Nanoparticle-Based Dry Powder Inhaler Containing Ciprofloxacin for Enhanced Targeted Antibacterial Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of the NS by Wet Milling
2.3. DPI Formulations by Spray Drying
2.4. Preparation of the Physical Mixtures
2.5. Determination of the Drug Content
2.6. Laser Diffraction-Based Particle Size Measurement
2.7. Nanoparticle Tracking Analysis of the NS
2.8. Investigation of the Morphology of the Nanocrystals and the DPIs
2.9. Analysis of the Crystalline Structure
2.10. Thermoanalytical Measurement
2.11. Fourier-Transform Infrared Spectroscopy Investigation
2.12. Solubility Test
2.13. In Vitro Dissolution Test of the DPIs
2.14. In Vitro Aerodynamic Characterization
2.15. Aerodynamic Particle Size Analysis Using the Spraytec® Device
3. Results
3.1. Characterization of the NS
3.1.1. Results of the Laser Diffraction-Based Particle Size Distribution of the NS
3.1.2. Outcomes of the Nanoparticle Tracking Analysis of the NS
3.1.3. Morphology Results of the Dried NS
3.2. Characterization of the Dry Powder Inhaler Formulation
3.2.1. Outcomes of the Laser Diffraction-Based Particle Size Measurement
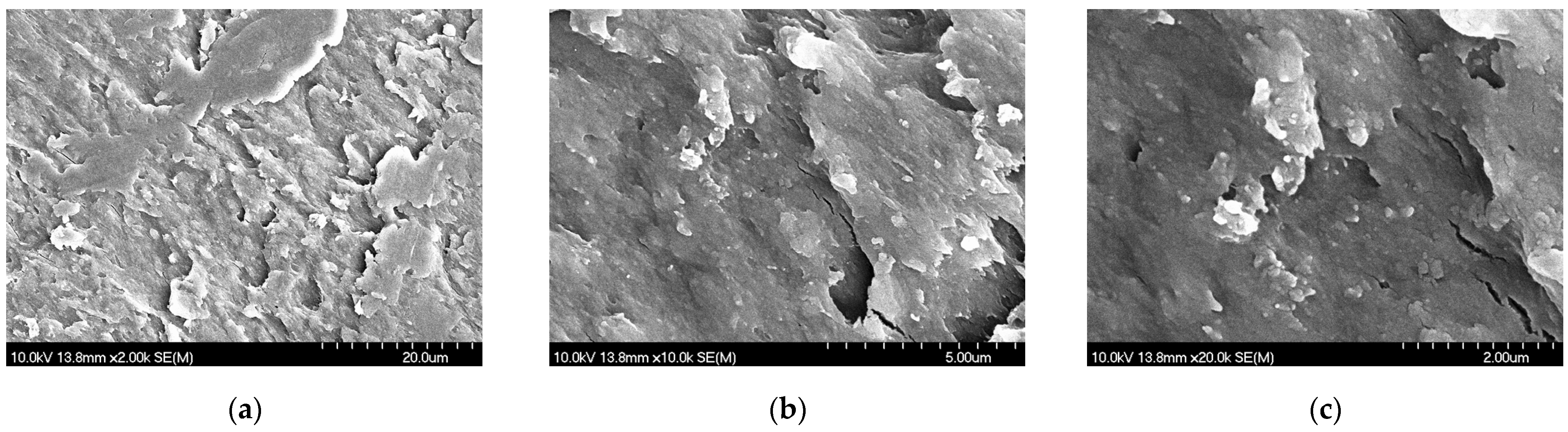
3.2.2. Findings of the Morphology Investigation
3.2.3. Characteristics of the Crystalline Structure
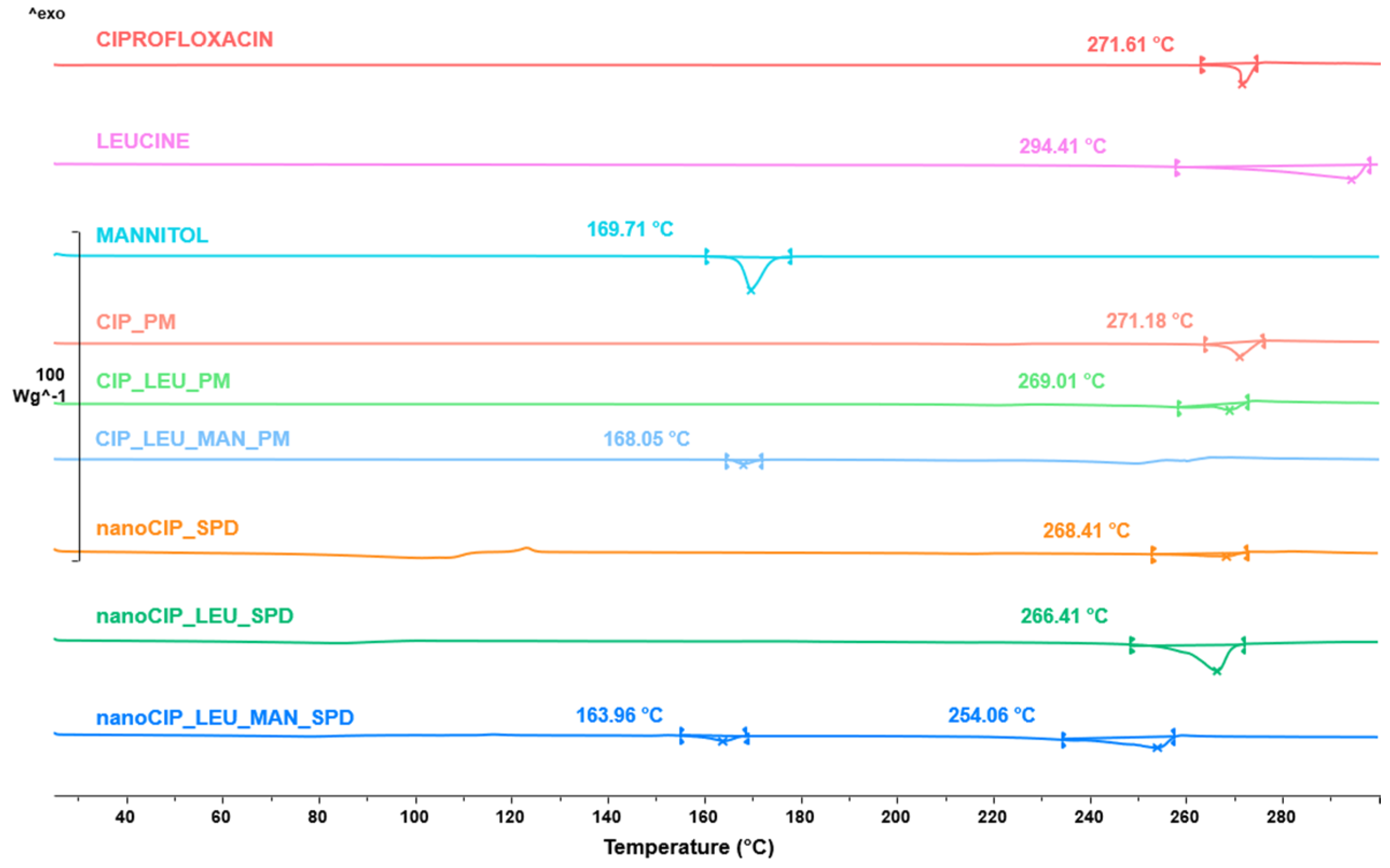
3.2.4. Results of the Thermoanalytical Measurement
3.2.5. Results of the FTIR Analysis
3.2.6. Effects of the Formulation on the Solubility
3.2.7. Results of the In Vitro Dissolution Test
3.2.8. Outcomes of the In Vitro Aerodynamic Characterization
3.2.9. Findings of the Aerodynamic Particle Size Analysis Using the Spraytec® Device
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gigliobianco, M.R.; Casadidio, C.; Censi, R.; Di Martino, P. Nanocrystals of Poorly Soluble Drugs: Drug Bioavailability and Physicochemical Stability. Pharmaceutics 2018, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, S. Nanotechnology Drug Delivery Market Size, Share, and Trends 2025 to 2034. Available online: https://www.precedenceresearch.com/nanotechnology-drug-delivery-market (accessed on 1 February 2025).
- Potočnik, J. Comission Recommendation of 18 October 2011 on the Definition of Nanomaterial. Off. J. Eur. Union 2011. [Google Scholar]
- U.S. Food and Drug Administration Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Available online: https://www.fda.gov/media/88423/download (accessed on 10 January 2025).
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for Oral and Parenteral Drug Delivery: A Perspective on Formulating Poorly-Water Soluble Compounds Using Wet Media Milling Technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Dahan, A.; Miller, J.M.; Amidon, G.L. Prediction of Solubility and Permeability Class Membership: Provisional BCS Classification of the World ’ s Top Oral Drugs. AAPS J. 2009, 11, 740–746. [Google Scholar] [CrossRef]
- Singhal, M.; Baumgartner, A.; Turunen, E.; van Veen, B.; Hirvonen, J.; Peltonen, L. Nanosuspensions of a Poorly Soluble Investigational Molecule ODM-106: Impact of Milling Bead Diameter and Stabilizer Concentration. Int. J. Pharm. 2020, 587, 119636. [Google Scholar] [CrossRef]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical Nanocrystals: Production by Wet Milling and Applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Fernández-García, R.; Fraguas-Sánchez, A.I. Nanomedicines for Pulmonary Drug Delivery: Overcoming Barriers in the Treatment of Respiratory Infections and Lung Cancer. Pharmaceutics 2024, 16, 1584. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guan, J.; Qin, L.; Zhang, X.; Mao, S. Physicochemical Properties Affecting the Fate of Nanoparticles in Pulmonary Drug Delivery. Drug Discov. Today 2020, 25, 150–159. [Google Scholar] [CrossRef]
- Thorley, A.J.; Tetley, T.D. New Perspectives in Nanomedicine. Pharmacol. Ther. 2013, 140, 176–185. [Google Scholar] [CrossRef]
- Ruge, C.C.; Kirch, J.; Lehr, C.M. Pulmonary Drug Delivery: From Generating Aerosols to Overcoming Biological Barriers-Therapeutic Possibilities and Technological Challenges. Lancet Respir. Med. 2013, 1, 402–413. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Wang, W.; Huang, Z.; Huang, Y.; Zhang, X.; Huang, J.; Cui, Y.; Yue, X.; Ma, C.; Fu, F.; Wang, W.; et al. Pulmonary Delivery Nanomedicines towards Circumventing Physiological Barriers: Strategies and Characterization Approaches. Adv. Drug Deliv. Rev. 2022, 185, 114309. [Google Scholar] [CrossRef]
- Donahue, N.D.; Acar, H.; Wilhelm, S. Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Deliv. Rev. 2019, 143, 68–96. [Google Scholar] [CrossRef]
- Torge, A.; Grützmacher, P.; Mücklich, F.; Schneider, M. The Influence of Mannitol on Morphology and Disintegration of Spray-Dried Nano-Embedded Microparticles. Eur. J. Pharm. Sci. 2017, 104, 171–179. [Google Scholar] [CrossRef]
- Bakand, S.; Hayes, A. Toxicological Considerations, Toxicity Assessment, and Risk Management of Inhaled Nanoparticles. Int. J. Mol. Sci. 2016, 17, 929. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Bakand, S. Toxicological Perspectives of Inhaled Therapeutics and Nanoparticles. Expert Opin. Drug Metab. Toxicol. 2014, 10, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Kole, E.; Jadhav, K.; Shirsath, N.; Dudhe, P.; Verma, R.K.; Chatterjee, A.; Naik, J. Nanotherapeutics for Pulmonary Drug Delivery: An Emerging Approach to Overcome Respiratory Diseases. J. Drug Deliv. Sci. Technol. 2023, 81, 104261. [Google Scholar] [CrossRef]
- Rusch, M.; Spielmeyer, A.; Zorn, H.; Hamscher, G. Biotransformation of Ciprofloxacin by Xylaria Longipes: Structure Elucidation and Residual Antibacterial Activity of Metabolites. Appl. Microbiol. Biotechnol. 2018, 102, 8573–8584. [Google Scholar] [CrossRef]
- Bush, N.G.; Diez-santos, I.; Abbott, L.R.; Maxwell, A. Quinolones: Mechanism, Lethality and Their Contributions to Antibiotic Resistance. Molecules 2020, 25, 5662. [Google Scholar] [CrossRef]
- Sharma, P.C.; Jain, A.; Jain, S.; Pahwa, R.; Yar, M.S. Ciprofloxacin: Review on Developments in Synthetic, Analytical, and Medicinal Aspects. J. Enzyme Inhib. Med. Chem. 2010, 25, 577–589. [Google Scholar] [CrossRef]
- Stass, H.; Weimann, B.; Nagelschmitz, J.; Rolinck-Werninghaus, C.; Staab, D. Tolerability and Pharmacokinetic Properties of Ciprofloxacin Dry Powder for Inhalation in Patients with Cystic Fibrosis: A Phase I, Randomized, Dose-Escalation Study. Clin. Ther. 2013, 35, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.E.; Manzo, R.H.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B. Biowaiver Monographs for Immediate Release Solid Oral Dosage Forms: Ciprofloxacin Hydrochloride. J. Pharm. Sci. 2011, 100, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Mcshane, P.J.; Weers, G.; Tarara, T.E.; Haynes, A.; Durbha, P.; Miller, D.P.; Mundry, T.; Operschall, E.; Elborn, J.S. Pulmonary Pharmacology & Therapeutics Cipro Fl Oxacin Dry Powder for Inhalation (Ciprofloxacin DPI): Technical Design and Features of an e Ffi Cient Drug—Device Combination. Pulm. Pharmacol. Ther. 2018, 50, 72–79. [Google Scholar] [CrossRef]
- Stass, H.; Nagelschmitz, J.; Willmann, S.; Delesen, H.; Gupta, A.; Baumann, S. Inhalation of a Dry Powder Ciprofloxacin Formulation in Healthy Subjects: A Phase I Study. Clin. Drug Investig. 2013, 33, 419–427. [Google Scholar] [CrossRef]
- Chin, W.W.L.; Parmentier, J.; Widzinski, M.; Tan, E.N.H.; Gokhale, R. A Brief Literature and Patent Review of Nanosuspensions to a Final Drug Product. J. Pharm. Sci. 2014, 103, 2980–2999. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Ambrus, R. Investigation of Physico-Chemical Stability and Aerodynamic Properties of Novel “Nano-in-Micro” Structured Dry Powder Inhaler System. Micromachines 2023, 14, 1348. [Google Scholar] [CrossRef]
- Scherließ, R.; Bock, S.; Bungert, N.; Neustock, A.; Valentin, L. Particle Engineering in Dry Powders for Inhalation. Eur. J. Pharm. Sci. 2022, 172, 106158. [Google Scholar] [CrossRef]
- Lechanteur, A.; Evrard, B. Influence of Composition and Spray-Drying Process Parameters on Carrier-Free DPI Properties and Behaviors in the Lung: A Review. Pharmaceutics 2020, 12, 55. [Google Scholar] [CrossRef]
- Cao, J.; Xu, Y.; Zhang, J.; Fang, T.; Wu, F.; Zhen, Y.; Yu, X.; Liu, Y.; Li, J.; Wang, D. “Nano-in-Micro” Structured Dry Powder Inhalers for Pulmonary Delivery: Advances and Challenges. J. Drug Deliv. Sci. Technol. 2024, 96, 105648. [Google Scholar] [CrossRef]
- Panthi, V.K.; Fairfull-smith, K.E.; Islam, N. Ciprofloxacin-Loaded Inhalable Formulations against Lower Respiratory Tract Infections: Challenges, Recent Advances, and Future Perspectives. Pharmaceutics 2024, 16, 648. [Google Scholar] [CrossRef]
- Feng, X.; Shi, Y.; Zhang, Y.; Lei, F.; Ren, R.; Tang, X. Opportunities and Challenges for Inhalable Nanomedicine Formulations in Respiratory Diseases: A Review. Int. J. Nanomed. 2024, 19, 1509–1538. [Google Scholar]
- Karimi, K.; Pallagi, E.; Szabó-Révész, P.; Csóka, I.; Ambrus, R. Development of a Microparticle-Based Dry Powder Inhalation Formulation of Ciprofloxacin Hydrochloride Applying the Quality by Design Approach. Drug Des. Dev. Ther. 2016, 10, 3331–3343. [Google Scholar] [CrossRef]
- Benke, E.; Winter, C.; Szabó-Révész, P.; Roblegg, E.; Ambrus, R. The Effect of Ethanol on the Habit and in Vitro Aerodynamic Results of Dry Powder Inhalation Formulations Containing Ciprofloxacin Hydrochloride. Asian J. Pharm. Sci. 2021, 16, 471–482. [Google Scholar] [CrossRef]
- Party, P.; Kókai, D.; Burián, K.; Nagy, A.; Hopp, B.; Ambrus, R. Development of Extra-Fine Particles Containing Nanosized Meloxicam for Deep Pulmonary Delivery: In Vitro Aerodynamic and Cell Line Measurements. Eur. J. Pharm. Sci. 2022, 176, 106247. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Klement, M.L.; Révész, P.S.; Ambrus, R. Preparation and Characterization of Ibuprofen Containing Nano—Embedded—Microparticles for Pulmonary Delivery. Pharmaceutics 2023, 15, 545. [Google Scholar] [CrossRef]
- Türeli, N.G.; Torge, A.; Juntke, J.; Schwarz, B.C.; Türeli, A.E.; Lehr, C.; Schneider, M. Ciprofloxacin-Loaded PLGA Nanoparticles against Cystic Fibrosis P. Aeruginosa Lung Infections. Eur. J. Pharm. Biopharm. 2017, 117, 363–371. [Google Scholar] [CrossRef]
- Ma, Y.; Cong, Z.; Wang, Y.; Gao, P. A Novel Multi-Drugs Ciprofloxacin-Curcumin-N-Acetylcysteine Co-Delivery System Based on Hybrid Nanocrystals for Dry Powder Inhalations. Next Nanotechnol. 2024, 6, 100084. [Google Scholar] [CrossRef]
- Guan, W.; Liu, Y.; Ding, S.; Wang, Y. Ciprofloxacin Nanocrystals and N—Acetylcysteine Co—Solidified Powders for Pulmonary Drug Delivery: Development and in Vitro and in Vivo Characterization. J. Nanoparticle Res. 2022, 24, 41. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Xue, L.; Guan, W.; Wang, Y. Pulmonary Multidrug Codelivery of Curcumin Nanosuspensions and Ciprofloxacin with N-Acetylcysteine for Lung Infection Therapy. J. Drug Deliv. Sci. Technol. 2023, 84, 104474. [Google Scholar] [CrossRef]
- Dillen, K.; Vandervoort, J.; Van Den Mooter, G.; Ludwig, A. Evaluation of Ciprofloxacin-Loaded Eudragit ® RS100 or RL100/PLGA Nanoparticles. Int. J. Pharm. 2006, 314, 72–82. [Google Scholar] [CrossRef]
- El-Gendy, N.; Desai, V.; Berkland, C. Agglomerates of Ciprofloxacin Nanoparticles Yield Fine Dry Powder Aerosols. J. Pharm. Innov. 2010, 5, 79–87. [Google Scholar] [CrossRef]
- Arnold, M.M.; Gorman, E.M.; Schieber, L.J.; Munson, E.J.; Berkland, C. NanoCipro Encapsulation in Monodisperse Large Porous PLGA Microparticles. J. Control Release 2007, 121, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Bera, H.; Wang, H.; Cun, D.; Feng, Y. Inhalable Ciprofloxacin/Polymyxin B Dry Powders in Respiratory Infection Therapy Inhalable Ciprofloxacin/Polymyxin B Dry Powders in Respiratory Infection Therapy. Acta Mater. Medica 2023, 2, 142–156. [Google Scholar] [CrossRef]
- Małgorzata, W.; Ostolska, I.; Szewczuk-Karpisz, K.; Chibowski, S.; Terpiłowski, K.; Gun’ko, V.I.; Zarko, V.I. Investigation of the Polyvinyl Alcohol Stabilization Mechanism and Adsorption Properties on the Surface. J. Nanoparticle Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Bartos, C.; Jójárt-Laczkovich, O.; Katona, G.; Budai-Szűcs, M.; Ambrus, R.; Bocsik, A.; Gróf, I.; Deli, M.A.; Szabó-Révész, P. Optimization of a Combined Wet Milling Process in Order to Produce Poly(Vinyl Alcohol) Stabilized Nanosuspension. Drug Des. Dev. Ther. 2018, 12, 1567–1580. [Google Scholar] [CrossRef]
- Wang, Y.; Kho, K.; Cheow, W.S.; Hadinoto, K. A Comparison between Spray Drying and Spray Freeze Drying for Dry Powder Inhaler Formulation of Drug-Loaded Lipid-Polymer Hybrid Nanoparticles. Int. J. Pharm. 2012, 424, 98–106. [Google Scholar] [CrossRef]
- Chvatal, A.; Alzhrani, R.; Tiwari, A.K.; Ambrus, R.; Szabó-Révész, P.; Boddu, S.H.S. Cytotoxicity of Inhalable Dry Powders in A549 Human Lung Cancer Cell Line. Farmacia 2018, 66, 172–175. [Google Scholar]
- Yue-xing, C.; Fei-fei, Y.; Han, W.; Tao-tao, F.; Chun-yu, L.; Li-hui, Q.; Yong-hong, L. The Effect of L-Leucine on the Stabilization and Inhalability of Spray-Dried Solid Lipid Nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 46, 474–481. [Google Scholar] [CrossRef]
- Chvatal, A.; Ambrus, R.; Party, P.; Katona, G.; Jójárt-Laczkovich, O.; Szabó-Révész, P.; Fattal, E.; Tsapis, N. Formulation and Comparison of Spray Dried Non-Porous and Large Porous Particles Containing Meloxicam for Pulmonary Drug Delivery. Int. J. Pharm. 2019, 559, 68–75. [Google Scholar] [CrossRef]
- Ordoubadi, M.; Shepard, K.B.; Wang, H.; Wang, Z.; Pluntze, A.M.; Churchman, J.P.; Vehring, R. On the Physical Stability of Leucine-Containing Spray-Dried Powders for Respiratory Drug Delivery. Pharmaceutics 2023, 15, 435. [Google Scholar] [CrossRef]
- Sibum, I.; Hagedoorn, P.; Kluitman, M.P.G.; Kloezen, M.; Frijlink, H.W.; Grasmeijer, F. Dispersibility and Storage Stability Optimization of High Dose Isoniazid Dry Powder Inhalation Formulations with L-Leucine or Trileucine. Pharmaceutics 2020, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Ke, W.; Yoon, R.; Chang, K.; Chan, H. Engineering the Right Formulation for Enhanced Drug Delivery. Adv. Drug Deliv. Rev. 2022, 191, 114561. [Google Scholar] [CrossRef] [PubMed]
- Party, P.; Piszman, Z.I.; Farkas, Á.; Ambrus, R. Comprehensive In Vitro and In Silico Aerodynamic Analysis of High-Dose Ibuprofen- and Mannitol-Containing Dry Powder Inhalers for the Treatment of Cystic Fibrosis. Pharmaceutics 2024, 16, 1465. [Google Scholar] [CrossRef]
- Hertel, N.; Birk, G.; Scherließ, R. Performance Tuning of Particle Engineered Mannitol in Dry Powder Inhalation Formulations. Int. J. Pharm. 2020, 586, 119592. [Google Scholar] [CrossRef] [PubMed]
- Bronchitol. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/bronchitol (accessed on 10 February 2025).
- Shetty, N.; Cipolla, D.; Park, H.; Zhou, Q.T. Physical Stability of Dry Powder Inhaler Formulations. Expert Opin. Drug Deliv. 2020, 17, 77–96. [Google Scholar] [CrossRef]
- Bartos, C. Optimization of a Combined Wet Milling Process to Produce Nanosuspension and Its Transformation into Surfactant-Free Solid Compositions to Increase the Product Stability and Drug Bioavailability. Ph.D. Thesis, University of Szeged, Szeged, Hungary, 2019. [Google Scholar]
- Benke, E.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. Stability Test of Novel Combined Formulated Dry Powder Inhalation System Containing Antibiotic: Physical Characterization and In Vitro–In Silico Lung Deposition Results. Drug Dev. Ind. Pharm. 2019, 45, 1369–1378. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Parlati, C. Respirable Microparticles of Aminoglycoside Antibiotics for Pulmonary Administration. Ph.D. Thesis, University of Parma, Parma, Italy, 2008. [Google Scholar]
- 2.9.3. Dissolution Test for Solid Dosage Forms. In European Pharmacopoeia 11.0; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2024; pp. 348–355.
- Fröhlich, E.; Mercuri, A.; Wu, S.; Salar-Behzadi, S. Measurements of Deposition, Lung Surface Area and Lung Fluid for Simulation of Inhaled Compounds. Front. Pharmacol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- 2.9.18. Preparation for Inhalation: Aerodynamic Assessment of Fine Particles. In European Pharmacopoeia 11.0; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2024; pp. 369–382.
- Levet, V.; Rosière, R.; Merlos, R.; Fusaro, L.; Berger, G.; Amighi, K.; Wauthoz, N. Development of Controlled-Release Cisplatin Dry Powders for Inhalation against Lung Cancers. Int. J. Pharm. 2016, 515, 209–220. [Google Scholar] [CrossRef]
- Dubey, R. Impact of Nanosuspension Technology on Drug Discovery and Development. Drug Deliv. Technol. 2006, 5, 67–71. [Google Scholar]
- Hou, J.; Ci, H.; Wang, P.; Wang, C.; Lv, B.; Miao, L.; You, G. Nanoparticle Tracking Analysis versus Dynamic Light Scattering: Case Study on the Effect of Ca2+ and Alginate on the Aggregation of Cerium Oxide Nanoparticles. J. Hazard. Mater. 2018, 360, 319–328. [Google Scholar] [CrossRef]
- Maguire, C.M.; Sillence, K.; Roesslein, M.; Hannell, C.; Suarez, G.; Sauvain, J.J.; Capracotta, S.; Contal, S.; Cambier, S.; El Yamani, N.; et al. Benchmark of Nanoparticle Tracking Analysis on Measuring Nanoparticle Sizing and Concentration. J. Micro Nano-Manuf. 2017, 5, 041002. [Google Scholar] [CrossRef]
- Chvatal, A.; Farkas, Á.; Balásházy, I.; Szabó-Révész, P.; Ambrus, R. Aerodynamic Properties and in Silico Deposition of Meloxicam Potassium Incorporated in a Carrier-Free DPI Pulmonary System. Int J Pharm 2017, 520, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Pallagi, E.; Karimi, K.; Ambrus, R.; Szabó-Révész, P.; Csóka, I. New Aspects of Developing a Dry Powder Inhalation Formulation Applying the Quality-by-Design Approach. Int. J. Pharm. 2016, 511, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Das, S.C.; Khadka, P.; Shah, R.; McGill, S.; Smyth, H.D.C. Nanomedicine in Pulmonary Delivery; Elsevier Inc.: Amsterdam, The Netherlands, 2021; ISBN 9780128204665. [Google Scholar]
- Karimi, K.; Katona, G.; Csóka, I.; Ambrus, R. Physicochemical Stability and Aerosolization Performance of Dry Powder Inhalation System Containing Ciprofloxacin Hydrochloride. J. Pharm. Biomed. Anal. 2018, 148, 73–79. [Google Scholar] [CrossRef]
- Böhm, B.H.L.; Müller, R.H. Lab-Scale Production Unit Design for Nanosuspensions of Sparingly Soluble Cytotoxic Drugs. Pharm. Sci. Technol. Today 1999, 2, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Cirz, R.T.; Neill, B.M.O.; Hammond, J.A.; Head, S.R.; Romesberg, F.E. Defining the Pseudomonas Aeruginosa SOS Response and Its Role in the Global Response to the Antibiotic Ciprofloxacin. J. Bacteriol. 2006, 188, 7101–7110. [Google Scholar] [CrossRef]
- Chalkley, L.J.; Koornhof, H.J. Antimicrobial Activity of Ciprofloxacin against Pseudomonas Aeruginosa, Escherichia Coli, and Staphylococcus Aureus Determined by the Killing Curve Method: Antibiotic Comparisons and Synergistic Interactions. Antimicrob. Agents Chemother. 1985, 28, 331–342. [Google Scholar]
- Phase, A.I.; Stass, H.; Staab, D. Safety and Pharmacokinetics of Ciprofloxacin Dry Powder for Inhalation in Cystic Fibrosis: A Phase I, Randomized, Single-Dose, Dose-Escalation Study Heino. J. Aerosol Med. Pulm. Drug Deliv. 2014, 27, 1–10. [Google Scholar] [CrossRef]
- Sharif, S.; Muneer, S.; Izake, E.L.; Islam, N. Impact of Leucine and Magnesium Stearate on the Physicochemical Properties and Aerosolization Behavior of Wet Milled Inhalable Ibuprofen Microparticles for Developing Dry Powder Inhaler Formulation. Pharmaceutics 2023, 15, 674. [Google Scholar] [CrossRef]
- Chapman, K.R.; Fogarty, C.M.; Peckitt, C.; Lassen, C. Delivery Characteristics and Patients ’ Handling of Two Single-Dose Dry-Powder Inhalers Used in COPD. Int. J. COPD 2011, 6, 353–363. [Google Scholar]
- Huang, Y.; Tang, H.; Meng, X.; Liu, D.; Liu, Y.; Chen, B. Highly Drug-Loaded Nanoaggregate Microparticles for Pulmonary Delivery of Cyclosporin A Highly Drug-Loaded Nanoaggregate Microparticles for Pulmonary Delivery of Cyclosporin A. Int. J. Nanomed. 2024, 19, 7529–7546. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.; Mahboubi, A.; Kobarfard, F.; Vatanara, A.; Mortazavi, S.A. Optimization of a Dry Powder Inhaler of Ciprofloxacin-Loaded Polymeric Nanomicelles by Spray Drying Process. Pharm. Dev. Technol. 2019, 24, 584–592. [Google Scholar] [CrossRef]
- Sabuj, M.Z.R.; Rashid, M.A.; Dargaville, T.R.; Islam, N. Stability of Inhaled Ciprofloxacin-Loaded Poly(2-Ethyl-2-Oxazoline) Nanoparticle Dry Powder Inhaler Formulation in High Stressed Conditions. Pharmaceuticals 2022, 15, 1223. [Google Scholar] [CrossRef] [PubMed]
- Adi, H.; Young, P.M.; Chan, H.K.; Agus, H.; Traini, D. Co-Spray-Dried Mannitol-Ciprofloxacin Dry Powder Inhaler Formulation for Cystic Fibrosis and Chronic Obstructive Pulmonary Disease. Eur. J. Pharm. Sci. 2010, 40, 239–247. [Google Scholar] [CrossRef]
- Razuc, M.; Piña, J.; Ramírez-Rigo, M.V. Optimization of Ciprofloxacin Hydrochloride Spray-Dried Microparticles for Pulmonary Delivery Using Design of Experiments. AAPS PharmSciTech 2018, 19, 3085–3096. [Google Scholar] [CrossRef]
- Cayli, Y.A.; Sahin, S.; Buttini, F.; Balducci, A.G.; Montanari, S.; Vural, I.; Oner, L.; Akdag, Y.; Sahin, S.; Buttini, F.; et al. Dry Powders for the Inhalation of Ciprofloxacin or Levofloxacin Combined with a Mucolytic Agent for Cystic Fibrosis Patients. Drug Dev. Ind. Pharm. 2017, 43, 1378–1389. [Google Scholar] [CrossRef]






| Sample Name | CIP (g) | PVA (g) | LEU (g) | MAN (g) | API Content (%) |
|---|---|---|---|---|---|
| nanoCIP_SPD | 2.00 | 0.90 | 0.00 | 0.00 | 66.04 ± 4.37 |
| nanoCIP_LEU_SPD | 2.00 | 0.90 | 0.40 | 0.00 | 40.11 ± 5.85 |
| nanoCIP_LEU_MAN_SPD | 2.00 | 0.90 | 0.40 | 0.25 | 31.94 ± 1.73 |
| CIP_PM | 2.00 | 0.90 | 0.00 | 0.00 | 68.97 |
| CIP_LEU_PM | 2.00 | 0.90 | 0.40 | 0.00 | 60.61 |
| CIP_LEU_MAN_PM | 2.00 | 0.90 | 0.40 | 0.25 | 56.34 |
| Sample Name | D10 (µm) | D50 (µm) | D90 (µm) | Span | SSA (m2/g) |
|---|---|---|---|---|---|
| nanoCIP_SPD | 2.63 ± 0.20 | 5.66 ± 0.31 | 15.46 ± 0.50 | 2.27 ± 0.07 | 1.23 ± 0.03 |
| nanoCIP_LEU_SPD | 2.50 ± 0.05 | 4.86 ± 0.17 | 9.41 ± 0.64 | 1.42 ± 0.08 | 1.40 ± 0.04 |
| nanoCIP_LEU_MAN_SPD | 2.25 ± 0.12 | 4.59 ± 0.09 | 9.18 ± 0.44 | 1.51 ± 0.10 | 1.52 ± 0.06 |
| Sample Name | MMAD (µm) | FPF by Size (%) | FPF by Stage (%) | EF (%) | GSD |
|---|---|---|---|---|---|
| nanoCIP_LEU_SPD | 3.25 ± 0.11 | 36.49 ± 4.89 | 41.85 ± 5.96 | 83.55 ± 0.01 | 1.99 ± 0.03 |
| nanoCIP_LEU_MAN_SPD | 3.71 ± 0.03 | 41.43 ± 2.88 | 49.56 ± 3.11 | 86.65 ± 0.08 | 1.78 ± 0.08 |
| Sample Name | D10 (µm) | D50 (µm) | D90 (µm) | Span | SSA (m2/g) |
|---|---|---|---|---|---|
| nanoCIP_LEU_SPD | 2.59 ± 0.05 | 4.91 ± 0.03 | 9.06 ± 0.06 | 1.32 ± 0.03 | 1.38 ± 0.01 |
| nanoCIP_LEU_MAN_SPD | 2.59 ± 0.04 | 5.22 ± 0.16 | 10.39 ± 0.56 | 1.49 ± 0.05 | 1.32 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Party, P.; Klement, M.L.; Gaudio, B.M.; Sorrenti, M.; Ambrus, R. Nanoparticle-Based Dry Powder Inhaler Containing Ciprofloxacin for Enhanced Targeted Antibacterial Therapy. Pharmaceutics 2025, 17, 486. https://doi.org/10.3390/pharmaceutics17040486
Party P, Klement ML, Gaudio BM, Sorrenti M, Ambrus R. Nanoparticle-Based Dry Powder Inhaler Containing Ciprofloxacin for Enhanced Targeted Antibacterial Therapy. Pharmaceutics. 2025; 17(4):486. https://doi.org/10.3390/pharmaceutics17040486
Chicago/Turabian StyleParty, Petra, Márk László Klement, Bianca Maria Gaudio, Milena Sorrenti, and Rita Ambrus. 2025. "Nanoparticle-Based Dry Powder Inhaler Containing Ciprofloxacin for Enhanced Targeted Antibacterial Therapy" Pharmaceutics 17, no. 4: 486. https://doi.org/10.3390/pharmaceutics17040486
APA StyleParty, P., Klement, M. L., Gaudio, B. M., Sorrenti, M., & Ambrus, R. (2025). Nanoparticle-Based Dry Powder Inhaler Containing Ciprofloxacin for Enhanced Targeted Antibacterial Therapy. Pharmaceutics, 17(4), 486. https://doi.org/10.3390/pharmaceutics17040486









