Alectinib-Loaded Chitosan–Alginate Nanoparticles: A Novel Synthesis Method with In Vitro and In Vivo Evaluations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. UV–Vis Spectroscopy of Alectinib, CANPs, and ACANPs
2.3. Development and Validation of HPLC Method for Alectinib Determination in Formulation
2.3.1. Chromatographic Conditions
2.3.2. Method Validation
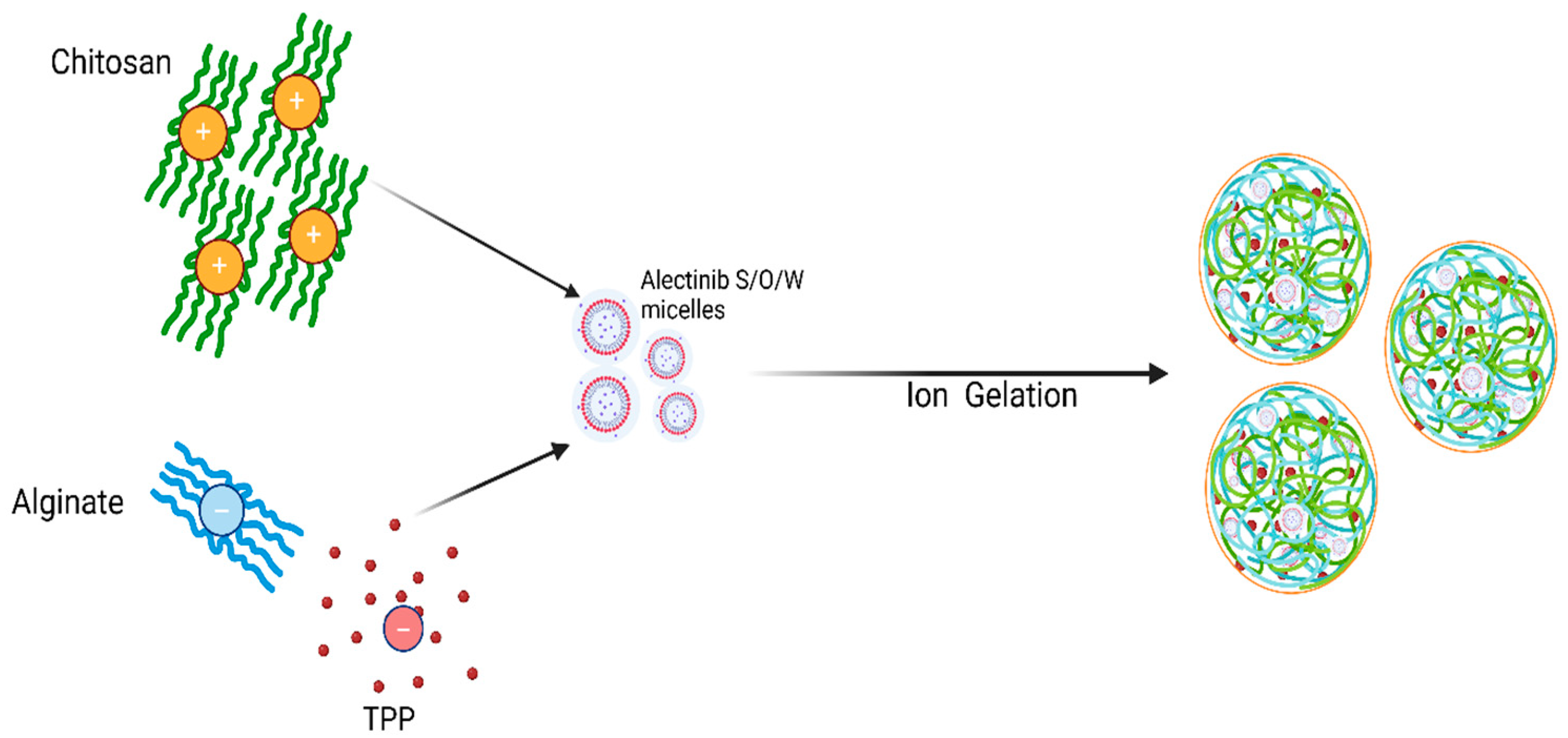
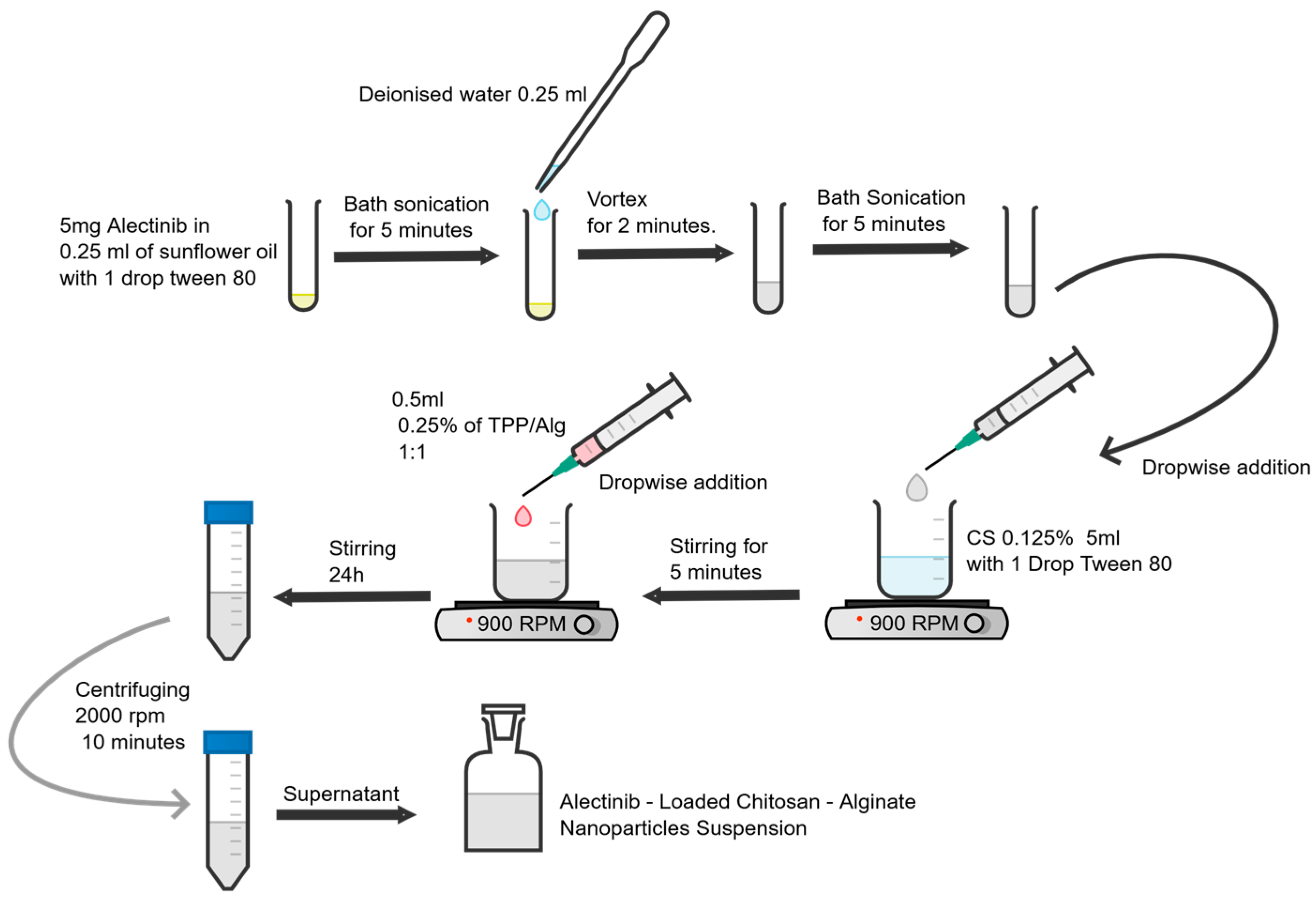
2.4. Preparation of Alectinib-Loaded Chitosan–Alginate Nanoparticles (ACANPs)
2.5. Optimization of Formulation
2.6. Characterization of Alectinib-Loaded Nanoparticles
2.6.1. Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential
2.6.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6.3. Differential Scanning Calorimetry (DSC)
2.6.4. Encapsulation Efficiency (EE%)
2.6.5. Stability Assessment
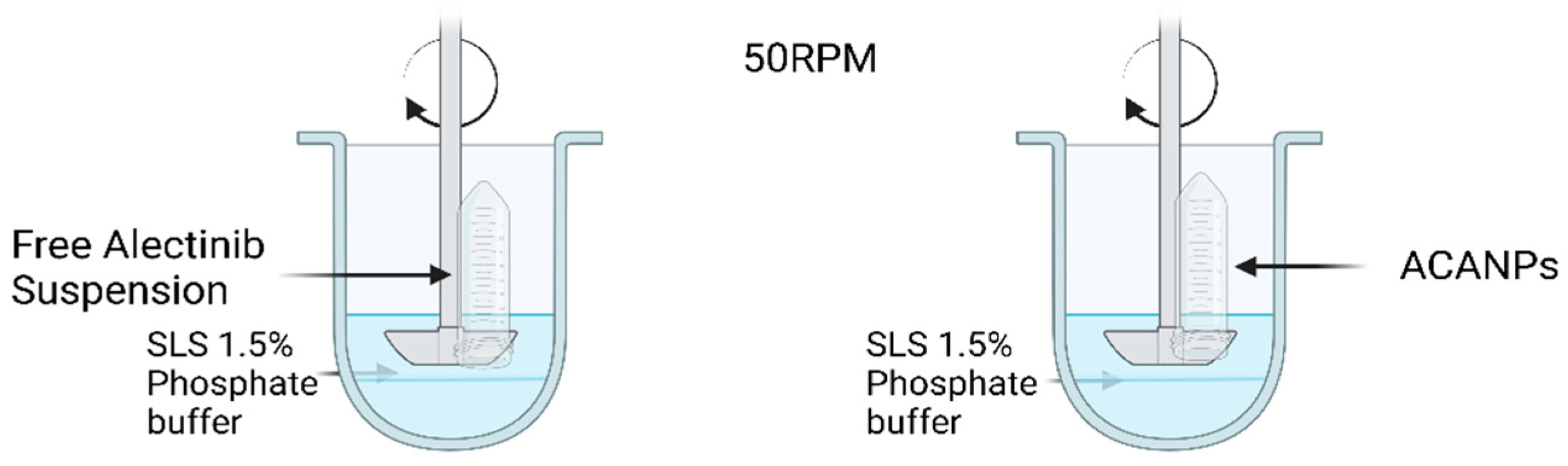
2.7. In Vitro Drug Release
2.8. In Vitro Cytotoxicity (MTT Assay)
2.9. Pharmacokinetic Investigation and Bioavailability Assessment
2.9.1. LC–MS/MS
2.9.2. Method Development
2.9.3. Animal Handling and Ethics
2.9.4. Dosing and Blood Sampling
2.9.5. Non-Compartmental Pharmacokinetic Analysis
2.9.6. Sample Preparation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Development and Validation of Alectinib Analytical Methods
3.1.1. UV–Vis Scan for Alectinib
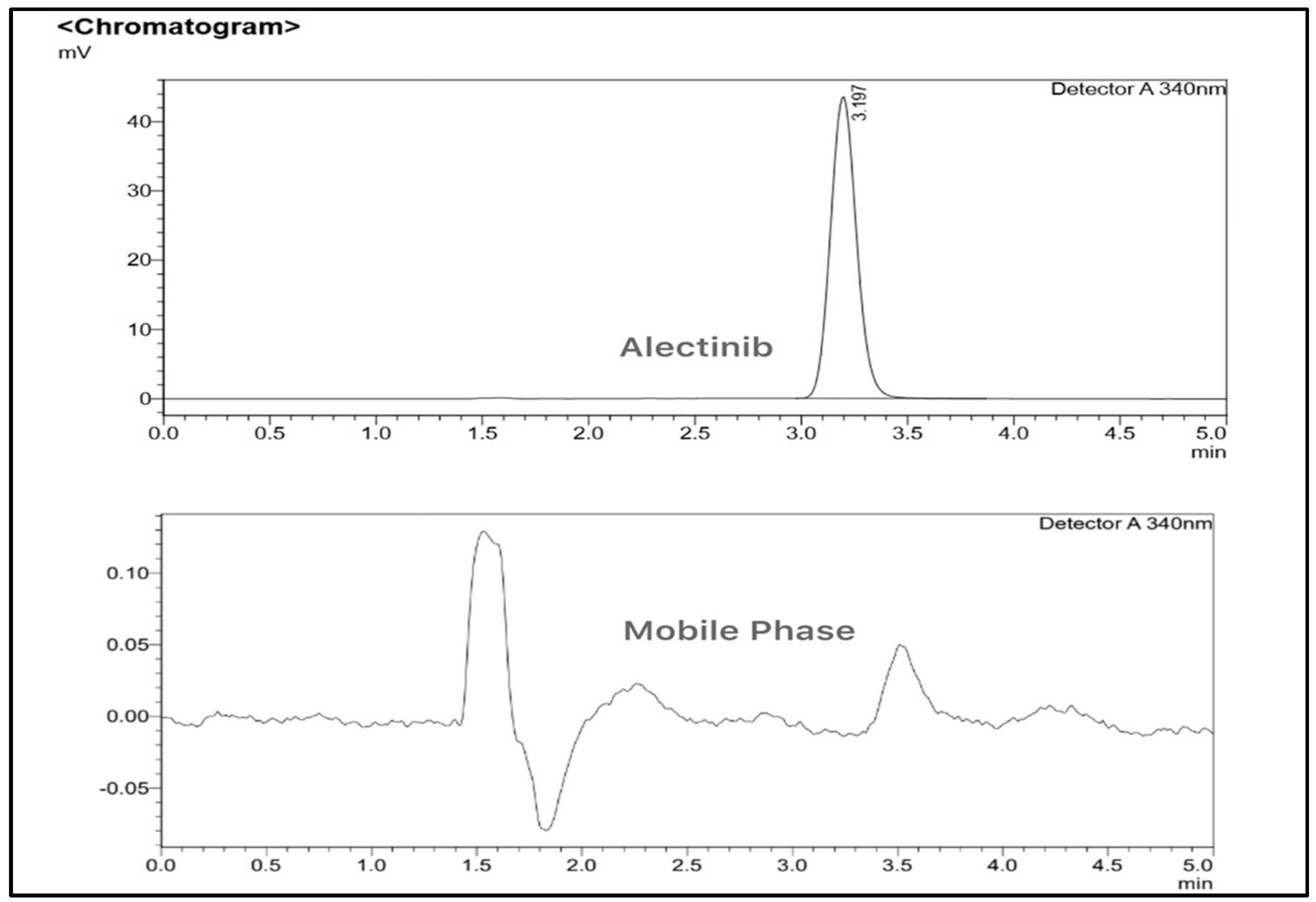
3.1.2. HPLC Method
Lower Limit of Quantification and Lower Limit of Detection
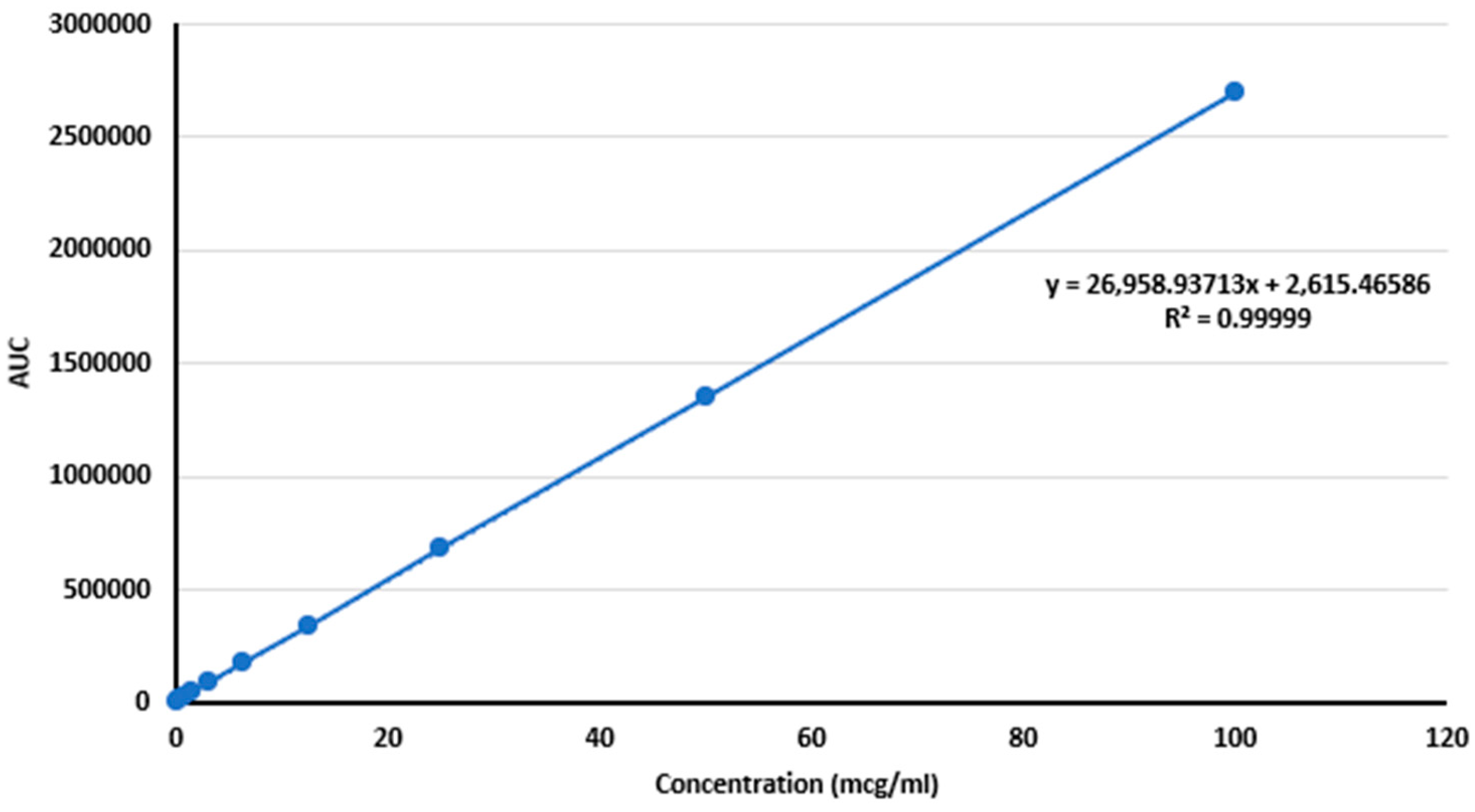
Linearity
Precision and Accuracy
Selectivity and Specificity
Recovery
3.2. Formulation and Optimization of Nanoparticles
3.3. Characterization of ACANPs (F13)
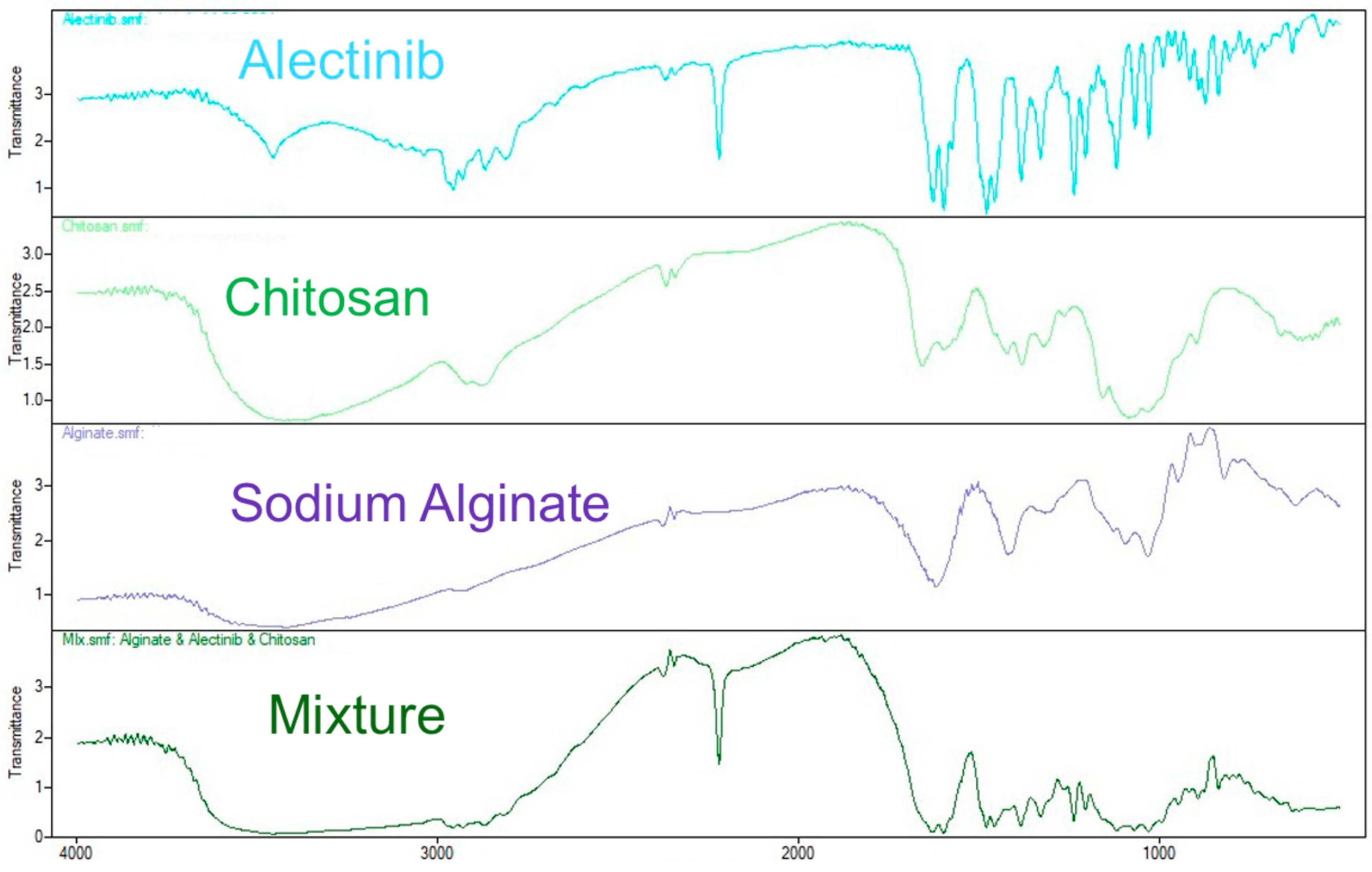
3.3.1. Results of FTIR Spectroscopy
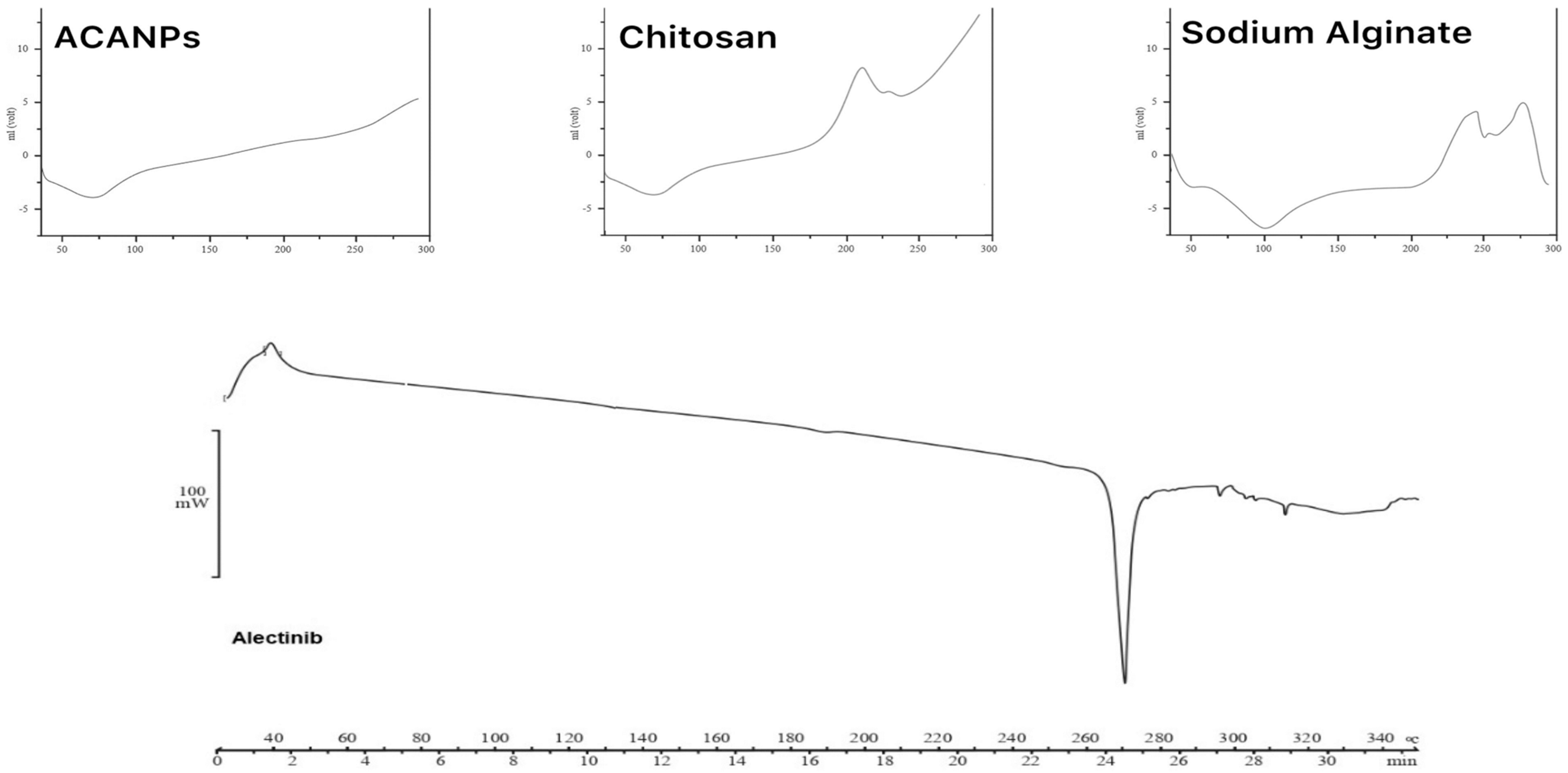
3.3.2. Results of Differential Scanning Calorimetry (DSC)
3.3.3. Results of Particle Size, Polydispersity Index, and Zeta Potential Measurements
3.3.4. Stability of the ACANPs
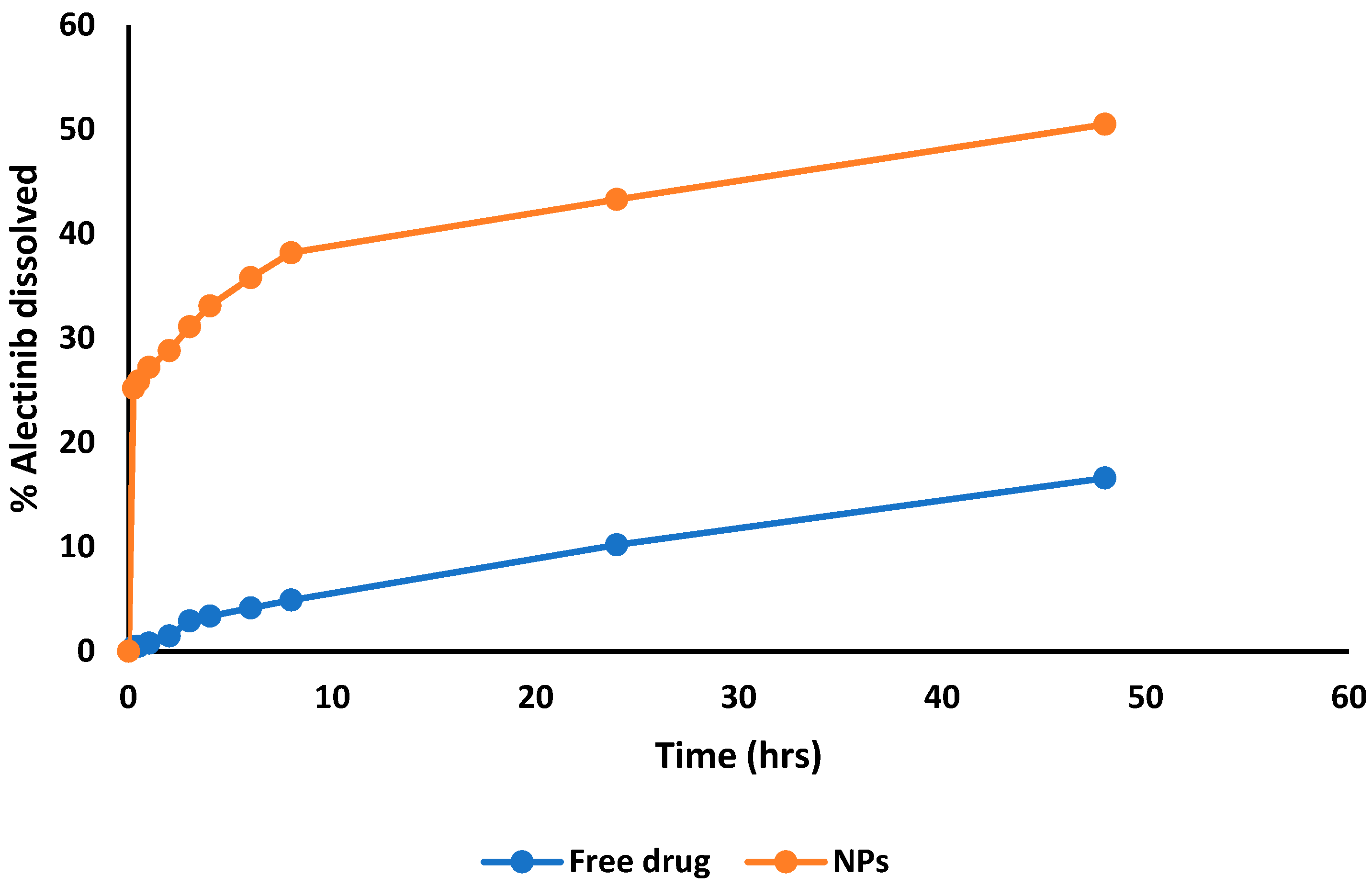
3.4. Results of In Vitro Drug Release
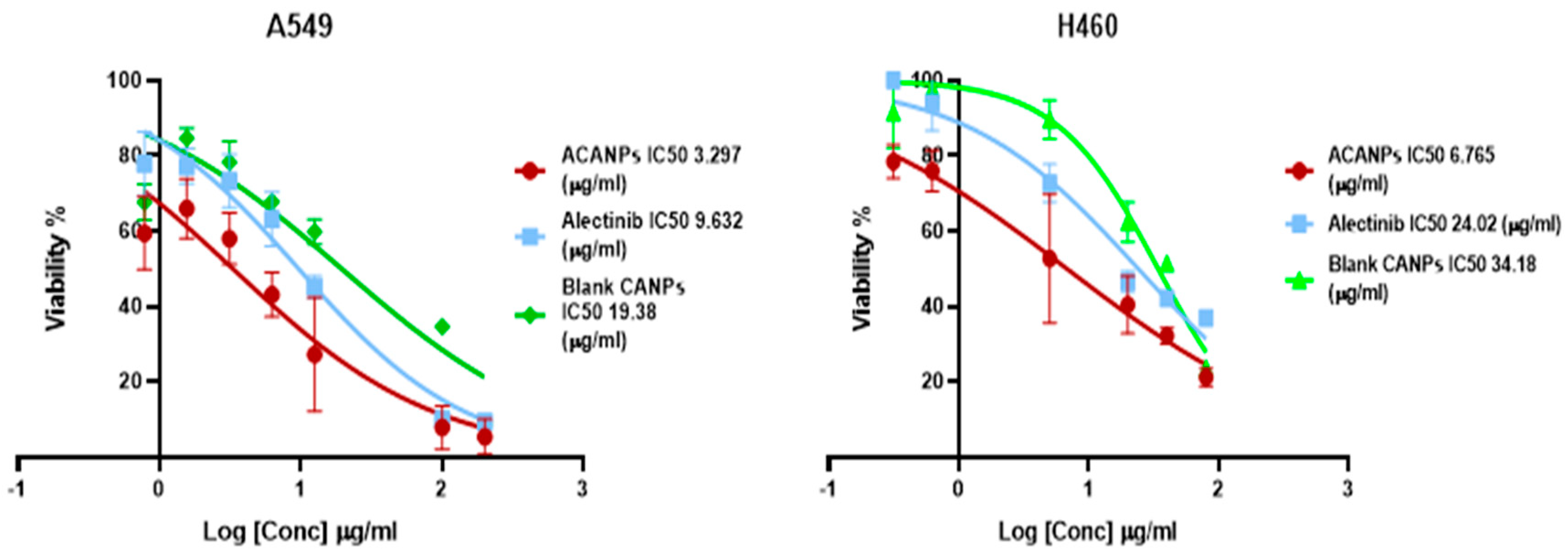
3.5. Assessment of Cell Culture Viability (MTT Assay)
3.6. LC–MS/MS Method for Measurement of Alectinib in Rat Plasma
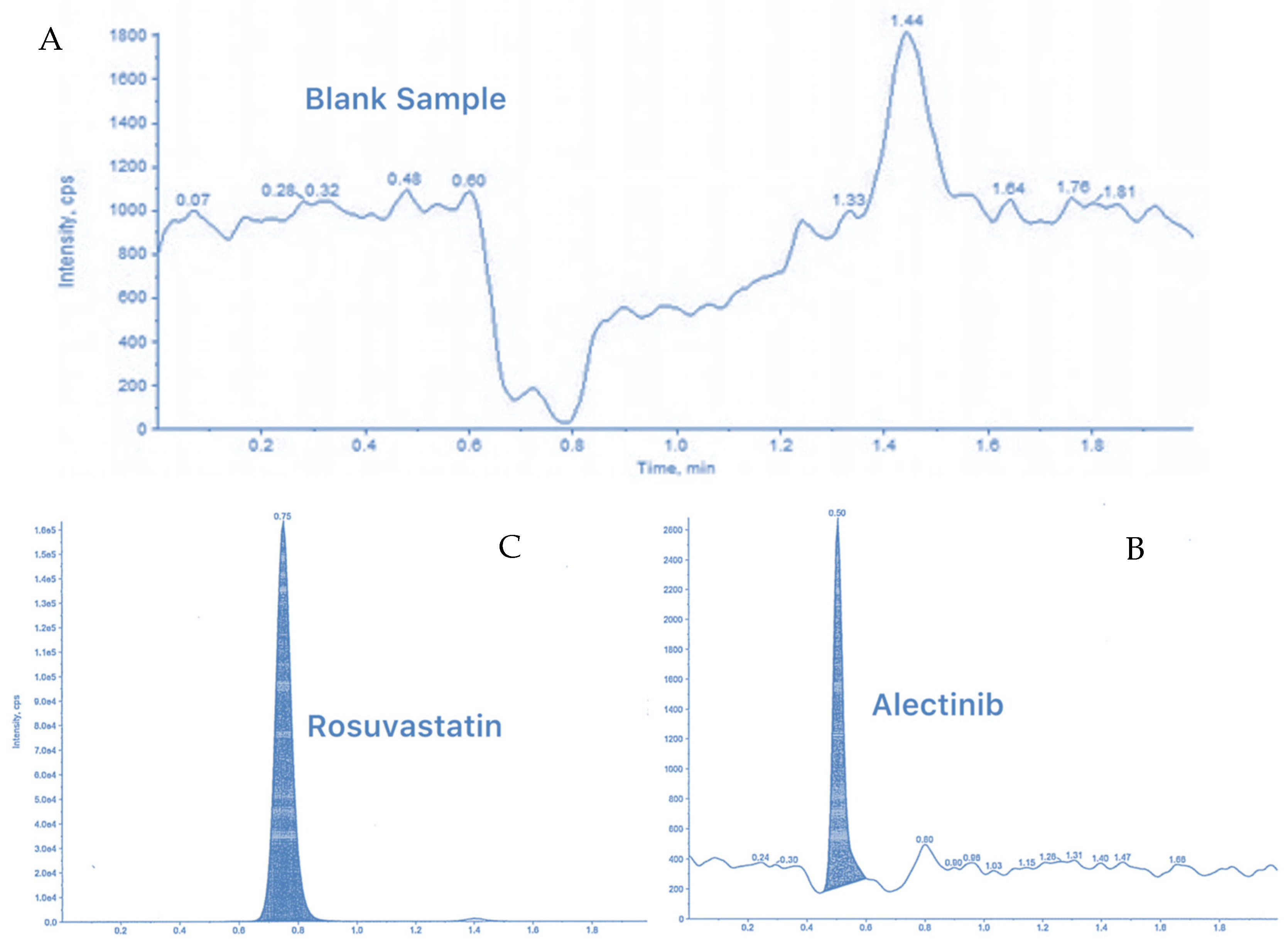
3.6.1. Selectivity and Specificity (LC-MS/MS Method)
3.6.2. Linearity (LC-MS/MS Method)
3.6.3. Precision and Accuracy (LC-MS/MS Method)
3.6.4. Recovery (LC-MS/MS Method)
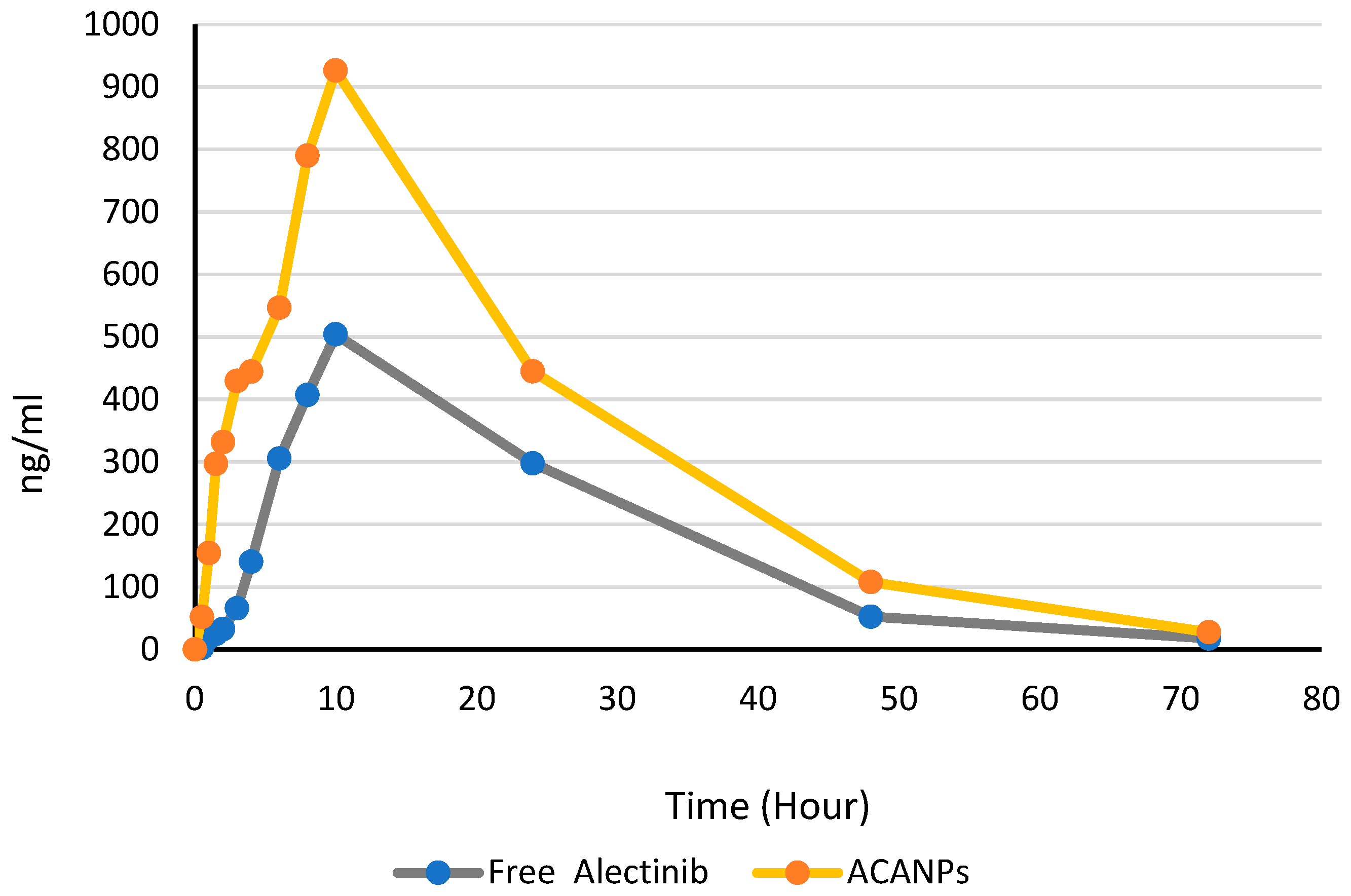
3.7. Pharmacokinetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2024. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2024-cancer-facts-figures.html (accessed on 2 September 2024).
- National Cancer Institute. Press Releases: 2024. Available online: https://www.cancer.gov/news-events/press-releases/2024 (accessed on 13 November 2024).
- Medical News Today. Cancer. Available online: https://www.medicalnewstoday.com/cancer (accessed on 22 October 2024).
- Centers for Disease Control and Prevention. Lung Cancer. Available online: https://www.cdc.gov/lung-cancer/ (accessed on 25 November 2024).
- Majeed, B.J.M.; Saadallah, M.A.; Al-Ani, I.H.; El-Tanani, M.K.; Al Azzam, K.M.; Abdallah, H.H.; Al-Hajji, F. Evaluation of Solubility, Dissolution Rate, and Oral Bioavailability of β-Cyclodextrin and Hydroxypropyl β-Cyclodextrin as Inclusion Complexes of the Tyrosine Kinase Inhibitor, Alectinib. Pharmaceuticals 2024, 17, 737. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Alectinib: EPAR—Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/alecensa-epar-product-information_en.pdf (accessed on 28 September 2024).
- Aldea, M.; Vasseur, D.; Teixidó, C.; Mezquita, L. Central nervous system progression and liquid biopsy in patients with oncogene-addicted non-small cell lung cancer treated with ALK/ROS1 inhibitors. Prec. Cancer Med. 2020, 3, 08036. [Google Scholar]
- Zou, Z.; Xing, P.; Hao, X.; Wang, Y.; Song, X.; Shan, L.; Zhang, C.; Liu, Z.; Ma, K.; Dong, G.; et al. Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases—a multicenter retrospective study. BMC Med. 2022, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves Alectinib as Adjuvant Treatment for ALK-Positive Non-Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-alectinib-adjuvant-treatment-alk-positive-non-small-cell-lung-cancer (accessed on 3 December 2024).
- U.S. Food and Drug Administration. FDA Approves Alectinib for Metastatic ALK-Positive Non-Small Cell Lung Cancer. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/alectinib-approved-alk-positive-metastatic-non-small-cell-lung-cancer-nsclc (accessed on 5 December 2024).
- Dutta, S.; Bhattacharya, S.; Ray, S.K. Chitosan: Preparation, properties and applications. J. Sci. Ind. Res. 2004, 63, 249–257. [Google Scholar]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar]
- Abasalizadeh, F.; Vaghefi Moghaddam, S.; Alizadeh, E.; Akbari, E.; Fazljou, S.M.B.; Torbati, M.; Akbarzadeh, A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020, 14, 8. [Google Scholar]
- Lai, J.; Azad, A.K.; Sulaiman, W.M.A.; Kumarasamy, V.; Alshehade, S.A. Alginate-based encapsulation fabrication technique for drug delivery: An updated review of particle type, formulation technique, pharmaceutical ingredient, and targeted delivery system. Pharmaceutics 2024, 16, 370. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar]
- Van Bavel, N.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. A simple method for synthesis of chitosan nanoparticles with ionic gelation and homogenization. Molecules 2023, 28, 4328. [Google Scholar] [CrossRef] [PubMed]
- Sawtarie, N.; Cai, Y.; Lapitsky, Y. Preparation of chitosan/tripolyphosphate nanoparticles with highly tunable size and low polydispersity. Colloids Surf. B Biointerfaces 2017, 157, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Nalini, T.; Basha, S.K.; Sadiq, A.M.M.; Kumari, V.S.; Kaviyarasu, K. Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Deliv. Sci. Technol. 2019, 52, 65–72. [Google Scholar]
- Sawant, A.; Kamath, S.; Kg, H.; Kulyadi, G.P. Solid-in-Oil-in-Water Emulsion: An Innovative Paradigm to Improve Drug Stability and Biological Activity. AAPS PharmSciTech 2021, 22, 199. [Google Scholar] [PubMed]
- Tamma, M.A.; Nsairat, H.; El-Tanani, M.; Madi, R. In vitro evaluation of lipidic nanocarriers for mebendazole delivery to improve anticancer activity. Drug Dev. Ind. Pharm. 2024, 50, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Alfreahat, I.; Nsairat, H.; Aldeeb, I.D.; Al-Samydai, A.; Alshaer, W. In Vitro Potentiation of Doxorubicin Cytotoxicity Utilizing Clarithromycin Loaded-PEGylated Liposomes. Technol. Cancer Res. Treat. 2025, 24, 15330338241312561. [Google Scholar] [CrossRef] [PubMed]
- Eid, M.M. Characterization of nanoparticles by FTIR and FTIR-microscopy. In Handbook of Consumer Nanoproducts; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Koshy, O.; Subramanian, L.; Thomas, S. Differential scanning calorimetry in nanoscience and nanotechnology. In Thermal and Rheological Measurement Techniques for Nanomaterials Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–122. [Google Scholar]
- Yousfan, A.; Al Rahwanji, M.J.; Hanano, A.; Al-Obaidi, H. A Comprehensive Study on Nanoparticle Drug Delivery to the Brain: Application of Machine Learning Techniques. Mol. Pharm. 2024, 21, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Madlool, D.T.; Al-Ani, I.; Ata, T.; Abu Dayyih, W. Solubility, pH-Solubility Profile, pH-Rate Profile, and Kinetic Stability of the Tyrosine Kinase Inhibitor, Alectinib. Pharmaceuticals 2024, 17, 776. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Samanta, M.; Raichur, A. Dual-drug delivery system based on in situ gel-forming nanosuspension of forskolin to enhance antiglaucoma efficacy. AAPS PharmSciTech 2010, 11, 322–335. [Google Scholar] [PubMed]
- Shapiro, A. How to Calculate IC50 for My Dose Response? Available online: https://www.researchgate.net/post/how_to_calculate_IC50_for_my_dose_response/57f6e2153d7f4b2a8f29e2f3/citation/download (accessed on 9 August 2024).
- Paya, J.; Díaz, P.; Montava, I.; Bonet-Aracil, M.; Bou-Belda, E. Chitosan Pretreatment for Cotton Dyeing with Black Tea. IOP Conf. Ser. Mater. Sci. Eng. 2017, 254, 112001. [Google Scholar]
- Krishnan, K.; Aono, M.; Tsuruoka, T. Thermally Stable Resistive Switching of a Polyvinyl Alcohol-Based Atomic Switch. J. Mater. Chem. C 2018, 6, 6460–6464. [Google Scholar] [CrossRef]
- Almeida e Sousa, L.; Dömötör, K.J.; Paiva, M. Drug-Polymer Solubility Determination: A New Thermodynamic Model Free from Lattice Theory Assumptions. Pharm. Res. 2019, 36, 175. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.; Thuy Nguyen, C.; Thi Thach, L.; Thi Tran, M.; Duc Mai, H.; Thi Thu Nguyen, T.; Duc Le, G.; Van Can, M.; Dai Tran, L.; Long Bach, G.; et al. Characterization of chitosan/alginate/lovastatin nanoparticles and investigation of their toxic effects in vitro and in vivo. Sci. Rep. 2020, 10, 909. [Google Scholar] [CrossRef]
- Bai, X.; Smith, Z.L.; Wang, Y.; Butterworth, S.; Tirella, A. Sustained drug release from smart nanoparticles in cancer therapy: A comprehensive review. Micromachines 2022, 13, 1623. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Chakraborty, S.; Bhattacharya, S.; Mishra, R.; Kundu, P.P. pH-sensitive chitosan/alginate core-shell nanoparticles for efficient and safe oral insulin delivery. Int. J. Biol. Macromol. 2015, 72, 640–648. [Google Scholar] [CrossRef] [PubMed]



| Formulation Code | Amount of Alectinib (mg) | CS/ALG/TPP Ratio | Solubilizing Agent/Amount | Method | EE% |
|---|---|---|---|---|---|
| F1 | 5 mg | 3:0:1 | DMSO/1 mL | Ion Gelation | 11 ± 2 |
| F2 | 5 mg | 4:0:1 | DMSO/1 mL | Ion Gelation | 4 ± 2.5 |
| F3 | 5 mg | 5:0:1 | DMSO/1 mL | Ion Gelation | 2 ± 1 |
| F4 | 5 mg | 5:0:1 | SLS/10 mg | Ion Gelation | 1.8 ± 1 |
| F5 | 5 mg | 5:0:1 | PEG 400/1 drop | Ion Gelation | 8 ± 4 |
| F6 | 5 mg | 5:0:1 | Chremophore Rh 40/3 drops | Ion Gelation | 20 ± 1 |
| F7 | 5 mg | 5:0:1 | Chremophore Rh 40 4 drops | Ion Gelation | 29 ± 5 |
| F8 | 5 mg | 5:0:1 | Chremophore Rh 40 5 drops | Ion Gelation | 31 ±2 |
| F9 | 5 mg | 5:1:1 | Chremophore Rh 40 5 drops | Ion Gelation | 35 ± 4 |
| F10 | 5 mg | 5:0:1 | 2 drops Tween 80 | Ion Gelation | 42 ± 5 |
| F11 | 5 mg | 5:0:1 | 3 drops Tween80 | Ion Gelation | 43 ± 3 |
| F12 | 5 mg | 5.0.1 | 2 drops Tween 80 | S/O/W Emulsification with Ion Gelation | 79 ± 4 |
| F13 | 5 mg | 5:1:1 | 2 drops of Tween 80 | S/O/W Emulsification with Ion Gelation | 97.1 ± 2 |
| Functional Group | Alectinib (cm−1) | Chitosan (cm−1) | Alginate (cm−1) | Physical Mixture (cm−1) | Observation |
|---|---|---|---|---|---|
| N–H stretching | 3454.51 | ~3400 | ~3400 | ~3410 | Broad band retained, no major shift |
| C≡N stretching | 2220 | - | - | 2118 | Slight shift, reduced intensity |
| C–H aromatic stretching | 3037.80 | - | - | 3035 (weak) | Retained |
| C=O (amide I, chitosan) | - | 1651 | - | 1627 | Minor shift, slight overlap |
| amide II (chitosan) | - | 1560 | - | 1555 (broad) | Small shift |
| COO− asymmetric (alginate) | - | - | 1620 | 1597 | Overlap with amide I |
| COO− symmetric (alginate) | - | - | 1417 | 1409 | Minor shift |
| C–O–C/Glycosidic linkage | - | 1150–1060 | 1028 | 1050–1030 | Retained, small shift |
| Nanoparticle Composition | Particle Size ± SD (nm) | Zeta Potential ± SD (mV) | PDI ± SD |
|---|---|---|---|
| F13 (ACANPs) | 161.1 ± 1.5 | 21.0 ± 0.32 | 0.233 ± 0.013 |
| Blank CANPs | 128.4 ± 3.2 | 10.0 ± 0.3 | 0.229 ± 0.011 |
| Lyophilized ACANPs | 161.9 ± 2.7 | 7.86 ± 0.4 | 0.296 ± 0.01 |
| Time (Day) | Particle Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 161.1 | 21.0 | 0.233 |
| 7 | 160.5 | 20.9 | 0.219 |
| 14 | 165.2 | 21.1 | 0.248 |
| 30 | 163.2 | 21.6 | 0.246 |
| 60 | 166.6 | 21.2 | 0.244 |
| 90 | 156.2 | 22.9 | 0.227 |
| Formula | Zero Order | First Order | Peppas | Higuchi | Hixon–Crowell |
|---|---|---|---|---|---|
| Free drug | 0.972 | 0.890 | 0.797 | 0.990 | 0.972 |
| ACANPs | 0.849 | 0.979 | 0.923 | 0.970 | 0.895 |
| Parameter | Free Alectinib | ACANPs |
|---|---|---|
| Cmax (ng/mL) | 504.1 ± 148 | 926.2 ± 166 |
| Tmax (hr) (median) | 10 | 10 |
| AUC0–72 (ng·h/mL) | 12,907.65 ± 3115 | 23,049.13 ± 5420 |
| AUC0- inf (ng·h/mL) | 13,216.80 ± 3917 | 23,528.78 ± 8269 |
| Kel (h−1) | 0.05662 ± 0.00984 | 0.057130 ± 0.00862 |
| t1/2 (h) | 12.24 ± 1.3 | 12.13 ± 1.5 |
| % Extrapolated AUC | 2.34% | 2.04% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ata, T.; Al-Ani, I.; Karameh, N.; Atta, M.R.; Dayyih, W.A. Alectinib-Loaded Chitosan–Alginate Nanoparticles: A Novel Synthesis Method with In Vitro and In Vivo Evaluations. Pharmaceutics 2025, 17, 492. https://doi.org/10.3390/pharmaceutics17040492
Ata T, Al-Ani I, Karameh N, Atta MR, Dayyih WA. Alectinib-Loaded Chitosan–Alginate Nanoparticles: A Novel Synthesis Method with In Vitro and In Vivo Evaluations. Pharmaceutics. 2025; 17(4):492. https://doi.org/10.3390/pharmaceutics17040492
Chicago/Turabian StyleAta, Tha’er, Israa Al-Ani, Nida Karameh, Mahmood R. Atta, and Wael Abu Dayyih. 2025. "Alectinib-Loaded Chitosan–Alginate Nanoparticles: A Novel Synthesis Method with In Vitro and In Vivo Evaluations" Pharmaceutics 17, no. 4: 492. https://doi.org/10.3390/pharmaceutics17040492
APA StyleAta, T., Al-Ani, I., Karameh, N., Atta, M. R., & Dayyih, W. A. (2025). Alectinib-Loaded Chitosan–Alginate Nanoparticles: A Novel Synthesis Method with In Vitro and In Vivo Evaluations. Pharmaceutics, 17(4), 492. https://doi.org/10.3390/pharmaceutics17040492










