Applications and Efficacy of Iron Oxide Nanoparticles in the Treatment of Brain Tumors
Abstract
1. Introduction: Challenges in Neuro-Oncology
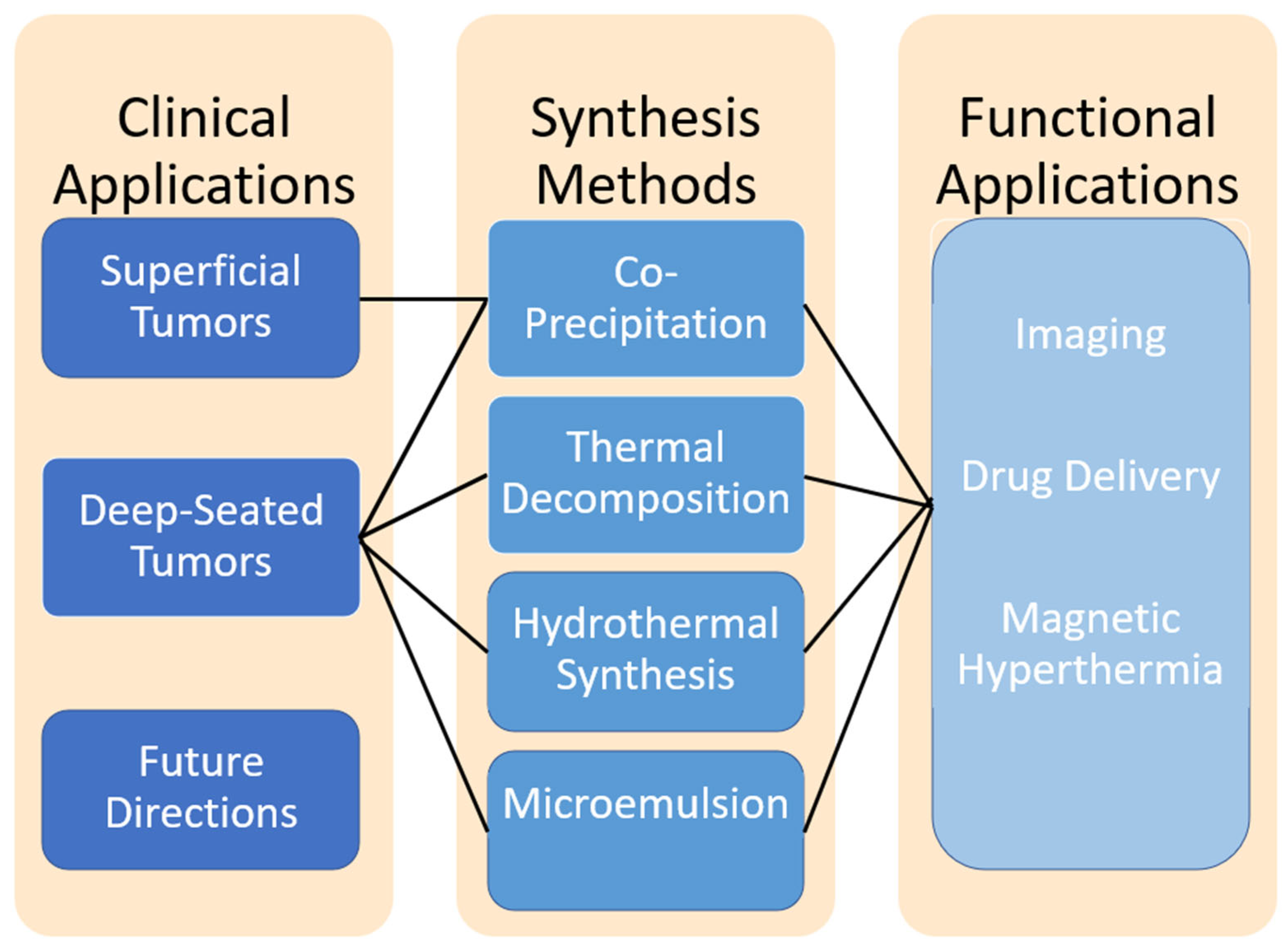
2. Overview of Iron Oxide Nanoparticle Synthesis for Neuro-Oncology Applications
2.1. Co-Precipitation Synthesis
- Superficial tumors are more accessible to externally applied magnetic fields, meaning that extremely precise size control or advanced surface modifications may not be as critical.
- Co-precipitation offers a cost-effective and scalable method, making it suitable for bulk nanoparticle production for superficial applications.
- The nanoparticles can still be functionalized after synthesis for targeting or therapeutic purposes, such as hyperthermia or drug delivery.
2.2. Thermal Decomposition
- Deep-seated tumors require more sophisticated and precise targeting due to the limited range of external magnetic fields and the difficulty in accessing the tumor site.
- Thermal decomposition produces monodisperse nanoparticles with a consistent size and surface properties, critical for achieving optimal localization in challenging environments.
- These nanoparticles are also amenable to post-synthesis functionalization for therapeutic or targeting purposes, such as hyperthermia or drug delivery.
2.3. Hydrothermal Synthesis
- Precise targeting is essential for deep-seated tumors due to their inaccessibility and the limited penetration of external magnetic fields.
- Hydrothermal synthesis is able to produce nanoparticles with consistent size and surface properties which optimize nanoparticle localization to specific regions of interest.
- This method has considerable means through which the nanoparticle design can be altered to suit precise applications.
2.4. Microemulsion Method
- This synthesis method allows for high control over the many facets of the nanoparticle products that allow for high specificity towards deep-seated tumor environments.
- Microemulsion synthesis is not cost or labor effective, making scalability for industrial use difficult to justify.
- Although microemulsion synthesis provides some control over specificity and uniformity, other methods reviewed here offer greater versatility and feasibility.
3. Safety and Biocompatibility in Brain Tumor Therapy
4. MR Imaging for Brain Tumor Visualization
5. Surface Functionalization and Targeted Delivery in Neuro-Oncology
5.1. Enhancing BBB Penetration
5.2. Surface Functionalization
6. Magnetic Hyperthermia in Brain Tumor Treatment
7. Discussion and Future Directions
Emerging Strategies in IONP-Based Drug Development
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BSA | Bovine serum albumin |
| DOX | Doxorubicin |
| FDA | Food and Drug Administration |
| hEGF | Human epidermal growth factor |
| hMSCs | Human mesenchymal stem cells |
| IONPs | Iron oxide nanoparticles |
| MHT | Magnetic hyperthermia therapy |
| MRI | Magnetic resonance imaging |
| PAPM | Poly(acrylic acid)–poly(methacrylic acid) |
| PEG | Polyethylene glycol |
| RF | Radio frequency |
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23 (Suppl. S2), iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.; Costa, A.; Osorio, L.; Lago, R.C.; Linhares, P.; Carvalho, B.; Caeiro, C. Current Standards of Care in Glioblastoma Therapy. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017. [Google Scholar]
- Maroon, J.C.; Bailes, J.E. Cryoprobe-assisted removal of spinal cord tumors: Technical note. Surg. Neurol. 1995, 43, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Horton, J.; Schoenfeld, D.; Salazer, O.; Perez-Tamayo, R.; Kramer, S.; Weinstein, A.; Nelson, J.S.; Tsukada, Y. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. A joint Radiation Therapy Oncology Group and Eastern Cooperative Oncology Group study. Cancer 1983, 52, 997–1007. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Wang, M.; Aldape, K.D.; Stupp, R.; Hegi, M.E.; Jaeckle, K.A.; Armstrong, T.S.; Wefel, J.S.; Won, M.; Blumenthal, D.T.; et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J. Clin. Oncol. 2013, 31, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Balana, C.; Vaz, M.A.; Manuel Sepulveda, J.; Mesia, C.; Del Barco, S.; Pineda, E.; Munoz-Langa, J.; Estival, A.; de Las Penas, R.; Fuster, J.; et al. A phase II randomized, multicenter, open-label trial of continuing adjuvant temozolomide beyond 6 cycles in patients with glioblastoma (GEINO 14-01). Neuro Oncol. 2020, 22, 1851–1861. [Google Scholar] [CrossRef]
- Zanganeh, S.; Hutter, G.; Spitler, R.; Lenkov, O.; Mahmoudi, M.; Shaw, A.; Pajarinen, J.S.; Nejadnik, H.; Goodman, S.; Moseley, M.; et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016, 11, 986–994. [Google Scholar] [CrossRef]
- Ling, D.; Hyeon, T. Chemical Design of Biocompatible Iron Oxide Nanoparticles for Medical Applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Besenhard, M.O.; Hodzic, A.; Sergides, A.; Bogart, L.K.; Gavriilidis, A.; Thanh, N.T.K. Unravelling the growth mechanism of the co-precipitation of iron oxide nanoparticles with the aid of synchrotron X-Ray diffraction in solution. Nanoscale 2019, 11, 6620–6628. [Google Scholar] [CrossRef]
- Ahn, T.; Kim, J.H.; Yang, H.-M.; Lee, J.; Kim, J.-D. Formation Pathways of Magnetite Nanoparticles by Coprecipitation Method. J. Phys. Chem. C 2012, 116, 6069–6076. [Google Scholar] [CrossRef]
- Khalil, M. Co-precipitation in aqueous solution synthesis of magnetite nanoparticles using iron (III) salts as precursors. Arab. J. Chem. 2015, 85, 279–284. [Google Scholar] [CrossRef]
- Ba-Abbad, M.M.; Benamour, A.; Ewis, D.; Mohammad, A.W.; Mahmoudi, E. Synthesis of Fe3O4 Nanoparticles with Different Shapes Through a Co-Precipitation Method and Their Application. JOM 2022, 74, 3531–3539. [Google Scholar] [CrossRef]
- de Souza, T.C.; Costa, A.F.S.; Vinhas, G.M.; Sarubbo, L.A. Synthesis of Iron Oxides and Influence on Final Sizes and Distribution in Bacterial Cellulose Applications. Polymers 2023, 15, 3284. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wu, Z.; Yu, T.; Jiang, C.; Kim, W.-S. Recent progress on magnetic iron oxide nanoparticles: Synthesis, surface functional strategies and biomedical applications. Sci. Technol. Adv. Mater. 2015, 16, 023501. [Google Scholar] [CrossRef] [PubMed]
- Ait Kerroum, M.A.; Essyed, A.; Iacovita, C.; Baaziz, W.; Ihiawakrim, D.; Mounkachi, O.; Hamedoun, M.; Benyoussef, A.; Benaissa, M.; Ersen, O. The effect of basic pH on the elaboration of ZnFe2O4 nanoparticles by co-precipitation method: Structural, magnetic and hyperthermia characterization. J. Magn. Magn. Mater. 2019, 478, 239–246. [Google Scholar] [CrossRef]
- Mürbe, J.; Rechtenbach, A.; Töpfer, J. Synthesis and physical characterization of magnetite nanoparticles for biomedical applications. Mater. Chem. Phys. 2008, 110, 426–433. [Google Scholar] [CrossRef]
- Hauser, A.K.; Mathias, R.; Anderson, K.W.; Hilt, J.Z. The effects of synthesis method on the physical and chemical properties of dextran coated iron oxide nanoparticles. Mater. Chem. Phys. 2015, 160, 177–186. [Google Scholar] [CrossRef]
- Basuki, J.S.; Jacquemin, A.; Esser, L.; Li, Y.; Boyer, C.; Davis, T.P. A block copolymer-stabilized co-precipitation approach to magnetic iron oxide nanoparticles for potential use as MRI contrast agents. Polym. Chem. 2014, 5, 2611–2620. [Google Scholar] [CrossRef]
- Chen, B.; Guo, Z.; Guo, C.; Mao, Y.; Qin, Z.; Ye, D.; Zang, F.; Lou, Z.; Zhang, Z.; Li, M.; et al. Moderate cooling coprecipitation for extremely small iron oxide as a pH dependent T1-MRI contrast agent. Nanoscale 2020, 12, 5521–5532. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin loaded PVA coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Vatasescu-Balcan, R.A.; Predoi, D.; Ungureanu, F.; Costache, M. Costache. Study of iron oxide nanoparticles coated with dextrin obtained by coprecipitation. J. Optoelectron. Adv. Mater. 2008, 10, 693–696. [Google Scholar]
- Ebadi, M.; Bullo, S.; Buskara, K.; Hussein, M.Z.; Fakurazi, S.; Pastorin, G. Release of a liver anticancer drug, sorafenib from its PVA/LDH- and PEG/LDH-coated iron oxide nanoparticles for drug delivery applications. Sci. Rep. 2020, 10, 21521. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, L.M.; Darwish, M.S.A. Functionalized iron oxide nanoparticles: Synthesis through ultrasonic-assisted co-precipitation and performance as hyperthermic agents for biomedical applications. Heliyon 2022, 8, e09654. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef]
- Ali, A.; Zafar, H.; Zia, M.; Ul Haq, I.; Phull, A.R.; Ali, J.S.; Hussain, A. Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2016, 9, 49–67. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, A.J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef]
- Kwon, S.G.; Hyeon, T. Formation Mechanisms of Uniform Nanocrystals via Hot-Injection and Heat-Up Methods. Small 2011, 7, 2685–2702. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Tarasevych, A.V.; Kukhar, V.P.; Boukherroub, R.; Szunerits, S. Recent advances in surface chemistry strategies for the fabrication of functional iron oxide based magnetic nanoparticles. Nanoscale 2013, 5, 10729–10752. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, Functionalization, and Biomedical Applications of Multifunctional Magnetic Nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef]
- Rezaei, B.; Yari, P.; Sanders, S.M.; Wang, H.; Chugh, V.K.; Liang, S.; Mostufa, S.; Xu, K.; Wang, J.-P.; Gómez-Pastora, J.; et al. Magnetic Nanoparticles: A Review on Synthesis, Characterization, Functionalization, and Biomedical Applications. Small 2024, 20, 2304848. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Martín, M.J.; Otarola, J.; Vakarelska, E.; Simeonov, V.; Lassalle, V.; Nedyalkova, M. Biomedical Applications of Iron Oxide Nanoparticles: Current Insights Progress and Perspectives. Pharmaceutics 2022, 14, 204. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.Q.; Shi, Y.N.; Zhu, Y.P.; Liu, Y.Q.; Gu, L.W.; Liu, D.D.; Ma, A.; Xia, F.; Guo, Q.Y.; Xu, C.C.; et al. Recent trends in preparation and biomedical applications of iron oxide nanoparticles. J. Nanobiotechnol. 2024, 22, 24. [Google Scholar] [CrossRef]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Hufschmid, R.; Arami, H.; Ferguson, R.M.; Gonzales, M.; Teeman, E.; Brush, L.N.; Browning, N.D.; Krishnan, K.M. Synthesis of phase-pure and monodisperse iron oxide nanoparticles by thermal decomposition. Nanoscale 2015, 7, 11142–11154. [Google Scholar] [CrossRef]
- Görke, M.; Okeil, S.; Menzel, D.; Semenenko, B.; Garnweitner, G. Tuning the Properties of Iron Oxide Nanoparticles in Thermal Decomposition Synthesis: A Comparative Study of the Influence of Temperature, Ligand Length and Ligand Concentration. Part. Part. Syst. Charact. 2024, 41, 2400059. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Lee, N.; Hyeon, T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Unni, M.; Uhl, A.M.; Savliwala, S.; Savitzky, B.H.; Dhavalikar, R.; Garraud, N.; Arnold, D.P.; Kourkoutis, L.F.; Andrew, J.S.; Rinaldi, C. Thermal Decomposition Synthesis of Iron Oxide Nanoparticles with Diminished Magnetic Dead Layer by Controlled Addition of Oxygen. ACS Nano 2017, 11, 2284–2303. [Google Scholar] [CrossRef]
- Ramimoghadam, D.; Bagheri, S.; Hamid, S.B.A. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. Recent advances in synthesis and surface modification of superparamagnetic iron oxide nanoparticles with silica. J. Magn. Magn. Mater. 2016, 416, 275–291. [Google Scholar] [CrossRef]
- Sathya, A.; Kalyani, S.; Ranoo, S.; Philip, J. One-step microwave-assisted synthesis of water-dispersible Fe3O4 magnetic nanoclusters for hyperthermia applications. J. Magn. Magn. Mater. 2017, 439, 107–113. [Google Scholar] [CrossRef]
- Aivazoglou, E.; Metaxa, E.; Hristoforou, E. Microwave-assisted synthesis of iron oxide nanoparticles in biocompatible organic environment. AIP Adv. 2017, 8, 048201. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Bilecka, I.; Niederberger, M. Microwave chemistry for inorganic nanomaterials synthesis. Nanoscale 2010, 2, 1358–1374. [Google Scholar] [CrossRef]
- Van der Eycken, E.V. Practical Microwave Synthesis for Organic Chemists.Strategies, Instruments, and Protocols. Edited by C. Oliver Kappe, Doris Dallinger and Shaun Murphree. Angew. Chem. Int. Ed. 2009, 48, 2828–2829. [Google Scholar] [CrossRef]
- Schütz, M.B.; Xiao, L.; Lehnen, T.; Fischer, T.; Mathur, S. Microwave-assisted synthesis of nanocrystalline binary and ternary metal oxides. Int. Mater. Rev. 2018, 63, 341–374. [Google Scholar] [CrossRef]
- Ozel, F.; Kockar, H.; Karaagac, O. Growth of Iron Oxide Nanoparticles by Hydrothermal Process: Effect of Reaction Parameters on the Nanoparticle Size. J. Supercond. Nov. Magn. 2015, 28, 823–829. [Google Scholar] [CrossRef]
- Ozel, F.; Kockar, H. Growth and characterizations of magnetic nanoparticles under hydrothermal conditions: Reaction time and temperature. J. Magn. Magn. Mater. 2015, 373, 213–216. [Google Scholar] [CrossRef]
- Wegmann, M.; Scharr, M. Synthesis of Magnetic Iron Oxide Nanoparticles. In Precision Medicine; Academic Press: Cambridge, MA, USA, 2018; pp. 145–181. [Google Scholar]
- Salvador, M.; Gutiérrez, G.; Noriega, S.; Moyano, A.; Blanco-López, M.C.; Matos, M. Microemulsion Synthesis of Superparamagnetic Nanoparticles for Bioapplications. Int. J. Mol. Sci. 2021, 22, 427. [Google Scholar] [CrossRef] [PubMed]
- Makovec, D.; Košak, A.; Žnidaršič, A.; Drofenik, M. The synthesis of spinel–ferrite nanoparticles using precipitation in microemulsions for ferrofluid applications. J. Magn. Magn. Mater. 2005, 289, 32–35. [Google Scholar] [CrossRef]
- Vidal-Vidal, J.; Rivas, J.; López-Quintela, M.A. Synthesis of monodisperse maghemite nanoparticles by the microemulsion method. Colloids Surf. A Physicochem. Eng. Asp. 2006, 288, 44–51. [Google Scholar] [CrossRef]
- Darbandi, M.; Stromberg, F.; Landers, J.; Reckers, N.; Sanyal, B.; Keune, W.; Wende, H. Nanoscale size effect on surface spin canting in iron oxide nanoparticles synthesized by the microemulsion method. J. Phys. D Appl. Phys. 2012, 45, 195001. [Google Scholar] [CrossRef]
- Overcast, W.B.; Davis, K.M.; Ho, C.Y.; Hutchins, G.D.; Green, M.A.; Graner, B.D.; Veronesi, M.C. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Curr. Oncol. Rep. 2021, 23, 34. [Google Scholar] [CrossRef]
- Wagner, B.; Drel, V.; Gorin, Y. Pathophysiology of gadolinium-associated systemic fibrosis. Am. J. Physiol. Ren. Physiol. 2016, 311, F1–F11. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, A.J.; Quattrocchi, C.C.; Mallio, C.A.; Dekkers, I.A. Ten years of gadolinium retention and deposition: ESMRMB-GREC looks backward and forward. Eur. Radiol. 2024, 34, 600–611. [Google Scholar] [CrossRef]
- Ringler, M.D.; Rhodes, N.G.; Ayers-Ringler, J.R.; Jakaitis, D.R.; McDonald, R.J.; Kallmes, D.F.; McDonald, J.S. Gadolinium retention within multiple rat organs after intra-articular administration of gadolinium-based contrast agents. Skelet. Radiol. 2021, 50, 1419–1425. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Akai, H.; Miyagawa, K.; Takahashi, K.; Mochida-Saito, A.; Kurokawa, K.; Takeda, H.; Tsuji, M.; Sugawara, H.; Yasaka, K.; Kunimatsu, A.; et al. Effects of Gadolinium Deposition in the Brain on Motor or Behavioral Function: A Mouse Model. Radiology 2021, 301, 409–416. [Google Scholar] [CrossRef]
- Abujudeh, H.H.; Kosaraju, V.K.; Kaewlai, R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: Experience with 32,659 injections. AJR Am. J. Roentgenol. 2010, 194, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Marino, M.; Smith, A.P.L.; Crowder, J.M.; Larsen, M.; Lowery, L.; Castle, J.; Hibberd, M.G.; Evans, P.M. Repeat and single dose administration of gadodiamide to rats to investigate concentration and location of gadolinium and the cell ultrastructure. Sci. Rep. 2021, 11, 13950. [Google Scholar] [CrossRef]
- Kasten, A.; Grüttner, C.; Kühn, J.P.; Bader, R.; Pasold, J.; Frerich, B. Comparative in vitro study on magnetic iron oxide nanoparticles for MRI tracking of adipose tissue-derived progenitor cells. PLoS ONE 2014, 9, e108055. [Google Scholar] [CrossRef] [PubMed]
- Ayers-Ringler, J.; McDonald, J.S.; Connors, M.A.; Fisher, C.R.; Han, S.; Jakaitis, D.R.; Scherer, B.; Tutor, G.; Wininger, K.M.; Dai, D.; et al. Neurologic Effects of Gadolinium Retention in the Brain after Gadolinium-based Contrast Agent Administration. Radiology 2022, 302, 676–683. [Google Scholar] [CrossRef]
- Strzeminska, I.; Factor, C.; Robert, P.; Grindel, A.L.; Comby, P.O.; Szpunar, J.; Corot, C.; Lobinski, R. Long-Term Evaluation of Gadolinium Retention in Rat Brain After Single Injection of a Clinically Relevant Dose of Gadolinium-Based Contrast Agents. Investig. Radiol. 2020, 55, 138–143. [Google Scholar] [CrossRef]
- Marcus, M.; Karni, M.; Baranes, K.; Levy, I.; Alon, N.; Margel, S.; Shefi, O. Iron oxide nanoparticles for neuronal cell applications: Uptake study and magnetic manipulations. J. Nanobiotechnol. 2016, 14, 37. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, Y.; Shih, G.; Zhang, Y.; Bohmart, A.; Hecht, E.M.; Prince, M.R. Effect of Renal Function on Gadolinium-Related Signal Increases on Unenhanced T1-Weighted Brain Magnetic Resonance Imaging. Investig. Radiol. 2016, 51, 677–682. [Google Scholar] [CrossRef]
- Foo, J.C.; Meinhardt, M.W.; Skorodumov, I.; Spanagel, R. Alcohol solution strength preference predicts compulsive-like drinking behavior in rats. Alcohol. Clin. Exp. Res. 2022, 46, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Roch, A.; Muller, R.; Gillis, P. Theory of proton relaxation induced by superparamagnetic particles. J. Chem. Phys. 1999, 110, 5403–5411. [Google Scholar] [CrossRef]
- Song, M.; Moon, W.K.; Kim, Y.; Lim, D.; Song, I.C.; Yoon, B.W. Labeling efficacy of superparamagnetic iron oxide nanoparticles to human neural stem cells: Comparison of ferumoxides, monocrystalline iron oxide, cross-linked iron oxide (CLIO)-NH2 and tat-CLIO. Korean J. Radiol. 2007, 8, 365–371. [Google Scholar] [CrossRef]
- Irrsack, E.; Schuller, J.; Petters, C.; Willmann, W.; Dringen, R.; Koch, M. Effects of Local Administration of Iron Oxide Nanoparticles in the Prefrontal Cortex, Striatum, and Hippocampus of Rats. Neurotox. Res. 2021, 39, 2056–2071. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Liou, G.G.; Pan, H.B.; Tseng, H.H.; Hung, Y.T.; Chou, C.P. Specific detection of CD133-positive tumor cells with iron oxide nanoparticles labeling using noninvasive molecular magnetic resonance imaging. Int. J. Nanomed. 2015, 10, 6997–7018. [Google Scholar] [CrossRef]
- Unterweger, H.; Janko, C.; Schwarz, M.; Dézsi, L.; Urbanics, R.; Matuszak, J.; Őrfi, E.; Fülöp, T.; Bäuerle, T.; Szebeni, J.; et al. Non-immunogenic dextran-coated superparamagnetic iron oxide nanoparticles: A biocompatible, size-tunable contrast agent for magnetic resonance imaging. Int. J. Nanomed. 2017, 12, 5223–5238. [Google Scholar] [CrossRef] [PubMed]
- Hachani, R.; Birchall, M.A.; Lowdell, M.W.; Kasparis, G.; Tung, L.D.; Manshian, B.B.; Soenen, S.J.; Gsell, W.; Himmelreich, U.; Gharagouzloo, C.A.; et al. Assessing cell-nanoparticle interactions by high content imaging of biocompatible iron oxide nanoparticles as potential contrast agents for magnetic resonance imaging. Sci. Rep. 2017, 7, 7850. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Chiappi, M.; Lazaro-Carrillo, A.; Rodríguez, M.J.; Chichón, F.J.; Crosbie-Staunton, K.; Prina-Mello, A.; Volkov, Y.; Villanueva, A.; Carrascosa, J.L. Characterization of interaction of magnetic nanoparticles with breast cancer cells. J. Nanobiotechnol. 2015, 13, 16. [Google Scholar] [CrossRef]
- Coricovac, D.E.; Moacă, E.A.; Pinzaru, I.; Cîtu, C.; Soica, C.; Mihali, C.V.; Păcurariu, C.; Tutelyan, V.A.; Tsatsakis, A.; Dehelean, C.A. Biocompatible Colloidal Suspensions Based on Magnetic Iron Oxide Nanoparticles: Synthesis, Characterization and Toxicological Profile. Front. Pharmacol. 2017, 8, 154. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Várallyay, C.G.; Manninger, S.; Solymosi, D.; Haluska, M.; Hunt, M.A.; Nesbit, G.; Stevens, A.; Jerosch-Herold, M.; Jacobs, P.M.; et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: A pilot study. Neurosurgery 2007, 60, 601–612. [Google Scholar] [CrossRef]
- Landry, R.; Jacobs, P.M.; Davis, R.; Shenouda, M.; Bolton, W.K. Pharmacokinetic Study of Ferumoxytol: A New Iron Replacement Therapy in Normal Subjects and Hemodialysis Patients. Am. J. Nephrol. 2005, 25, 400–410. [Google Scholar] [CrossRef]
- Ersoy, H.; Rybicki, F.J. Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J. Magn. Reson. Imaging 2007, 26, 1190–1197. [Google Scholar] [CrossRef]
- Uchiyama, M.K.; Toma, S.H.; Rodrigues, S.F.; Shimada, A.L.; Loiola, R.A.; Cervantes Rodríguez, H.J.; Oliveira, P.V.; Luz, M.S.; Rabbani, S.R.; Toma, H.E.; et al. Ultrasmall cationic superparamagnetic iron oxide nanoparticles as nontoxic and efficient MRI contrast agent and magnetic-targeting tool. Int. J. Nanomed. 2015, 10, 4731–4746. [Google Scholar] [CrossRef]
- Ali, A.A.A.; Shahror, R.A.; Chen, K.Y. Efficient Labeling Of Mesenchymal Stem Cells For High Sensitivity Long-Term MRI Monitoring In Live Mice Brains. Int. J. Nanomed. 2020, 15, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wen, W.; Wang, X.; Huang, D.; Cao, J.; Qi, X.; Shen, S. Ultrasmall iron oxide nanoparticles cause significant toxicity by specifically inducing acute oxidative stress to multiple organs. Part. Fibre Toxicol. 2022, 19, 24. [Google Scholar] [CrossRef]
- Kenzaoui, B.H.; Bernasconi, C.C.; Hofmann, H.; Juillerat-Jeanneret, L. Evaluation of uptake and transport of ultrasmall superparamagnetic iron oxide nanoparticles by human brain-derived endothelial cells. Nanomedicine 2012, 7, 39–53. [Google Scholar] [CrossRef]
- Petters, C.; Thiel, K.; Dringen, R. Lysosomal iron liberation is responsible for the vulnerability of brain microglial cells to iron oxide nanoparticles: Comparison with neurons and astrocytes. Nanotoxicology 2016, 10, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Bourrinet, P.; Bengele, H.H.; Bonnemain, B.; Dencausse, A.; Idee, J.M.; Jacobs, P.M.; Lewis, J.M. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Investig. Radiol. 2006, 41, 313–324. [Google Scholar] [CrossRef]
- Zou, J.; Wang, X.; Zhang, L.; Wang, J. Iron nanoparticles significantly affect the in vitro and in vivo expression of Id genes. Chem. Res. Toxicol. 2015, 28, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Královec, K.; Melounková, L.; Slováková, M.; Mannová, N.; Sedlák, M.; Bartáček, J.; Havelek, R. Disruption of Cell Adhesion and Cytoskeletal Networks by Thiol-Functionalized Silica-Coated Iron Oxide Nanoparticles. Int. J. Mol. Sci. 2020, 21, 9350. [Google Scholar] [CrossRef]
- Mai, T.; Hilt, J.Z. Magnetic nanoparticles: Reactive oxygen species generation and potential therapeutic applications. J. Nanoparticle Res. 2017, 19, 253. [Google Scholar] [CrossRef]
- Ahamed, M.; Alhadlaq, H.A.; Khan, M.A.M.; Akhtar, M.J. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. J. Nanoparticle Res. 2012, 15, 1225. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, J.; Liu, J.; Lin, G.; Xie, F.; Pang, X.; Pei, Y.; Cheng, Y.; Zhang, Y.; Lin, Z.; et al. Oxidative stress-driven DR5 upregulation restores TRAIL/Apo2L sensitivity induced by iron oxide nanoparticles in colorectal cancer. Biomaterials 2020, 233, 119753. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Salehzadeh, A.; Kafilzadeh, F.; Zaefizadeh, M. Evaluation of the effect of iron oxide nanoparticles functionalized by glucose and conjugated with coumarin (Fe3O4@Glu-Coumarin NPs) on the expression of CASP8, CASP9, p53, mTOR1, and MAPK1 genes in liver cancer cell line. Gene Rep. 2023, 33, 101818. [Google Scholar] [CrossRef]
- Kanda, T.; Nakai, Y.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Gadolinium deposition in the brain. Magn. Reson. Imaging 2016, 34, 1346–1350. [Google Scholar] [CrossRef]
- Habermeyer, J.; Boyken, J.; Harrer, J.; Canneva, F.; Ratz, V.; Moceri, S.; Admard, J.; Casadei, N.; Jost, G.; Bäuerle, T.; et al. Comprehensive phenotyping revealed transient startle response reduction and histopathological gadolinium localization to perineuronal nets after gadodiamide administration in rats. Sci. Rep. 2020, 10, 22385. [Google Scholar] [CrossRef] [PubMed]
- Irrsack, E.; Aydin, S.; Bleckmann, K.; Schuller, J.; Dringen, R.; Koch, M. Local Administrations of Iron Oxide Nanoparticles in the Prefrontal Cortex and Caudate Putamen of Rats Do Not Compromise Working Memory and Motor Activity. Neurotox. Res. 2023, 42, 6. [Google Scholar] [CrossRef] [PubMed]
- Taboada, E.; Rodríguez, E.; Roig, A.; Oró, J.; Roch, A.; Muller, R.N. Relaxometric and Magnetic Characterization of Ultrasmall Iron Oxide Nanoparticles with High Magnetization. Evaluation as Potential T1 Magnetic Resonance Imaging Contrast Agents for Molecular Imaging. Langmuir 2007, 23, 4583–4588. [Google Scholar] [CrossRef]
- Xiao, Y.-D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.-S.; Zhou, S.-K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Edelman, R.R.; Leloudas, N.; Ankenbrandt, W.J.; Walker, M.T.; Bobustuc, G.C.; Bailes, J.E.; Pruitt, A.A.; Koktzoglou, I. Dark Blood Contrast-Enhanced Brain MRI Using Echo-uT(1)RESS. J. Magn. Reson. Imaging 2024, 60, 789–797. [Google Scholar] [CrossRef]
- Tao, C.; Zheng, Q.; An, L.; He, M.; Lin, J.; Tian, Q.; Yang, S. T₁-Weight Magnetic Resonance Imaging Performances of Iron Oxide Nanoparticles Modified with a Natural Protein Macromolecule and an Artificial Macromolecule. Nanomaterials 2019, 9, 170. [Google Scholar] [CrossRef]
- Wei, H.; Wiśniowska, A.; Fan, J.; Harvey, P.; Li, Y.; Wu, V.; Hansen, E.C.; Zhang, J.; Kaul, M.G.; Frey, A.M.; et al. Single-nanometer iron oxide nanoparticles as tissue-permeable MRI contrast agents. Proc. Natl. Acad. Sci. USA 2021, 118, e2102340118. [Google Scholar] [CrossRef]
- Yang, L.; Afshari, M.J.; Ge, J.; Kou, D.; Chen, L.; Zhou, D.; Li, C.; Wu, S.; Zhang, L.; Zeng, J.; et al. Functionalized Ultrasmall Iron Oxide Nanoparticles for T(1)-Weighted Magnetic Resonance Imaging of Tumor Hypoxia. Molecules 2022, 27, 6929. [Google Scholar] [CrossRef]
- Christoforidis, G.A.; Yang, M.; Kontzialis, M.S.; Larson, D.G.; Abduljalil, A.; Basso, M.; Yang, W.; Ray-Chaudhury, A.; Heverhagen, J.; Knopp, M.V.; et al. High resolution ultra high field magnetic resonance imaging of glioma microvascularity and hypoxia using ultra-small particles of iron oxide. Investig. Radiol. 2009, 44, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.B.; Varallyay, C.G.; Horvath, A.; Bashir, M.R.; Choyke, P.L.; Daldrup-Link, H.E.; Dosa, E.; Finn, J.P.; Gahramanov, S.; Harisinghani, M.; et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017, 92, 47–66. [Google Scholar] [CrossRef]
- Rad, A.M.; Arbab, A.S.; Iskander, A.S.; Jiang, Q.; Soltanian-Zadeh, H. Quantification of superparamagnetic iron oxide (SPIO)-labeled cells using MRI. J. Magn. Reson. Imaging 2007, 26, 366–374. [Google Scholar] [CrossRef]
- Zhang, Z.; Mascheri, N.; Dharmakumar, R.; Fan, Z.; Paunesku, T.; Woloschak, G.; Li, D. Superparamagnetic iron oxide nanoparticle-labeled cells as an effective vehicle for tracking the GFP gene marker using magnetic resonance imaging. Cytotherapy 2009, 11, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ariza de Schellenberger, A.; Kratz, H.; Farr, T.D.; Löwa, N.; Hauptmann, R.; Wagner, S.; Taupitz, M.; Schnorr, J.; Schellenberger, E.A. Labeling of mesenchymal stem cells for MRI with single-cell sensitivity. Int. J. Nanomed. 2016, 11, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
- Jarzyna, P.A.; Deddens, L.H.; Kann, B.H.; Ramachandran, S.; Calcagno, C.; Chen, W.; Gianella, A.; Dijkhuizen, R.M.; Griffioen, A.W.; Fayad, Z.A.; et al. Tumor angiogenesis phenotyping by nanoparticle-facilitated magnetic resonance and near-infrared fluorescence molecular imaging. Neoplasia 2012, 14, 964–973. [Google Scholar] [CrossRef][Green Version]
- Melemenidis, S.; Jefferson, A.; Ruparelia, N.; Akhtar, A.M.; Xie, J.; Allen, D.; Hamilton, A.; Larkin, J.R.; Perez-Balderas, F.; Smart, S.C.; et al. Molecular Magnetic Resonance Imaging of Angiogenesis In Vivo using Polyvalent Cyclic RGD-Iron Oxide Microparticle Conjugates. Theranostics 2015, 5, 515–529. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Aksenov, D.P.; Doubovikov, E.D. Diffusion constraints in neuroprotection: Implications for clinical trials. Front. Pharmacol. 2025, 16, 1542431. [Google Scholar] [CrossRef]
- Enteshari Najafabadi, R.; Kazemipour, N.; Esmaeili, A.; Beheshti, S.; Nazifi, S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 2018, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Mi, G.; Bhattacharya, S.; Nayar, S.; Webster, T.J. Optimizing superparamagnetic iron oxide nanoparticles as drug carriers using an in vitro blood-brain barrier model. Int. J. Nanomed. 2016, 11, 5371–5379. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Yakovleva, L.Y.; Marchenko, Y.Y.; Dobrodumov, A.V.; Mikhrina, A.L.; Martynova, M.G.; Bystrova, O.A.; Yakovenko, I.V.; Ischenko, A.M. Superparamagnetic iron oxide nanoparticles conjugated with epidermal growth factor (SPION-EGF) for targeting brain tumors. Int. J. Nanomed. 2014, 9, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Salehnia, Z.; Shahbazi-Gahrouei, D.; Akbarzadeh, A.; Baradaran, B.; Farajnia, S.; Naghibi, M. Synthesis and characterisation of iron oxide nanoparticles conjugated with epidermal growth factor receptor (EGFR) monoclonal antibody as MRI contrast agent for cancer detection. IET Nanobiotechnol. 2019, 13, 400–406. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Yakovleva, L.Y.; Nikolaev, B.P.; Marchenko, Y.Y.; Dobrodumov, A.V.; Onokhin, K.V.; Onokhina, Y.S.; Selkov, S.A.; Mikhrina, A.L.; Guzhova, I.V.; et al. Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma. Neuro Oncol. 2014, 16, 38–49. [Google Scholar] [CrossRef]
- Hölig, P.; Bach, M.; Völkel, T.; Nahde, T.; Hoffmann, S.; Müller, R.; Kontermann, R.E. Novel RGD lipopeptides for the targeting of liposomes to integrin-expressing endothelial and melanoma cells. Protein Eng. Des. Sel. 2004, 17, 433–441. [Google Scholar] [CrossRef]
- Wu, T.; Ding, X.; Su, B.; Soodeen-Lalloo, A.K.; Zhang, L.; Shi, J.Y. Magnetic resonance imaging of tumor angiogenesis using dual-targeting RGD10-NGR9 ultrasmall superparamagnetic iron oxide nanoparticles. Clin. Transl. Oncol. 2018, 20, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi Kamalabadi, M.; Neshastehriz, A.; Ghaznavi, H.; Amini, S.M. Folate functionalized gold-coated magnetic nanoparticles effect in combined electroporation and radiation treatment of HPV-positive oropharyngeal cancer. Med. Oncol. 2022, 39, 196. [Google Scholar] [CrossRef]
- Liang, P.C.; Chen, Y.C.; Chiang, C.F.; Mo, L.R.; Wei, S.Y.; Hsieh, W.Y.; Lin, W.L. Doxorubicin-modified magnetic nanoparticles as a drug delivery system for magnetic resonance imaging-monitoring magnet-enhancing tumor chemotherapy. Int. J. Nanomed. 2016, 11, 2021–2037. [Google Scholar] [CrossRef]
- Patel, P.; Alghamdi, A.; Shaw, G.; Legge, C.; Glover, M.; Freeman, D.; Hodgetts, H.; Wilson, E.; Howard, F.; Staniland, S.; et al. Development of a Personalised Device for Systemic Magnetic Drug Targeting to Brain Tumours. Nanotheranostics 2023, 7, 102–116. [Google Scholar] [CrossRef]
- Sun, C.; Fang, C.; Stephen, Z.; Veiseh, O.; Hansen, S.; Lee, D.; Ellenbogen, R.G.; Olson, J.; Zhang, M. Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine 2008, 3, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Sundaram, S.K.; Teeguarden, J.G.; Riley, B.J.; Fifield, L.S.; Jacobs, J.M.; Addleman, S.R.; Kaysen, G.A.; Moudgil, B.M.; Weber, T.J. Adsorbed proteins influence the biological activity and molecular targeting of nanomaterials. Toxicol. Sci. 2007, 100, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-circulating and target-specific nanoparticles: Theory to practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, S.; Ogawara, K.; Fukuoka, Y.; Higaki, K.; Kimura, T. Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int. J. Pharm. 2007, 342, 215–221. [Google Scholar] [CrossRef]
- Camner, P.; Lundborg, M.; Låstbom, L.; Gerde, P.; Gross, N.; Jarstrand, C. Experimental and calculated parameters on particle phagocytosis by alveolar macrophages. J. Appl. Physiol. 2002, 92, 2608–2616. [Google Scholar] [CrossRef]
- Chen, H.; Wang, L.; Yeh, J.; Wu, X.; Cao, Z.; Wang, Y.A.; Zhang, M.; Yang, L.; Mao, H. Reducing non-specific binding and uptake of nanoparticles and improving cell targeting with an antifouling PEO-b-PgammaMPS copolymer coating. Biomaterials 2010, 31, 5397–5407. [Google Scholar] [CrossRef]
- Cole, A.J.; David, A.E.; Wang, J.; Galbán, C.J.; Hill, H.L.; Yang, V.C. Polyethylene glycol modified, cross-linked starch-coated iron oxide nanoparticles for enhanced magnetic tumor targeting. Biomaterials 2011, 32, 2183–2193. [Google Scholar] [CrossRef]
- Foy, S.P.; Manthe, R.L.; Foy, S.T.; Dimitrijevic, S.; Krishnamurthy, N.; Labhasetwar, V. Optical imaging and magnetic field targeting of magnetic nanoparticles in tumors. ACS Nano 2010, 4, 5217–5224. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Ikeda, H.; Ishii, A.; Sano, K.; Chihara, H.; Arai, D.; Abekura, Y.; Nishi, H.; Ono, M.; Saji, H.; Miyamoto, S. Activatable fluorescence imaging of macrophages in atherosclerotic plaques using iron oxide nanoparticles conjugated with indocyanine green. Atherosclerosis 2018, 275, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Mu, Q.; Revia, R.; Wang, K.; Tian, B.; Lin, G.; Lee, W.; Hong, Y.K.; Zhang, M. Iron oxide-carbon core-shell nanoparticles for dual-modal imaging-guided photothermal therapy. J. Control. Release 2018, 289, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Gneveckow, U.; Jordan, A.; Scholz, R.; Brüß, V.; Waldöfner, N.; Ricke, J.; Feussner, A.; Hildebrandt, B.; Rau, B.; Wust, P. Description and characterization of the novel hyperthermia- and thermoablation-system for clinical magnetic fluid hyperthermia. Med. Phys. 2004, 31, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Pu, P.-y.; Zhang, Y.-z.; Jiang, D.-h. Apoptosis induced by hyperthermia in human glioblastoma cell line and murine glioblastoma. Chin. J. Cancer Res. 2000, 12, 257–262. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. J. Neuro-Oncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Usov, N.A. Iron Oxide Nanoparticles for Magnetic Hyperthermia. SPIN 2019, 09, 1940001. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Hurley, K.R.; Zhang, J.; Jeon, S.; Ring, H.L.; Hogan, C.; Haynes, C.L.; Garwood, M.; Bischof, J.C. Accounting for biological aggregation in heating and imaging of magnetic nanoparticles. Technology 2014, 02, 214–228. [Google Scholar] [CrossRef]
- Nemati, Z.; Alonso Masa, J.; Martinez, L.; Khurshid, H.; Garaio, E.; Garcia, J.; Phan, M.-H.; Srikanth, H. Enhanced Magnetic Hyperthermia in Iron Oxide Nano-Octopods: Size and Anisotropy Effects. J. Phys. Chem. C 2016, 120, 8370–8379. [Google Scholar] [CrossRef]
- Mérida, F.; Chiu-Lam, A.; Bohórquez, A.C.; Maldonado-Camargo, L.; Pérez, M.-E.; Pericchi, L.; Torres-Lugo, M.; Rinaldi, C. Optimization of synthesis and peptization steps to obtain iron oxide nanoparticles with high energy dissipation rates. J. Magn. Magn. Mater. 2015, 394, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Nemati, Z.; Alonso, J.; Rodrigo, I.; Das, R.; Garaio, E.; García, J.Á.; Orue, I.; Phan, M.-H.; Srikanth, H. Improving the Heating Efficiency of Iron Oxide Nanoparticles by Tuning Their Shape and Size. J. Phys. Chem. C 2018, 122, 2367–2381. [Google Scholar] [CrossRef]
- Singh, A.; Kumar, P.; Pathak, S.; Jain, K.; Garg, P.; Pant, M.; Mahapatro, A.K.; Rath, D.; Wang, L.; Kim, S.-K.; et al. A threefold increase in SAR performance for magnetic hyperthermia by compositional tuning in zinc-substituted iron oxide superparamagnetic nanoparticles with superior biocompatibility. J. Alloys Compd. 2023, 968, 171868. [Google Scholar] [CrossRef]
- Myrovali, E.; Maniotis, N.; Makridis, A.; Terzopoulou, A.; Ntomprougkidis, V.; Simeonidis, K.; Sakellari, D.; Kalogirou, O.; Samaras, T.; Salikhov, R.; et al. Arrangement at the nanoscale: Effect on magnetic particle hyperthermia. Sci. Rep. 2016, 6, 37934. [Google Scholar] [CrossRef] [PubMed]
- Mehdaoui, B.; Meffre, A.; Lacroix, L.M.; Carrey, J.; Lachaize, S.; Gougeon, M.; Respaud, M.; Chaudret, B. Large specific absorption rates in the magnetic hyperthermia properties of metallic iron nanocubes. J. Magn. Magn. Mater. 2010, 322, L49–L52. [Google Scholar] [CrossRef]
- Fatima, H.; Charinpanitkul, T.; Kim, K.-S. Fundamentals to Apply Magnetic Nanoparticles for Hyperthermia Therapy. Nanomaterials 2021, 11, 1203. [Google Scholar] [CrossRef] [PubMed]
- Kahil, H.; Faramawy, A.; El-Sayed, H.; Abdel-Sattar, A. Magnetic Properties and SAR for Gadolinium-Doped Iron Oxide Nanoparticles Prepared by Hydrothermal Method. Crystals 2021, 11, 1153. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Silvestri, N.; Fernandez-Cabada, T.; Marinaro, F.; Fernandes, S.; Fiorito, S.; Miscuglio, M.; Serantes, D.; Ruta, S.; Livesey, K.; et al. Exploiting Unique Alignment of Cobalt Ferrite Nanoparticles, Mild Hyperthermia, and Controlled Intrinsic Cobalt Toxicity for Cancer Therapy. Adv. Mater. 2020, 32, e2003712. [Google Scholar] [CrossRef]
- Khurshid, H.; Alonso, J.; Nemati, Z.; Phan, M.H.; Mukherjee, P.; Fdez-Gubieda, M.L.; Barandiarán, J.M.; Srikanth, H. Anisotropy effects in magnetic hyperthermia: A comparison between spherical and cubic exchange-coupled FeO/Fe3O4 nanoparticles. J. Appl. Phys. 2015, 117, 17A337. [Google Scholar] [CrossRef]
- Thanh, T.D.; Manh, D.H.; Phong, L.T.H.; Bach, T.N.; Nam, P.H.; Anh, N.T.N.; Ky, V.H.; Le, N.T.H.; Thi, T.M. Iron oxide nanoparticles synthesized by coprecipitation method: Impacts of zinc doping and surface functionalization by polyvinylpyrrolidone on the magnetic properties and heating efficiency. Ceram. Int. 2025, 51, 1448–1455. [Google Scholar] [CrossRef]
- Oroskhani, N.; Amini, S.M.; Shirvalilou, S.; Khodaie, M.; Mahdavi, S.R. Anti-Proliferative Activity of Poloxamer Cobalt Ferrite Nanoparticles against Human Prostate Cancer (DU-145) Cells: In-Vitro Study. IET Nanobiotechnol. 2024, 2024, 8929168. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Zheng, K.; Deng, L.; Yang, L.; Zhang, N.; Xu, C.; Ran, H.; Wang, Z.; Wang, Z.; et al. Injectable PLGA/Fe3O4 implants carrying cisplatin for synergistic magnetic hyperthermal ablation of rabbit VX2 tumor. PLoS ONE 2017, 12, e0177049. [Google Scholar] [CrossRef]
- Shirvalilou, S.; Khoei, S.; Esfahani, A.J.; Kamali, M.; Shirvaliloo, M.; Sheervalilou, R.; Mirzaghavami, P. Magnetic Hyperthermia as an adjuvant cancer therapy in combination with radiotherapy versus radiotherapy alone for recurrent/progressive glioblastoma: A systematic review. J. Neuro-Oncol. 2021, 152, 419–428. [Google Scholar] [CrossRef]
- Kampinga, H.H.; Dikomey, E. Hyperthermic radiosensitization: Mode of action and clinical relevance. Int. J. Radiat. Biol. 2001, 77, 399–408. [Google Scholar] [CrossRef]
- Krawczyk, P.M.; Eppink, B.; Essers, J.; Stap, J.; Rodermond, H.; Odijk, H.; Zelensky, A.; van Bree, C.; Stalpers, L.J.; Buist, M.R.; et al. Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc. Natl. Acad. Sci. USA 2011, 108, 9851–9856. [Google Scholar] [CrossRef]
- Karakatsanis, A.; Daskalakis, K.; Stålberg, P.; Olofsson, H.; Andersson, Y.; Eriksson, S.; Bergkvist, L.; Wärnberg, F. Superparamagnetic iron oxide nanoparticles as the sole method for sentinel node biopsy detection in patients with breast cancer. Br. J. Surg. 2017, 104, 1675–1685. [Google Scholar] [CrossRef]
- Lee, D.; Sohn, J.; Kirichenko, A. Quantifying Liver Heterogeneity via R2*-MRI with Super-Paramagnetic Iron Oxide Nanoparticles (SPION) to Characterize Liver Function and Tumor. Cancers 2022, 14, 5269. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutiérrez, L.; Morales, M.P.; Böhm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Leiner, T.; Ho, K.Y.J.A.M.; Ho, V.B.; Bongartz, G.; Mali, W.P.T.M.; Rasch, W.; van Engelshoven, J.M.A. Multicenter phase-II trial of safety and efficacy of NC100150 for steady-state contrast-enhanced peripheral magnetic resonance angiography. Eur. Radiol. 2003, 13, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Weishaupt, D.; Rühm, S.G.; Binkert, C.A.; Schmidt, M.; Patak, M.A.; Steybe, F.; McGill, S.; Debatin, J.F. Equilibrium-Phase MR Angiography of the Aortoiliac and Renal Arteries Using a Blood Pool Contrast Agent. Am. J. Roentgenol. 2000, 175, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Montiel Schneider, M.G.; Lassalle, V.L. Magnetic iron oxide nanoparticles as novel and efficient tools for atherosclerosis diagnosis. Biomed. Pharmacother. 2017, 93, 1098–1115. [Google Scholar] [CrossRef]
- Taupitz, M.; Wagner, S.; Schnorr, J.; Kravec, I.; Pilgrimm, H.; Bergmann-Fritsch, H.; Hamm, B. Phase I Clinical Evaluation of Citrate-coated Monocrystalline Very Small Superparamagnetic Iron Oxide Particles as a New Contrast Medium for Magnetic Resonance Imaging. Investig. Radiol. 2004, 39, 394–405. [Google Scholar] [CrossRef]
- Wagner, M.; Wagner, S.; Schnorr, J.; Schellenberger, E.; Kivelitz, D.; Krug, L.; Dewey, M.; Laule, M.; Hamm, B.; Taupitz, M. Coronary MR angiography using citrate-coated very small superparamagnetic iron oxide particles as blood-pool contrast agent: Initial experience in humans. J. Magn. Reson. Imaging 2011, 34, 816–823. [Google Scholar] [CrossRef]
- Teshome, M.; Wei, C.; Hunt, K.K.; Thompson, A.; Rodriguez, K.; Mittendorf, E.A. Use of a Magnetic Tracer for Sentinel Lymph Node Detection in Early-Stage Breast Cancer Patients: A Meta-analysis. Ann. Surg. Oncol. 2016, 23, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Engberink, R.D.O.; van der Pol, S.M.A.; Walczak, P.; van der Toorn, A.; Viergever, M.A.; Dijkstra, C.D.; Bulte, J.W.M.; de Vries, H.E.; Blezer, E.L.A. Magnetic Resonance Imaging of Monocytes Labeled with Ultrasmall Superparamagnetic Particles of Iron Oxide Using Magnetoelectroporation in an Animal Model of Multiple Sclerosis. Mol. Imaging 2010, 9, 268–277. [Google Scholar] [CrossRef]
- Blumenstein, I.; Shanbhag, S.; Langguth, P.; Kalra, P.A.; Zoller, H.; Lim, W. Newer formulations of intravenous iron: A review of their chemistry and key safety aspects–hypersensitivity, hypophosphatemia, and cardiovascular safety. Expert. Opin. Drug Saf. 2021, 20, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Tassa, C.; Shaw, S.Y.; Weissleder, R. Dextran-Coated Iron Oxide Nanoparticles: A Versatile Platform for Targeted Molecular Imaging, Molecular Diagnostics, and Therapy. Acc. Chem. Res. 2011, 44, 842–852. [Google Scholar] [CrossRef]
- Jin, R.; Lin, B.; Li, D.; Ai, H. Superparamagnetic iron oxide nanoparticles for MR imaging and therapy: Design considerations and clinical applications. Curr. Opin. Pharmacol. 2014, 18, 18–27. [Google Scholar] [CrossRef]
- Lima-Tenório, M.K.; Gómez Pineda, E.A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Magnetic nanoparticles: In vivo cancer diagnosis and therapy. Int. J. Pharm. 2015, 493, 313–327. [Google Scholar] [CrossRef]
- Nardecchia, S.; Sánchez-Moreno, P.; de Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical Trials of Thermosensitive Nanomaterials: An Overview. Nanomaterials 2019, 9, 191. [Google Scholar] [CrossRef] [PubMed]
- Lapusan, R.; Borlan, R.; Focsan, M. Advancing MRI with magnetic nanoparticles: A comprehensive review of translational research and clinical trials. Nanoscale Adv. 2024, 6, 2234–2259. [Google Scholar] [CrossRef]
- NanoTherm Therapy System. Available online: https://magforce.de/ (accessed on 18 February 2025).
- Egea-Benavente, D.; Ovejero, J.G.; Morales, M.D.P.; Barber, D.F. Understanding MNPs Behaviour in Response to AMF in Biological Milieus and the Effects at the Cellular Level: Implications for a Rational Design That Drives Magnetic Hyperthermia Therapy toward Clinical Implementation. Cancers 2021, 13, 4583. [Google Scholar] [CrossRef]
- Grauer, O.; Jaber, M.; Heß, K.; Weckesser, M.; Schwindt, W.; Maring, S.; Stummer, W.; Wölfer, J. RTHP-22. inflammatory response after modified nanotherm and radiotherapy of recurrent glioblastoma. Jpn. Soc. Neuro-Oncol. 2016, 18, vi178–vi179. [Google Scholar] [CrossRef]
- Marcus, M.; Smith, A.; Maswadeh, A.; Shemesh, Z.; Zak, I.; Motiei, M.; Schori, H.; Margel, S.; Sharoni, A.; Shefi, O. Magnetic Targeting of Growth Factors Using Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 707. [Google Scholar] [CrossRef] [PubMed]
- Yellen, B.B.; Forbes, Z.G.; Halverson, D.S.; Fridman, G.; Barbee, K.A.; Chorny, M.; Levy, R.; Friedman, G. Targeted drug delivery to magnetic implants for therapeutic applications. J. Magn. Magn. Mater. 2005, 293, 647–654. [Google Scholar] [CrossRef]
- Doubovikov, E.D.; Aksenov, D.P. Brain Tissue Oxygen Dynamics Under Localised Hypoxia in the Awake State and the Physical Neuroprotective Effects of General Anaesthesia. In Oxygen Transport to Tissue XLV; Springer: Cham, Switzerland, 2024; Volume 1463, pp. 35–39. [Google Scholar] [CrossRef]
- Chorny, M.; Fishbein, I.; Yellen, B.B.; Alferiev, I.S.; Bakay, M.; Ganta, S.; Adamo, R.; Amiji, M.; Friedman, G.; Levy, R.J. Targeting stents with local delivery of paclitaxel-loaded magnetic nanoparticles using uniform fields. Proc. Natl. Acad. Sci. USA 2010, 107, 8346–8351. [Google Scholar] [CrossRef]
- Pislaru, S.V.; Harbuzariu, A.; Agarwal, G.; Witt, T.; Gulati, R.; Sandhu, N.P.; Mueske, C.; Kalra, M.; Simari, R.D.; Sandhu, G.S. Magnetic forces enable rapid endothelialization of synthetic vascular grafts. Circulation 2006, 114 (Suppl. S1), I314–I318. [Google Scholar] [CrossRef]
- Pislaru, S.V.; Harbuzariu, A.; Gulati, R.; Witt, T.; Sandhu, N.P.; Simari, R.D.; Sandhu, G.S. Magnetically Targeted Endothelial Cell Localization in Stented Vessels. J. Am. Coll. Cardiol. 2006, 48, 1839–1845. [Google Scholar] [CrossRef]
- Consigny, P.M.; Silverberg, D.A.; Vitali, N.J. Use of Endothelial Cells Containing Superparamagnetic Microspheres to Improve Endothelial Cell Delivery to Arterial Surfaces after Angioplasty. J. Vasc. Interv. Radiol. 1999, 10, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Polyak, B.; Fishbein, I.; Chorny, M.; Alferiev, I.; Williams, D.; Yellen, B.; Friedman, G.; Levy, R.J. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc. Natl. Acad. Sci. USA 2008, 105, 698–703. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef]


| Synthesis Method | Available Alterations | Industrial Scalability | Neuro-Oncology Applications | Clinical Feasibility |
|---|---|---|---|---|
| Co-Precipitation | Limited size control
| Promising for large scale
| Superficial brain tumors | Yes. Scalable, simple, and low-cost. Limited precision may restrict use to applications where deep targeting is not required. |
| Thermal Decomposition | Precise control of size, shape, and uniformity
| Challenging for large-scale
| Deep-seated tumors | Yes (conditionally). Highly controlled nanoparticles ideal for precision applications like deep brain targeting, but scale up cost is a challenge. |
| Hydrothermal | Moderate size control
| Very promising for industrial-scale
| Deep-seated tumors | Yes. Scalable and flexible, suitable for clinical production and adaptation. |
| Microemulsion | Precise size control
| Not producible at an industrial scale
| Limited use in neuro-oncology (conceptually applicable to deep tumors) | No. Cost and complexity make it impractical for clinical production. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varalli, L.; Berlet, R.; Abenojar, E.; McDaid, J.; Gascoigne, D.A.; Bailes, J.; Aksenov, D.P. Applications and Efficacy of Iron Oxide Nanoparticles in the Treatment of Brain Tumors. Pharmaceutics 2025, 17, 499. https://doi.org/10.3390/pharmaceutics17040499
Varalli L, Berlet R, Abenojar E, McDaid J, Gascoigne DA, Bailes J, Aksenov DP. Applications and Efficacy of Iron Oxide Nanoparticles in the Treatment of Brain Tumors. Pharmaceutics. 2025; 17(4):499. https://doi.org/10.3390/pharmaceutics17040499
Chicago/Turabian StyleVaralli, London, Reed Berlet, EC Abenojar, John McDaid, David A. Gascoigne, Julian Bailes, and Daniil P. Aksenov. 2025. "Applications and Efficacy of Iron Oxide Nanoparticles in the Treatment of Brain Tumors" Pharmaceutics 17, no. 4: 499. https://doi.org/10.3390/pharmaceutics17040499
APA StyleVaralli, L., Berlet, R., Abenojar, E., McDaid, J., Gascoigne, D. A., Bailes, J., & Aksenov, D. P. (2025). Applications and Efficacy of Iron Oxide Nanoparticles in the Treatment of Brain Tumors. Pharmaceutics, 17(4), 499. https://doi.org/10.3390/pharmaceutics17040499








