Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine
Abstract
1. Introduction
2. Materials and Methods
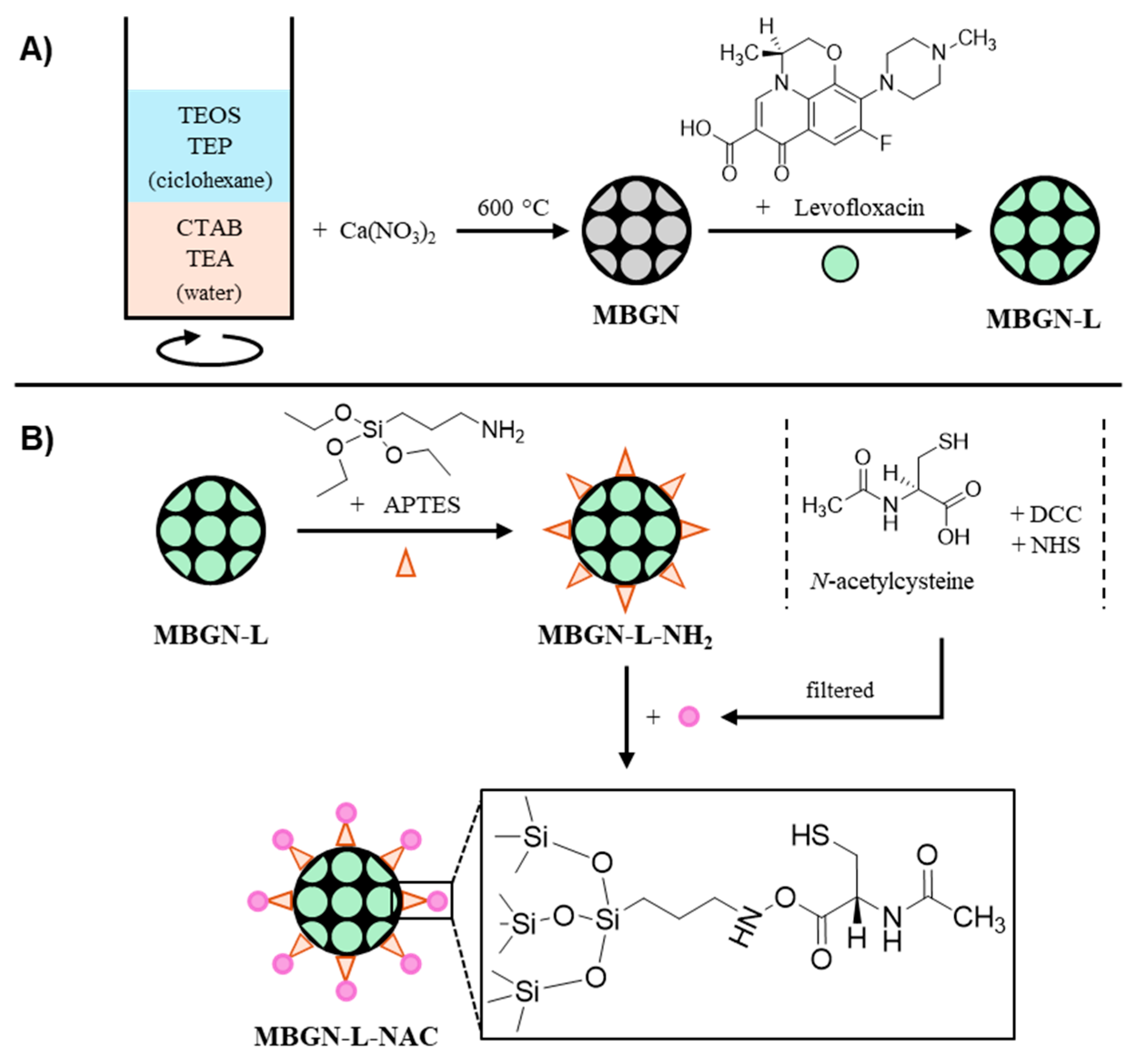
2.1. Synthetic Procedure
2.2. Antibiotic Loading
2.3. N-Acetylcysteine (NAC) Anchoring
2.4. Characterization Techniques
2.5. In Vitro Bioactivity Assay
2.6. Levofloxacin Release Study
2.7. In Vitro Antimicrobial Evaluation
2.7.1. Biofilm Formation and Treatment with Nanoparticles
2.7.2. Live/Dead Bacteria Study
2.8. In Vitro Cell Culture Studies with MC3T3-E1 Pre-Osteoblasts
2.8.1. In Vitro Cell Proliferation Assay
2.8.2. Determination of Reactive Oxygen Species (ROS)
2.8.3. In Vitro Cell Differentiation Assay
2.8.4. In Vitro Mineralization Assay
2.8.5. Confocal Laser Scanning Microscopy
2.9. Statistical Analysis
3. Results
3.1. Synthesis and Characterization of the Nanosystems
3.2. In Vitro Bioactivity Assay
3.3. Levofloxacin Release Study
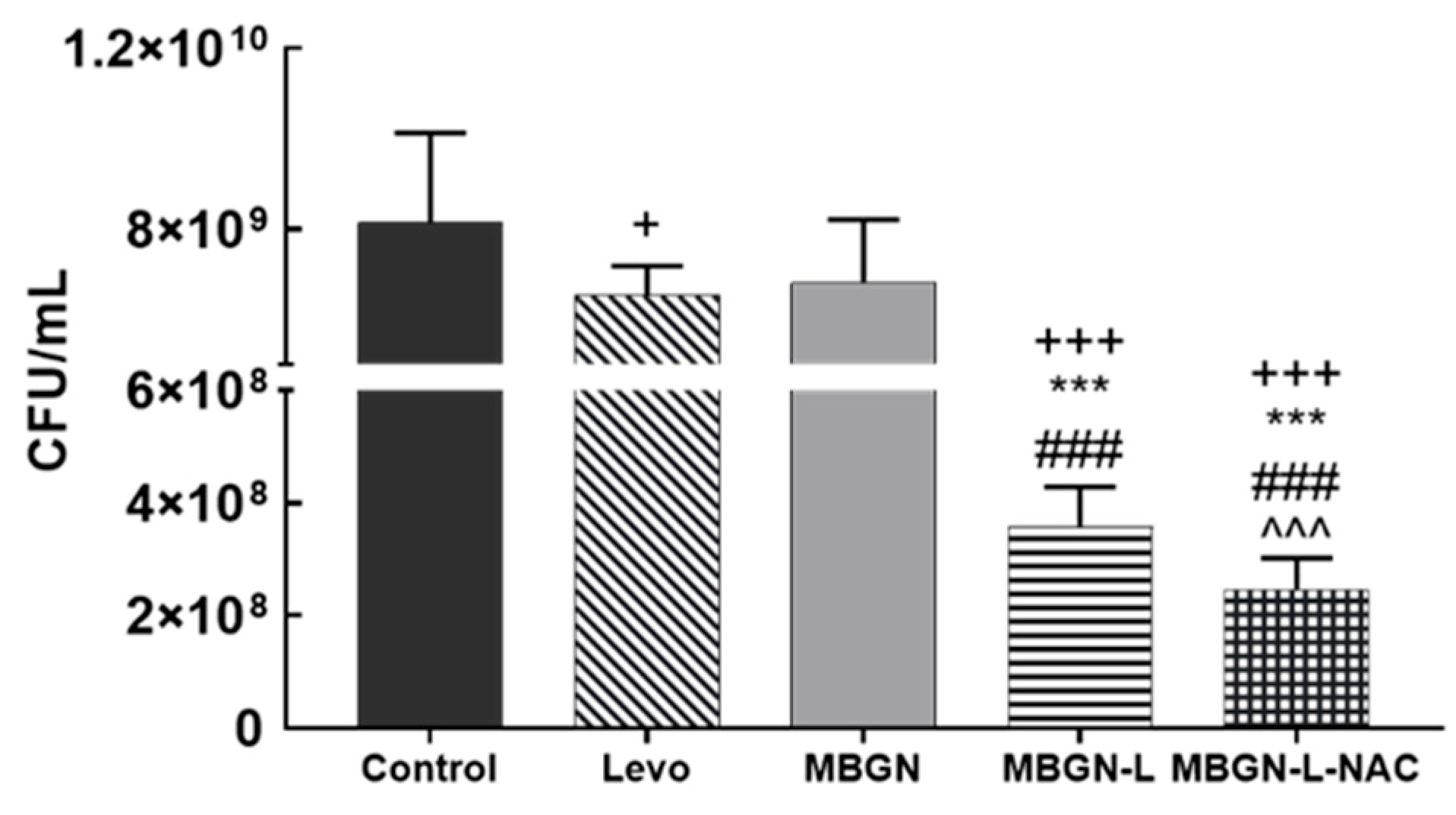
3.4. In Vitro Antimicrobial Evaluation
3.5. In Vitro Cell Culture Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, R. Periprosthetic Joint Infection. N. Engl. J. Med. 2023, 388, 251–262. [Google Scholar] [CrossRef]
- Dufour, D.; Leung, V.; Lévesque, C.M. Bacterial Biofilm: Structure, Function, and Antimicrobial Resistance. Endod. Top. 2010, 22, 2–16. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Lee, S.F.; Davey, L. Disulfide Bonds: A Key Modification in Bacterial Extracytoplasmic Proteins. J. Dent. Res. 2017, 96, 1465–1473. [Google Scholar] [CrossRef]
- Davis, R.; Bryson, H.M. Levofloxacin. Drugs 1994, 47, 677–700. [Google Scholar] [CrossRef]
- Barman, S.; Kurnaz, L.B.; Leighton, R.; Hossain, M.W.; Decho, A.W.; Tang, C. Intrinsic Antimicrobial Resistance: Molecular Biomaterials to Combat Microbial Biofilms and Bacterial Persisters. Biomaterials 2024, 311, 122690. [Google Scholar] [CrossRef]
- Huang, C.; Yu, M.; Li, H.; Wan, X.; Ding, Z.; Zeng, W.; Zhou, Z. Research Progress of Bioactive Glass and Its Application in Orthopedics. Adv. Mater. Interfaces 2021, 8, 2100606. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, K.; Boccaccini, A.R. Multi-Functional Silica-Based Mesoporous Materials for Simultaneous Delivery of Biologically Active Ions and Therapeutic Biomolecules. Acta Biomater. 2021, 129, 1–17. [Google Scholar] [CrossRef]
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Schmitz, S.I.; Moghaddam, A.; Boccaccini, A.R. Osteogenic Properties of Manganese-Doped Mesoporous Bioactive Glass Nanoparticles. J. Biomed. Mater. Res. Part A 2020, 108, 1806–1815. [Google Scholar] [CrossRef]
- Westhauser, F.; Wilkesmann, S.; Nawaz, Q.; Hohenbild, F.; Rehder, F.; Saur, M.; Fellenberg, J.; Moghaddam, A.; Ali, M.S.; Peukert, W.; et al. Effect of Manganese, Zinc, and Copper on the Biological and Osteogenic Properties of Mesoporous Bioactive Glass Nanoparticles. J. Biomed. Mater. Res. Part A 2021, 109, 1457–1467. [Google Scholar] [CrossRef]
- Marovic, D.; Haugen, H.J.; Negovetic Mandic, V.; Par, M.; Zheng, K.; Tarle, Z.; Boccaccini, A.R. Incorporation of Copper-Doped Mesoporous Bioactive Glass Nanospheres in Experimental Dental Composites: Chemical and Mechanical Characterization. Materials 2021, 14, 2611. [Google Scholar] [CrossRef]
- Zheng, K.; Balasubramanian, P.; Paterson, T.E.; Stein, R.; MacNeil, S.; Fiorilli, S.; Vitale-Brovarone, C.; Shepherd, J.; Boccaccini, A.R. Ag Modified Mesoporous Bioactive Glass Nanoparticles for Enhanced Antibacterial Activity in 3D Infected Skin Model. Mater. Sci. Eng. C 2019, 103, 109764. [Google Scholar] [CrossRef]
- Besheli, N.H.; Verbakel, J.; Hosseini, M.; Andrée, L.; Joosten, B.; Walboomers, X.F.; Cambi, A.; Yang, F.; Leeuwenburgh, S.C. Cellular Uptake of Modified Mesoporous Bioactive Glass Nanoparticles for Effective Intracellular Delivery of Therapeutic Agents. Int. J. Nanomed. 2023, 18, 1599–1612. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Li, L.; Zhao, W.; Li, L.; Gao, J.; Ruan, M.; Shi, J. Functionalization and Bioactivity in Vitro of Mesoporous Bioactive Glasses. J. Non-Cryst. Solids 2008, 354, 3799–3805. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, T.-H.; Kim, M.; Eltohamy, M.; Won, J.-E.; Lee, E.-J.; Kim, H.-W. Capacity of Mesoporous Bioactive Glass Nanoparticles to Deliver Therapeutic Molecules. Nanoscale 2012, 4, 7475–7488. [Google Scholar] [CrossRef]
- Ilyas, K.; Singer, L.; Akhtar, M.A.; Bourauel, C.P.; Boccaccini, A.R. Boswellia Sacra Extract-Loaded Mesoporous Bioactive Glass Nano Particles: Synthesis and Biological Effects. Pharmaceutics 2022, 14, 126. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Gomez-Cerezo, M.N.; Cao, Y.; Lei, C.; Dai, H.; Walsh, L.J.; Ivanovski, S.; Xu, C. A Comparative Study of Mesoporous Silica and Mesoporous Bioactive Glass Nanoparticles as Non-Viral MicroRNA Vectors for Osteogenesis. Pharmaceutics 2022, 14, 2302. [Google Scholar] [CrossRef]
- El-Fiqi, A.; Kim, J.-H.; Kim, H.-W. Osteoinductive Fibrous Scaffolds of Biopolymer/Mesoporous Bioactive Glass Nanocarriers with Excellent Bioactivity and Long-Term Delivery of Osteogenic Drug. ACS Appl. Mater. Interfaces 2015, 7, 1140–1152. [Google Scholar] [CrossRef]
- Sui, B.; Liu, X.; Sun, J. Dual-Functional Dendritic Mesoporous Bioactive Glass Nanospheres for Calcium Influx-Mediated Specific Tumor Suppression and Controlled Drug Delivery in Vivo. ACS Appl. Mater. Interfaces 2018, 10, 23548–23559. [Google Scholar] [CrossRef]
- Fan, W.; Wu, C.; Han, P.; Zhou, Y.; Xiao, Y. Porous Ca–Si-Based Nanospheres: A Potential Intra-Canal Disinfectant-Carrier for Infected Canal Treatment. Mater. Lett. 2012, 81, 16–19. [Google Scholar] [CrossRef]
- Arcos, D.; Gómez-Cerezo, N.; Saiz-Pardo, M.; de Pablo, D.; Ortega, L.; Enciso, S.; Fernández-Tomé, B.; Díaz-Güemes, I.; Sánchez-Margallo, F.M.; Casarrubios, L.; et al. Injectable Mesoporous Bioactive Nanoparticles Regenerate Bone Tissue under Osteoporosis Conditions. Acta Biomater. 2022, 151, 501–511. [Google Scholar] [CrossRef]
- Sui, B.; Zhong, G.; Sun, J. Drug-Loadable Mesoporous Bioactive Glass Nanospheres: Biodistribution, Clearance, BRL Cellular Location and Systemic Risk Assessment via (45)Ca Labelling and Histological Analysis. Sci. Rep. 2016, 6, 33443. [Google Scholar] [CrossRef]
- Cui, Y.; Hong, S.; Jiang, W.; Li, X.; Zhou, X.; He, X.; Liu, J.; Lin, K.; Mao, L. Engineering Mesoporous Bioactive Glasses for Emerging Stimuli-Responsive Drug Delivery and Theranostic Applications. Bioact. Mater. 2024, 34, 436–462. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and Potential Therapeutic Uses for N-Acetylcysteine: The Need for Conversion to Intracellular Glutathione for Antioxidant Benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef]
- Hurst, G.A.; Shaw, P.B.; LeMaistre, C.A. Laboratory and Clinical Evaluation of the Mucolytic Properties of Acetylcysteine. Am. Rev. Respir. Dis. 1967, 96, 962–970. [Google Scholar]
- Olofsson, A.-C.; Hermansson, M.; Elwing, H. N-Acetyl-L-Cysteine Affects Growth, Extracellular Polysaccharide Production, and Bacterial Biofilm Formation on Solid Surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef]
- Sempere, J.; Llamosí, M.; Román, F.; Lago, D.; González-Camacho, F.; Pérez-García, C.; Yuste, J.; Domenech, M. Clearance of Mixed Biofilms of Streptococcus pneumoniae and Methicillin-Susceptible/Resistant Staphylococcus aureus by Antioxidants N-Acetyl-l-Cysteine and Cysteamine. Sci. Rep. 2022, 12, 6668. [Google Scholar] [CrossRef]
- Drago, L.; De Vecchi, E.; Mattina, R.; Romanò, C.L. Activity of N-Acetyl-L-Cysteine against Biofilm of Staphylococcus aureus and Pseudomonas Aeruginosa on Orthopedic Prosthetic Materials. Int. J. Artif. Organs 2013, 36, 39–46. [Google Scholar] [CrossRef]
- Blasi, F.; Page, C.; Rossolini, G.M.; Pallecchi, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. The Effect of N-Acetylcysteine on Biofilms: Implications for the Treatment of Respiratory Tract Infections. Respir. Med. 2016, 117, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Aiyer, A.; Manoharan, A.; Paino, D.; Farrell, J.; Whiteley, G.S.; Kriel, F.H.; Glasbey, T.O.; Manos, J.; Das, T. Disruption of Biofilms and Killing of Burkholderia Cenocepacia from Cystic Fibrosis Lung Using an Antioxidant-Antibiotic Combination Therapy. Int. J. Antimicrob. Agents 2021, 58, 106372. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. GLUTATHIONE. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox Regulation of the Immune Response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, J.; Li, Y.; Lv, X.; Zhou, H.; Wang, H.; Xu, Y.; Wang, C.; Wang, J.; Liu, Z. ROS-Scavenging Hydrogel to Promote Healing of Bacteria Infected Diabetic Wounds. Biomaterials 2020, 258, 120286. [Google Scholar] [CrossRef]
- Xie, W.; Chen, X.; Li, Y.; Miao, G.; Wang, G.; Tian, T.; Zeng, L.; Chen, X. Facile Synthesis and in Vitro Bioactivity of Radial Mesoporous Bioactive Glass with High Phosphorus and Calcium Content. Adv. Powder Technol. 2020, 31, 3307–3317. [Google Scholar] [CrossRef]
- García, A.; González, B.; Harvey, C.; Izquierdo-Barba, I.; Vallet-Regí, M. Effective Reduction of Biofilm through Photothermal Therapy by Gold Core@shell Based Mesoporous Silica Nanoparticles. Microporous Mesoporous Mater. 2021, 328, 111489. [Google Scholar] [CrossRef]
- Algar, W.R.; Prasuhn, D.E.; Stewart, M.H.; Jennings, T.L.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. The Controlled Display of Biomolecules on Nanoparticles: A Challenge Suited to Bioorthogonal Chemistry. Bioconjug. Chem. 2011, 22, 825–858. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Particle Technology Series; Springer Netherlands: Dordrecht, The Netherlands, 2004; Volume 16, ISBN 978-90-481-6633-6. [Google Scholar]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Lv, X.; Yuan, L.; Rao, C.; Wu, X.; Qing, X.; Weng, X. Structure and Near-Infrared Spectral Properties of Mesoporous Silica for Hyperspectral Camouflage Materials. Infrared Phys. Technol. 2023, 129, 104558. [Google Scholar] [CrossRef]
- Sinha, R.K.; Biswas, P. Structural Elucidation of Levofloxacin and Ciprofloxacin Using Density Functional Theory and Raman Spectroscopy with Inexpensive Lab-Built Setup. J. Mol. Struct. 2020, 1222, 128946. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kang, M.-S.; Mahapatra, C.; Kim, H.-W. Effect of Aminated Mesoporous Bioactive Glass Nanoparticles on the Differentiation of Dental Pulp Stem Cells. PLoS ONE 2016, 11, e0150727. [Google Scholar] [CrossRef]
- Sharma, K.P. Medical Nutritional and Biochemical Role of N-Acetyl-L-Cysteine and Its Spectrophotometric Determination by Complexion with RU (III) and Characterization by Elemental Analysis, FTIR, ESR, NMR, TGA, DTA Proposed Structure of the Complex. J. Chem. Appl. Biochem. 2017, 4, 122. [Google Scholar]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous Silica Nanoparticles Decorated with Polycationic Dendrimers for Infection Treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Shi, G.; Chen, X.; Wang, H.; Wang, S.; Guo, X.; Zhang, X. Activity of Sitafloxacin against Extracellular and Intracellular Staphylococcus aureus in Vitro and in Vivo: Comparison with Levofloxacin and Moxifloxacin. J. Antibiot. 2012, 65, 229–236. [Google Scholar] [CrossRef]
- Aguilar-Colomer, A.; Colilla, M.; Izquierdo-Barba, I.; Jiménez-Jiménez, C.; Mahillo, I.; Esteban, J.; Vallet-Regí, M. Impact of the Antibiotic-Cargo from MSNs on Gram-Positive and Gram-Negative Bacterial Biofilms. Microporous Mesoporous Mater. 2021, 311, 110681. [Google Scholar] [CrossRef]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic Joint Infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef]
- Li, C.; Renz, N.; Trampuz, A. Management of Periprosthetic Joint Infection. Hip Pelvis 2018, 30, 138–146. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, Y.; Zhang, Y.; Li, F.; Shen, Y.; Qin, L.; Huang, W. Current Status and Perspectives of Diagnosis and Treatment of Periprosthetic Joint Infection. Infect. Drug Resist. 2024, 17, 2417–2429. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Izquierdo-Barba, I.; Portolés, M.T.; Vallet-Regí, M. Biocompatibility and Levofloxacin Delivery of Mesoporous Materials. Eur. J. Pharm. Biopharm. 2013, 84, 115–124. [Google Scholar] [CrossRef]
- Wang, Y.; Baeyens, W.; Huang, C.; Fei, G.; He, L.; Ouyang, J. Enhanced Separation of Seven Quinolones by Capillary Electrophoresis with Silica Nanoparticles as Additive. Talanta 2009, 77, 1667–1674. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Doadrio, J.C.; Hernández, A.; Portolés, M.T.; Izquierdo-Barba, I.; Vallet-Regí, M. Multifunctional pH Sensitive 3D Scaffolds for Treatment and Prevention of Bone Infection. Acta Biomater. 2018, 65, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Schneider, C.; Suk, J.S.; Cone, R.; Hanes, J. Mucus Penetrating Nanoparticles: Biophysical Tool and Method of Drug and Gene Delivery. Adv. Mater. 2012, 24, 3887–3894. [Google Scholar] [CrossRef] [PubMed]
- Pedre, B.; Barayeu, U.; Ezeriņa, D.; Dick, T.P. The Mechanism of Action of N-Acetylcysteine (NAC): The Emerging Role of H2S and Sulfane Sulfur Species. Pharmacol. Ther. 2021, 228, 107916. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Lai, S.K.; Boylan, N.J.; Dawson, M.R.; Boyle, M.P.; Hanes, J. Rapid Transport of Muco-Inert Nanoparticles in Cystic Fibrosis Sputum Treated with N.-Acetyl Cysteine. Nanomedicine 2011, 6, 365–375. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D Scaffold with Effective Multidrug Sequential Release against Bacteria Biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered Mesoporous Bioactive Glasses for Bone Tissue Regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Ji, H.; Liu, Y.; Zhao, X.; Zhang, M. N-Acetyl-L-Cysteine Enhances the Osteogenic Differentiation and Inhibits the Adipogenic Differentiation through up Regulation of Wnt 5a and down Regulation of PPARG in Bone Marrow Stromal Cells. Biomed. Pharmacother. 2011, 65, 369–374. [Google Scholar] [CrossRef]
- Yamada, M.; Tsukimura, N.; Ikeda, T.; Sugita, Y.; Att, W.; Kojima, N.; Kubo, K.; Ueno, T.; Sakurai, K.; Ogawa, T. N-Acetyl Cysteine as an Osteogenesis-Enhancing Molecule for Bone Regeneration. Biomaterials 2013, 34, 6147–6156. [Google Scholar] [CrossRef]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive Development of the Rat Osteoblast Phenotype in Vitro: Reciprocal Relationships in Expression of Genes Associated with Osteoblast Proliferation and Differentiation during Formation of the Bone Extracellular Matrix. J. Cell. Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer Cell Killing via ROS: To Increase or Decrease, That Is the Question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef]
- Meng, Z.; Liu, J.; Feng, Z.; Guo, S.; Wang, M.; Wang, Z.; Li, Z.; Li, H.; Sui, L. N-Acetylcysteine Regulates Dental Follicle Stem Cell Osteogenesis and Alveolar Bone Repair via ROS Scavenging. Stem Cell Res. Ther. 2022, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Casarrubios, L.; Gómez-Cerezo, N.; Feito, M.J.; Vallet-Regí, M.; Arcos, D.; Portolés, M.T. Ipriflavone-Loaded Mesoporous Nanospheres with Potential Applications for Periodontal Treatment. Nanomaterials 2020, 10, 2573. [Google Scholar] [CrossRef]
- Mody, N.; Parhami, F.; Sarafian, T.A.; Demer, L.L. Oxidative Stress Modulates Osteoblastic Differentiation of Vascular and Bone Cells. Free Radic. Biol. Med. 2001, 31, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Han, L.; Martin-Millan, M.; O’Brien, C.A.; Manolagas, S.C. Oxidative Stress Antagonizes Wnt Signaling in Osteoblast Precursors by Diverting Beta-Catenin from T Cell Factor- to Forkhead Box O-Mediated Transcription. J. Biol. Chem. 2007, 282, 27298–27305. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-T.; Shih, Y.-R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.-H. Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes during Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Marques-Carvalho, A.; Kim, H.-N.; Almeida, M. The Role of Reactive Oxygen Species in Bone Cell Physiology and Pathophysiology. Bone Rep. 2023, 19, 101664. [Google Scholar] [CrossRef]
- Schoppa, A.M.; Chen, X.; Ramge, J.-M.; Vikman, A.; Fischer, V.; Haffner-Luntzer, M.; Riegger, J.; Tuckermann, J.; Scharffetter-Kochanek, K.; Ignatius, A. Osteoblast Lineage Sod2 Deficiency Leads to an Osteoporosis-like Phenotype in Mice. Dis. Model. Mech. 2022, 15, dmm049392. [Google Scholar] [CrossRef]
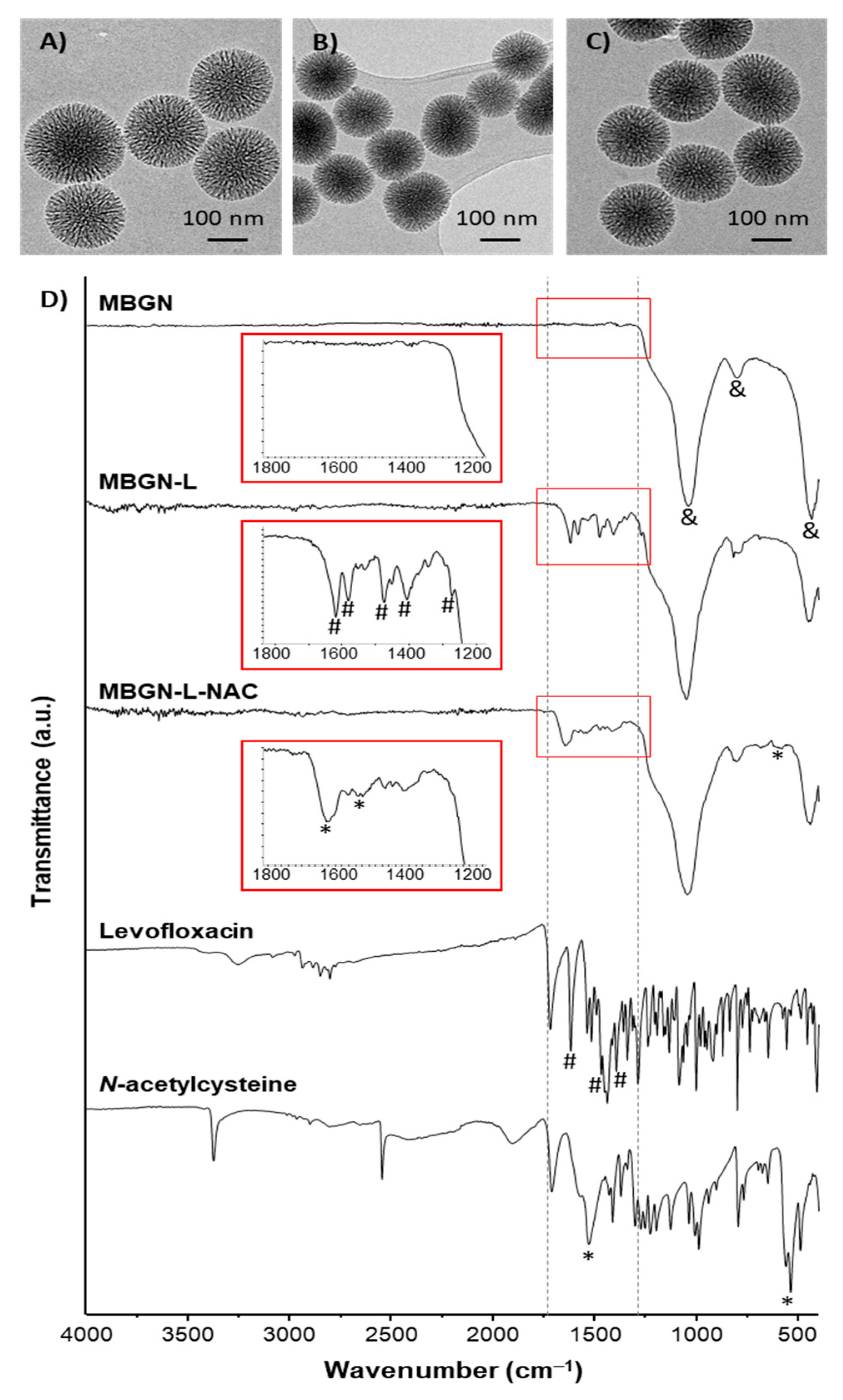







| %C | %N | %S | |
|---|---|---|---|
| MBGN | 0.7 ± 0.4 | <0.5 | - |
| MBGN-L | 11.0 ± 0.4 | 2.2 ± 0.3 | - |
| MBGN-L-NAC | 10.5 ± 0.4 | 2.9 ± 0.3 | 1.1 ± 0.4 |
| Surface Area [m2/g] | Pore Size [nm] | Pore Volume [cm3/g] | |
|---|---|---|---|
| MBGN | 348.9 | 8.0 | 0.6 |
| MBGN-L | 206.9 | 8.5 | 0.3 |
| MBGN-L-NAC | 144.8 | 6.7 | 0.2 |
| A | k | d | R2 | |
|---|---|---|---|---|
| MBGN-L | 31.76 | 0.16 | 0.9 | 0.995 |
| MBGN-L-NAC | 42.75 | 0.52 | 1 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo-Montalvo, A.; Gómez-Cerezo, N.; Cicuéndez, M.; González, B.; Izquierdo-Barba, I.; Arcos, D. Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine. Pharmaceutics 2025, 17, 519. https://doi.org/10.3390/pharmaceutics17040519
Polo-Montalvo A, Gómez-Cerezo N, Cicuéndez M, González B, Izquierdo-Barba I, Arcos D. Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine. Pharmaceutics. 2025; 17(4):519. https://doi.org/10.3390/pharmaceutics17040519
Chicago/Turabian StylePolo-Montalvo, Alberto, Natividad Gómez-Cerezo, Mónica Cicuéndez, Blanca González, Isabel Izquierdo-Barba, and Daniel Arcos. 2025. "Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine" Pharmaceutics 17, no. 4: 519. https://doi.org/10.3390/pharmaceutics17040519
APA StylePolo-Montalvo, A., Gómez-Cerezo, N., Cicuéndez, M., González, B., Izquierdo-Barba, I., & Arcos, D. (2025). Osteogenic and Antibacterial Response of Levofloxacin-Loaded Mesoporous Nanoparticles Functionalized with N-Acetylcysteine. Pharmaceutics, 17(4), 519. https://doi.org/10.3390/pharmaceutics17040519










