Stability and Efficacy of Mucoadhesive Eye Drops Containing Olopatadine HCl: Physicochemical, Functional, and Preclinical In Vivo Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation of OCH Polysaccharide Eye Drops
2.4. Physical Appearance, pH, and Osmolality Measurements
2.5. Surface Tension Measurement
2.6. Rheological Measurements
2.7. In Vitro Mucoadhesive Strength
2.8. Stability Study of the Tested Eye Drops
2.9. Drug Quantification
2.10. In Vivo Efficacy Study
2.11. Statistical Analysis
3. Results and Discussion
3.1. Physical Appearance, pH, and Osmolality Measurements
3.2. Surface Tension
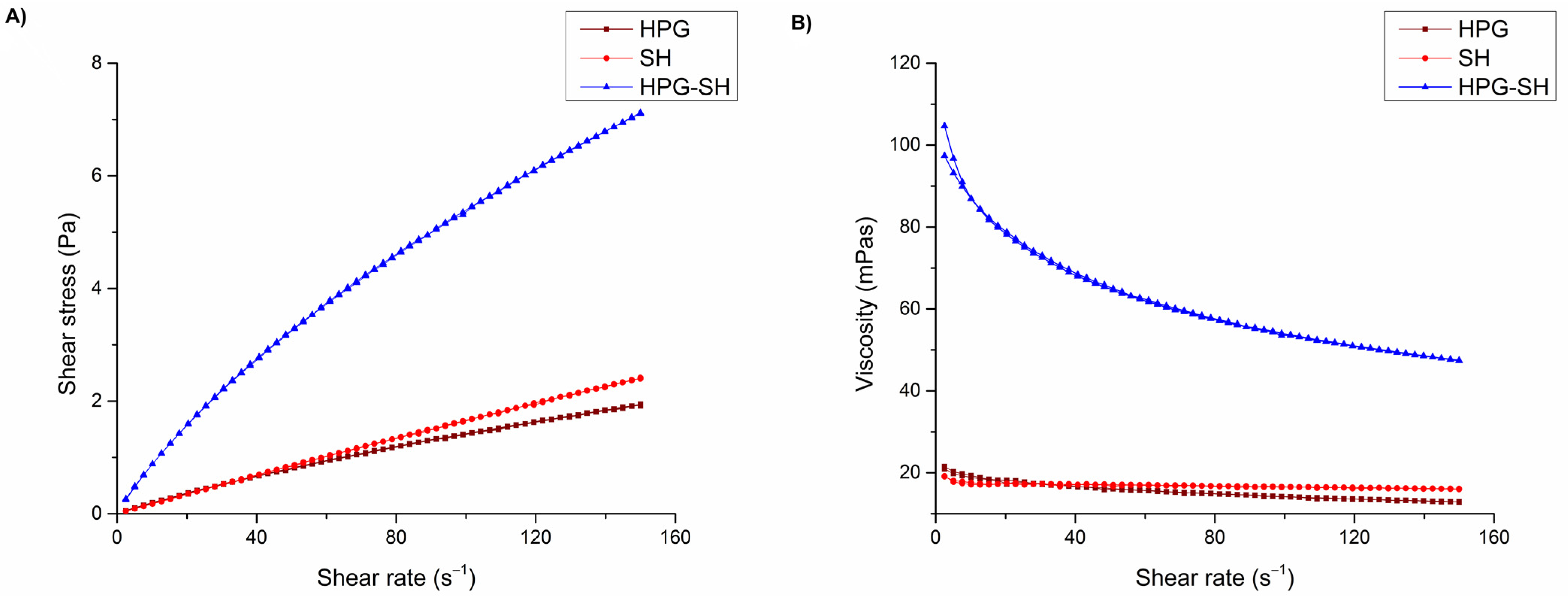
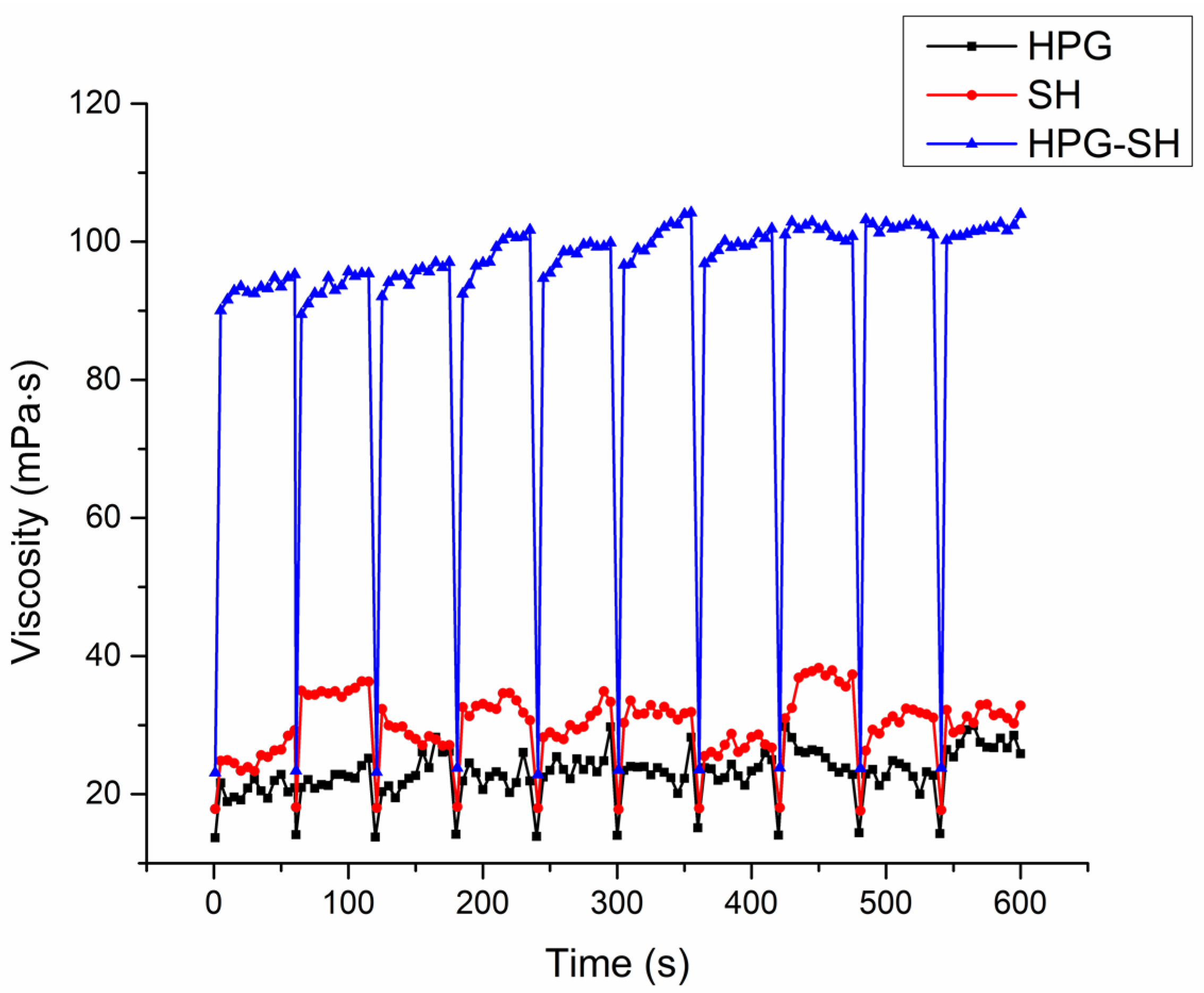
3.3. Rheological Characterization
3.4. Mucoadhesive Strength of the Tested Eye Drops
3.5. Preliminary Stability of the Tested Eye Drops
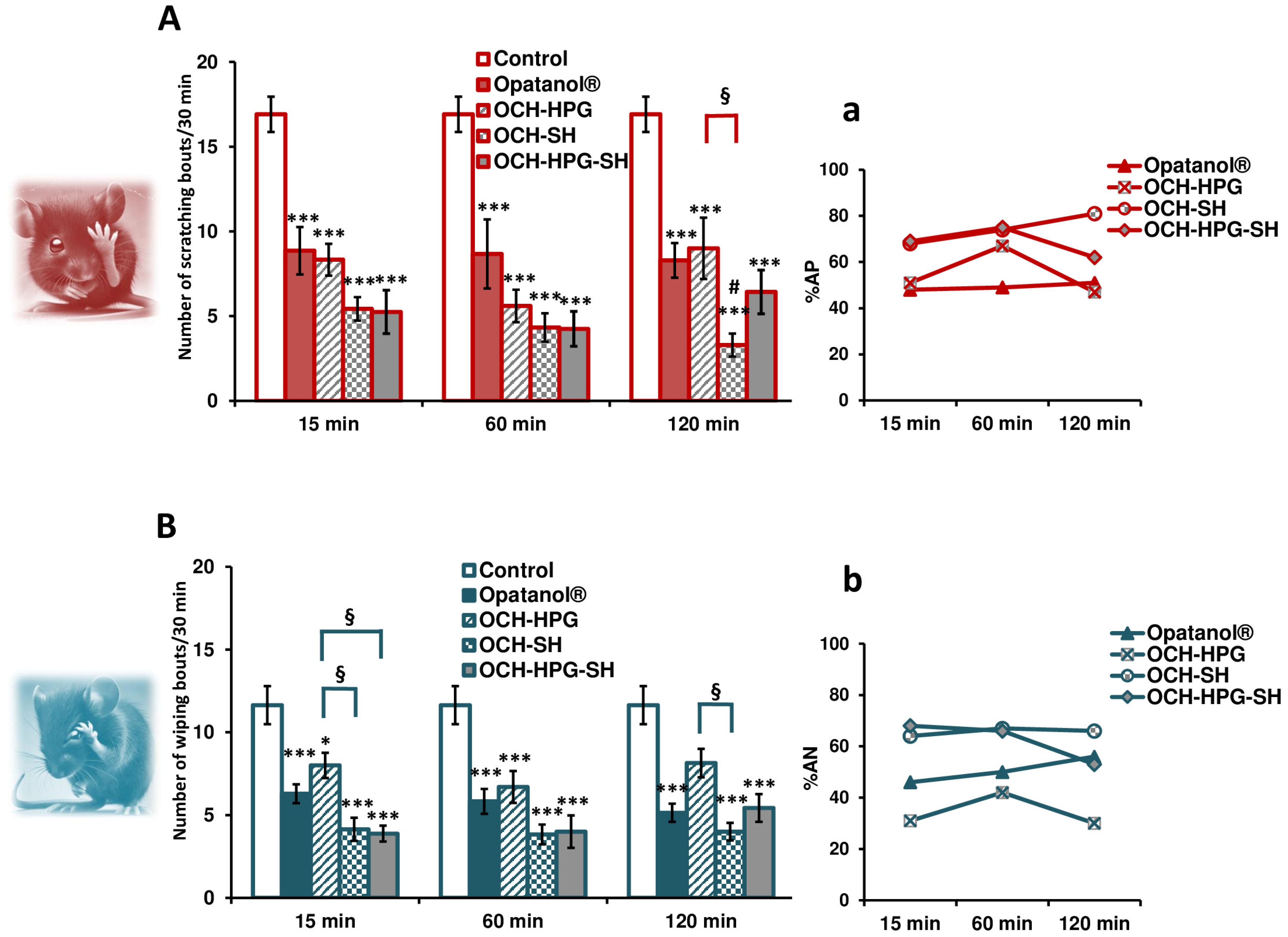
3.6. In Vivo Efficacy Study
3.7. Limitations and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPG | Hydroxypropyl guar gum |
| SH | Sodium hyaluronate |
| OCH | Olopatadine hydrochloride |
| BAC | Benzalkonium chloride |
| HPLC | High-performance liquid chromatography |
| %AP | Percentage of antipruritic activity |
| %AN | Percentage of antinociceptive activity |
References
- Leonardi, A.; Quintieri, L.; Presa, I.J.; LLoves, J.M.; Montero, J.; Benítez-del-Castillo, J.M.; Lestón, F.J.S.; González-Mancebo, E.; Asero, R.; Groblewska, A. Allergic conjunctivitis management: Update on ophthalmic solutions. Curr. Allergy Asthma Rep. 2024, 24, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Güven, U.M.; Başaran, E. In vitro-in vivo evaluation of olopatadine incorporated chitosan nanoparticles for the treatment of ocular allergy. J. Drug Deliv. Sci. Technol. 2021, 64, 102518. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Račić, A.; Čalija, B.; Milić, J.; Dukovski, B.J.; Lovrić, J.; Dobričić, V.; Micov, A.; Vuković, M.; Stepanović-Petrović, R.; Krajišnik, D. Formulation of olopatadine hydrochloride viscous eye drops–physicochemical, biopharmaceutical and efficacy assessment using in vitro and in vivo approaches. Eur. J. Pharm. Sci. 2021, 166, 105906. [Google Scholar] [CrossRef]
- Koutsoviti, M.; Siamidi, A.; Pavlou, P.; Vlachou, M. Recent advances in the excipients used for modified ocular drug delivery. Materials 2021, 14, 4290. [Google Scholar] [CrossRef]
- Lee, S.; Jung, M.-Y.; Park, C.Y. Development of a conjunctival contact–type drug delivery device for latanoprost using hyaluronic acid. Drug Deliv. 2025, 32, 2459775. [Google Scholar] [CrossRef]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of approaches for increasing ophthalmic bioavailability for eye drop formulations. AAPS PharmSciTech 2021, 22, 107. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Cheng, Y.; Brown, K.M.; Prud’homme, R.K. Characterization and intermolecular interactions of hydroxypropyl guar solutions. Biomacromolecules 2002, 3, 456–461. [Google Scholar] [CrossRef]
- The European Pharmacopoeia (Ph. Eur.), 11th ed.; Council of Europe: Strasbourg, France, 2023.
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.; Cairns, D. Preformulation of cysteamine gels for treatment of the ophthalmic complications in cystinosis. Int. J. Pharm. 2016, 515, 575–582. [Google Scholar] [CrossRef]
- Destruel, P.-L.; Zeng, N.; Seguin, J.; Douat, S.; Rosa, F.; Brignole-Baudouin, F.; Dufay, S.; Dufay-Wojcicki, A.; Maury, M.; Mignet, N. Novel in situ gelling ophthalmic drug delivery system based on gellan gum and hydroxyethylcellulose: Innovative rheological characterization, in vitro and in vivo evidence of a sustained precorneal retention time. Int. J. Pharm. 2020, 574, 118734. [Google Scholar] [CrossRef] [PubMed]
- Dukovski, B.J.; Ljubica, J.; Kocbek, P.; Kučuk, M.S.; Krtalić, I.; Hafner, A.; Pepić, I.; Lovrić, J. Towards the development of a biorelevant in vitro method for the prediction of nanoemulsion stability on the ocular surface. Int. J. Pharm. 2023, 633, 122622. [Google Scholar] [CrossRef] [PubMed]
- Račić, A.; Dukovski, B.J.; Lovrić, J.; Dobričić, V.; Vučen, S.; Micov, A.; Stepanović-Petrović, R.; Tomić, M.; Pecikoza, U.; Bajac, J. Synergism of polysaccharide polymers in antihistamine eye drops: Influence on physicochemical properties and in vivo efficacy. Int. J. Pharm. 2024, 655, 124033. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, A.; Lewandowicz, J.; Rojewska, M.; Krüger, K.; Lulek, J.; Prochaska, K. Study of viscoelastic, sorption and mucoadhesive properties of selected polymer blends for biomedical applications. J. Mol. Liq. 2022, 361, 119623. [Google Scholar] [CrossRef]
- Fathalla, Z.M.; Vangala, A.; Longman, M.; Khaled, K.A.; Hussein, A.K.; El-Garhy, O.H.; Alany, R.G. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: Design, characterisation, toxicity and transcorneal permeation studies. Eur. J. Pharm. Biopharm. 2017, 114, 119–134. [Google Scholar] [CrossRef]
- Huang, C.-C.; Kim, Y.S.; Olson, W.P.; Li, F.; Guo, C.; Luo, W.; Huang, A.J.; Liu, Q. A histamine-independent itch pathway is required for allergic ocular itch. J. Allergy Clin. Immunol. 2016, 137, 1267–1270.e1266. [Google Scholar] [CrossRef]
- Huang, C.-C.; Yang, W.; Guo, C.; Jiang, H.; Li, F.; Xiao, M.; Davidson, S.; Yu, G.; Duan, B.; Huang, T. Anatomical and functional dichotomy of ocular itch and pain. Nat. Med. 2018, 24, 1268–1276. [Google Scholar] [CrossRef]
- Le Brun, P.; Kramer, I.; Smith, J.; Woerdenbag, H. (Eds.) Practical Pharmaceutics: An International Guideline for the Preparation, Care and Use of Medicinal Products, 2nd ed.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Hotujac Grgurević, M.; Juretić, M.; Hafner, A.; Lovrić, J.; Pepić, I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev. Ind. Pharm. 2017, 43, 275–282. [Google Scholar] [CrossRef]
- Brockman, H.; Graff, G.; Spellman, J.; Yanni, J. A comparison of the effects of olopatadine and ketotifen on model membranes. Acta Ophthalmol. Scand. 2000, 78, 10–15. [Google Scholar] [CrossRef]
- Ribeiro, W.; Mata, J.L.; Saramago, B. Effect of concentration and temperature on surface tension of sodium hyaluronate saline solutions. Langmuir 2007, 23, 7014–7017. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Silva, C.; Torres, M.; Díaz-Varela, D.; Hilliou, L.; Argence, H. Surface tension and refractive index of guar and tragacanth gums aqueous dispersions at different polymer concentrations, polymer ratios and temperatures. Food Hydrocoll. 2012, 28, 284–290. [Google Scholar] [CrossRef]
- Nagyova, B.; Tiffany, J. Components responsible for the surface tension of human tears. Curr. Eye Res. 1999, 19, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hom, M.M.; Nguyen, A.L.; Bielory, L. Allergic conjunctivitis and dry eye syndrome. Ann. Allergy Asthma Immunol. 2012, 108, 163–166. [Google Scholar] [CrossRef]
- Leonardi, A.; Modugno, R.L.; Salami, E. Allergy and dry eye disease. Ocul. Immunol. Inflamm. 2021, 29, 1168–1176. [Google Scholar] [CrossRef]
- Pahuja, P.; Arora, S.; Pawar, P. Ocular drug delivery system: A reference to natural polymers. Expert Opin. Drug Deliv. 2012, 9, 837–861. [Google Scholar] [CrossRef]
- Zhang, L.-M.; Kong, T. Aqueous polysaccharide blends based on hydroxypropyl guar gum and carboxymethyl cellulose: Synergistic viscosity and thixotropic properties. Colloid Polym. Sci. 2006, 285, 145–151. [Google Scholar] [CrossRef]
- Jiménez, M.M.; Fresno, M.J.; Ramírez, A. Rheological Study of Binary Gels with Carbopol® UltrezTM 10 and Hyaluronic Acid. Chem. Pharm. Bull. 2007, 55, 1157–1163. [Google Scholar] [CrossRef]
- Krstonošić, V.S.; Sazdanić, D.B.; Ćirin, D.M.; Nikolić, I.R.; Hadnađev, M.S.; Atanacković Krstonošić, M.T. Characterization of Oil-in-Water Emulsions Prepared with Triblock Copolymer Poloxamer 407 and Low-Molecular-Mass Surfactant Mixtures as Carriers of Grape Pomace Waste Polyphenols. Pharmaceutics 2024, 16, 578. [Google Scholar] [CrossRef]
- Xu, X.; Al-Ghabeish, M.; Rahman, Z.; Krishnaiah, Y.S.; Yerlikaya, F.; Yang, Y.; Manda, P.; Hunt, R.L.; Khan, M.A. Formulation and process factors influencing product quality and in vitro performance of ophthalmic ointments. Int. J. Pharm. 2015, 493, 412–425. [Google Scholar] [CrossRef]
- Ehrmann, K.; Francis, I.; Stapleton, F. A novel instrument to quantify the tension of upper and lower eyelids. Contact Lens Anterior Eye 2001, 24, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Lin, S.-Y.; Cheng, W.-J.; Sheu, M.-T.; Chung, C.-Y.; Hsu, C.-H.; Lin, H.-L. Poloxamer sols endowed with in-situ gelability and mucoadhesion by adding hypromellose and hyaluronan for prolonging corneal retention and drug delivery. Drug Deliv. 2023, 30, 2158964. [Google Scholar] [CrossRef] [PubMed]
- Račić, A.; Čalija, B.; Milić, J.; Milašinović, N.; Krajišnik, D. Development of polysaccharide-based mucoadhesive ophthalmic lubricating vehicles: The effect of different polymers on physicochemical properties and functionality. J. Drug Deliv. Sci. Technol. 2019, 49, 50–57. [Google Scholar] [CrossRef]
- Hägerström, H.; Edsman, K. Limitations of the rheological mucoadhesion method: The effect of the choice of conditions and the rheological synergism parameter. Eur. J. Pharm. Sci. 2003, 18, 349–357. [Google Scholar] [CrossRef]
- Xu, X.; Shen, Y.; Wang, W.; Sun, C.; Li, C.; Xiong, Y.; Tu, J. Preparation and in vitro characterization of thermosensitive and mucoadhesive hydrogels for nasal delivery of phenylephrine hydrochloride. Eur. J. Pharm. Biopharm. 2014, 88, 998–1004. [Google Scholar] [CrossRef]
- Unated States Pharmacopeia. USP Monographs, Olopatadine Hydrochloride Ophthalmic Solution; USP-NF; Unated States Pharmacopeia: Rockville, MD, USA, 2024. [Google Scholar]
- Bindal, A.; Narsimhan, G.; Hem, S.L.; Kulshreshtha, A. Effect of steam sterilization on the rheology of polymer solutions. Pharm. Dev. Technol. 2003, 8, 219–228. [Google Scholar] [CrossRef]
- Schmelz, M.; Schmidt, R.; Weidner, C.; Hilliges, M.; Torebjork, H.; Handwerker, H.O. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003, 89, 2441–2448. [Google Scholar] [CrossRef]
| Composition % (w/V) | Formulation Code | ||
|---|---|---|---|
| HPG | SH | HPG-SH | |
| OCH | 0.1 | 0.1 | 0.1 |
| HPG | 0.25 | - | 0.25 |
| SH | - | 0.4 | 0.4 |
| NaCl | 0.6 | 0.7 | 0.7 |
| Na2HPO4 | 0.5 | 0.5 | 0.5 |
| BAC | 0.01 | 0.01 | 0.01 |
| Aqua purificata | q.s. ad 100 mL | q.s. ad 100 mL | q.s. ad 100 mL |
| Formulation Code | |||
|---|---|---|---|
| HPG | SH | HPG-SH | |
| Appearance | Colorless | Colorless | Colorless |
| Transparency (%) * | 96.7 | 100.0 | 98.4 |
| pH * | 5.5 ± 0.2 ## | 5.6 ± 0.2 | 5.3 ± 0.1 |
| Osmolality * (mOsmol/kg) | 261 ± 1 ## | 359 ± 2 | 378 ± 2 |
| Viscosity (mPa·s) * at 150 s−1 | 12.89 ± 0.08 | 16.04 ± 0.10 | 47.37 ± 0.93 |
| Surface tension # (mN/m) | 34.3 ± 0.3 | 39.5 ± 0.1 | 34.2 ± 0.1 |
| Viscosity * (mPa·s) at 20 °C | Formulation Code | ||
|---|---|---|---|
| HPG | SH | HPG-SH | |
| 25 s−1 | 17.56 ± 0.18 | 17.09 ± 0.02 | 75.05 ± 1.15 |
| 50 s−1 | 16.07 ± 0.06 | 17.05 ± 0.01 | 64.60 ± 1.30 |
| 100 s−1 | 14.13 ± 0.06 | 16.47 ± 0.14 | 53.53 ± 0.54 |
| 150 s−1 | 12.89 ± 0.08 | 16.04 ± 0.10 | 47.37 ± 0.93 |
| K (Pa·sn) | 0.0335 ± 0.0018 | 0.0205 ± 0.0001 | 0.1826 ± 0.0029 |
| n | 0.8114 ± 0.0122 | 0.9521 ± 0.0025 | 0.7331 ± 0.0007 |
| Formulation Code | |||
|---|---|---|---|
| HPG | SH | HPG-SH | |
| Mucoadhesive strength [mN] * | 4.40 ± 0.30 | 4.37 ± 0.07 | 5.05 ± 0.21 |
| Work of adhesion [mJ] * | 0.021 ± 0.004 | 0.022 ± 0.004 | 0.031 ± 0.006 |
| Parameter | Storage at 22 ± 2 °C | Storage at 4 ± 1 °C | |||||
|---|---|---|---|---|---|---|---|
| HPG | SH | HPG-SH | HPG | SH | HPG-SH | ||
| Initially | Appearance and clarity | Clear | Clear | Clear | Clear | Clear | Clear |
| pH | 5.2 | 5.6 | 5.3 | 5.2 | 5.6 | 5.3 | |
| Osmolality (mOsm/kg) | 261 | 359 | 378 | 261 | 359 | 378 | |
| Drug content (%) * | 106.15 | 101.13 | 111.06 | 109.03 | 104.52 | 114.11 | |
| 3 months | Appearance and clarity | Clear | Clear | Clear | Clear | Clear | Clear |
| pH | 5.2 | 5.6 | 5.3 | 5.2 | 5.4 | 5.3 | |
| Osmolality (mOsm/kg) | 261 | 359 | 378 | 259 | 355 | 368 | |
| Drug content (%) # | 106.15 | 101.13 | 111.06 | 99.08 | 94.23 | 103.51 | |
| 6 months | Appearance and clarity | Clear | Clear | Clear | Clear | Clear | Clear |
| pH | 5.3 | 5.6 | 5.3 | 5.2 | 5.6 | 5.4 | |
| Osmolality (mOsm/kg) | 256 | 355 | 379 | 258 | 353 | 372 | |
| Drug content (%) # | 90.57 | 106.93 | 89.19 | 95.41 | 90.38 | 94.74 | |
| 12 months | Appearance and clarity | Clear | Clear | Clear | Clear | Clear | Clear |
| pH | 5.2 | 5.6 | 5.3 | 5.1 | 5.5 | 5.3 | |
| Osmolality (mOsm/kg) | 262 | 357 | 377 | 260 | 351 | 377 | |
| Drug content (%) # | 94.62 | 98.11 | 90.00 | 98.90 | 98.46 | 107.63 | |
| Parameter | Formulation Code | |||
|---|---|---|---|---|
| HPG | SH | HPG-SH | ||
| Before CT | Appearance and clarity | Clear | Clear | Clear |
| pH | 5.2 | 5.6 | 5.3 | |
| Osmolality (mOsm/kg) | 261 | 359 | 378 | |
| Drug content (%) * | 106.15 | 101.13 | 111.06 | |
| Apparent viscosity (at 25 s−1) | 77.6 | 77.2 | 94.2 | |
| After CT | Appearance and clarity | Clear | Clear | Clear |
| pH | 5.2 | 5.5 | 5.3 | |
| Osmolality (mOsm/kg) | 261 | 359 | 388 | |
| Drug content (%) # | 108.49 | 109.90 | 100.00 | |
| Apparent viscosity (at 25 s−1) | 81.0 | 80.1 | 52.6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Račić, A.; Krstonošić, V.; Micov, A.; Pecikoza, U.; Dobričić, V.; Turković, E.; Krajišnik, D. Stability and Efficacy of Mucoadhesive Eye Drops Containing Olopatadine HCl: Physicochemical, Functional, and Preclinical In Vivo Assessment. Pharmaceutics 2025, 17, 517. https://doi.org/10.3390/pharmaceutics17040517
Račić A, Krstonošić V, Micov A, Pecikoza U, Dobričić V, Turković E, Krajišnik D. Stability and Efficacy of Mucoadhesive Eye Drops Containing Olopatadine HCl: Physicochemical, Functional, and Preclinical In Vivo Assessment. Pharmaceutics. 2025; 17(4):517. https://doi.org/10.3390/pharmaceutics17040517
Chicago/Turabian StyleRačić, Anđelka, Veljko Krstonošić, Ana Micov, Uroš Pecikoza, Vladimir Dobričić, Erna Turković, and Danina Krajišnik. 2025. "Stability and Efficacy of Mucoadhesive Eye Drops Containing Olopatadine HCl: Physicochemical, Functional, and Preclinical In Vivo Assessment" Pharmaceutics 17, no. 4: 517. https://doi.org/10.3390/pharmaceutics17040517
APA StyleRačić, A., Krstonošić, V., Micov, A., Pecikoza, U., Dobričić, V., Turković, E., & Krajišnik, D. (2025). Stability and Efficacy of Mucoadhesive Eye Drops Containing Olopatadine HCl: Physicochemical, Functional, and Preclinical In Vivo Assessment. Pharmaceutics, 17(4), 517. https://doi.org/10.3390/pharmaceutics17040517








