxicity in Rats with Special Emphasis on the Functional and Histopathological Changes. Toxicol. Rep. 2019, 6, 625–631. [Google Scholar] [PubMed]
- Aboulthana, W.M.; Youssef, A.M.; Seif, M.M.; Osman, N.M.; Sahu, R.K.; Ismael, M.; El-Baz, H.A.; Omar, N.I. Comparative Study between Croton tiglium Seeds and Moringa oleifera Leaves Extracts, after Incorporating Silver Nanoparticles, on Murine Brains. Egypt. J. Chem. 2021, 64, 1709–1731. [Google Scholar]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar]
- Kumar, A.; P, N.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; K, S.; et al. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Jin, Y.; Hu, D.; Chen, Q.; Shi, C.; Ye, J.; Dai, Z.; Lu, Y. Water-based green and sustainable extraction protocols for value-added compounds from natural resources. Curr. Opin. Green Sustain. Chem. 2023, 40, 100757. [Google Scholar] [CrossRef]


| Extracts | Major Phyto-Constituents | Antioxidant Activities | |||
|---|---|---|---|---|---|
| Total Phenolic Content (mg Gallic Acid Equivalent/100 g) | Total Condensed Tannins (μg/mL) | Total Flavonoid (mg Quercetin Equivalent/100 g) | TAC (mg Gallic Acid/g) | IRP (µg/mL) | |
| Methanolic | 173.27 ± 1.19 | 77.01 ± 0.53 | 44.01 ± 0.30 | 303.23 ± 2.08 | 295.48 ± 2.08 |
| Aqueous | 138.62 ± 0.95 | 61.61 ± 0.42 | 35.20 ± 0.24 | 242.58 ± 1.66 | 234.83 ± 1.66 |
| Acetone | 80.59 ± 0.55 | 35.82 ± 0.25 | 20.47 ± 0.14 | 141.04 ± 0.97 | 133.29 ± 0.97 |
| Ethyl Acetate | 50.41 ± 0.34 | 22.40 ± 0.15 | 12.80 ± 0.09 | 88.21 ± 0.60 | 80.46 ± 0.60 |
| STD(Ascorbic Acid) | - | - | - | 84.50 ± 0.13 | 76.75 ± 0.13 |
| Extracts | Inhibition (%) | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|---|
| DPPH | ABTS | NO | DPPH | ABTS | NO | |
| Methanolic | 53.31 ± 0.36 | 57.06 ± 0.36 | 47.56 ± 0.36 | 5.32 ± 0.02 | 4.68 ± 0.03 | 7.03 ± 0.04 |
| Aqueous | 42.65 ± 0.29 | 46.40 ± 0.29 | 36.90 ± 0.29 | 6.69 ± 0.06 | 5.75 ± 0.03 | 9.07 ± 0.05 |
| Acetone | 24.80 ± 0.17 | 28.55 ± 0.17 | 19.05 ± 0.17 | 11.54 ± 0.04 | 9.42 ± 0.06 | 17.56± 0.12 |
| Ethyl Acetate | 15.51 ± 0.11 | 19.26 ± 0.11 | 9.76 ± 0.11 | 18.25 ± 0.07 | 13.88 ± 0.06 | 34.28 ± 0.30 |
| STD (Ascorbic Acid) | 66.50 ± 0.11 | 70.25 ± 0.11 | 60.75 ± 0.11 | 4.29 ± 0.03 | 3.80 ± 0.04 | 5.51 ± 0.02 |
| Extracts | Anti-Alzheimer Activity | Anti-Diabetic Activity | ||||
|---|---|---|---|---|---|---|
| AChE | α-Amylase | α-Glucosidase | ||||
| Inhibition (%) | IC50 (µg/mL | Inhibition (%) | IC50 (µg/mL | Inhibition (%) | IC50 (µg/mL) | |
| Methanolic | 51.34 ± 0.35 | 5.35 ± 0.04 | 54.35 ± 0.35 | 4.24 ± 0.03 | 44.10 ± 0.35 | 2.87 ± 0.05 |
| Aqueous | 41.07 ± 0.28 | 6.68 ± 0.05 | 44.19 ± 0.28 | 5.22 ± 0.04 | 33.94 ± 0.28 | 3.73 ± 0.06 |
| Acetone | 23.88 ± 0.16 | 11.50 ± 0.09 | 27.19 ± 0.16 | 8.48 ± 0.06 | 16.94 ± 0.16 | 7.47 ± 0.14 |
| Ethyl Acetate | 14.94 ± 0.10 | 18.38 ± 0.14 | 18.34 ± 0.10 | 12.57 ± 0.09 | 8.09 ± 0.10 | 15.64 ± 0.33 |
| STD | Donepezil | Acarbose | ||||
| 65.15 ± 0.12 | 4.21 ± 0.02 | 66.90 ± 0.10 | 3.45 ± 0.03 | 56.65 ± 0.10 | 2.23 ± 0.02 | |
| Activities | STD | Methanolic | Aqueous | Acetone | Ethyl Acetate | ||
|---|---|---|---|---|---|---|---|
| Anti-arthritic activity | Protein Denaturation (%) | 53.52 ± 0.08 (Diclofenac Sodium) | 43.48 ± 0.28 | 35.35 ± 0.22 | 21.75 ± 0.13 | 14.67 ± 0.08 | |
| Proteinase | (%) | 50.82 ± 0.08 (Diclofenac Sodium) | 40.78 ± 0.28 | 32.65 ± 0.22 | 19.05 ± 0.13 | 11.97 ± 0.08 | |
| IC50 (µg/mL) | 6.29 ± 0.02 (Diclofenac Sodium) | 7.84 ± 0.04 | 9.79 ± 0.05 | 16.77 ± 0.08 | 26.68 ± 0.13 | ||
| Anti-inflammatory activity | COX-1 | (%) | 68.61 ± 0.11 (Indomethacin) | 55.05 ± 0.38 | 44.08 ± 0.30 | 25.72 ± 0.17 | 16.17 ± 0.11 |
| IC50 (µg/mL) | 5.72 ± 0.02 (Indomethacin) | 7.13 ± 0.08 | 8.90 ± 0.10 | 15.26 ± 0.18 | 24.28 ± 0.28 | ||
| COX-2 | (%) | 70.86 ± 0.11 (Indomethacin) | 57.30 ± 0.38 | 46.33 ± 0.30 | 27.97 ± 0.17 | 18.42 ± 0.11 | |
| IC50 (µg/mL) | 4.26 ± 0.03 (Indomethacin) | 5.27 ± 0.04 | 6.52 ± 0.05 | 10.79 ± 0.08 | 16.39 ± 0.11 | ||
| 5-LOX | (%) | 55.71 ± 0.11 (Zileuton) | 50.15 ± 0.38 | 39.18 ± 0.30 | 20.82 ± 0.17 | 11.27 ± 0.11 | |
| IC50 (µg/mL) | 7.21 ± 0.03 (Zileuton) | 8.01 ± 0.07 | 10.26 ± 0.09 | 19.31 ± 0.18 | 27.35 ± 0.05 | ||
| Extracts | The Median Inhibitory Concentrations (IC50) | |||||
|---|---|---|---|---|---|---|
| HepG-2 | Caco-2 | A549 | ||||
| Caspase-3 (pg/mL) | Bcl-2 (ng/mL) | Caspase-3 (pg/mL) | Bcl-2 (ng/mL) | Caspase-3 (pg/mL) | Bcl-2 (ng/mL) | |
| DMSO | 108.79 ± 0.85 | 11.15 ± 0.15 | 94.81 ± 0.14 | 5.74 ± 0.08 | 88.67 ± 0.12 | 12.58 ± 0.05 |
| Methanolic | 310.05 ± 2.42 | 4.95 ± 0.07 | 270.21 ± 0.41 | 2.55 ± 0.04 | 252.72 ± 0.34 | 5.59 ± 0.02 |
| Aqueous | 212.14 ± 1.66 | 6.76 ± 0.09 | 222.80 ± 0.34 | 3.28 ± 0.05 | 208.38 ± 0.28 | 7.19 ± 0.03 |
| Acetone | 201.26 ± 1.57 | 8.92 ± 0.12 | 175.40 ± 0.26 | 4.59 ± 0.06 | 164.05 ± 0.22 | 10.06 ± 0.04 |
| Ethyl Acetate | 157.75 ± 1.23 | 10.62 ± 0.14 | 137.47 ± 0.21 | 5.47 ± 0.08 | 128.58 ± 0.17 | 11.98 ± 0.04 |
| STD (Doxorubicin) | 418.84 ± 3.27 | 2.90 ± 0.04 | 365.02 ± 0.55 | 1.49 ± 0.02 | 341.39 ± 0.46 | 3.27 ± 0.01 |
| C. | Methanolic | Aqueous | Acetone | Ethyl Acetate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 900 (mg/kg) | 450 (mg/kg) | 833.33 (mg/kg) | 416.67 (mg/kg) | 700 (mg/kg) | 350 (mg/kg) | 562.50 (mg/kg) | 281.25 (mg/kg) | |||
| Formed Elements | RBCs (106/µL) | 7.38 ± 0.02 | 7.39 ± 0.02 | 7.37 ± 0.02 | 7.38 ± 0.02 | 7.40 ± 0.02 | 7.41 ± 0.02 | 7.43 ± 0.02 | 5.62 ± 0.02 a | 6.46 ± 0.02 ab |
| HB (g/dL) | 16.24 ± 0.06 | 16.27 ± 0.06 | 16.22 ± 0.06 | 16.25 ± 0.06 | 16.29 ± 0.06 | 16.32 ± 0.06 | 16.35 ± 0.06 | 12.36 ± 0.05 a | 14.22 ± 0.06 ab | |
| HCT (%) | 45.07 ± 0.05 | 45.16 ± 0.05 | 45.02 ± 0.05 | 45.11 ± 0.05 | 45.20 ± 0.05 | 45.28 ± 0.05 | 45.37 ± 0.05 | 34.31 ± 0.04 a | 39.46 ± 0.04 ab | |
| PLT (103/µL) | 489.27 ± 1.60 | 490.24 ± 1.60 | 488.68 ± 1.60 | 489.64 ± 1.60 | 490.60 ± 1.60 | 491.57 ± 1.61 | 492.54 ± 1.61 | 372.43 ± 1.22 a | 428.30 ± 1.40 ab | |
| WBCs (103/µL) | 9.38 ± 0.01 | 9.39 ± 0.01 | 9.36 ± 0.01 | 9.38 ± 0.01 | 9.40 ± 0.01 | 9.42 ± 0.01 | 9.44 ± 0.01 | 7.14 ± 0.01 a | 8.21 ± 0.01 ab | |
| Differential Count | Lymp. (103/µL) | 8.13 ± 0.01 | 8.15 ± 0.01 | 8.12 ± 0.01 | 8.14 ± 0.01 | 8.15 ± 0.01 | 8.17 ± 0.01 | 8.18 ± 0.01 | 6.19 ± 0.01 a | 7.12 ± 0.01 ab |
| Mono. (103/µL) | 0.74 ± 0.01 | 0.74 ± 0.01 | 0.74 ± 0.01 | 0.74 ± 0.01 | 0.74 ± 0.01 | 0.74 ± 0.01 | 0.74 ± 0.01 | 0.56 ± 0.01 a | 0.65 ± 0.01 ab | |
| Gran. (103/µL) | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.50 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.38 ± 0.01 a | 0.44 ± 0.01 ab | |
| C. | Methanolic | Aqueous | Acetone | Ethyl Acetate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 900 (mg/kg) | 450 (mg/kg) | 833.33 (mg/kg) | 416.67 (mg/kg) | 700 (mg/kg) | 350 (mg/kg) | 562.50 (mg/kg) | 281.25 (mg/kg) | |||
| Liver | ALT (U/L) | 43.55 ± 0.01 | 43.64 ± 0.01 | 43.50 ± 0.01 | 43.58 ± 0.01 | 43.67 ± 0.01 | 43.76 ± 0.01 | 43.84 ± 0.01 | 63.02 ± 0.02 a | 54.80 ± 0.01 ab |
| AST (U/L) | 63.16 ± 0.01 | 63.28 ± 0.01 | 63.08 ± 0.01 | 63.20 ± 0.01 | 63.33 ± 0.01 | 63.45 ± 0.01 | 63.58 ± 0.01 | 91.39 ± 0.01 a | 79.47 ± 0.01 ab | |
| ALP (U/L) | 97.23 ± 0.01 | 97.42 ± 0.01 | 97.11 ± 0.01 | 97.30 ± 0.01 | 97.50 ± 0.01 | 97.69 ± 0.01 | 97.88 ± 0.01 | 140.70 ± 0.02 a | 122.35 ± 0.01 ab | |
| GGT (U/L) | 24.51 ± 0.01 | 24.56 ± 0.01 | 24.48 ± 0.01 | 24.53 ± 0.01 | 24.58 ± 0.01 | 24.63 ± 0.01 | 24.67 ± 0.01 | 35.47 ± 0.01 a | 30.84 ± 0.01 ab | |
| Kidney | Urea (mg/dL) | 38.45 ± 0.02 | 38.52 ± 0.02 | 38.40 ± 0.02 | 38.48 ± 0.02 | 38.55 ± 0.02 | 38.63 ± 0.02 | 38.70 ± 0.02 | 55.64 ± 0.03 a | 48.38 ± 0.02 ab |
| Creat. (mg/dL) | 1.26 ± 0.01 | 1.26 ± 0.01 | 1.26 ± 0.01 | 1.26 ± 0.01 | 1.26 ± 0.01 | 1.27 ± 0.01 | 1.27 ± 0.01 | 1.82 ± 0.01 a | 1.59 ± 0.01 ab | |
| BUN (mg/dL) | 8.79 ± 0.03 | 8.81 ± 0.03 | 8.78 ± 0.03 | 8.80 ± 0.03 | 8.82 ± 0.03 | 8.84 ± 0.03 | 8.85 ± 0.03 | 12.73 ± 0.04 a | 11.07 ± 0.03 ab | |
| T. Protein (g/dL) | 8.30 ± 0.01 | 8.32 ± 0.01 | 8.29 ± 0.01 | 8.31 ± 0.01 | 8.32 ± 0.01 | 8.34 ± 0.01 | 8.36 ± 0.01 | 5.35 ± 0.01 a | 6.69 ± 0.01 ab | |
| Albumin (g/dL) | 3.96 ± 0.01 | 3.96 ± 0.01 | 3.95 ± 0.01 | 3.96 ± 0.01 | 3.97 ± 0.01 | 3.98 ± 0.01 | 3.98 ± 0.01 | 2.55 ± 0.01 a | 3.19 ± 0.01 ab | |
| Heart | CK (U/L) | 70.56 ± 0.03 | 70.70 ± 0.03 | 70.48 ± 0.03 | 70.62 ± 0.03 | 70.76 ± 0.03 | 70.90 ± 0.03 | 71.03 ± 0.03 | 102.11 ± 0.04 a | 88.79 ± 0.03 ab |
| LDH (U/L) | 225.12 ± 0.08 | 225.56 ± 0.08 | 224.84 ± 0.08 | 225.29 ± 0.08 | 225.73 ± 0.08 | 226.17 ± 0.08 | 226.62 ± 0.08 | 325.77 ± 0.12 a | 283.27 ± 0.10 ab | |
| Lipid | TC (mg/dL) | 89.81 ± 0.02 | 89.99 ± 0.02 | 89.70 ± 0.02 | 89.88 ± 0.02 | 90.05 ± 0.02 | 90.23 ± 0.02 | 90.41 ± 0.02 | 129.96 ± 0.03 a | 113.01 ± 0.02 ab |
| T.Gs (mg/dL) | 89.82 ± 0.01 | 90.00 ± 0.01 | 89.71 ± 0.01 | 89.89 ± 0.01 | 90.06 ± 0.01 | 90.24 ± 0.01 | 90.42 ± 0.01 | 129.98 ± 0.02 a | 113.02 ± 0.02 ab | |
| HDL-c (mg/dL) | 18.75 ± 0.02 | 18.79 ± 0.02 | 18.73 ± 0.02 | 18.77 ± 0.02 | 18.80 ± 0.02 | 18.84 ± 0.02 | 18.88 ± 0.02 | 9.14 ± 0.01 a | 13.60 ± 0.02 ab | |
| LDL-c (mg/dL) | 53.09 ± 0.02 | 53.20 ± 0.02 | 53.03 ± 0.02 | 53.13 ± 0.02 | 53.24 ± 0.02 | 53.34 ± 0.02 | 53.45 ± 0.02 | 76.83 ± 0.03 a | 66.81 ± 0.03 ab | |
| C. | Methanolic | Aqueous | Acetone | Ethyl Acetate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 900 (mg/kg) | 450 (mg/kg) | 833.33 (mg/kg) | 416.67 (mg/kg) | 700 (mg/kg) | 350 (mg/kg) | 562.50 (mg/kg) | 281.25 (mg/kg) | |||
| Liver | TAC (µmol/g) | 10.35 ± 0.01 | 10.37 ± 0.01 | 10.39 ± 0.01 | 10.41 ± 0.01 | 10.43 ± 0.01 | 10.45 ± 0.01 | 10.47 ± 0.01 | 6.70 ± 0.01 a | 8.38 ± 0.01 ab |
| GSH (mg/g tissue) | 144.51 ± 0.06 | 144.79 ± 0.06 | 145.08 ± 0.06 | 145.37 ± 0.06 | 145.65 ± 0.06 | 145.94 ± 0.06 | 146.23 ± 0.06 | 93.58 ± 0.04 a | 116.98 ± 0.05 ab | |
| SOD (IU/g tissue) | 54.95 ± 0.01 | 55.06 ± 0.01 | 55.17 ± 0.01 | 55.28 ± 0.01 | 55.39 ± 0.01 | 55.50 ± 0.01 | 55.61 ± 0.01 | 35.59 ± 0.01 a | 44.49 ± 0.01 ab | |
| CAT (IU/g tissue) | 94.35 ± 0.02 | 94.54 ± 0.02 | 94.73 ± 0.02 | 94.91 ± 0.02 | 95.10 ± 0.02 | 95.29 ± 0.02 | 95.47 ± 0.02 | 61.10 ± 0.01 a | 76.38 ± 0.02 ab | |
| GPx (IU/g tissue) | 73.84 ± 0.02 | 73.98 ± 0.02 | 74.13 ± 0.02 | 74.28 ± 0.02 | 74.42 ± 0.02 | 74.57 ± 0.02 | 74.72 ± 0.02 | 47.82 ± 0.01 a | 59.77 ± 0.02 ab | |
| Kidney | TAC (µmol/g) | 9.20 ± 0.01 | 9.22 ± 0.01 | 9.24 ± 0.01 | 9.26 ± 0.01 | 9.28 ± 0.01 | 9.30 ± 0.01 | 9.31 ± 0.01 | 9.35 ± 0.01 | 9.33 ± 0.01 |
| GSH (mg/g tissue) | 128.30 ± 0.06 | 128.55 ± 0.06 | 128.81 ± 0.06 | 129.06 ± 0.06 | 129.31 ± 0.06 | 129.57 ± 0.06 | 129.82 ± 0.06 | 130.34 ± 0.06 | 130.08 ± 0.06 | |
| SOD (IU/g tissue) | 48.80 ± 0.01 | 48.90 ± 0.01 | 48.99 ± 0.01 | 49.09 ± 0.01 | 49.19 ± 0.01 | 49.29 ± 0.01 | 49.38 ± 0.01 | 49.58 ± 0.01 | 49.48 ± 0.01 | |
| CAT (IU/g tissue) | 83.78 ± 0.02 | 83.94 ± 0.02 | 84.11 ± 0.02 | 84.27 ± 0.02 | 84.44 ± 0.02 | 84.61 ± 0.02 | 84.77 ± 0.02 | 85.11 ± 0.02 | 84.94 ± 0.02 | |
| GPx (IU/g tissue) | 65.57 ± 0.02 | 65.69 ± 0.02 | 65.82 ± 0.02 | 65.95 ± 0.02 | 66.08 ± 0.02 | 66.21 ± 0.02 | 66.34 ± 0.02 | 66.61 ± 0.02 | 66.48 ± 0.02 | |
| Spleen | TAC (µmol/g) | 8.95 ± 0.01 | 8.97 ± 0.01 | 8.99 ± 0.01 | 9.01 ± 0.01 | 9.03 ± 0.01 | 9.04 ± 0.01 | 9.06 ± 0.01 | 5.80 ± 0.01 a | 7.25 ± 0.01 ab |
| GSH (mg/g tissue) | 124.76 ± 0.05 | 125.01 ± 0.05 | 125.25 ± 0.05 | 125.50 ± 0.05 | 125.75 ± 0.06 | 125.99 ± 0.06 | 126.24 ± 0.06 | 80.80 ± 0.04 a | 100.99 ± 0.04 ab | |
| SOD (IU/g tissue) | 47.46 ± 0.01 | 47.55 ± 0.01 | 47.65 ± 0.01 | 47.74 ± 0.01 | 47.83 ± 0.01 | 47.93 ± 0.01 | 48.02 ± 0.01 | 30.73 ± 0.01 a | 38.42 ± 0.01 ab | |
| CAT (IU/g tissue) | 81.47 ± 0.02 | 81.63 ± 0.02 | 81.79 ± 0.02 | 81.95 ± 0.02 | 82.11 ± 0.02 | 82.27 ± 0.02 | 82.44 ± 0.02 | 52.76 ± 0.01 a | 65.95 ± 0.01 ab | |
| GPx (IU/g tissue) | 63.76 ± 0.02 | 63.89 ± 0.02 | 64.01 ± 0.02 | 64.14 ± 0.02 | 64.26 ± 0.02 | 64.39 ± 0.02 | 64.52 ± 0.02 | 41.29 ± 0.01 a | 51.61 ± 0.01 ab | |
| C. | Methanolic | Aqueous | Acetone | Ethyl Acetate | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 900 (mg/kg) | 450 (mg/kg) | 833.33 (mg/kg) | 416.67 (mg/kg) | 700 (mg/kg) | 350 (mg/kg) | 562.50 (mg/kg) | 281.25 (mg/kg) | |||
| Liver | TNF-α(pg/g tissue) | 215.24 ± 0.72 | 215.66 ± 0.73 | 216.09 ± 0.73 | 216.51 ± 0.73 | 216.94 ± 0.73 | 217.37 ± 0.73 | 217.80 ± 0.73 | 340.31 ± 1.14 a | 272.24 ± 0.92 ab |
| IL-6 (pg/g tissue) | 256.50 ± 0.51 | 257.01 ± 0.51 | 257.51 ± 0.51 | 258.02 ± 0.51 | 258.53 ± 0.51 | 259.04 ± 0.51 | 259.55 ± 0.51 | 405.54 ± 0.80 a | 324.43 ± 0.64 ab | |
| AChE (ng/g tissue) | 1.27 ± 0.01 | 1.27 ± 0.01 | 1.27 ± 0.01 | 1.27 ± 0.01 | 1.28 ± 0.01 | 1.28 ± 0.01 | 1.28 ± 0.01 | 2.00 ± 0.01 a | 1.60 ± 0.01 ab | |
| Kidney | TNF-α (pg/g tissue) | 190.04 ± 0.28 | 190.41 ± 0.28 | 190.79 ± 0.28 | 191.16 ± 0.28 | 191.54 ± 0.28 | 191.92 ± 0.28 | 192.30 ± 0.29 | 193.05 ± 0.29 | 192.67 ± 0.29 |
| IL-6 (pg/g tissue) | 228.90 ± 0.45 | 229.35 ± 0.45 | 229.81 ± 0.45 | 230.26 ± 0.45 | 230.71 ± 0.46 | 231.17 ± 0.46 | 231.62 ± 0.46 | 232.54 ± 0.46 | 232.08 ± 0.46 | |
| AChE (ng/g tissue) | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.89 ± 0.01 | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.90 ± 0.01 | 0.90 ± 0.01 | |
| Spleen | TNF-α (pg/g tissue) | 83.78 ± 0.47 | 83.95 ± 0.47 | 84.11 ± 0.47 | 84.28 ± 0.47 | 84.44 ± 0.47 | 84.61 ± 0.47 | 84.78 ± 0.47 | 132.46 ± 0.74 a | 105.97 ± 0.59 ab |
| IL-6 (pg/g tissue) | 99.08 ± 0.17 | 99.27 ± 0.17 | 99.47 ± 0.17 | 99.66 ± 0.18 | 99.86 ± 0.18 | 100.06 ± 0.18 | 100.25 ± 0.18 | 156.65 ± 0.28 a | 125.32 ± 0.22 ab | |
| AChE (ng/g tissue) | 6.25 ± 0.01 | 6.26 ± 0.01 | 6.27 ± 0.01 | 6.29 ± 0.01 | 6.30 ± 0.01 | 6.31 ± 0.01 | 6.32 ± 0.01 | 9.88 ± 0.01 a | 7.90 ± 0.01 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naglah, A.M.; Almehizia, A.A.; Al-Omar, M.A.; Al-Wasidi, A.S.; Mohamed, M.H.; Alsobeai, S.M.; Hassan, A.S.; Aboulthana, W.M. Investigations of In Vitro Anti-Acetylcholinesterase, Anti-Diabetic, Anti-Inflammatory, and Anti-Cancer Efficacy of Garden Cress (Lepidium sativum Linn.) Seed Extracts, as Well as In Vivo Biochemical and Hematological Assays. Pharmaceutics 2025, 17, 446. https://doi.org/10.3390/pharmaceutics17040446
Naglah AM, Almehizia AA, Al-Omar MA, Al-Wasidi AS, Mohamed MH, Alsobeai SM, Hassan AS, Aboulthana WM. Investigations of In Vitro Anti-Acetylcholinesterase, Anti-Diabetic, Anti-Inflammatory, and Anti-Cancer Efficacy of Garden Cress (Lepidium sativum Linn.) Seed Extracts, as Well as In Vivo Biochemical and Hematological Assays. Pharmaceutics. 2025; 17(4):446. https://doi.org/10.3390/pharmaceutics17040446
Chicago/Turabian StyleNaglah, Ahmed M., Abdulrahman A. Almehizia, Mohamed A. Al-Omar, Asma S. Al-Wasidi, Mayada H. Mohamed, Sanad M. Alsobeai, Ashraf S. Hassan, and Wael M. Aboulthana. 2025. "Investigations of In Vitro Anti-Acetylcholinesterase, Anti-Diabetic, Anti-Inflammatory, and Anti-Cancer Efficacy of Garden Cress (Lepidium sativum Linn.) Seed Extracts, as Well as In Vivo Biochemical and Hematological Assays" Pharmaceutics 17, no. 4: 446. https://doi.org/10.3390/pharmaceutics17040446
APA StyleNaglah, A. M., Almehizia, A. A., Al-Omar, M. A., Al-Wasidi, A. S., Mohamed, M. H., Alsobeai, S. M., Hassan, A. S., & Aboulthana, W. M. (2025). Investigations of In Vitro Anti-Acetylcholinesterase, Anti-Diabetic, Anti-Inflammatory, and Anti-Cancer Efficacy of Garden Cress (Lepidium sativum Linn.) Seed Extracts, as Well as In Vivo Biochemical and Hematological Assays. Pharmaceutics, 17(4), 446. https://doi.org/10.3390/pharmaceutics17040446








