N-Acetylcysteine to Reduce Kidney and Liver Injury Associated with Drug-Resistant Tuberculosis Treatment
Abstract
:1. Introduction
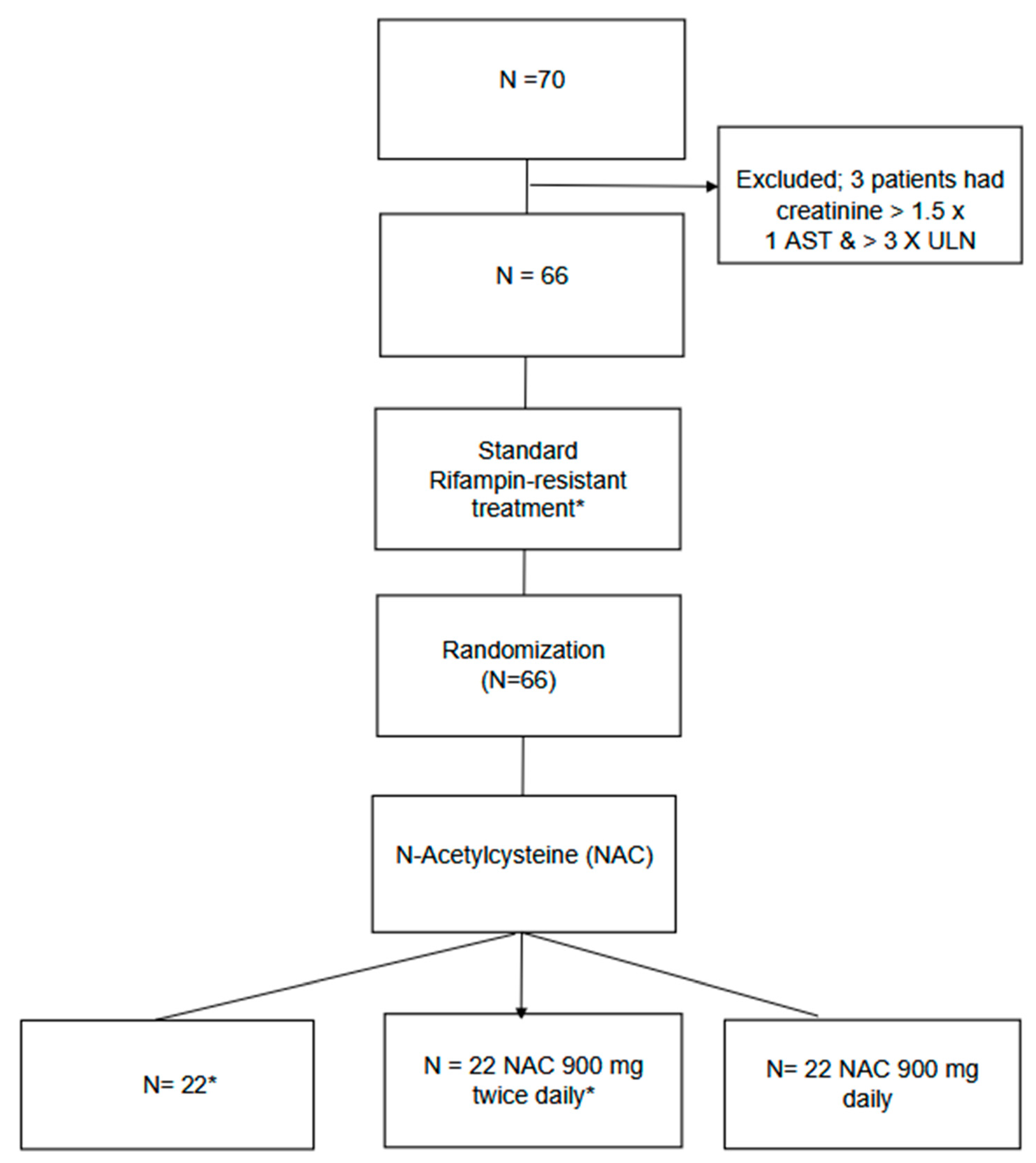
2. Materials and Methods
2.1. Ethical Considerations
Consent for Publications
2.2. Interventions
2.3. Outcome Measures
2.4. Statistical Analysis
Metadata
3. Results
3.1. Incidence of Adverse Events
3.2. Time to Individual Adverse Events
4. Discussions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Tuberculosis Report. 2023. Available online: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (accessed on 6 May 2024).
- WHO Announces Updated Definitions of Extensively Drug-Resistant Tuberculosis. Available online: https://www.who.int/news/item/27-01-2021-who-announces-updated-definitions-of-extensively-drug-resistant-tuberculosis (accessed on 14 December 2023).
- Ormerod, L.P. Multidrug-resistant tuberculosis (MDR-TB): Epidemiology, prevention and treatment. Br. Med. Bull. 2005, 73–74, 17–24. [Google Scholar] [CrossRef] [PubMed]
- WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment—Drug-Resistant Tuberculosis Treatment, 2022 Update. World Health Organization. Available online: http://www.ncbi.nlm.nih.gov/books/NBK588564/ (accessed on 16 November 2023).
- Jang, J.G.; Chung, J.H. Diagnosis and treatment of multidrug-resistant tuberculosis. Yeungnam Univ. J. Med. 2020, 37, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kushemererwa, O.; Nuwagira, E.; Kiptoo, J.; Yadesa, T.M. Adverse drug reactions and associated factors in multidrug-resistant tuberculosis: A retrospective review of patient medical records at Mbarara Regional Referral Hospital, Uganda. SAGE Open Med. 2023, 11, 20503121231171350. [Google Scholar] [CrossRef]
- Coleman, J.J.; Pontefract, S.K. Adverse drug reactions. Clin. Med. 2016, 16, 481–485. [Google Scholar] [CrossRef]
- Nunn, A.J.; Phillips, P.P.; Meredith, S.K.; Chiang, C.-Y.; Conradie, F.; Dalai, D.; Van Deun, A.; Dat, P.-T.; Langley, I.; Master, I.; et al. A Trial of a Shorter Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med. 2019, 380, 1201–1213. [Google Scholar] [CrossRef]
- Conradie, F.; Bagdasaryan, T.R.; Borisov, S.; Howell, P.; Mikiashvili, L.; Ngubane, N.; Samoilova, A.; Skornykova, S.; Tudor, E.; Variava, E.; et al. Bedaquiline–Pretomanid–Linezolid Regimens for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 810–823. [Google Scholar] [CrossRef]
- Bezu, H.; Seifu, D.; Yimer, G.; Mebrhatu, T. Prevalence and Risk Factors of Adverse Drug Reactions Associated Multidrug Resistant Tuberculosis Treatments in Selected Treatment Centers in Addis Ababa Ethiopia. J. Tuberc. Res. 2014, 2, 144–154. [Google Scholar] [CrossRef]
- TB Prevalence in Tanzania|National Tuberculosis & Leprosy Programme. Available online: https://ntlp.go.tz/tuberculosis/tb-prevalence-in-tanzania/ (accessed on 15 November 2023).
- Mpagama, S.G.; Mvungi, H.C.; Mbelele, P.M.; Semvua, H.H.; Liyoyo, A.A.; de Guex, K.P.; Sloan, D.; Kibiki, G.S.; Boeree, M.; Phillips, P.P.J.; et al. Protocol for a feasibility randomized controlled trial to evaluate the efficacy, safety and tolerability of N-acetylcysteine in reducing adverse drug reactions among adults treated for multidrug-resistant tuberculosis in Tanzania. Pilot Feasibility Stud. 2023, 9, 55. [Google Scholar] [CrossRef]
- Karimi, P.; Guantai, A.; Kigondu, C.; Ogaro, T. Adverse Drug Reactions Among Patients Being Treated For Multi-Drug Resistant Tuberculosis in Nairobi City County Health Facilities. Pharmaceut. J. Kenya 2017, 23, 61–65. [Google Scholar]
- Lan, Z.; Ahmad, N.; Baghaei, P.; Barkane, L.; Benedetti, A.; Brode, S.K.; Brust, J.C.M.; Campbell, J.R.; Chang, V.W.L.; Falzon, D.; et al. Drug-associated adverse events in the treatment of multidrug-resistant tuberculosis: An individual patient data meta-analysis. Lancet Respir. Med. 2020, 8, 383–394. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, A.; Gupta, N. Adverse Drug Reactions with First-Line and Second-Line Drugs in Treatment of Tuberculosis. Ann. Natl. Acad. Med. Sci. 2021, 57, 15–35. [Google Scholar] [CrossRef]
- Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Mapamba, D.A.; Sauli, E.; Mrema, L.; Lalashowi, J.; Magombola, D.; Buza, J.; Olomi, W.; Wallis, R.S.; Ntinginya, N.E. Impact of N-Acetyl Cysteine (NAC) on Tuberculosis (TB) Patients—A Systematic Review. Antioxidants 2022, 11, 2298. [Google Scholar] [CrossRef] [PubMed]
- Ejigu, D.A.; Abay, S.M. N-Acetyl Cysteine as an Adjunct in the Treatment of Tuberculosis. Tuberc. Res. Treat. 2020, 2020, 5907839. [Google Scholar] [CrossRef] [PubMed]
- Mahakalkar, S.; Nagrale, D.; Gaur, S.; Urade, C.; Murhar, B.; Turankar, A. N-acetylcysteine as an add-on to Directly Observed Therapy Short-I therapy in fresh pulmonary tuberculosis patients: A randomized, placebo-controlled, double-blinded study. Perspect. Clin. Res. 2017, 8, 132–136. [Google Scholar] [CrossRef]
- Safe, I.P.; Lacerda, M.V.G.; Printes, V.S.; Marins, A.F.P.; Rabelo, A.L.R.; Costa, A.A.; Tavares, M.A.; Jesus, J.S.; Souza, A.B.; Beraldi-Magalhães, F.; et al. Safety and efficacy of N-acetylcysteine in hospitalized patients with HIV-associated tuberculosis: An open-label, randomized, phase II trial (RIPENACTB Study). PLoS ONE 2020, 15, e0235381. [Google Scholar] [CrossRef]
- Huang, J.W.; Lahey, B.; Clarkin, O.J.; Kong, J.; Clark, E.; Kanji, S.; McCudden, C.; Akbari, A.; Chow, B.J.; Shabana, W.; et al. A Systematic Review of the Effect of N-Acetylcysteine on Serum Creatinine and Cystatin C Measurements. Kidney Int. Rep. 2021, 6, 396–403. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, J.; Lei, L.; Xue, Y.; Liu, L.; Huang, H.; Chen, S.; Liu, Y.; Lin, Y.; Tao, J.; et al. Effect of N-acetylcysteine on prevention of contrast-associated acute kidney injury in patients with STEMI undergoing primary percutaneous coronary intervention: A systematic review and meta-analysis of randomised controlled trials. BMJ Open 2020, 10, e039009. [Google Scholar] [CrossRef]
- Xie, W.; Liang, X.; Lin, Z.; Liu, M.; Ling, Z. Latest Clinical Evidence About Effect of Acetylcysteine on Preventing Contrast-Induced Nephropathy in Patients Undergoing Angiography: A Meta-Analysis. Angiology 2021, 72, 105–121. [Google Scholar] [CrossRef]
- Ahmed, S.; Rao, N.A. Role of N-acetylcysteine(NAC) in preventing development of anti tuberculosis therapy(ATT) induced liver injury in pulmonary tuberculosis(PTB) patients, a simple randomized single blind clinical trial. Eur. Respir. J. 2020, 56 (Suppl. S64), 5301. [Google Scholar] [CrossRef]
- Sanabria-Cabrera, J.; Tabbai, S.; Niu, H.; Alvarez-Alvarez, I.; Licata, A.; Björnsson, E.; Andrade, R.J.; Lucena, M.I. N-Acetylcysteine for the Management of Non-Acetaminophen Drug-Induced Liver Injury in Adults: A Systematic Review. Front. Pharmacol. 2022, 13, 876868. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, D.; Usharani, P.; Paramjyothi, G.; Subbalaxmi, M.; Sireesha, K.; Ali, M.A. A study to evaluate the hepatoprotective effect of N- acetylcysteine on anti tuberculosis drug induced hepatotoxicity and quality of life. Indian J. Tuberc. 2023, 70, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Kalolo, A.; Lalashowi, J.; Pamba, D.; Shayo, P.J.; Gitige, C.; Mvungi, H.; Ntagazwa, W.; Lekule, I.; Kisonga, R.; Mleoh, L.; et al. Implementation of the ‘Removed Injectable modified Short-course regimens for EXpert Multidrug Resistant Tuberculosis’ (RISE study) in Tanzania: A protocol for a mixed-methods process evaluation. BMJ Open 2022, 12, e054434. [Google Scholar] [CrossRef]
- Santana-Santos, E.; Gowdak, L.H.W.; Gaiotto, F.A.; Puig, L.B.; Hajjar, L.A.; Zeferino, S.P.; Drager, L.F.; Shimizu, M.H.M.; Bortolotto, L.A.; De Lima, J.J. High Dose of N-Acetylcystein Prevents Acute Kidney Injury in Chronic Kidney Disease Patients Undergoing Myocardial Revascularization. Ann. Thorac. Surg. 2014, 97, 1617–1623. [Google Scholar] [CrossRef]
- Javaherforooshzadeh, F.; Shaker, Z.; Rashidi, M.; Akhondzadeh, R.; Hayati, F. The effect of N-acetyl cysteine injection on renal function after coronary artery bypass graft surgery: A randomized double blind clinical trial. J. Cardiothorac. Surg. 2021, 16, 161. [Google Scholar] [CrossRef]
- Modarresi, A.; Ziaie, S.; Salamzadeh, J.; Sahraei, Z.; Nafar, M.; Panahi, Y.; Parvin, M.; Einollahi, B. Study of The Effects of N-Acetylcysteine on Oxidative Stress Status of Patients on Maintenance-Hemodialysis Undergoing Cadaveric Kidney Transplantation. Iran J. Pharm. Res. 2017, 16, 1631–1638. [Google Scholar] [PubMed]
- Hernández-Cruz, E.Y.; Aparicio-Trejo, O.E.; Hammami, F.A.; Bar-Shalom, D.; Tepel, M.; Pedraza-Chaverri, J.; Scholze, A. N-acetylcysteine in Kidney Disease: Molecular Mechanisms, Pharmacokinetics, and Clinical Effectiveness. Kidney Int. Rep. 2024, 9, 2883–2903. [Google Scholar] [CrossRef]
- Cepaityte, D.; Leivaditis, K.; Varouktsi, G.; Roumeliotis, A.; Roumeliotis, S.; Liakopoulos, V. N-Acetylcysteine: More than preventing contrast-induced nephropathy in uremic patients—Focus on the antioxidant and anti-inflammatory properties. Int. Urol. Nephrol. 2023, 55, 1481–1492. [Google Scholar] [CrossRef]
- Efrati, S.; Dishy, V.; Averbukh, M.; Blatt, A.; Krakover, R.; Weisgarten, J.; Morrow, J.D.; Stein, M.C.; Golik, A. The effect of N-acetylcysteine on renal function, nitric oxide, and oxidative stress after angiography. Kidney Int. 2003, 64, 2182–2187. [Google Scholar] [CrossRef]
- Tan, Y.K.; Luo, H.; Kang, G.S.; Teoh, K.L.; Kofidis, T. N-Acetylcysteine’s Renoprotective Effect in Cardiac Surgery: A Systematic Review and Meta-Analysis. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 138–145. [Google Scholar] [CrossRef]
- Nyang’wa, B.-T.; Berry, C.; Kazounis, E.; Motta, I.; Parpieva, N.; Tigay, Z.; Solodovnikova, V.; Liverko, I.; Moodliar, R.; Dodd, M.; et al. A 24-Week, All-Oral Regimen for Rifampin-Resistant Tuberculosis. N. Engl. J. Med. 2022, 387, 2331–2343. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.W.; Qadeer, A.; Munawar, K.; Qureshi, S.S.; Khan, M.T.; Abdullah, A.; Bano, S.; Shad, Z.S. Determining the Incidence of Acute Kidney Injury Using the RIFLE Criteria in the Medical Intensive Care Unit in a Tertiary Care Hospital Setting in Pakistan. Cureus 2019, 11, e4071. [Google Scholar] [CrossRef]
- Yaqub, S.; Hashmi, S.; Kazmi, M.K.; Ali, A.A.; Dawood, T.; Sharif, H. A Comparison of AKIN, KDIGO, and RIFLE Definitions to Diagnose Acute Kidney Injury and Predict the Outcomes after Cardiac Surgery in a South Asian Cohort. Cardiorenal Med. 2022, 12, 29–38. [Google Scholar] [CrossRef]
- Acute Kidney Injury—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S0140673619325632?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0140673619325632%3Fshowall%3Dtrue&referrer=https:%2F%2Fpubmed.ncbi.nlm.nih.gov%2F (accessed on 29 June 2024).
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, S.; Eftekhari, P.; Tabarsi, P.; Fahimi, F.; Raoufy, M.R.; Masjedi, M.R.; Velayati, A.A. Protective effect of N-acetylcysteine on antituberculosis drug-induced hepatotoxicity. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Hatami, B.; Abdi, S.; Pourhoseingholi, M.A.; Eghlimi, H.; Rabbani, A.H.; Masoumi, M.; Hajimohammadebrahim-Ketabforoush, M. The effects of N-acetylcysteine on hepatic, hematologic, and renal parameters in cirrhotic patients: A randomized controlled trial. Gastroenterol. Hepatol Bed Bench. 2023, 16, 432–440. [Google Scholar]
- Popescu, M.; Bratu, A.; Agapie, M.; Borjog, T.; Jafal, M.; Sima, R.-M.; Orban, C. The Use and Potential Benefits of N-Acetylcysteine in Non-Acetaminophen Acute Liver Failure: An Etiology-Based Review. Biomedicines 2024, 12, 676. [Google Scholar] [CrossRef]
- Yew, W.W.; Leung, C.C. Antituberculosis drugs and hepatotoxicity. Respirology 2006, 11, 699–707. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Zhang, T.; Wang, Q.; Xie, W. Drug-Induced Liver Injury from Anti-Tuberculosis Treatment: A Retrospective Cohort Study. Med. Sci. Monit. 2020, 26, e920350-1–e920350-8. [Google Scholar] [CrossRef]
- Warmelink, I.; Hacken, N.H.T.; van der Werf, T.S.; van Altena, R. Weight loss during tuberculosis treatment is an important risk factor for drug-induced hepatotoxicity. Br. J. Nutr. 2011, 105, 400–408. [Google Scholar] [CrossRef]
- Guerini, M.; Condrò, G.; Friuli, V.; Maggi, L.; Perugini, P. N-acetylcysteine (NAC) and Its Role in Clinical Practice Management of Cystic Fibrosis (CF): A Review. Pharmaceuticals 2022, 15, 217. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.C.; Noonan, P.K.; Sanabria, C.; Peacock, W.F. Effervescent N-Acetylcysteine Tablets versus Oral Solution N-Acetylcysteine in Fasting Healthy Adults: An Open-Label, Randomized, Single-Dose, Crossover, Relative Bioavailability Study. Curr. Ther. Res. 2016, 83, 1–7. [Google Scholar] [CrossRef]
- Singh, S.; Allwood, B.; Chiyaka, T.; Kleyhans, L.; Naidoo, C.; Moodley, S.; Theron, G.; Segal, L. Immunologic and imaging signatures in post tuberculosis lung disease. Tuberculosis 2022, 136, 102244. [Google Scholar] [CrossRef] [PubMed]
- Ravimohan, S.; Kornfeld, H.; Weissman, D.; Bisson, G.P. Tuberculosis and lung damage: From epidemiology to pathophysiology. Eur. Respir. Rev. 2018, 27, 170077. [Google Scholar] [CrossRef] [PubMed]
- Malefane, L.; Maarman, G. Post-tuberculosis lung disease and inflammatory role players: Can we characterise the myriad inflammatory pathways involved to gain a better understanding? Chem. Interactions 2023, 387, 110817. [Google Scholar] [CrossRef]
- Wallis, R.S.; Sabi, I.; Lalashowi, J.; Bakuli, A.; Mapamba, D.; Olomi, W.; Siyame, E.; Ngaraguza, B.; Chimbe, O.; Charalambous, S.; et al. Adjunctive N-Acetylcysteine and Lung Function in Pulmonary Tuberculosis. NEJM Evid. 2024, 3, evidoa2300332. [Google Scholar] [CrossRef]
- Calverley, P.; Rogliani, P.; Papi, A. Safety of N-Acetylcysteine at High Doses in Chronic Respiratory Diseases: A Review. Drug Saf. 2021, 44, 273–290. [Google Scholar] [CrossRef]
- Oldham, J.M.; Ma, S.-F.; Martinez, F.J.; Anstrom, K.J.; Raghu, G.; Schwartz, D.A.; Valenzi, E.; Witt, L.; Lee, C.; Vij, R.; et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 1475–1482. [Google Scholar] [CrossRef]
- Magner, K.; Ilin, J.V.; Clark, E.G.; Kong, J.W.Y.; Davis, A.; Hiremath, S. Meta-analytic Techniques to Assess the Association Between N-acetylcysteine and Acute Kidney Injury After Contrast Administration. JAMA Netw. Open 2022, 5, e2220671. [Google Scholar] [CrossRef]
- McCudden, C.; Clark, E.G.; Akbari, A.; Kong, J.; Kanji, S.; Hiremath, S. N-Acetylcysteine Interference With Creatinine Measurement: An In Vitro Analysis. Kidney Int. Rep. 2021, 6, 1973–1976. [Google Scholar] [CrossRef]

| Treatment Arm (N = 66) | Control (N = 22) | Daily (N = 22) | Twice-Daily (N = 22) |
|---|---|---|---|
| Age ± SD | 47.8 ± 12.4 | 47.5 ± 12.3 | 47.7 ± 12.3 |
| BMI ± SD | 19.11 ± 2.7 | 19.05 ± 2.7 | 19.12 ± 2.69 |
| Gender male (%) | 17 (77) | 18 (81) | 18 (81) |
| Smoking (%) | 9 (41) | 9 (41) | 9 (41) |
| HIV (%) | 5 (23) | 2 (9) | 4 (18) |
| AST median (IQR) | 27.8 (22.1–40.1) | 28.4 (21.3–34.8) | 34.8 (24.0–69.0) |
| ALT median (IQR) | 21.8 (10.1–28.7) | 20.3 (13.8–27.4) | 26.6 (22.1–30.8) |
| Creatinine median (IQR) | 76.4 (63.0–90.0) | 79.9 (62.8–94.4) | 82.8 (69.1–106.0) |
| Hemoglobin median (IQR) | 13.5 (12.2–15.1) | 14 (12.7–14.9) | 13.0 (11.18–15.0) |
| System |
TAEs in Standard Treatment Group |
Total Patients with at Least One Event in Standard Treatment Group (N = 22) |
TAEs in NAC Daily Group |
Total Patients with at Least One Event in Daily (N = 22) |
TAEs Twice-Daily (AEs) |
Total Patients with at Least one Event in Twice-Daily (N = 22) | TAEs in All Patients Across all Groups | Total Patients with at Least One AE Across All Groups (N = 66) | p-Value | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| central nervous | 0 | 0 | 1 | 1 (5%) | 1 | 1 (5%) | 2 | 2 | 1.000 | 1.000 |
| visual | 0 | 0 | 2 | 2 (9%) | 0 | 0 | 2 | 2 | 0.323 | 0.323 |
| endocrine | 2 | 2 (9) % | 1 | 1 (5%) | 0 | 0 | 3 | 3 | 0.767 | 0.767 |
|
gastro intestinal tract | 5 | 3 (14%) | 4 | 4 (18%) | 11 | 3 (14%) | 20 | 10 | 0.578 | 0.578 |
| hepatic | 2 | 2 (9%) | 2 | 2 (9%) | 2 | 2 (9%) | 6 | 6 | 1.000 | 1.000 |
| renal | 16 | 10 (45%) | 11 | 6 (27%) | 6 | 4 (18%) | 33 | 20 | 0.134 | 0.134 |
| muscular-skeletal | 15 | 6 (27%) | 24 | 6 (27%) | 19 | 7 (32%) | 58 | 19 | 0.703 | 0.703 |
| skin | 3 | 3 (14%) | 1 | 1 (5%) | 0 | 0 | 4 | 3 | 0.312 | 0.312 |
| hematology | 9 | 7 (32%) | 9 | 7 (32%) | 12 | 9 (40%) | 30 | 23 | 0.715 | 0.715 |
| total AEs | 52 (32%) | 55 (35%) | 51 (32%) | 158 | 1.000 | 1.000 | ||||
| SAEs grade 3 or above | ||||||||||
| renal | 4 | 3 (14%) | 3 | 1 (5%) | 1 | 1 (5%) | 8 | 0.606 | 0.606 | |
| hepatic | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| hematology | 2 | 1 (5%) | 1 | 1 (5%) | 3 | 2 (9%) | 4 | 1.000 | 1.000 | |
| Total SAEs | 6 | 4 | 4 | 0.901 | 0.901 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meadows, I.; Mvungi, H.; Salim, K.; Kaswaga, O.; Mbelele, P.; Liyoyo, A.; Semvua, H.; Ngoma, A.; Heysell, S.K.; Mpagama, S.G. N-Acetylcysteine to Reduce Kidney and Liver Injury Associated with Drug-Resistant Tuberculosis Treatment. Pharmaceutics 2025, 17, 516. https://doi.org/10.3390/pharmaceutics17040516
Meadows I, Mvungi H, Salim K, Kaswaga O, Mbelele P, Liyoyo A, Semvua H, Ngoma A, Heysell SK, Mpagama SG. N-Acetylcysteine to Reduce Kidney and Liver Injury Associated with Drug-Resistant Tuberculosis Treatment. Pharmaceutics. 2025; 17(4):516. https://doi.org/10.3390/pharmaceutics17040516
Chicago/Turabian StyleMeadows, Idu, Happiness Mvungi, Kassim Salim, Oscar Kaswaga, Peter Mbelele, Alphonce Liyoyo, Hadija Semvua, Athumani Ngoma, Scott K. Heysell, and Stellah G. Mpagama. 2025. "N-Acetylcysteine to Reduce Kidney and Liver Injury Associated with Drug-Resistant Tuberculosis Treatment" Pharmaceutics 17, no. 4: 516. https://doi.org/10.3390/pharmaceutics17040516
APA StyleMeadows, I., Mvungi, H., Salim, K., Kaswaga, O., Mbelele, P., Liyoyo, A., Semvua, H., Ngoma, A., Heysell, S. K., & Mpagama, S. G. (2025). N-Acetylcysteine to Reduce Kidney and Liver Injury Associated with Drug-Resistant Tuberculosis Treatment. Pharmaceutics, 17(4), 516. https://doi.org/10.3390/pharmaceutics17040516





