Targeting DLL3: Innovative Strategies for Tumor Treatment
Abstract
1. Introduction
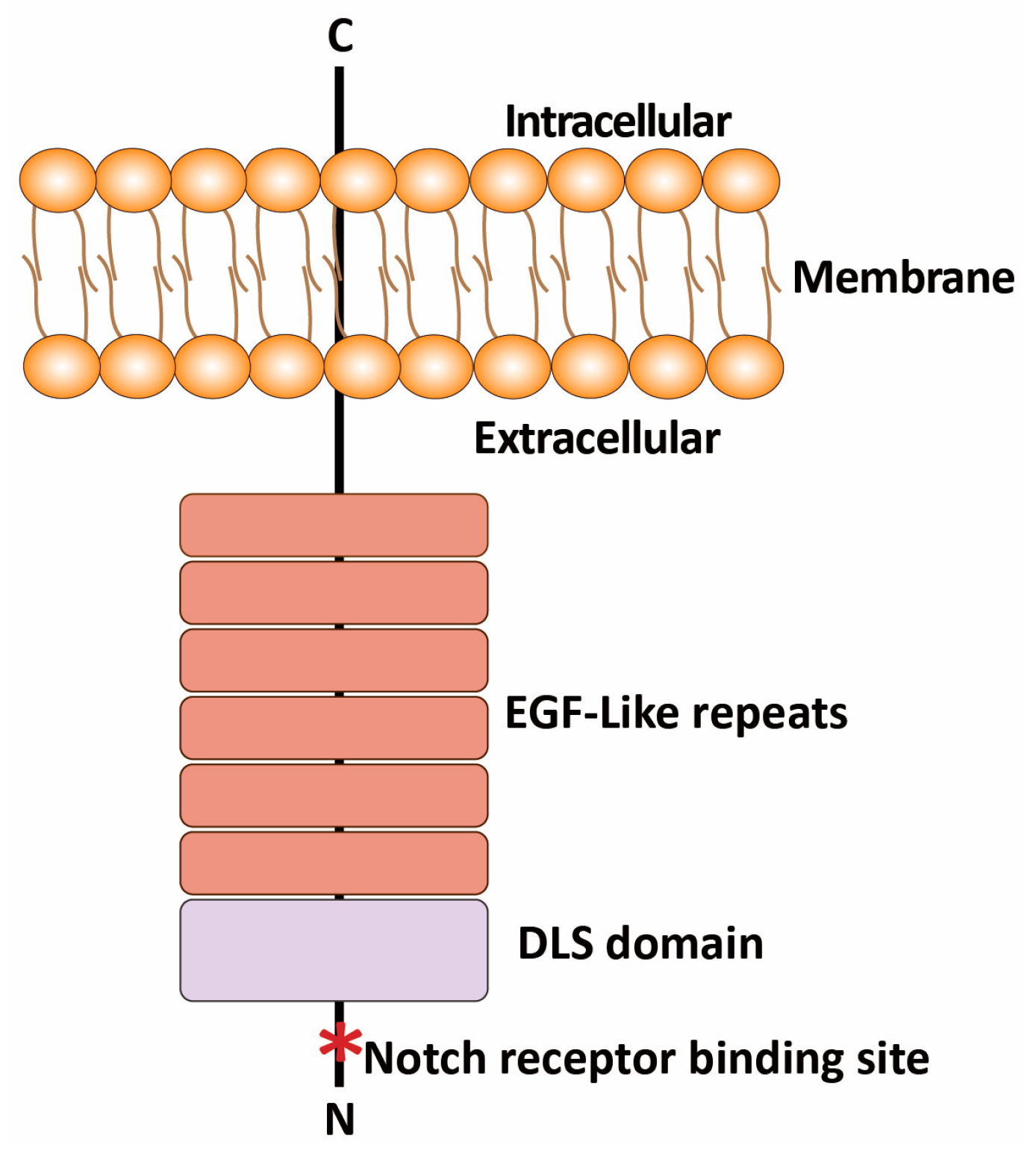
2. Biological Characteristics of DLL3
2.1. Structure and Expression of DLL3
2.2. Role of DLL3 in Notch Signaling Pathway
3. DLL3 Mechanism of Action Inducing Cancer Progression and Treatment
3.1. DLL3 in Tumorigenesis and Progression
3.2. Interaction of DLL3 with Other Oncogenic Signaling Pathways
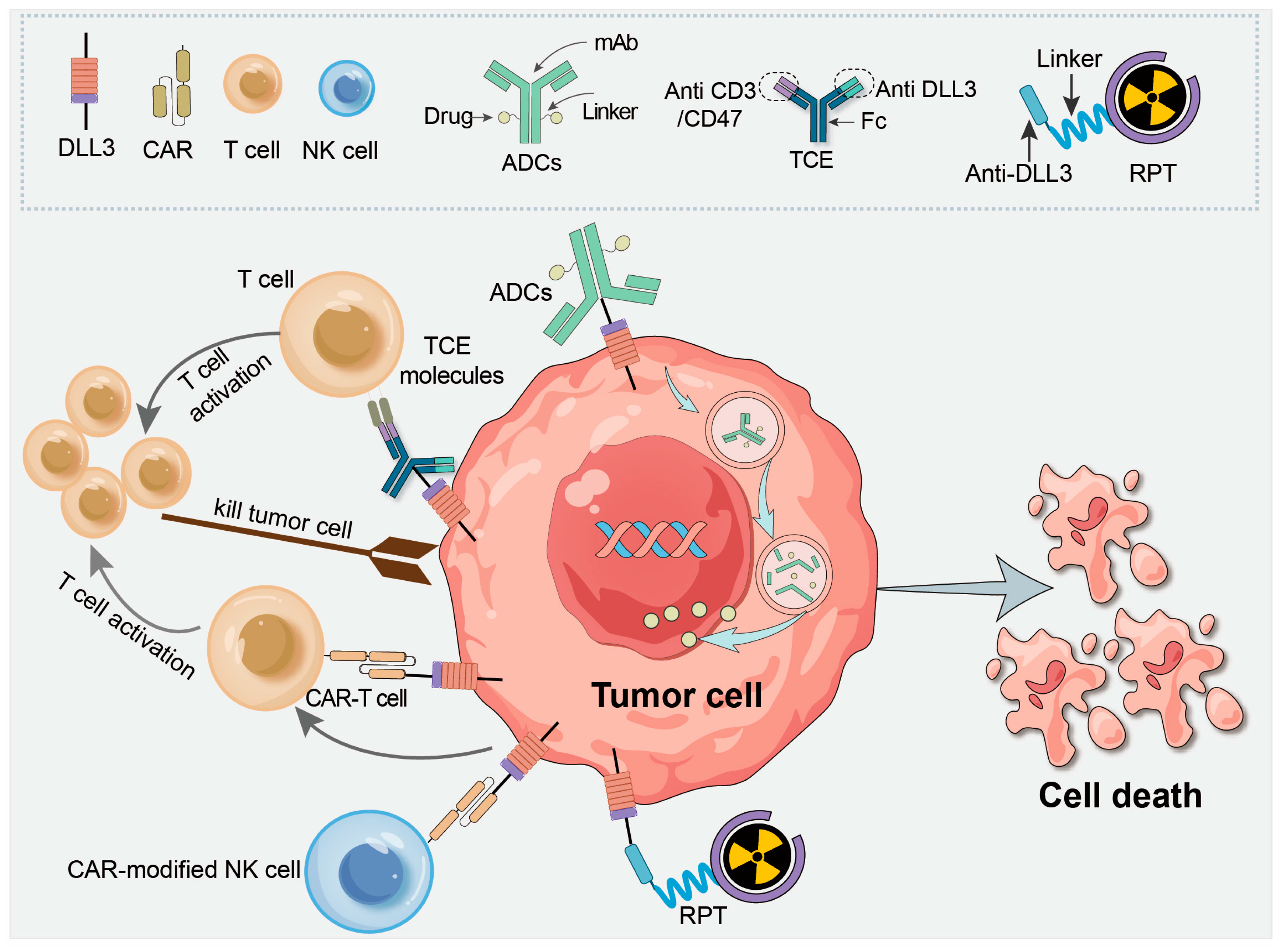
4. DLL3 as a Therapeutic Target in Cancer Treatment
4.1. ADCs
4.2. T Cell Engager (TCE) Molecules
4.3. CAR-T Therapy
4.4. CAR-Modified Natural Killer (NK) Cells
4.5. Radiopharmaceutical Therapy Targeting DLL3
5. Clinical Challenges in Targeting DLL3
5.1. Tumor Heterogeneity and Variability in DLL3 Expression
5.2. Resistance to DLL3-Targeted Therapies
5.3. Adverse Effects and Barriers Associated with DLL3-Targeted Agents
6. Future Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Richiardone, E.; Jorge, J.; Polónia, B.; Xavier, C.P.; Salaroglio, I.C.; Riganti, C.; Vasconcelos, M.H.; Corbet, C.; Sarmento-Ribeiro, A.B. Impact of cancer metabolism on therapy resistance—Clinical implications. Drug Resist. Updat. 2021, 59, 100797. [Google Scholar] [CrossRef] [PubMed]
- Barry, S.T.; Gabrilovich, D.I.; Sansom, O.J.; Campbell, A.D.; Morton, J.P. Therapeutic targeting of tumour myeloid cells. Nat. Rev. Cancer 2023, 23, 216–237. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef]
- Allen, F.; Maillard, I. Therapeutic Targeting of Notch Signaling: From Cancer to Inflammatory Disorders. Front. Cell Dev. Biol. 2021, 9, 649205. [Google Scholar] [CrossRef]
- Saunders, L.R.; Bankovich, A.J.; Anderson, W.C.; Aujay, M.A.; Bheddah, S.; Black, K.; Desai, R.; Escarpe, P.A.; Hampl, J.; Laysang, A.; et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci. Transl. Med. 2015, 7, 302ra136. [Google Scholar] [CrossRef]
- Ranallo, N.; Bocchini, M.; Menis, J.; Pilotto, S.; Severi, S.; Liverani, C.; Bongiovanni, A. Delta-like ligand 3 (DLL3): An attractive actionable target in tumors with neuroendocrine origin. Expert. Rev. Anticancer Ther. 2022, 22, 597–603. [Google Scholar] [CrossRef]
- Furuta, M.; Kikuchi, H.; Shoji, T.; Takashima, Y.; Kikuchi, E.; Kikuchi, J.; Kinoshita, I.; Dosaka-Akita, H.; Sakakibara-Konishi, J. DLL3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci. 2019, 110, 1599–1608. [Google Scholar] [CrossRef]
- Matsuo, K.; Taniguchi, K.; Hamamoto, H.; Inomata, Y.; Komura, K.; Tanaka, T.; Lee, S.; Uchiyama, K. Delta-like canonical Notch ligand 3 as a potential therapeutic target in malignancies: A brief overview. Cancer Sci. 2021, 112, 2984–2992. [Google Scholar] [CrossRef] [PubMed]
- Ladi, E.; Nichols, J.T.; Ge, W.; Miyamoto, A.; Yao, C.; Yang, L.-T.; Boulter, J.; Sun, Y.E.; Kintner, C.; Weinmaster, G. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J. Cell. Biol. 2005, 170, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Dunwoodie, S.L.; Clements, M.; Sparrow, D.B.; Sa, X.; Conlon, R.A.; Beddington, R.S.P. Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development 2002, 129, 1795–1806. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, W.; Li, S.; Wu, J.; Hu, X.; Wang, H.; Chi, K.; Zhuo, M.; Lin, D. Heterogeneity of molecular subtyping and therapy-related marker expression in primary tumors and paired lymph node metastases of small cell lung cancer. Virchows Arch. 2024, 486, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, J.; Jin, X.; Zhang, C.; Zhu, C.; Lv, M.; Chen, S.; Du, X.; Feng, G. The predictive value of delta-like3 and serum NSE in evaluating chemotherapy response and prognosis in patients with advanced small cell lung carcinoma: An observational study. Medicine 2024, 103, e38487. [Google Scholar] [CrossRef]
- Domvri, K.; Yaremenko, A.V.; Apostolopoulos, A.; Petanidis, S.; Karachrysafi, S.; Pastelli, N.; Papamitsou, T.; Papaemmanouil, S.; Lampaki, S.; Porpodis, K. Expression patterns and clinical implications of PDL1 and DLL3 biomarkers in small cell lung cancer retrospectively studied: Insights for therapeutic strategies and survival prediction. Heliyon 2024, 10, e27208. [Google Scholar] [CrossRef]
- Vitorino, P.; Chuang, C.-H.; Iannello, A.; Zhao, X.; Anderson, W.; Ferrando, R.; Zhang, Z.; Madhavan, S.; Karsunky, H.; Saunders, L.R. Rova-T enhances the anti-tumor activity of anti-PD1 in a murine model of small cell lung cancer with endogenous Dll3 expression. Transl. Oncol. 2021, 14, 100883. [Google Scholar] [CrossRef]
- Ye, J.-B.; Wen, J.-J.; Wu, D.-L.; Hu, B.-X.; Luo, M.-Q.; Lin, Y.-Q.; Ning, Y.-S.; Li, Y. Elevated DLL3 in stomach cancer by tumor-associated macrophages enhances cancer-cell proliferation and cytokine secretion of macrophages. Gastroenterol. Rep. 2021, 10, goab052. [Google Scholar] [CrossRef]
- Spino, M.; Kurz, S.C.; Chiriboga, L.; Serrano, J.; Zeck, B.; Sen, N.; Patel, S.; Shen, G.; Vasudevaraja, V.; Tsirigos, A.; et al. Cell Surface Notch Ligand DLL3 is a Therapeutic Target in Isocitrate Dehydrogenase-mutant Glioma. Clin. Cancer Res. 2019, 25, 1261–1271. [Google Scholar] [CrossRef]
- Matsuo, K.; Taniguchi, K.; Hamamoto, H.; Ito, Y.; Futaki, S.; Inomata, Y.; Shima, T.; Asakuma, M.; Lee, S.; Tanaka, K.; et al. Delta-like 3 localizes to neuroendocrine cells and plays a pivotal role in gastrointestinal neuroendocrine malignancy. Cancer Sci. 2019, 110, 3122–3131. [Google Scholar] [CrossRef]
- Koshkin, V.S.; Garcia, J.A.; Reynolds, J.P.; Elson, P.; Magi-Galluzzi, C.; McKenney, J.K.; Isse, K.; Bishop, E.; Saunders, L.R.; Balyimez, A.; et al. Transcriptomic and Protein Analysis of Small-cell Bladder Cancer (SCBC) Identifies Prognostic Biomarkers and DLL3 as a Relevant Therapeutic Target. Clin. Cancer Res. 2019, 25, 210–221. [Google Scholar] [CrossRef]
- Yuan, C.; Chang, K.; Xu, C.; Li, Q.; Du, Z. High expression of DLL3 is associated with a poor prognosis and immune infiltration in invasive breast cancer patients. Transl. Oncol. 2021, 14, 101080. [Google Scholar] [CrossRef] [PubMed]
- Alì, G.; Di Stefano, I.; Poma, A.M.; Ricci, S.; Proietti, A.; Davini, F.; Lucchi, M.; Melfi, F.; Fontanini, G. Prevalence of Delta-Like Protein 3 in a Consecutive Series of Surgically Resected Lung Neuroendocrine Neoplasms. Front. Oncol. 2021, 11, 729765. [Google Scholar] [CrossRef]
- Puca, L.; Gavyert, K.; Sailer, V.; Conteduca, V.; Dardenne, E.; Sigouros, M.; Isse, K.; Kearney, M.; Vosoughi, A.; Fernandez, L.; et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci. Transl. Med. 2019, 11, eaav0891. [Google Scholar] [CrossRef] [PubMed]
- Obermayr, E.; Agreiter, C.; Schuster, E.; Fabikan, H.; Weinlinger, C.; Baluchova, K.; Hamilton, G.; Hochmair, M.; Zeillinger, R. Molecular Characterization of Circulating Tumor Cells Enriched by A Microfluidic Platform in Patients with Small-Cell Lung Cancer. Cells 2019, 8, 880. [Google Scholar] [CrossRef] [PubMed]
- Messaritakis, I.; Nikolaou, M.; Koinis, F.; Politaki, E.; Koutsopoulos, A.; Lagoudaki, E.; Vetsika, E.K.; Georgoulias, V.; Kotsakis, A. Characterization of DLL3-positive circulating tumor cells (CTCs) in patients with small cell lung cancer (SCLC) and evaluation of their clinical relevance during front-line treatment. Lung Cancer 2019, 135, 33–39. [Google Scholar] [CrossRef]
- Welsch, E.; Holzer, B.; Schuster, E.; Fabikan, H.; Weinlinger, C.; Hauptmann-Repitz, E.; Illini, O.; Hochmair, M.J.; Fischer, M.B.; Weiss, E.; et al. Prognostic significance of circulating tumor cells and tumor related transcripts in small cell lung cancer: A step further to clinical implementation. Int. J. Cancer 2024, 154, 2189–2199. [Google Scholar] [CrossRef]
- Shrestha, P.; Kao, S.; Cheung, V.K.; Cooper, W.A.; van Zandwijk, N.; Rasko, J.E.J.; Yeo, D. Circulating tumor cells: Advancing personalized therapy in small cell lung cancer patients. Mol. Oncol. 2024. [Google Scholar] [CrossRef]
- Su, P.-L.; Chakravarthy, K.; Furuya, N.; Brownstein, J.; Yu, J.; Long, M.; Carbone, D.; Li, Z.; He, K. DLL3-guided therapies in small-cell lung cancer: From antibody-drug conjugate to precision immunotherapy and radioimmunotherapy. Mol. Cancer 2024, 23, 97. [Google Scholar] [CrossRef]
- Mullendore, M.E.; Koorstra, J.-B.; Li, Y.-M.; Offerhaus, G.J.; Fan, X.; Henderson, C.M.; Matsui, W.; Eberhart, C.G.; Maitra, A.; Feldmann, G. Ligand-dependent Notch signaling is involved in tumor initiation and tumor maintenance in pancreatic cancer. Clin. Cancer Res. 2009, 15, 2291–2301. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Xu, H.; Han, N.; Chu, Q.; Yu, S.; Chen, Y.; Wu, K. Meta-analysis reveals the correlation of Notch signaling with non-small cell lung cancer progression and prognosis. Sci. Rep. 2015, 5, 10338. [Google Scholar] [CrossRef]
- Zhang, Y.; Tacheva-Grigorova, S.K.; Sutton, J.; Melton, Z.; Mak, Y.S.; Lay, C.; Smith, B.A.; Sai, T.; Van Blarcom, T.; Sasu, B.J.; et al. Allogeneic CAR T Cells Targeting DLL3 Are Efficacious and Safe in Preclinical Models of Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Leonetti, A.; Facchinetti, F.; Minari, R.; Cortellini, A.; Rolfo, C.D.; Giovannetti, E.; Tiseo, M. Notch pathway in small-cell lung cancer: From preclinical evidence to therapeutic challenges. Cell. Oncol. 2019, 42, 261–273. [Google Scholar] [CrossRef]
- Kim, J.W.; Ko, J.H.; Sage, J. DLL3 regulates Notch signaling in small cell lung cancer. iScience 2022, 25, 105603. [Google Scholar] [CrossRef]
- Rudin, C.M.; Reck, M.; Johnson, M.L.; Blackhall, F.; Hann, C.L.; Yang, J.C.-H.; Bailis, J.M.; Bebb, G.; Goldrick, A.; Umejiego, J.; et al. Emerging therapies targeting the delta-like ligand 3 (DLL3) in small cell lung cancer. J. Hematol. Oncol. 2023, 16, 66. [Google Scholar] [CrossRef]
- Yao, J.; Bergsland, E.; Aggarwal, R.; Aparicio, A.; Beltran, H.; Crabtree, J.S.; Hann, C.L.; Ibrahim, T.; Byers, L.A.; Sasano, H.; et al. DLL3 as an Emerging Target for the Treatment of Neuroendocrine Neoplasms. Oncologist 2022, 27, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Kudoh, S.; Ichimura, T.; Fujino, K.; Hassan, W.A.M.A.; Udaka, N. Small cell lung cancer, an epithelial to mesenchymal transition (EMT)-like cancer: Significance of inactive Notch signaling and expression of achaete-scute complex homologue 1. Hum. Cell 2017, 30, 1–10. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Gao, H.; Wan, D.; Li, C.; Li, Z.; Zhang, Y. EGFL7 participates in regulating biological behavior of growth hormone-secreting pituitary adenomas via Notch2/DLL3 signaling pathway. Tumor Biol. 2017, 39, 1010428317706203. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Li, F.; Zhang, L. Knockdown of Delta-like 3 restricts lipopolysaccharide- induced inflammation, migration and invasion of A2058 melanoma cells via blocking Twist1-mediated epithelial-mesenchymal transition. Life Sci. 2019, 226, 149–155. [Google Scholar] [CrossRef]
- Jia, D.; Underwood, J.; Xu, Q.; Xie, Q. NOTCH2/NOTCH3/DLL3/MAML1/ADAM17 signaling network is associated with ovarian cancer. Oncol. Lett. 2019, 17, 4914–4920. [Google Scholar] [CrossRef]
- Tanaka, K.; Isse, K.; Fujihira, T.; Takenoyama, M.; Saunders, L.; Bheddah, S.; Nakanishi, Y.; Okamoto, I. Prevalence of Delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer 2018, 115, 116–120. [Google Scholar] [CrossRef]
- Shirasawa, M.; Yoshida, T.; Shiraishi, K.; Goto, N.; Yagishita, S.; Imabayashi, T.; Matsumoto, Y.; Masuda, K.; Shinno, Y.; Okuma, Y.; et al. Tumor microenvironment-mediated immune profiles and efficacy of anti-PD-L1 antibody plus chemotherapy stratified by DLL3 expression in small-cell lung cancer. Br. J. Cancer 2023, 129, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, K.; Liu, Z.; Wang, T.; Shi, F.; Zhang, Y.; Su, J.; Jia, Y. Upregulated delta-like protein 3 expression is a diagnostic and prognostic marker in endometrial cancer: A retrospective study. Medicine 2018, 97, e13442. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, J.E.; Khan, J.F.; Godfrey, W.D.; Lopez, A.V.; Ciampricotti, M.; Rudin, C.M.; Brentjens, R.J. IL-18-secreting CAR T cells targeting DLL3 are highly effective in small cell lung cancer models. J. Clin. Investig. 2023, 133, e166028. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Liu, Q.; Li, J.; Zhang, Q.; Zhuang, Z.; Yao, X.; Liu, C.; Li, Y.; Cao, L.; et al. DAPT, a γ-Secretase Inhibitor, Suppresses Tumorigenesis, and Progression of Growth Hormone-Producing Adenomas by Targeting Notch Signaling. Front. Oncol. 2019, 9, 809. [Google Scholar] [CrossRef]
- Aiello, N.M.; Kang, Y. Context-dependent EMT programs in cancer metastasis. J. Exp. Med. 2019, 216, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cao, D.; Sha, J.; Zhu, X.; Han, S. DLL3 is regulated by LIN28B and miR-518d-5p and regulates cell proliferation, migration and chemotherapy response in advanced small cell lung cancer. Biochem. Biophys. Res. Commun. 2019, 514, 853–860. [Google Scholar] [CrossRef]
- Penton, A.L.; Leonard, L.D.; Spinner, N.B. Notch signaling in human development and disease. Semin. Cell Dev. Biol. 2012, 23, 450–457. [Google Scholar] [CrossRef]
- Shao, S.; Zhao, X.; Zhang, X.; Luo, M.; Zuo, X.; Huang, S.; Wang, Y.; Gu, S.; Zhao, X. Notch1 signaling regulates the epithelial-mesenchymal transition and invasion of breast cancer in a Slug-dependent manner. Mol. Cancer 2015, 14, 28. [Google Scholar] [CrossRef]
- Turchi, L.; Debruyne, D.N.; Almairac, F.; Virolle, V.; Fareh, M.; Neirijnck, Y.; Burel-Vandenbos, F.; Paquis, P.; Junier, M.-P.; Van Obberghen-Schilling, E.; et al. Tumorigenic potential of miR-18A* in glioma initiating cells requires NOTCH-1 signaling. Stem Cells 2013, 31, 1252–1265. [Google Scholar] [CrossRef]
- Hu, B.; Nandhu, M.S.; Sim, H.; Agudelo-Garcia, P.A.; Saldivar, J.C.; Dolan, C.E.; Mora, M.E.; Nuovo, G.J.; Cole, S.E.; Viapiano, M.S. Fibulin-3 promotes glioma growth and resistance through a novel paracrine regulation of Notch signaling. Cancer Res. 2012, 72, 3873–3885. [Google Scholar] [CrossRef] [PubMed]
- Ingenwerth, M.; Brandenburg, T.; Führer-Sakel, D.; Goetz, M.; Weber, F.; Dralle, H.; Schildhaus, H.-U.; Schmid, K.W.; Theurer, S. DLL3 (delta-like protein 3) expression correlates with stromal desmoplasia and lymph node metastases in medullary thyroid carcinomas. Endocr. Connect. 2021, 10, 283–289. [Google Scholar] [CrossRef]
- Song, P.; Gao, Z.; Bao, Y.; Chen, L.; Huang, Y.; Liu, Y.; Dong, Q.; Wei, X. Wnt/β-catenin signaling pathway in carcinogenesis and cancer therapy. J. Hematol. Oncol. 2024, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Ayyanan, A.; Civenni, G.; Ciarloni, L.; Morel, C.; Mueller, N.; Lefort, K.; Mandinova, A.; Raffoul, W.; Fiche, M.; Dotto, G.P.; et al. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc. Natl. Acad. Sci. USA 2006, 103, 3799–3804. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.-M.; Yan, X.-C.; Liang, L.; Wang, L.; Liu, Y.; Duan, J.-L.; Yang, Z.-Y.; Chang, T.-F.; Ruan, B.; Zheng, Q.-J.; et al. The Notch ligand delta-like 3 promotes tumor growth and inhibits Notch signaling in lung cancer cells in mice. Biochem. Biophys. Res. Commun. 2017, 483, 488–494. [Google Scholar] [CrossRef]
- Li, W.; Ye, L.; Huang, Y.; Zhou, F.; Wu, C.; Wu, F.; He, Y.; Li, X.; Wang, H.; Xiong, A.; et al. Characteristics of Notch signaling pathway and its correlation with immune microenvironment in SCLC. Lung Cancer 2022, 167, 25–33. [Google Scholar] [CrossRef]
- Rudin, C.M.; Pietanza, M.C.; Bauer, T.M.; Ready, N.; Morgensztern, D.; Glisson, B.S.; Byers, L.A.; Johnson, M.L.; Burris, H.A., 3rd; Robert, F.; et al. SCRX16-001 investigators Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: A first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 2017, 18, 42–51. [Google Scholar] [CrossRef]
- Morgensztern, D.; Besse, B.; Greillier, L.; Santana-Davila, R.; Ready, N.; Hann, C.L.; Glisson, B.S.; Farago, A.F.; Dowlati, A.; Rudin, C.M.; et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results from the Phase II TRINITY Study. Clin. Cancer Res. 2019, 25, 6958–6966. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Hong, D.S.; Hann, C.L.; Farago, A.F.; Beltran, H.; Waqar, S.N.; Hendifar, A.E.; Anthony, L.B.; Taylor, M.H.; Bryce, A.H.; et al. A phase I/II study of rovalpituzumab tesirine in delta-like 3-expressing advanced solid tumors. NPJ Precis. Oncol. 2021, 5, 74. [Google Scholar] [CrossRef]
- Blackhall, F.; Jao, K.; Greillier, L.; Cho, B.C.; Penkov, K.; Reguart, N.; Majem, M.; Nackaerts, K.; Syrigos, K.; Hansen, K.; et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared with Topotecan as Second-Line Therapy in DLL3-High SCLC: Results from the Phase 3 TAHOE Study. J. Thorac. Oncol. 2021, 16, 1547–1558. [Google Scholar] [CrossRef]
- Johnson, M.L.; Zvirbule, Z.; Laktionov, K.; Helland, A.; Cho, B.C.; Gutierrez, V.; Colinet, B.; Lena, H.; Wolf, M.; Gottfried, M.; et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients with Extensive-Stage-SCLC: Results from the Phase 3 MERU Study. J. Thorac. Oncol. 2021, 16, 1570–1581. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, Y.; Yao, J.; Yang, J.; Qiu, Y.; Zhu, Z.; Hua, H. DB-1314, a novel DLL3-targeting ADC with DNA topoisomerase I inhibitor, exhibits promising safety profile and therapeutic efficacy in preclinical small cell lung cancer models. J. Transl. Med. 2024, 22, 766. [Google Scholar] [CrossRef]
- Guo, Q.; Gao, B.; Song, R.; Li, W.; Zhu, S.; Xie, Q.; Lou, S.; Wang, L.; Shen, J.; Zhao, T.; et al. FZ-AD005, a Novel DLL3-Targeted Antibody-Drug Conjugate with Topoisomerase I Inhibitor, Shows Potent Antitumor Activity in Preclinical Models. Mol. Cancer Ther. 2024, 23, 1367–1377. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Paz-Ares, L.; Champiat, S.; Champiat, S.; Lai, W.V.; Lai, W.V.; Izumi, H.; Izumi, H.; Govindan, R.; Govindan, R.; et al. Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J. Clin. Oncol. 2023, 41, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, W.; Li, Z.; Wu, J.; Huang, X.; Li, J.; Zhang, X.; Ye, X. Delta-like ligand 3 in small cell lung cancer: Potential mechanism and treatment progress. Crit. Rev. Oncol. 2023, 191, 104136. [Google Scholar] [CrossRef]
- Matthies, K.; Crouse-Zeineddini, J. Target cell line characterization reveals changes in expression of a key antigen that impacts T cell dependent cellular cytotoxicity assay performance. J. Immunol. Methods 2022, 509, 113326. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.R.; Das, M. Small Cell Lung Cancer: Emerging Targets and Strategies for Precision Therapy. Cancers 2023, 15, 4016. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-J.; Cho, B.C.; Felip, E.; Korantzis, I.; Ohashi, K.; Majem, M.; Juan-Vidal, O.; Handzhiev, S.; Izumi, H.; Lee, J.-S.; et al. DeLLphi-301 Investigators Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 2063–2075. [Google Scholar] [CrossRef]
- Wermke, M.; Felip, E.; Gambardella, V.; Kuboki, Y.; Morgensztern, D.; Hamed, Z.O.; Liu, M.; Studeny, M.; Owonikoko, T.K. Phase I trial of the DLL3/CD3 bispecific T-cell engager BI 764532 in DLL3-positive small-cell lung cancer and neuroendocrine carcinomas. Futur. Oncol. 2022, 18, 2639–2649. [Google Scholar] [CrossRef]
- Molloy, M.E.; Aaron, W.H.; Barath, M.; Bush, M.C.; Callihan, E.C.; Carlin, K.; Cremin, M.; Evans, T.; Guerrero, M.G.; Hemmati, G.; et al. HPN328, a Trispecific T Cell-Activating Protein Construct Targeting DLL3-Expressing Solid Tumors. Mol. Cancer Ther. 2024, 23, 1294–1304. [Google Scholar] [CrossRef]
- Zhou, D.; Byers, L.A.; Sable, B.; Smit, M.D.; Sadraei, N.H.; Dutta, S.; Upreti, V.V. Clinical Pharmacology Profile of AMG 119, the First Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting Delta-Like Ligand 3 (DLL3), in Patients with Relapsed/Refractory Small Cell Lung Cancer (SCLC). J. Clin. Pharmacol. 2024, 64, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Elahi, R.; Heidary, A.H.; Hadiloo, K.; Esmaeilzadeh, A. Chimeric Antigen Receptor-Engineered Natural Killer (CAR NK) Cells in Cancer Treatment; Recent Advances and Future Prospects. Stem Cell Rev. Rep. 2021, 17, 2081–2106. [Google Scholar] [CrossRef]
- Wang, L.; Dou, M.; Ma, Q.; Yao, R.; Liu, J. Chimeric antigen receptor (CAR)-modified NK cells against cancer: Opportunities and challenges. Int. Immunopharmacol. 2019, 74, 105695. [Google Scholar] [CrossRef]
- Montagner, I.M.; Penna, A.; Fracasso, G.; Carpanese, D.; Pietà, A.D.; Barbieri, V.; Zuccolotto, G.; Rosato, A. Anti-PSMA CAR-engineered NK-92 Cells: An Off-the-shelf Cell Therapy for Prostate Cancer. Cells 2020, 9, 1382. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Huang, W.; Guo, Y.; Zhou, Y.; Zhi, C.; Chen, J.; Li, J.; He, J.; Lian, H.; Zhou, J.; et al. CAR NK-92 cells targeting DLL3 kill effectively small cell lung cancer cells in vitro and in vivo. J. Leukoc. Biol. 2022, 112, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.M.; Tendler, S.; Carter, L.M.; Sharma, S.K.; Samuels, Z.V.; Mandleywala, K.; Korsen, J.A.; Reyes, A.M.D.; Piersigilli, A.; Travis, W.D.; et al. Radioimmunotherapy Targeting Delta-like Ligand 3 in Small Cell Lung Cancer Exhibits Antitumor Efficacy with Low Toxicity. Clin. Cancer Res. 2022, 28, 1391–1401. [Google Scholar] [CrossRef]
- Korsen, J.A.; Gutierrez, J.A.; Tully, K.M.; Carter, L.M.; Samuels, Z.V.; Khitrov, S.; Poirier, J.T.; Rudin, C.M.; Chen, Y.; Morris, M.J.; et al. Delta-like ligand 3-targeted radioimmunotherapy for neuroendocrine prostate cancer. Proc. Natl. Acad. Sci. USA 2022, 119, e2203820119. [Google Scholar] [CrossRef]
- Tendler, S.; Dunphy, M.P.; Agee, M.; O’Donoghue, J.; Aly, R.G.; Choudhury, N.J.; Kesner, A.; Kirov, A.; Mauguen, A.; Baine, M.K.; et al. Imaging with [89Zr]Zr-DFO-SC16.56 anti-DLL3 antibody in patients with high-grade neuroendocrine tumours of the lung and prostate: A phase 1/2, first-in-human trial. Lancet Oncol. 2024, 25, 1015–1024. [Google Scholar] [CrossRef]
- Chou, J.; Egusa, E.A.; Wang, S.; Badura, M.L.; Lee, F.; Bidkar, A.P.; Zhu, J.; Shenoy, T.; Trepka, K.; Robinson, T.M.; et al. Immunotherapeutic Targeting and PET Imaging of DLL3 in Small-Cell Neuroendocrine Prostate Cancer. Cancer Res. 2023, 83, 301–315. [Google Scholar] [CrossRef]
- Zhang, Y.; Shang, L.; Han, J.; Shen, X.; Liu, H.; Yang, J.; Shi, H. Biological and immunological significance of DLL3 expression in different tumor tissues: A pan-cancer analysis. Aging 2023, 15, 3427–3441. [Google Scholar] [CrossRef]
- Gay, C.M.; Stewart, C.A.; Park, E.M.; Diao, L.; Groves, S.M.; Heeke, S.; Nabet, B.Y.; Fujimoto, J.; Solis, L.M.; Lu, W.; et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39, 346–360.e7. [Google Scholar] [CrossRef]
- Keogh, A.; Finn, S.; Radonic, T. Emerging Biomarkers and the Changing Landscape of Small Cell Lung Cancer. Cancers 2022, 14, 3772. [Google Scholar] [CrossRef]
- Mizoguchi, M.; Yoshimoto, K.; Ma, X.; Guan, Y.; Hata, N.; Amano, T.; Nakamizo, A.; Suzuki, S.O.; Iwaki, T.; Sasaki, T. Molecular characteristics of glioblastoma with 1p/19q co-deletion. Brain Tumor Pathol. 2012, 29, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, Y.; Maemura, K.; Tanaka, Y.; Hirata, A.; Futaki, S.; Hamamoto, H.; Taniguchi, K.; Hayashi, M.; Uchiyama, K.; Shibata, M.-A.; et al. Expression of delta-like 3 is downregulated by aberrant DNA methylation and histone modification in hepatocellular carcinoma. Oncol. Rep. 2018, 39, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Noor, H.; Whittaker, S.; McDonald, K.L. DLL3 expression and methylation are associated with lower-grade glioma immune microenvironment and prognosis. Genomics 2022, 114, 110289. [Google Scholar] [CrossRef]
- Hamamoto, H.; Maemura, K.; Matsuo, K.; Taniguchi, K.; Tanaka, Y.; Futaki, S.; Takeshita, A.; Asai, A.; Hayashi, M.; Hirose, Y.; et al. Delta-like 3 is silenced by HBx via histone acetylation in HBV-associated HCCs. Sci. Rep. 2018, 8, 4842. [Google Scholar] [CrossRef] [PubMed]
- Sethu, S.; Govindappa, K.; Alhaidari, M.; Pirmohamed, M.; Park, K.; Sathish, J. Immunogenicity to biologics: Mechanisms, prediction and reduction. Arch. Immunol. Ther. Exp. 2012, 60, 331–344. [Google Scholar] [CrossRef]
- Baeuerle, P.A.; Wesche, H. T-cell-engaging antibodies for the treatment of solid tumors: Challenges and opportunities. Curr. Opin. Oncol. 2022, 34, 552–558. [Google Scholar] [CrossRef]
- Davda, J.; Declerck, P.; Hu-Lieskovan, S.; Hickling, T.P.; Jacobs, I.A.; Chou, J.; Salek-Ardakani, S.; Kraynov, E. Immunogenicity of immunomodulatory, antibody-based, oncology therapeutics. J. Immunother. Cancer 2019, 7, 105. [Google Scholar] [CrossRef]
- Mejstríková, E.; Hrusak, O.; Borowitz, M.J.; Whitlock, J.A.; Brethon, B.; Trippett, T.M.; Zugmaier, G.; Gore, L.; von Stackelberg, A.; Locatelli, F. CD19-negative relapse of pediatric B-cell precursor acute lymphoblastic leukemia following blinatumomab treatment. Blood Cancer J. 2017, 7, 659. [Google Scholar] [CrossRef]
- Duell, J.; Dittrich, M.; Bedke, T.; Mueller, T.; Eisele, F.; Rosenwald, A.; Rasche, L.; Hartmann, E.; Dandekar, T.; Einsele, H.; et al. Frequency of regulatory T cells determines the outcome of the T-cell-engaging antibody blinatumomab in patients with B-precursor ALL. Leukemia 2017, 31, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, L.A.; Mazza, T.; Fabrizio, F.P.; Sparaneo, A.; D’Alessandro, V.; Tancredi, A.; Trombetta, D.; Centra, F.; Muscarella, S.P.; Di Micco, C.M.; et al. Neuroendocrine-Related Circulating Transcripts in Small-Cell Lung Cancers: Detection Methods and Future Perspectives. Cancers 2021, 13, 1339. [Google Scholar] [CrossRef]
- Zhou, Y.; Penny, H.L.; Kroenke, M.A.; Bautista, B.; Hainline, K.; Chea, L.S.; Parnes, J.; Mytych, D.T. Immunogenicity assessment of bispecific antibody-based immunotherapy in oncology. J. Immunother. Cancer 2022, 10, e004225. [Google Scholar] [CrossRef]
- Kobold, S.; Pantelyushin, S.; Rataj, F.; Berg, J.V. Rationale for Combining Bispecific T Cell Activating Antibodies With Checkpoint Blockade for Cancer Therapy. Front. Oncol. 2018, 8, 285. [Google Scholar] [CrossRef]
- Chen, X.; Amar, N.; Zhu, Y.; Wang, C.; Xia, C.; Yang, X.; Wu, D.; Feng, M. Combined DLL3-targeted bispecific antibody with PD-1 inhibition is efficient to suppress small cell lung cancer growth. J. Immunother. Cancer 2020, 8, e000785. [Google Scholar] [CrossRef]
- Truong, N.T.H.; Gargett, T.; Brown, M.P.; Ebert, L.M. Effects of Chemotherapy Agents on Circulating Leukocyte Populations: Potential Implications for the Success of CAR-T Cell Therapies. Cancers 2021, 13, 2225. [Google Scholar] [CrossRef] [PubMed]
- Bargh, J.D.; Isidro-Llobet, A.; Parker, J.S.; Spring, D.R. Cleavable linkers in antibody-drug conjugates. Chem. Soc. Rev. 2019, 48, 4361–4374. [Google Scholar] [CrossRef]
- Sedykh, S.; Prinz, V.V.; Buneva, V.N.; Nevinsky, G. Bispecific antibodies: Design, therapy, perspectives. Drug Des. Devel Ther. 2018, 12, 195–208. [Google Scholar] [CrossRef]
- Garbayo, E.; Pascual-Gil, S.; Rodríguez-Nogales, C.; Saludas, L.; de Mendoza, A.E.; Blanco-Prieto, M.J. Nanomedicine and drug delivery systems in cancer and regenerative medicine. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1637. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnology 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Guo, Y.; Xu, Y.; Wang, X.; Chen, J.; Wu, X. Combination of micelles and liposomes as a promising drug delivery system: A review. Drug Deliv. Transl. Res. 2023, 13, 2767–2789. [Google Scholar] [CrossRef] [PubMed]


| Agent | Targets | Conditions | Phase | Trial ID | Sponsor |
|---|---|---|---|---|---|
| ADC | |||||
| FZ-AD005 | DLL3/Topoisomerase I Inhibitor | Advanced Solid Tumor SCLC, LCNC | I | NCT06424665 | Zhangjiang Bio-Pharmaceutical |
| ZL1310 | DLL3 | SCLC | I | NCT06179069 | Zai Lab (Shanghai) |
| TCE | |||||
| tarlatamab (AMG757) | DLL3/CD3 | ES-SCLC | 1b | NCT05361395 a | Amgen Inc. |
| LS-SCLC, SCLC | III | NCT06117774 | |||
| ES-SCLC, SCLC | III | NCT06211036 | |||
| BI764532 | DLL3/CD3 | SCLC, Advanced NEC | I | NCT05879978 b | Boehringer Ingelhelm |
| SCLC, Other Neoplasms | I | NCT04429087 | |||
| Relapsed/Refractory ES-SCLC, NEC | II | NCT05882058 c | |||
| Advanced NEC | I | NCT06132113 d | |||
| SCLC | 1b | NCT05990738 e | |||
| SCLC | I | NCT06077500 f | |||
| Glioma | 1b | NCT05916313 | |||
| SCLC, NEC | I | NCT05963867 g | |||
| QLS31904 | DLL3/CD3 | Advanced Solid Tumor | I | NCT05461287 | Qilu Pharmaceutical |
| PT-217 | DLL3/CD47 | Relapsed/Tefractory NEC | I/II | NCT05652686 | Phanes Therapeutics |
| RO7616789 | DLL3/CD3/CD137 | SCLC, NEC | I | NCT05619744 | Hoffmann-La Roche |
| HPN328 | DLL3/CD3/albumin | Advanced Tumors | I/II | NCT00471727 | Harpoon Therapeutics |
| ZG006 | DLL3/DLL3/CD3 | SCLC, NEC | I/II | NCT05978284 | Suzhou Zelgen Bio-pharmaceuticals |
| CAR-T | |||||
| AMG119 | DLL3/CD28/4-1BB/CD3 | SCLC | I (suspended) | NCT03392064 | Amgen |
| LB-2102 | DLL3/DLL3 | ES-SCLC, Lung LCNC | I | NCT05680922 | Legend Biotech USA Inc |
| CAR-NK | |||||
| NK-92 | DLL3 | ES-SCLC | I | NCT05507593 | Tianjin Cancer Hospital |
| RPT | |||||
| [177Lu]Lu-DTPA-CHX-A”-SC16 | DLL3 | NEC | I/II | NCT04199741 | Memorial Sloan Kettering Cancer Center |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zheng, T.; Xu, D.; Sun, C.; Huang, D.; Liu, X. Targeting DLL3: Innovative Strategies for Tumor Treatment. Pharmaceutics 2025, 17, 520. https://doi.org/10.3390/pharmaceutics17040520
Wang H, Zheng T, Xu D, Sun C, Huang D, Liu X. Targeting DLL3: Innovative Strategies for Tumor Treatment. Pharmaceutics. 2025; 17(4):520. https://doi.org/10.3390/pharmaceutics17040520
Chicago/Turabian StyleWang, Hui, Tong Zheng, Dan Xu, Chao Sun, Daqing Huang, and Xiongxiong Liu. 2025. "Targeting DLL3: Innovative Strategies for Tumor Treatment" Pharmaceutics 17, no. 4: 520. https://doi.org/10.3390/pharmaceutics17040520
APA StyleWang, H., Zheng, T., Xu, D., Sun, C., Huang, D., & Liu, X. (2025). Targeting DLL3: Innovative Strategies for Tumor Treatment. Pharmaceutics, 17(4), 520. https://doi.org/10.3390/pharmaceutics17040520






