The Development and Characterization of an Andiroba Oil-Based Nanoemulsion (Carapa guianensis, Aubl.): Insights into Its Physico-Chemical Features and In Vitro Potential Healing Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Andiroba Fixed Oil Characterization
2.2.1. Gas Chromatography–Mass Spectrometry (GC-MS)
2.2.2. Nuclear Magnetic Resonance (NMR) Analysis
2.2.3. Peroxide and Acidity Index
2.2.4. Methods for Determining Antioxidant Activity
2.2.5. Total Phenolic Content and Total Carotenoids
2.3. Development of Andiroba Fixed Oil-Based Nanoemulsion (NeAnd)
2.4. Physicochemical Characterization of NeAnd
2.4.1. Colloidal Characteristics
2.4.2. Colloidal Stability of NeAnd over Time and at Different pHs
2.4.3. Morphology of NeAnd by Transmission Electron Microscopy (TEM)
2.4.4. Infrared Spectrophotometry (FTIR) Analysis of NeAnd
2.4.5. Raman Analysis of NeAnd
2.5. Cell Culture
2.5.1. Cytotoxicity Assay
2.5.2. Scratch Assay
2.6. Statistical Analyses
3. Results
3.1. Chemical Characterization of Andiroba Oil
3.1.1. Nuclear Magnetic Resonance (NMR) Analysis of Andiroba Oil
3.1.2. Antioxidant Activity, Total Phenolic Content, and Carotenoid Content of Andiroba Oil
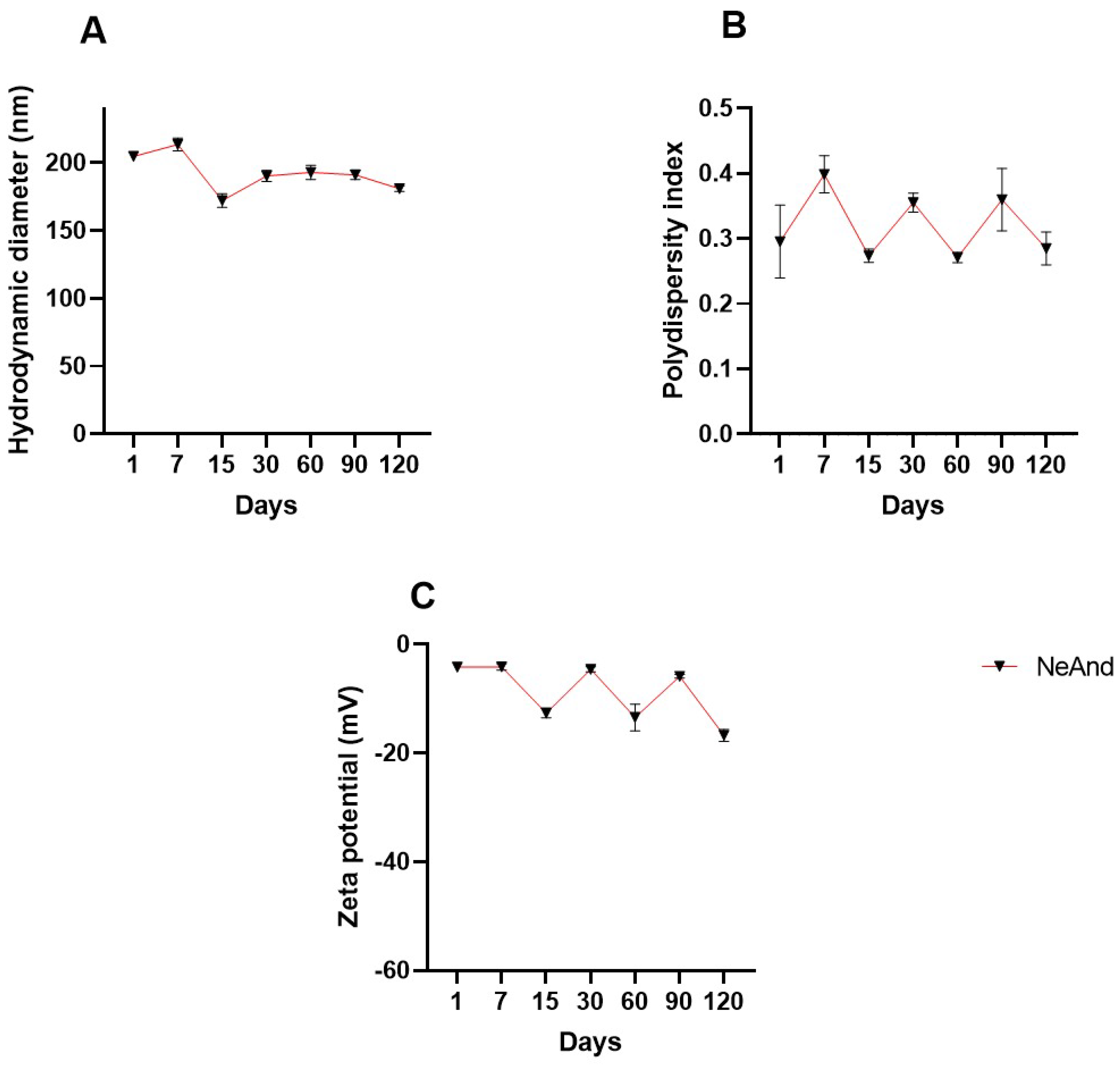
3.2. Hydrodynamic Diameter, Polydispersity Index, and Zeta Potential of NeAnd
3.2.1. Stability Evaluation of NeAnd Under pH Stress
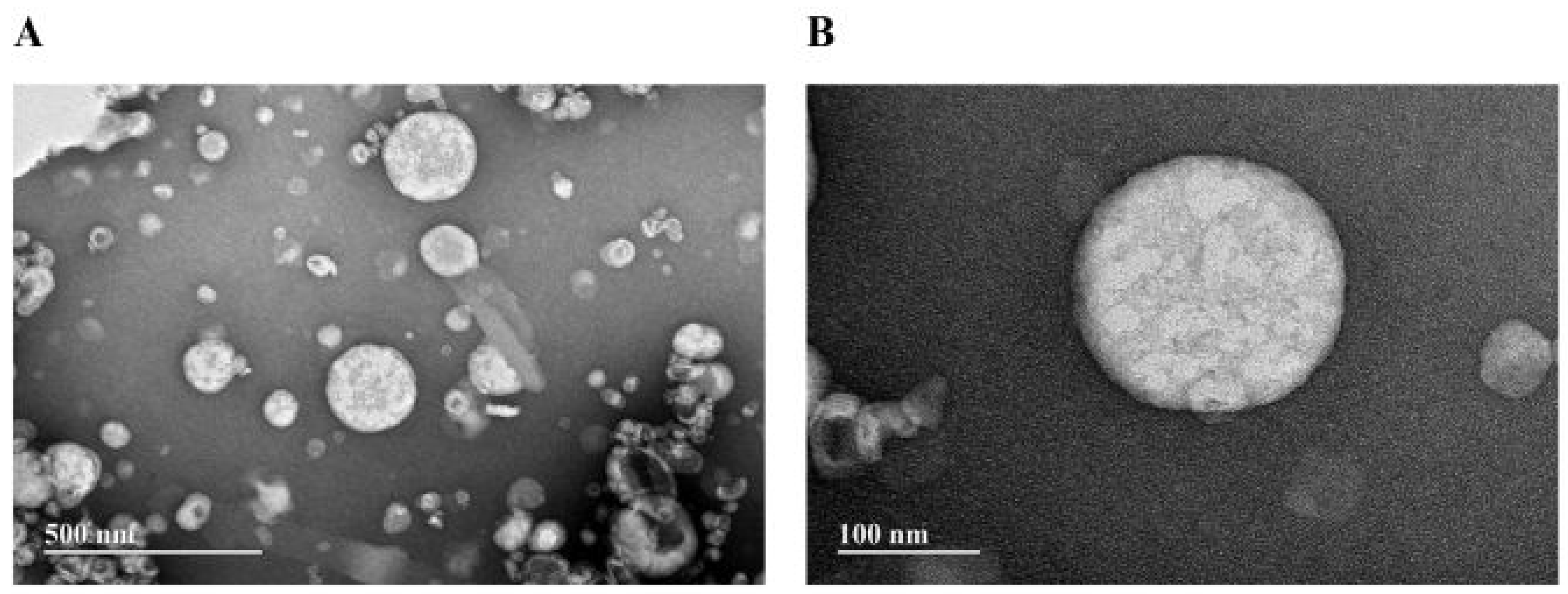
3.2.2. Transmission Electron Microscopy (TEM) of NeAnd
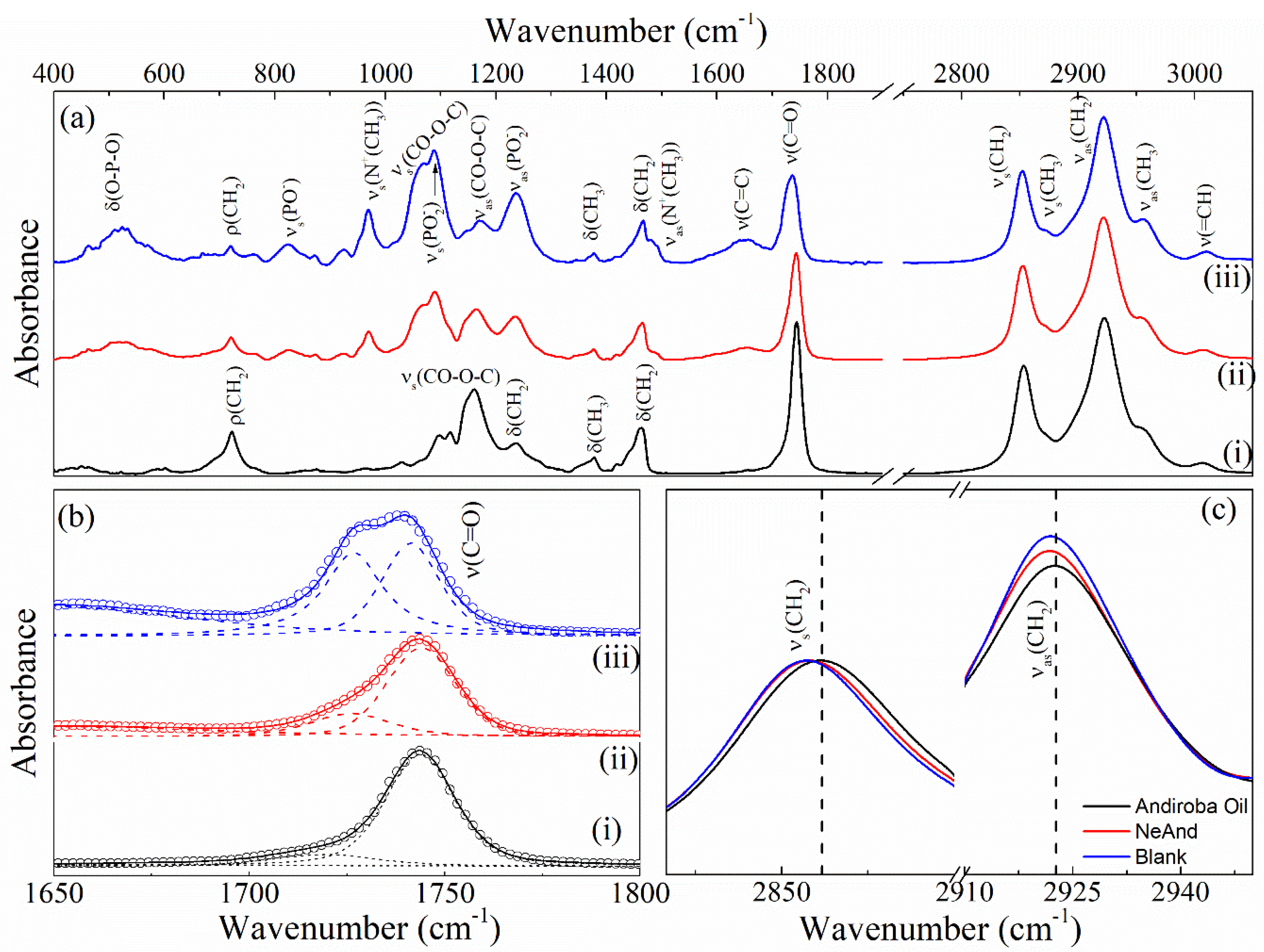
3.2.3. Infrared Spectra (FTIR) of NeAnd
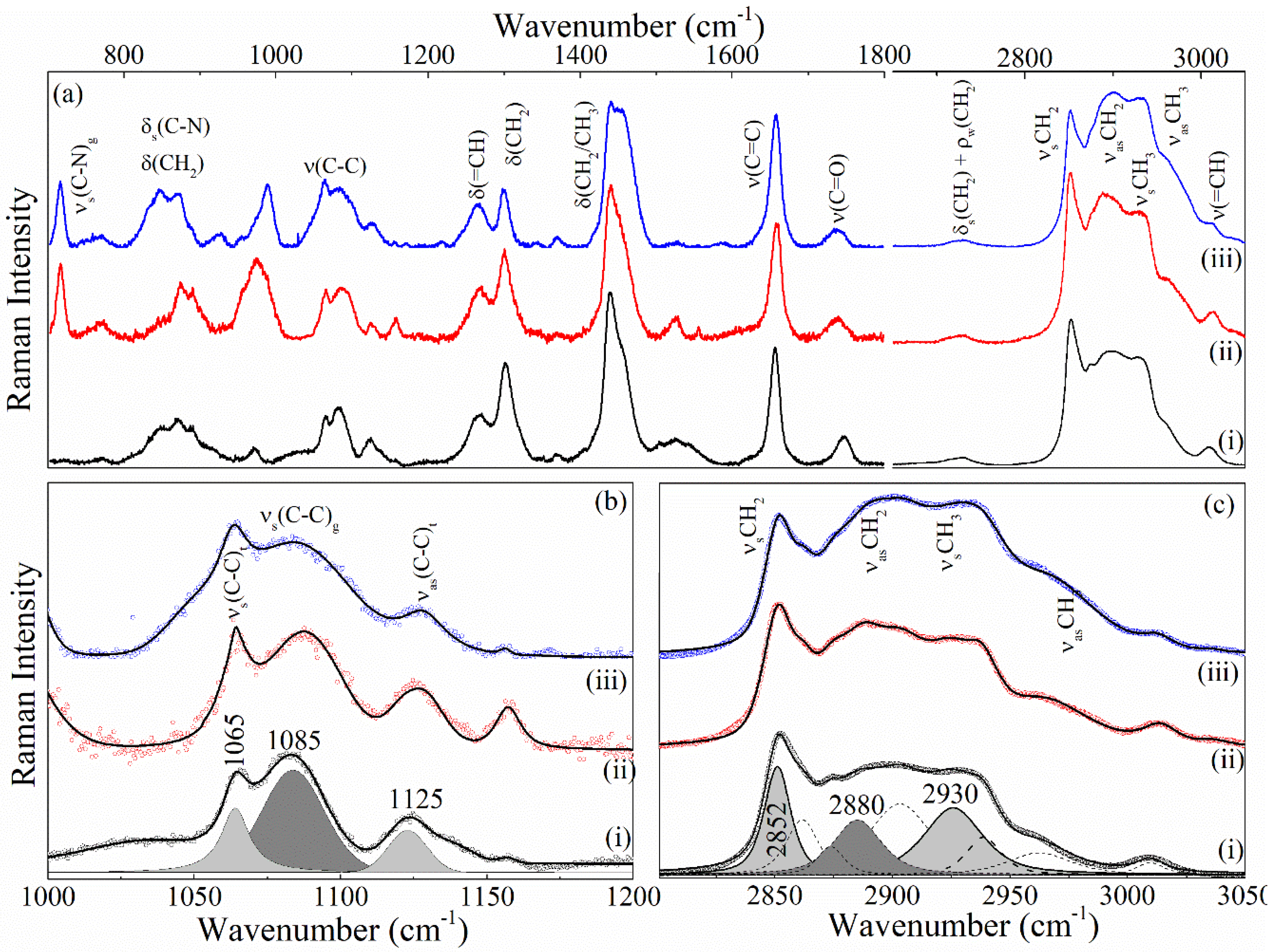
3.2.4. Raman of NeAnd
3.3. Cytotoxicity of NeAnd in Human Keratinocytes In Vitro
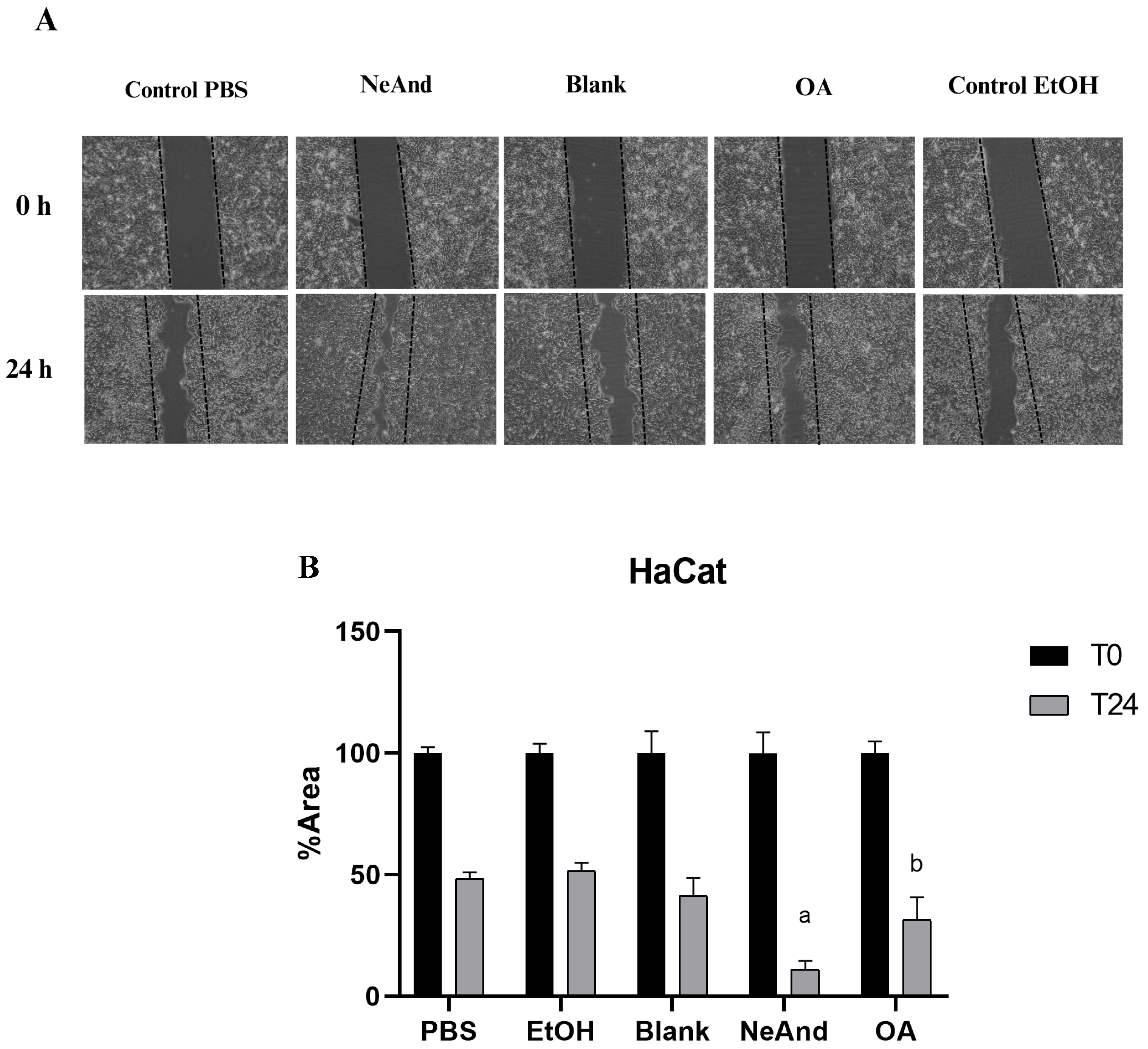
3.4. Scratch Assay in Keratinocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Dias, K.K.B.; Cardoso, A.L.; Costa, K.M.P.; de Souza, A.A.F.; Passos, M.F.; Costa, C.E.F.; Rocha-Filho, G.N.; Andrade, E.H.A.; Luque, R.; Nascimento, L.A.S.; et al. Biological activities from andiroba (Carapa guianensis Aublet.) and its biotechnological applications: A systematic review. Arab. J. Chem. 2023, 16, 104629. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Jiang, T.; Li, Q.; Qiu, J.; Du, S.; Xu, X.; Wu, Z.; Yang, X.; Chen, Z.; Chen, T. Nanobiotechnology: Applications in Chronic Wound Healing. Int. J. Nanomed. 2022, 17, 3125–3145. [Google Scholar] [CrossRef]
- Chopra, H.; Mohanta, Y.K.; Mahanta, S.; Mohanta, T.K.; Singh, I.; Avula, S.K.; Mallick, S.P.; Rabaan, A.A.; AlSaihati, H.; Alsayyah, A.; et al. Recent updates in nanotechnological advances for wound healing: A narrative review. Nanotechnol. Rev. 2023, 12, 20230129. [Google Scholar] [CrossRef]
- Lucca, L.G.; Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga, V.F., Jr.; Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory Effect from a Hydrogel Containing Nanoemulsified Copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech 2018, 19, 522–530. [Google Scholar] [CrossRef]
- Blanco-Fernandez, B.; Castaño, O.; Mateos-Timoneda, M.Á.; Engel, E.; Pérez-Amodio, S. Nanotechnology approaches in chronic wound healing. Adv. Wound Care 2020, 9, 585–600. [Google Scholar] [CrossRef]
- Bajerski, L.; Michels, L.R.; Colomé, L.M.; Bender, E.A.; Freddo, R.J.; Bruxel, F.; Haas, S.E. The use of Brazilian vegetable oils in nanoemulsions: An update on preparation and biological applications. Braz. J. Pharm. Sci. 2016, 52, 347–363. [Google Scholar] [CrossRef]
- Giácomo, R.G.; Pereira, M.G.G.; Silva, C.F.; Gaia-Gomes, J.H. Litter and carbon deposition in secondary forest, Sabia and Andiroba plantations. Floresta 2017, 47, 187–196. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Fernandes, M.M.P.; Mesquita, M.N.; Cruz, A.C.L.; Pelizzari, C.; Neves, E.C.; Peruquetti, R.C.; Berón, M.M.; Viott, A.M.; Souza, S.F. Therapeutic laser with or without andiroba oil in treating cutaneous wounds by second intention in Wistar rats. Acta Vet. Bras. 2021, 15, 316–322. [Google Scholar] [CrossRef]
- Chia, C.Y.; Medeiros, A.D.; Corraes, A.M.S.; Manso, J.E.F.; Silva, C.S.C.; Takiya, C.M.; Vanz, R.L. Healing effect of andiroba-based emulsion in cutaneous wound healing via modulation of inflammation and transforming growth factor beta 3. Acta Cir. Bras. 2018, 33, 1000–1015. [Google Scholar] [CrossRef]
- Souza, B.A.A.; Braga, L.A.; Lopes, R.L.O.; Ribeiro-Junior, R.F.G.; Nascimento, L.N.S.; Cavalcante, L.C.C.; Monteiro, A.M.; Couteiro, R.P.; Yasojima, E.Y.; Hamoy, M. Effects of Andiroba oil (Carapa guianensis) on wound healing in alloxan-diabetic rats. Int. Arch. Med. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Silva, C.E.S.; Santos, O.J.; Ribas-Filho, J.M.; Tabushi, F.I.; Kume, M.H.; Jukonis, L.B.; Cella, I.F. Effect of Carapa guianensis Aublet (Andiroba) and Orbignya phalerata (Babassu) in colonic healing in rats. Ver. Cor. Bras. Cir. 2023, 42, 399–406. [Google Scholar] [CrossRef]
- Araújo, A.L.; Teixeira, F.A.; Lacerda, T.F.; Flecher, M.A.; Souza, V.R.C.; Coelho, C.S. Effects of topical application of pure and ozonized andiroba oil on experimentally induced wounds in horses. Braz. J. Vet. Res. Anim. Sci. 2017, 54, 66–74. [Google Scholar] [CrossRef]
- Ombredane, A.S.; Araújo, V.H.S.; Borges, C.O.; Costa, P.L.; Landim, M.G.; Pinheiro, A.C.; Szlachetka, I.O.; Benedito, L.E.C.; Espindola, L.S.; Dias, D.J.S.; et al. Nanoemulsion-based systems as a promising approach for enhancing the antitumoral activity of pequi oil (Caryocar brasilense Cambess.) in breast cancer cells. J. Drug. Deliv. Sci. Technol. 2020, 58, 101819. [Google Scholar] [CrossRef]
- Allaw, M.; Manconi, M.; Caboni, P.; Bacchetta, G.; Escribano-Ferrer, E.; Peris, J.E.; Nacher, A.; Diez-Sales, O.; Manca, M.L. Formulation of liposomes loading lentisk oil to ameliorate topical delivery, attenuate oxidative stress damage and improve cell migration in scratch assay. Biomed. Pharmacother. 2021, 144, 112351. [Google Scholar] [CrossRef]
- Morganti, P. Bionanotechnology & Bioeconomy for a Greener Development. J. Appl. Cosmetol. 2015, 33, 51–65. [Google Scholar]
- Nasser, K.; Petru-Cristian, N. View of Nanotechnology: A Tiny Solution for the Big Challenges in Agriculture. Int. J. Res. Adv. Agric. Sci. 2023, 2, 14–26. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; American Oil Chemists’ Society: Champaign, IL, USA, 2004. [Google Scholar]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Pérez-Jiménez, J.; Saura-Calixto, F.; Mancini-Filho, J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010, 121, 996–1002. [Google Scholar] [CrossRef]
- Medina, M.B. Determination of the total phenolics in juices and superfruits by a novel chemical method. J. Funct. Foods. 2011, 3, 79–87. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. A Guide to Carotenoid Analysis in Foods; ILSI Human Nutrition Institute, ILSI PRESS: Washington, DC, USA, 2001; ISBN 1-57881-072-8. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Liakopoulou, A.; Mourelatou, E.; Hatziantoniou, S. Exploitation of traditional healing properties, using the nanotechnology’s advantages: The case of curcumin. Toxicol. Rep. 2021, 8, 1143–1155. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Figueiroa, F.T.; Clavijo, C.; Arbeláez, P.; Cruz, J.C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- Santos, O.N.A.; Folegatti, M.V.; Dutra, L.M.; Andrade, I.P.S.; Fanaya, E.D., Jr.; Lena, B.P.; Barison, A.; Santos, A.D.C. Tracking lipid profiles of Jatropha curcas L. seeds under different pruning types and water managements by low-field and HR-MAS NMR spectroscopy. Ind. Crops Prod. 2017, 109, 918–922. [Google Scholar] [CrossRef]
- Miyake, Y.; Yokomizo, K.; Matsuzaki, N. Determination of unsaturated fatty acid composition by high-resolution nuclear magnetic resonance spectroscopy. JAOCS 1998, 75, 1091–1094. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar] [CrossRef]
- Santana, F.B.; Mazivila, S.J.; Gontijo, L.C.; Neto, W.B.; Poppi, R.J. Rapid Discrimination Between Authentic and Adulterated Andiroba Oil Using FTIR-HATR Spectroscopy and Random Forest. Food Anal. Methods 2018, 11, 1927–1935. [Google Scholar] [CrossRef]
- Lozano-Garzón, K.; Orduz-Díaz, L.L.; Guerrero-Perilla, C.; Quintero-Mendoza, W.; Carrillo, M.P.; Cardona-Jaramillo, J.E.C. Comprehensive Characterization of Oils and Fats of Six Species from the Colombian Amazon Region with Industrial Potential. Biomolecules 2023, 13, 985. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Sena, I.S.; Magalhães, K.F.; Oliveira, S.L.; Ferreira, I.M.; Porto, A.L.M. Amazon Oils from Andiroba (Carapa sp.) and Babassu (Orbignya sp.) for Preparation Biodiesel by Enzymatic Catalysis. Curr. Biotechnol. 2018, 7, 428–437. [Google Scholar] [CrossRef]
- Viera, W.; Shinohara, T.; Samaniego, I.; Sanada, A.; Terada, N.; Ron, L.; Suárez-Tapia, A.; Koshio, K. Phytochemical Composition and Antioxidant Activity of Passiflora spp. Germplasm Grown in Ecuador. Plants 2022, 11, 328. [Google Scholar] [CrossRef]
- Barros, H.E.A.; Natarelli, C.V.L.; Tavares, I.M.C.; Oliveira, A.L.M.; Araújo, A.B.S.; Pereira, J.; Carvalho, E.E.N.; Vilas-Boas, E.V.B.; Franco, M. Nutritional Clustering of Cookies Developed with Cocoa Shell, Soy, and Green Banana Flours Using Exploratory Methods. Food Bioproc. Technol. 2020, 13, 1566–1578. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Al-Bar, O.A.M.; Al-Najada, A.R. Chemical modification of curcumin: Solubility and antioxidant capacity. Int. J. Food Prop. 2017, 20, 718–724. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Ascrizzi, R.; Flamini, G.; Kilinçarslan, O.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Kala, N.S.; Ramasubbu, R. Chemical composition, antimicrobial and antioxidant properties of essential oils of Trichopus zeylanicus ssp. travancoricus. Indian J. Nat. Prod. Resour. 2021, 12, 570–577. [Google Scholar]
- da Costa, C.A.R.; Machado, G.G.L.; Rodrigues, L.J.; Barros, H.E.A.; Natarelli, C.V.L.; Vilas-Boas, E.V.B. Phenolic compounds profile and antioxidant activity of purple passion fruit’s pulp, peel and seed at different maturation stages. Sci. Hortic. 2023, 321, 112244. [Google Scholar] [CrossRef]
- John, S.; Monica, S.J.; Priyadarshini, S.; Sivaraj, C.; Arumugam, P. Antioxidant and Antibacterial Activities of Beta vulgaris L. Peel Extracts. Int. J. Pharm. Sci. Res. 2017, 5, 1974–1979. [Google Scholar] [CrossRef]
- Ribeiro, P.P.C.; Damasceno, K.S.F.S.C.; Veras, B.O.; Oliveira, J.R.S.; Lima, V.L.M.; Assis, C.R.D.; Silva, M.V.; Júnior, F.C.S.; Assis, C.F.; Padilha, C.E.A.; et al. Chemical and biological activities of faveleira (Cnidoscolus quercifolius Pohl) seed oil for potential health applications. Food Chem. 2021, 337, 127771. [Google Scholar] [CrossRef]
- Seck, I.; Hosu, A.; Cimpoiu, C.; Ndoye, S.F.; Ba, L.A.; Sall, C.; Seck, M. Phytochemicals content, screening and antioxidant/pro-oxidant activities of Carapa procera (barks) (Meliaceae). S. Afr. J. Bot. 2021, 137, 369–376. [Google Scholar] [CrossRef]
- Araujo-Lima, C.F.; Fernandes, A.S.; Gomes, E.M.; Oliveira, L.L.; Macedo, A.F.; Antoniassi, R.; Wilhelm, A.E.; Aiub, C.A.F.; Felzenszwalb, I. Antioxidant activity and genotoxic assessment of crabwood (andiroba, Carapa guianensis Aublet) seed oils. Oxidative Med. Cell Longev. 2018, 2018, 3246719. [Google Scholar] [CrossRef] [PubMed]
- Vasco, C.; Ruales, J.; Kamal-Eldin, A. Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chem. 2008, 111, 816–823. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Castón, M.J. Nutritional Importance of Carotenoids and Their Effect on Liver Health: A Review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Miller, A.P.; Coronel, J.; Amengual, J. The role of β-carotene and vitamin A in atherogenesis: Evidences from preclinical and clinical studies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2020, 1865, 158635. [Google Scholar] [CrossRef]
- Fadilah, N.I.M.; Phang, S.J.; Kamaruzaman, N.; Salleh, A.; Zawani, M.; Sanyal, A.; Maarof, M.; Fauzi, M.B. Antioxidant Biomaterials in Cutaneous Wound Healing and Tissue Regeneration: A Critical Review. Antioxidants 2023, 12, 787. [Google Scholar] [CrossRef]
- Klang, V.; Valenta, C. Lecithin-based nanoemulsions. J. Drug Deliv. Sci. Technol. 2011, 21, 55–76. [Google Scholar] [CrossRef]
- Fuentes, K.; Matamala, C.; Martínez, N.; Zúñiga, R.N.; Troncoso, E. Comparative Study of Physicochemical Properties of Nanoemulsions Fabricated with Natural and Synthetic Surfactants. Processes 2021, 9, 2002. [Google Scholar] [CrossRef]
- Choi, S.J.; McClements, D.J. Nanoemulsions as delivery systems for lipophilic nutraceuticals: Strategies for improving their formulation, stability, functionality and bioavailability. Food Sci. Biotechnol. 2020, 29, 149–168. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Chanamai, R.; Horn, G.; McClements, D.J. Influence of oil polarity on droplet growth in oil-in-water emulsions stabilized by a weakly adsorbing biopolymer or a nonionic surfactant. J. Colloid Interface Sci. 2002, 247, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Avramescu, M.L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2016, 24, 1553–1564. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, H.; Yang, X.; Jia, M.; Li, Y.; Huang, Y.; Lin, J.; Wu, S.; Hou, Z. Mitomycin C-soybean phosphatidylcholine complex-loaded self-assembled PEG-Lipid-PLA hybrid nanoparticles for targeted drug delivery and dual-controlled drug release. Mol. Pharm. 2014, 11, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Ahangar, L.E.; Movassaghi, K.; Yaghoobi, F. The pH Role in Nanotechnology, Electrochemistry, and Nano-Drug Delivery. Iran. J. Chem. Chem. Eng. 2022, 41, 2175–2188. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential–what they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Ferreira, B.S.; Almeida, C.G.; Hyaric, M.L.; Oliveira, V.E.; Edwards, H.G.M.; Oliveira, L.F.C. Raman spectroscopic investigation of carotenoids in oils from Amazonian products. Spectrosc. Lett. 2013, 46, 122–127. [Google Scholar] [CrossRef]
- Akita, C.; Kawaguchi, T.; Kaneko, F. Structural Study on Polymorphism of Cis-Unsaturated Triacylglycerol: Triolein. J. Phys. Chem. B 2006, 110, 4346–4353. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Membrane lipid phase transitions and phase organization studied by Fourier transform infrared spectroscopy. Biochim. Biophys. Acta 2013, 1828, 2347–2358. [Google Scholar] [CrossRef]
- Silva, D.F.; Lima, K.T.; Bastos, G.N.; Oliveira, J.A.R.; Nascimento, L.A.S.; Costa, C.E.F.; Filho, G.N.R.; Concha, V.O.C.; Passos, M.F. PCL/Andiroba Oil (Carapa guianensis Aubl.) Hybrid Film for Wound Healing Applications. Polymers 2021, 13, 1591. [Google Scholar] [CrossRef]
- Lewis, R.N.A.H.; McElhaney, R.N. Fourier Transform Infrared Spectroscopy in the Study of Lipid Phase Transitions in Model and Biological Membranes. Methods Mol. Biol. 2007, 400, 207–226. [Google Scholar] [CrossRef]
- Rohman, A.; Man, Y.B.C. The use of Fourier transform mid infrared (FT-MIR) spectroscopy for detection and quantification of adulteration in virgin coconut oil. Food Chem. 2011, 129, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Tantipolphan, R.; Rades, T.; Strachan, C.J.; Gordon, K.C.; Medlicott, N.J. Analysis of lecithin–cholesterol mixtures using Raman spectroscopy. J. Pharm. Biomed. Anal. 2006, 41, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Rousseau, D. Molecular order and thermodynamics of the solid–liquid transition in triglycerides via Raman spectroscopy. Phys. Chem. Chem. Phys. 2008, 10, 4606–4613. [Google Scholar] [CrossRef]
- Csiszár, A.; Koglin, E.; Meier, R.J.; Klumpp, E. The phase transition behavior of 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC) model membrane influenced by 2,4-dichlorophenol—An FT-Raman Spectroscopy Study. Chem. Phys. Lipids 2006, 139, 115–124. [Google Scholar] [CrossRef]
- Milhomem-Paixão, S.S.R.; Fascineli, M.L.; Muehlmann, L.A.; Melo, K.M.; Salgado, H.L.C.; Joanitti, G.A.; Pieczarka, J.C.; Azevedo, R.B.; Santos, A.S.; Grisolia, C.K. Andiroba Oil (Carapa guianensis Aublet) Nanoemulsions: Development and Assessment of Cytotoxicity, Genotoxicity, and Hematotoxicity. J. Nanomater. 2017, 17, 362046. [Google Scholar] [CrossRef]
- Porfírio-Dias, C.L.; Melo, K.M.; Bastos, C.E.M.C.; Ferreira, T.A.A.; Azevedo, L.F.C.; Salgado, H.L.; Santos, A.S.; Rissino, J.D.; Nagamachi, C.Y.; Pieczarka, J.C. Andiroba oil (Carapa guianensis Aubl.) shows cytotoxicity but no mutagenicity in the ACPP02 gastric cancer cell line. J. Appl. Toxicol. 2020, 40, 1060–1066. [Google Scholar] [CrossRef]
- Fonseca, A.S.A.D.; Monteiro, I.S.; Santos, C.R.; Carneiro, M.L.B.; Morais, S.S.; Araújo, P.L.; Santana, T.F.; Joanitti, G.A. Effects of andiroba oil (Carapa guianensis Aublet) on the immune system in inflammation and wound healing: A scoping review. J. Ethnopharmacol. 2024, 327, 118004. [Google Scholar] [CrossRef]
- Tuji, F.M.; Figueiredo, P.B.A.; Cavalcante, G.H.S.; Burbano, R.M.R. Evaluation of The Anti-Inflammatory Action of Andiroba Oil—Carapa guianensis aubl (Meliceae) in Oral Mucositis. In Pharmacological Studies in Natural Oral Care; Chauhan, D.N., Singh, P.R., Chauhan, N.S., Shah, K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2023. [Google Scholar] [CrossRef]
- Cardoso, C.R.B.; Souza, M.A.; Ferro, E.A.V.; Favoreto, S., Jr.; Pena, J.D.O. Influence of topical administration of n-3 and n-6 essential and n-9 nonessential fatty acids on the healing of cutaneous wounds. Wound Repair Regen. 2004, 12, 235–243. [Google Scholar] [CrossRef]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of oleic and linoleic acids on the inflammatory phase of wound healing in rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]




| Parameter | Value Obtained | |
|---|---|---|
| Phosphomolybdenum complex | 741.47 ± 23.23 | mg/100 g |
| DPPH• | 41.28 ± 1.45 | discoloration % |
| TPC | 338.92 ± 8.55 | mg/100 g |
| α-Carotene | 1.88 ± 0.28 | μg/g |
| β-Carotene | 1.86 ± 0.32 | μg/g |
| δ-Carotene | 1.76 ± 0.51 | μg/g |
| γ-Carotene | 2.05 ± 0.42 | μg/g |
| Lycopene | 1.15 ± 0.50 | μg/g |
| Total carotenoids | 8.69 ± 1.42 | μg/g |
| Samples | I1065/I1085 | I1125/I1085 | I2880/I2850 | I2880/I2930 |
|---|---|---|---|---|
| Andiroba oil | 0.63 | 0.40 | 0.38 | 0.81 |
| Blank | 1.12 | 0.60 | 0.50 | 1.06 |
| NeAnd | 1.02 | 0.59 | 0.52 | 1.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, I.d.S.; Fonseca, A.S.A.; dos Santos, C.R.; de Carvalho, J.P.S.; da Silva, S.W.; Veiga-Junior, V.F.; Ribeiro, R.; Vieira, I.J.C.; Nogueira, T.S.R.; da Costa, C.A.R.; et al. The Development and Characterization of an Andiroba Oil-Based Nanoemulsion (Carapa guianensis, Aubl.): Insights into Its Physico-Chemical Features and In Vitro Potential Healing Effects. Pharmaceutics 2025, 17, 498. https://doi.org/10.3390/pharmaceutics17040498
Monteiro IdS, Fonseca ASA, dos Santos CR, de Carvalho JPS, da Silva SW, Veiga-Junior VF, Ribeiro R, Vieira IJC, Nogueira TSR, da Costa CAR, et al. The Development and Characterization of an Andiroba Oil-Based Nanoemulsion (Carapa guianensis, Aubl.): Insights into Its Physico-Chemical Features and In Vitro Potential Healing Effects. Pharmaceutics. 2025; 17(4):498. https://doi.org/10.3390/pharmaceutics17040498
Chicago/Turabian StyleMonteiro, Isolda de Sousa, Aimê Stefany Alves Fonseca, Carolina Ramos dos Santos, João Paulo Santos de Carvalho, Sebastião William da Silva, Valdir F. Veiga-Junior, Rayssa Ribeiro, Ivo José Curcino Vieira, Thalya Soares Ribeiro Nogueira, Carlos Alexandre Rocha da Costa, and et al. 2025. "The Development and Characterization of an Andiroba Oil-Based Nanoemulsion (Carapa guianensis, Aubl.): Insights into Its Physico-Chemical Features and In Vitro Potential Healing Effects" Pharmaceutics 17, no. 4: 498. https://doi.org/10.3390/pharmaceutics17040498
APA StyleMonteiro, I. d. S., Fonseca, A. S. A., dos Santos, C. R., de Carvalho, J. P. S., da Silva, S. W., Veiga-Junior, V. F., Ribeiro, R., Vieira, I. J. C., Nogueira, T. S. R., da Costa, C. A. R., Machado, G. G. L., Souza, L. R., Boas, E. V. B. V., Morais, S. S., Almeida, J. R. G. d. S., Dutra, L. M., Santos, V. L. d. A., Silva, A. O., Sousa, M. H., ... Joanitti, G. A. (2025). The Development and Characterization of an Andiroba Oil-Based Nanoemulsion (Carapa guianensis, Aubl.): Insights into Its Physico-Chemical Features and In Vitro Potential Healing Effects. Pharmaceutics, 17(4), 498. https://doi.org/10.3390/pharmaceutics17040498










