Pickering Double Emulsions Stabilized with Chitin Nanocrystals and Myristic Acid-Functionalized Silica Nanoparticles for Curcumin and Chlorogenic Acid Co-Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chitin Nanocrystal (ChNC) Preparation
2.3. Characterization of Chitin Nanocrystals (ChNCs)
2.3.1. Size of ChNCs
2.3.2. Wettability of ChNCs
2.3.3. Microstructure of ChNCs
2.3.4. ζ-Potential of ChNCs
2.3.5. X-Ray Diffraction
2.4. Preparation of W1/O Emulsions
2.5. Preparation of Pickering DE-ChNC and DE-ChNC-C14
2.6. Characterization of DEs Formulated Under Optimal Homogenization Parameters
2.6.1. Oil Droplet Size and Size Distribution, Microscopic Structure, Gravitational Stability, and ζ-Potential
2.6.2. Encapsulation Efficiency (EE) and Encapsulation Stability (ES)
2.6.3. Rheological Behavior
2.7. Release of Curcumin, Chlorogenic Acid, and Fatty Acids During In Vitro Digestion
2.8. Statistical Analysis
3. Results and Discussion
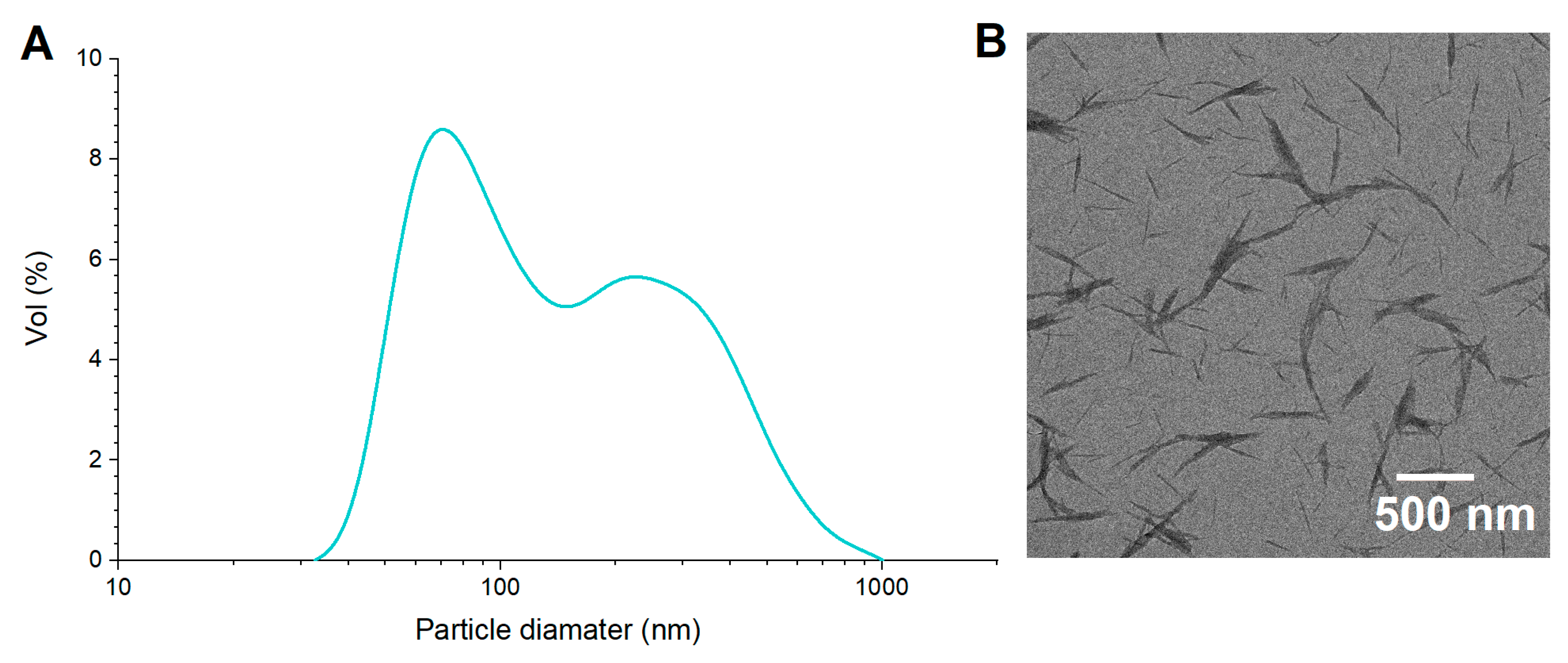
3.1. Characterization of ChNCs
3.2. Preparation and Characterization of DEs
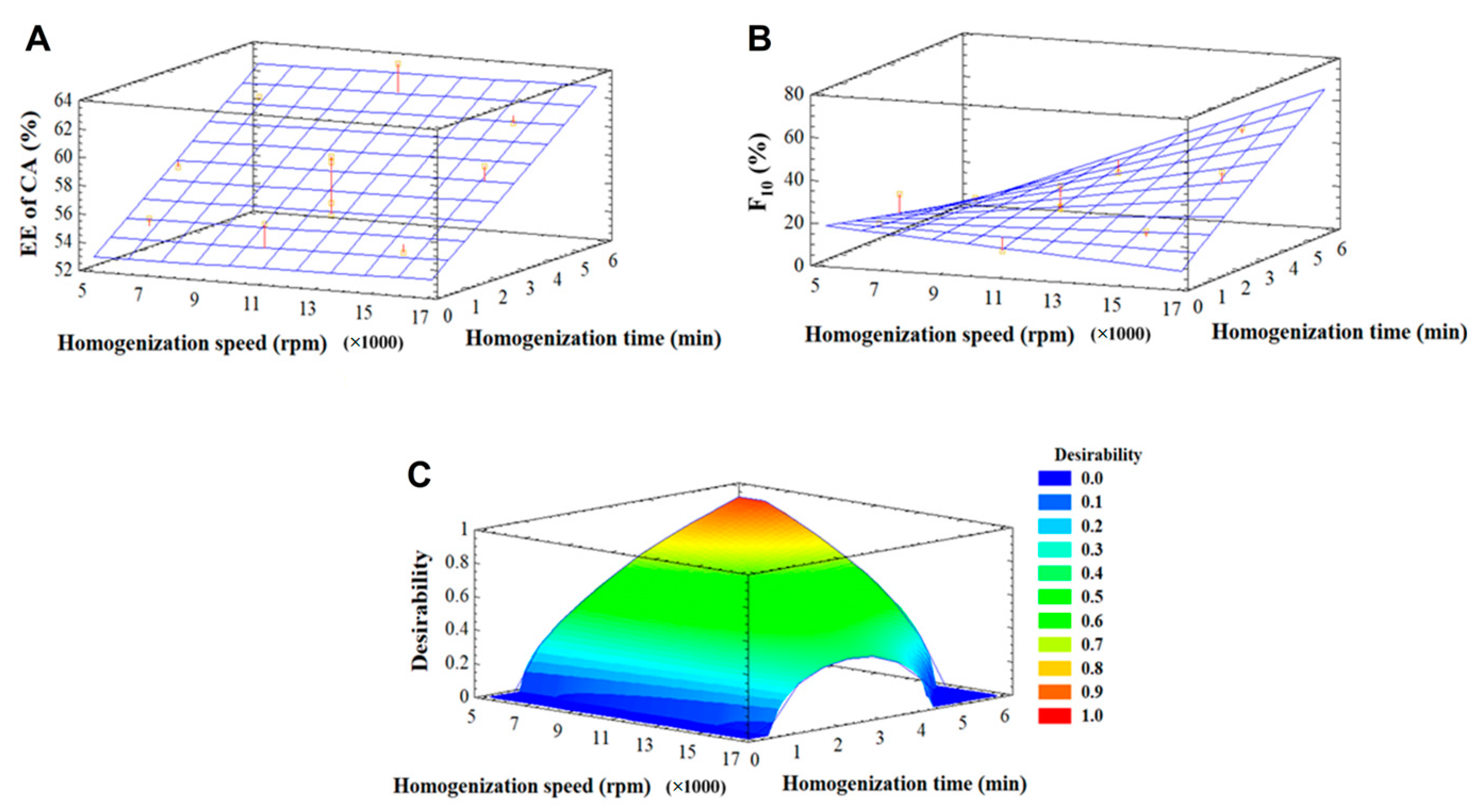
3.2.1. Optimization of Homogenization Parameters
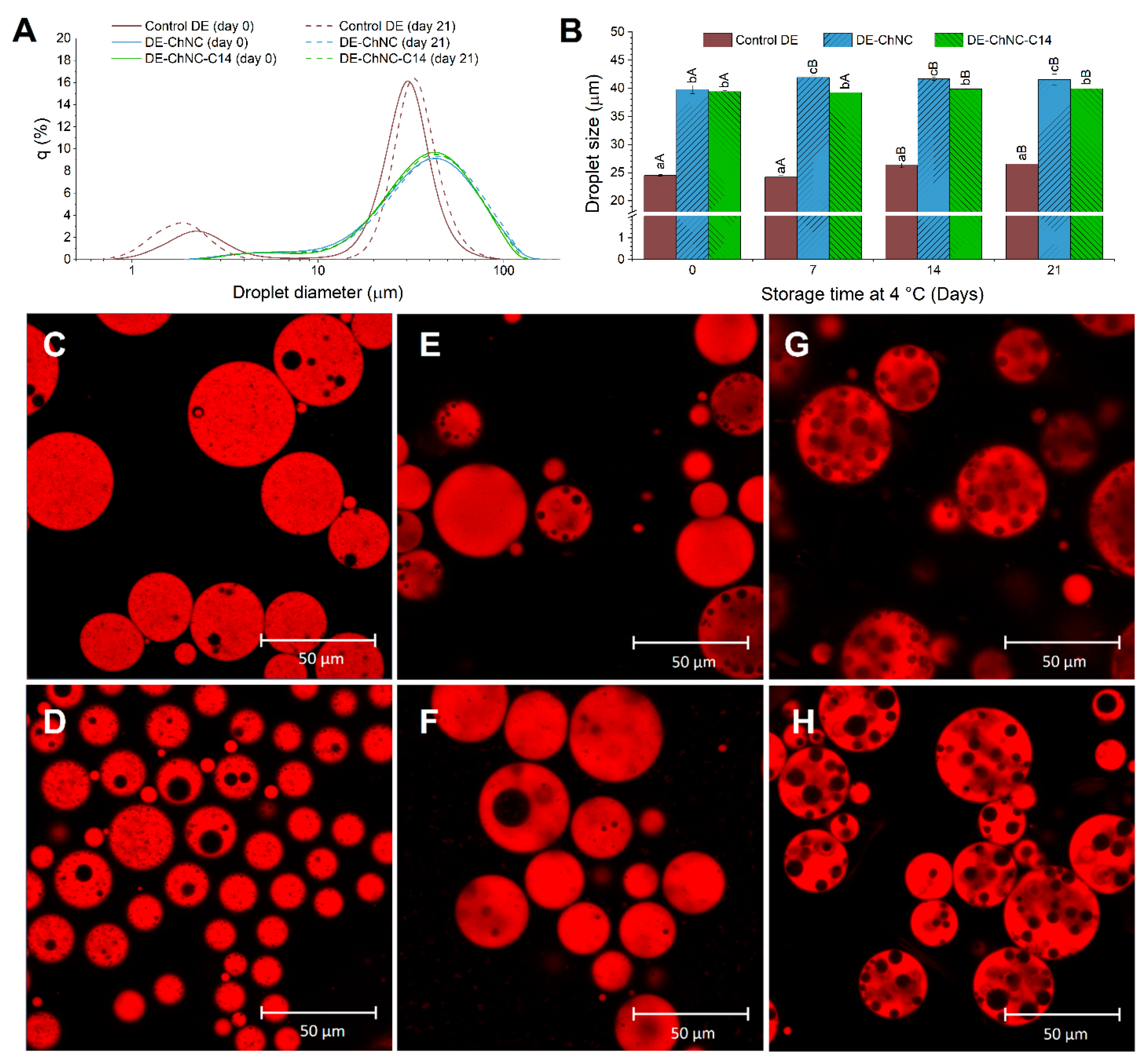
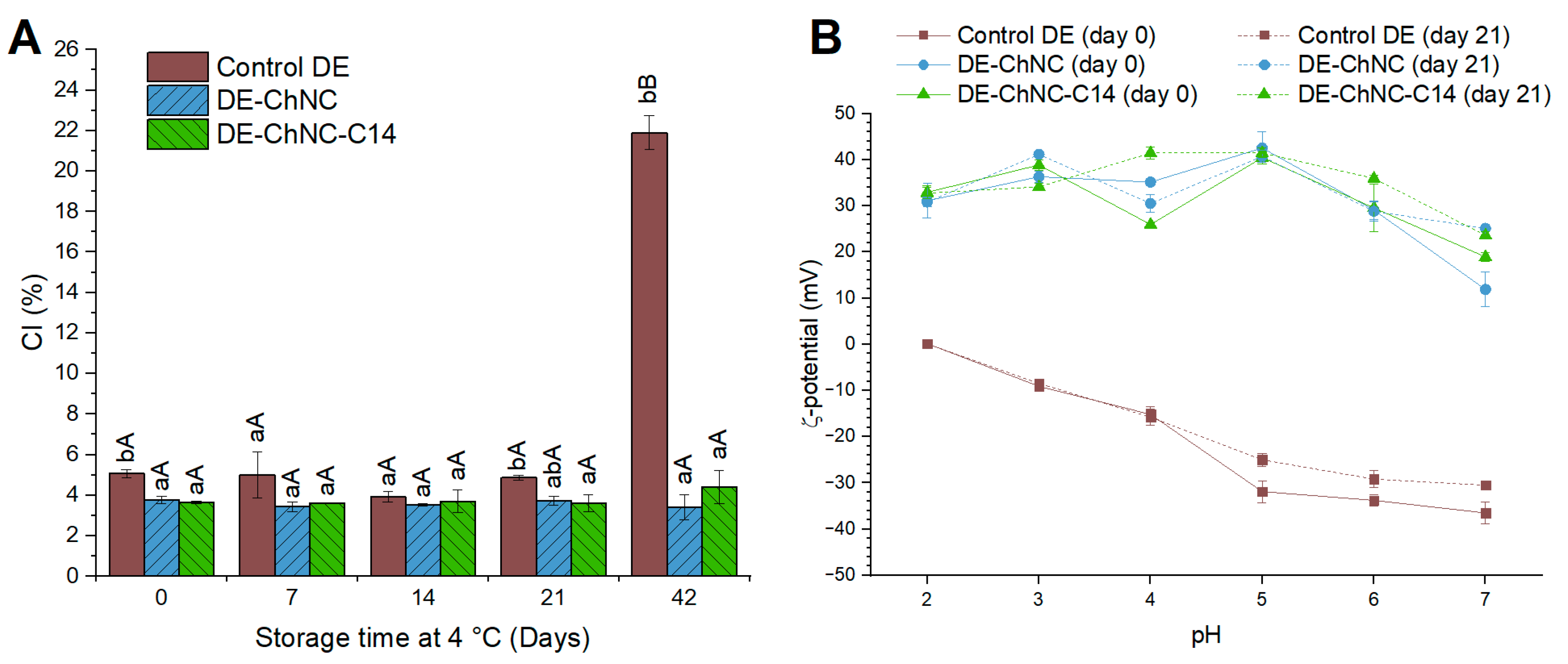
3.2.2. Oil Droplet Size and Distribution, Microscopic Structure, Gravitational Stability, and ζ-Potential
3.2.3. Loading Capacity (LC), Encapsulation Efficiency (EE), and Stability (ES)
3.2.4. Rheological Behavior
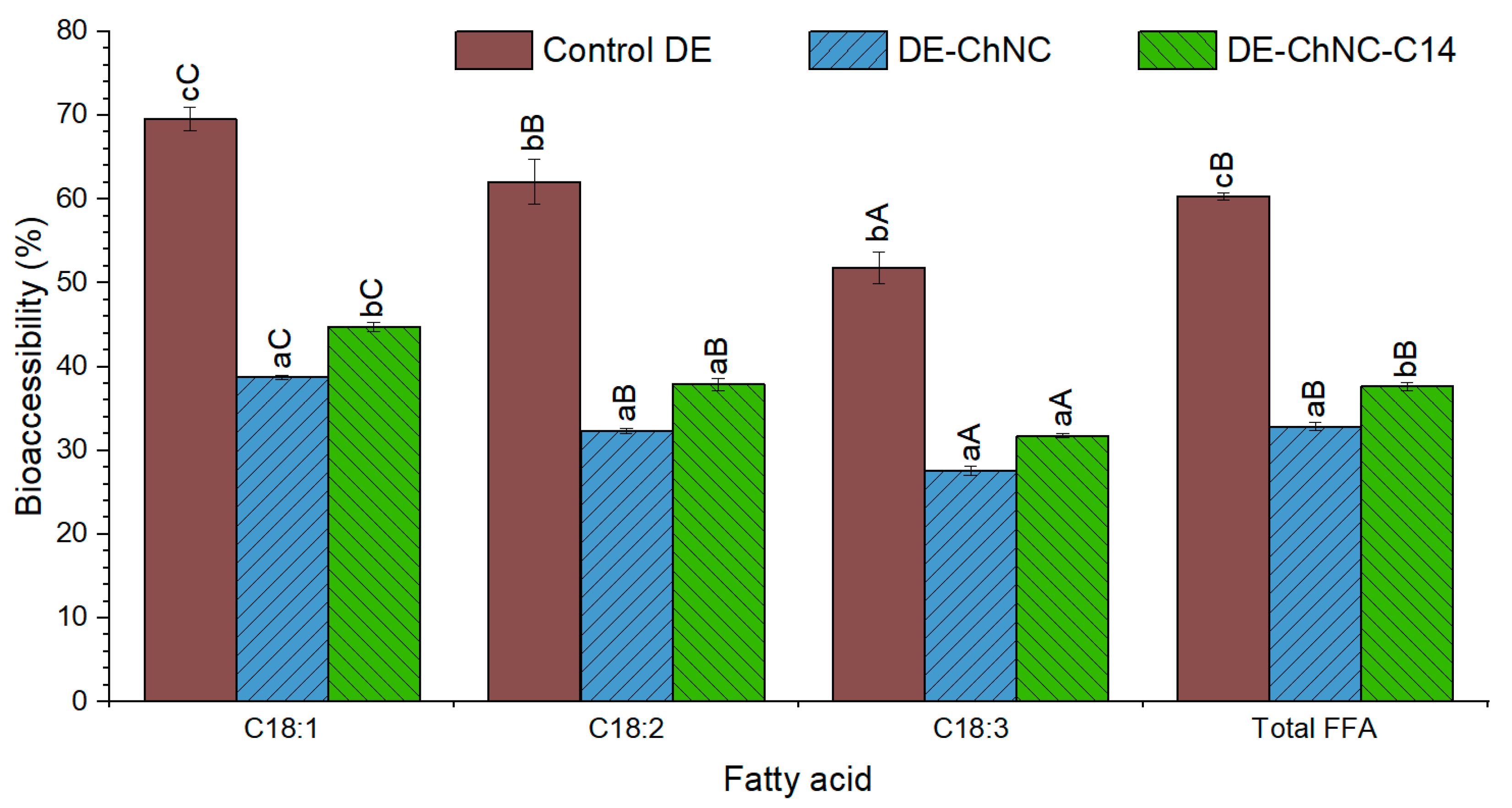
3.3. Release of Curcumin, Chlorogenic Acid, and Fatty Acids During In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DEs | Double emulsions |
| SE | Simple W1/O emulsion |
| F10 | Fraction of oil droplets exhibiting sizes smaller than 10 µm |
| FFAs | Free fatty acids |
| SSF | Simulated salivary fluid |
| SGF | Simulated gastric fluid |
| SIF | Simulated intestinal fluid |
| D4,3 | Volume mean diameter |
| ϑoil/water | Contact angle among the particles, water, and oil |
| CA | Chlorogenic acid |
| W1 | Internal aqueous phase |
| W2 | External aqueous phase |
| W1:O | Inner interface |
| O:W2 | Outer interface |
| ChNCs | Chitin nanocrystals |
| SNPs-C14 | Myristic acid-functionalized silica nanoparticles |
| DE-ChNC | Double emulsion stabilized with polyglycerol polyricinoleate at the inner interface and chitin nanocrystals at the outer interface. |
| DE-ChNC-C14 | Double emulsion stabilized with myristic acid-functionalized silica nanoparticles at the inner interface and chitin nanocrystals at the outer interface |
| LC | Loading capacity |
| EE | Encapsulation efficiency |
| ES | Encapsulation stability |
| DLS | Dynamic light scattering |
| CLSM | Confocal laser scanning microscopy |
| TEM | Transmission electron microscopy |
References
- Muschiolik, G.; Dickinson, E. Double Emulsions Relevant to Food Systems: Preparation, Stability, and Applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Luo, T.; Wei, Z. Recent Progress in Food-Grade Double Emulsions: Fabrication, Stability, Applications, and Future Trends. Food Front. 2023, 4, 1622–1642. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, R.; Kumar, V.; Kumar, S.; Gehlot, R.; Aggarwal, P. New Insights into Water-in-Oil-in-Water (W/O/W) Double Emulsions: Properties, Fabrication, Instability Mechanism, and Food Applications. Trends Food Sci. Technol. 2022, 128, 22–37. [Google Scholar] [CrossRef]
- Aditya, N.P.; Aditya, S.; Yang, H.; Kim, H.W.; Park, S.O.; Ko, S. Co-Delivery of Hydrophobic Curcumin and Hydrophilic Catechin by a Water-in-Oil-in-Water Double Emulsion. Food Chem. 2015, 173, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.; Abdullah, N.; Tian, W.; Song, M.; Cao, Y.; Xiao, J. Co-Delivery of EGCG and Lycopene via a Pickering Double Emulsion Induced Synergistic Hypolipidemic Effect. Food Funct. 2022, 13, 3419–3430. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, C.; Yan, H.; Wang, P.; Yu, S.; Zhang, T.; Yin, Z. Preparation and Characterization of GX-50 and Vitamin C Co-Encapsulated Microcapsules by a Water-in-Oil-in-Water (W1/O/W2) Double Emulsion-Complex Coacervation Method. Langmuir 2023, 39, 13863–13875. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, Y.; Hou, Y.; Wang, H.; Tan, M. Fabrication of Novel W/O/W Emulsion Gels Using Beeswax Stabilized W/O: Preparation, Characterization and Co-Delivery of Phycocyanin and Astaxanthin. Food Biosci. 2024, 57, 103536. [Google Scholar] [CrossRef]
- Zhao, S.; Deng, X.; Wang, Y.; Chen, S.; Liu, X.; Liu, F. Co-Delivery of Hydrophobic β-Carotene and Hydrophilic Riboflavin by Novel Water-in-Oleic Acid-in-Water (W/OA/W) Emulsions. Food Chem. 2024, 432, 137224. [Google Scholar] [CrossRef]
- Li, W.; Wang, W.; Yong, C.; Lan, Y.; Huang, Q.; Xiao, J. Effects of the Distribution Site of Crystallizable Emulsifiers on the Gastrointestinal Digestion Behavior of Double Emulsions. J. Agric. Food Chem. 2022, 70, 5115–5125. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, J.; Cheng, Y.; Huang, Q. Recent Advances in Pickering Double Emulsions and Potential Applications in Functional Foods: A Perspective Paper. Foods 2023, 12, 992. [Google Scholar] [CrossRef]
- McClements, D.J. Encapsulation, Protection, and Release of Hydrophilic Active Components: Potential and Limitations of Colloidal Delivery Systems. Adv. Colloid Interface Sci. 2015, 219, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, W.W.; Lim, H.P.; Low, L.E.; Tey, B.T.; Chan, E.S. Food-Grade Pickering Emulsions for Encapsulation and Delivery of Bioactives. Trends Food Sci. Technol. 2020, 100, 320–332. [Google Scholar] [CrossRef]
- Boostani, S.; Sarabandi, K.; Tarhan, O.; Rezaei, A.; Assadpour, E.; Rostamabadi, H.; Falsafi, S.R.; Tan, C.; Zhang, F.; Jafari, S.M. Multiple Pickering Emulsions Stabilized by Food-Grade Particles as Innovative Delivery Systems for Bioactive Compounds. Adv. Colloid Interface Sci. 2024, 328, 103174. [Google Scholar] [CrossRef]
- Rayees, R.; Gani, A.; Noor, N.; Ayoub, A.; Ashraf, Z.U. General Approaches to Biopolymer-Based Pickering Emulsions. Int. J. Biol. Macromol. 2024, 267, 131430. [Google Scholar] [CrossRef] [PubMed]
- Tenorio-Garcia, E.; Araiza-Calahorra, A.; Simone, E.; Sarkar, A. Recent Advances in Design and Stability of Double Emulsions: Trends in Pickering Stabilization. Food Hydrocoll. 2022, 128, 107601. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, E.; Zhu, G.; Zhou, R.; Niu, Y. Preparation, Characterization and Rheological Behavior of Chitosan Nanocapsule Emulsion Encapsulated Tuberose Fragrance. Pol. J. Chem. Technol. 2016, 18, 1–8. [Google Scholar] [CrossRef]
- Ngasotter, S.; Sampath, L.; Xavier, K.A.M. Nanochitin: An Update Review on Advances in Preparation Methods and Food Applications. Carbohydr. Polym. 2022, 291, 119627. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; Lv, S.; Liu, J.; Muriel Mundo, J.L.; Bai, L.; Rojas, O.J.; McClements, D.J. Nanochitin-Stabilized Pickering Emulsions: Influence of Nanochitin on Lipid Digestibility and Vitamin Bioaccessibility. Food Hydrocoll. 2020, 106, 105878. [Google Scholar] [CrossRef]
- Zhou, H.; Dai, T.; Liu, J.; Tan, Y.; Bai, L.; Rojas, O.J.; McClements, D.J. Chitin Nanocrystals Reduce Lipid Digestion and β-Carotene Bioaccessibility: An in-Vitro INFOGEST Gastrointestinal Study. Food Hydrocoll. 2021, 113, 106494. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, S.; Liu, Y.; Liu, L.; Yu, J.; Fan, Y. In Vitro Digestion Properties of Different Chitin Nanofibrils Stabilized Lipid Emulsions. Food Hydrocoll. 2023, 139, 108512. [Google Scholar] [CrossRef]
- Ngasotter, S.; Xavier, K.A.M.; Meitei, M.M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S.K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health—A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Garti, N.; Aserin, A.; Tiunova, I.; Binyamin, H. Double Emulsions of Water-in-Oil-in-Water Stabilized by α-Form Fat Microcrystals. Part 1: Selection of Emulsifiers and Fat Microcrystalline Particles. JAOCS J. Am. Oil Chem. Soc. 1999, 76, 383–389. [Google Scholar] [CrossRef]
- Frasch-Melnik, S.; Spyropoulos, F.; Norton, I.T. W1/O/W2 Double Emulsions Stabilised by Fat Crystals—Formulation, Stability and Salt Release. J. Colloid Interface Sci. 2010, 350, 178–185. [Google Scholar] [CrossRef]
- Spyropoulos, F.; Duffus, L.J.; Smith, P.; Norton, I.T. Impact of Pickering Intervention on the Stability of W1/O/W2 Double Emulsions of Relevance to Foods. Langmuir 2019, 47, 15137–15150. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Toledo, J.; Herrera, J.; Morales, J.; Robert, P.; Oyarzun-Ampuero, F.; Giménez, B. Bioaccessibility of Chlorogenic Acid and Curcumin Co-Encapsulated in Double Emulsions with the Inner Interface Stabilized by Functionalized Silica Nanoparticles. Food Chem. 2024, 445, 138828. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-evaluation of Silicon Dioxide (E 551) as a Food Additive. EFSA J. 2018, 16, e05088. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA). Food Additives Permitted for Direct Addition to Food for Human Consump-tion-Silicon Dioxide. Code of Federal Regulations. (CFR) Title 21, Chapter I, Subchapter B, Part 172, Subpart E, Section 172.480. 2018. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-E/section-172.480 (accessed on 10 April 2025).
- Lee, J.A.; Kim, M.K.; Song, J.H.; Jo, M.R.; Yu, J.; Kim, K.M.; Kim, Y.R.; Oh, J.M.; Choi, S.J. Biokinetics of Food Additive Silica Nanoparticles and Their Interactions with Food Components. Colloids Surf. B Biointerfaces 2017, 150, 384–392. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Tovar, C.A.; Montero, P.; Gómez-Guillén, M.C. Structural, Viscoelastic, and Emulsifying Properties of Shrimp. Chitin Nanowhisker Dispersions as a Function of Acidic PHs. J. Food Eng. 2023, 351, 111519. [Google Scholar] [CrossRef]
- Stark, A.H.; Reifen, R.; Crawford, M.A. Past and Present Insights on Alpha-linolenic Acid and the Omega-3 Fatty Acid Family. Crit. Rev. Food Sci. Nutr. 2016, 56, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhao, T.; Yang, W.W.; Wang, G.H.; Yu, H.; Zhao, H.X.; Yang, C.; Sun, L.X. Comparative Pharmacokinetics of Chlorogenic Acid after Oral Administration in Rats. J. Pharm. Anal. 2011, 1, 270–274. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Steward, W.P.; Gescher, A.J. Rapid Analysis of Curcumin and Curcumin Metabolites in Rat Biomatrices Using a Novel Ultraperformance Liquid Chromatography (UPLC) Method. J. Agric. Food Chem. 2009, 57, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Abbaspourrad, A. Phycocyanin-rich water-in-oil-in-water (W1/O/W2) double emulsion with nanosized particles: Improved color stability against light exposure. Colloids Surf. B Biointerfaces 2022, 220, 112930. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Ng, N.; Chen, P.X.; Ghazani, S.M.; Wright, A.J.; Marangoni, A.; Goff, H.D.; Joye, I.J.; Rogers, M.A. Lipid Digestion of Oil-in-Water Emulsions Stabilized with Low Molecular Weight Surfactants. Food Funct. 2019, 10, 8195–8207. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Huan, S.; Bai, L.; Ketola, A.; Shi, X.; Zhang, X.; Ketoja, J.A.; Rojas, O.J. High Internal Phase Oil-in-Water Pickering Emulsions Stabilized by Chitin Nanofibrils: 3D Structuring and Solid Foam. ACS Appl. Mater. Interfaces 2020, 12, 11240–11251. [Google Scholar] [CrossRef]
- Jia, X.; Ma, P.; Taylor, K.S.Y.; He, Y.; Mao, Y.; Wang, Q. Innovative Production of Phosphoric Acid-Hydrolyzed Chitin Nanocrystals for Pickering Emulsion Stabilization. Food Biosci. 2024, 60, 104308. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Zou, Y.; Yu, J.; Liu, L.; Fan, Y. Comparison of Cellulose and Chitin Nanofibers on Pickering Emulsion Stability—Investigation of Size and Surface Wettability Contribution. Int. J. Biol. Macromol. 2023, 235, 123754. [Google Scholar] [CrossRef]
- Ben Cheikh, F.; Ben Mabrouk, A.; Magnin, A.; Putaux, J.L.; Boufi, S. Chitin Nanocrystals as Pickering Stabilizer for O/W Emulsions: Effect of the Oil Chemical Structure on the Emulsion Properties. Colloids Surf. B Biointerfaces 2021, 200, 111604. [Google Scholar] [CrossRef]
- Low, L.E.; Siva, S.P.; Ho, Y.K.; Chan, E.S.; Tey, B.T. Recent Advances of Characterization Techniques for the Formation, Physical Properties and Stability of Pickering Emulsion. Adv. Colloid Interface Sci. 2020, 277, 102117. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Rojas, O.J.; McClements, D.J. Recent Innovations in Emulsion Science and Technology for Food Applications. J. Agric. Food Chem. 2021, 69, 8944–8963. [Google Scholar] [CrossRef]
- Peng, G.; Wu, D. Insight into Different Roles of Chitin Nanocrystals and Cellulose Nanocrystals towards Stabilizing Pickering Emulsions. Food Hydrocoll. 2022, 131, 107808. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-Water Emulsions Stabilized by Chitin Nanocrystal Particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Chiu, N.; Tarrega, A.; Parmenter, C.; Hewson, L.; Wolf, B.; Fisk, I.D. Optimisation of octinyl succinic anhydride starch stablised W1/O/W2 emulsions for oral destablisation of encapsulated salt and enhanced saltiness. Food Hydrocoll. 2017, 69, 450–458. [Google Scholar] [CrossRef]
- Cui, F.; Han, S.; Wang, J.; McClements, D.J.; Liu, X.; Liu, F. Co-Delivery of Curcumin and Epigallocatechin Gallate in W/O/W Emulsions Stabilized by Protein Fibril-Cellulose Complexes. Colloids Surf. B Biointerfaces 2023, 222, 113072. [Google Scholar] [CrossRef]
- Surh, J.; Decker, E.A.; McClements, D.J. Influence of PH and Pectin Type on Properties and Stability of Sodium-Caseinate Stabilized Oil-in-Water Emulsions. Food Hydrocoll. 2006, 20, 607–618. [Google Scholar] [CrossRef]
- Xiao, J.; Lu, X.; Huang, Q. Double Emulsion Derived from Kafirin Nanoparticles Stabilized Pickering Emulsion: Fabrication, Microstructure, Stability and In Vitro Digestion profile. Food Hydrocoll. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; Yin, J.; Chang, F.; Wang, C.; He, X.; Huang, Q.; Zhang, B. Anthocyanin-loaded Double Pickering Emulsion Stabilized by Octenylsuccinate Quinoa Starch: Preparation, Stability and In Vitro Gastrointestinal Digestion. Int. J. Biol. Macromol. 2019, 152, 1233–1241. [Google Scholar] [CrossRef]
- Dhanasekaran, S.; Rameshthangam, P.; Venkatesan, S.; Singh, S.K.; Vijayan, S.R. In Vitro and In Silico Studies of Chitin and Chitosan Based Nanocarriers for Curcumin and Insulin Delivery. J. Polym. Environ. 2018, 26, 4095–4113. [Google Scholar] [CrossRef]
- Tang, X.Y.; Wang, Z.M.; Meng, H.C.; Lin, J.W.; Guo, X.M.; Zhang, T.; Chen, H.L.; Lei, C.Y.; Yu, S.J. Robust W/O/W Emulsion Stabilized by Genipin-Cross-Linked Sugar Beet Pectin-Bovine Serum Albumin Nanoparticles: Co-encapsulation of Betanin and Curcumin. J. Agric. Food Chem. 2021, 69, 1318–1328. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhao, J.; Zhong, W.; Huang, C.; Zhi, Z.; Pang, J.; Wu, C. Preparation and Characterization of Fish Oil Pickering Emulsions Stabilized by Resveratrol-Loaded Gliadin/Chitin Nanocrystal Composite Nanoparticles. J. Agric. Food Chem. 2023, 72, 9436–9444. [Google Scholar] [CrossRef]
- Seshadri, R.; Weiss, J.; Hulbert, G.J.; Mount, J. Ultrasonic processing influences rheological and optical properties of high-methoxyl pectin dispersions. Food Hydrocoll. 2003, 17, 191–197. [Google Scholar] [CrossRef]
- Torlopov, M.A.; Vaseneva, I.N.; Mikhaylov, V.I.; Martakov, I.S.; Legki, P.V.; Sitnikov, P.A. Chitin Nanocrystals/Alginate Complex for Tuning Stability, Rheology and Bioavailability of Cholecalciferol in Pickering Emulsions. Int. J. Biol. Macromol. 2024, 264, 130671. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Degradation Kinetics of Chlorogenic Acid at Various PH Values and Effects of Ascorbic Acid and Epigallocatechin Gallate on Its Stability under Alkaline Conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Hynes, M.J.; O’Coinceanainn, M. The Kinetics and Mechanisms of Reactions of Iron(III) with Caffeic Acid, Chlorogenic Acid, Sinapic Acid, Ferulic Acid and Naringin. J. Inorg. Biochem. 2004, 98, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jeon, H.; Myeong, J.; Kwon, C.W.; Chang, P.S. Influence of Creamer Addition on Chlorogenic Acid Bioaccessibility and Antioxidant Activity of Instant Coffee during in Vitro Digestion. LWT 2021, 151, 112178. [Google Scholar] [CrossRef]
- Bai, L.; Lv, S.; Xiang, W.; Huan, S.; McClements, D.J.; Rojas, O.J. Oil-in-Water Pickering Emulsions via Microfluidization with Cellulose Nanocrystals: 2. In Vitro Lipid Digestion. Food Hydrocoll. 2019, 96, 709–716. [Google Scholar] [CrossRef]
- Paredes-Toledo, J.; Herrera, J.; Díaz-Calderón, P.; Robert, P.; Giménez, B. In Vitro Gastrointestinal Release of Chlorogenic Acid and Curcumin Co-Encapsulated in Double Emulsions with the Outer Interface Stabilized by Cellulose Nanocrystals. Colloids Interfaces 2024, 8, 24. [Google Scholar] [CrossRef]


| Encapsulation Efficiency (EE) and Stability (ES) (%) | |||||
|---|---|---|---|---|---|
| Double Emulsion (DE) | Day 0 | Day 7 | Day 14 | Day 21 | |
| CA | Control DE | 26.0 ± 0.1 aA | 31.7 ± 0.8 aB | 32.6 ± 0.3 aB | 24.9 ± 0.5 aA |
| DE-ChNC | 45.0 ± 2.7 bB | 38.6 ± 3.6 aAB | 38.1 ± 1.1 aAB | 32.9 ± 0.6 bA | |
| DE-ChNC-C14 | 91.4 ± 0.9 cB | 52.0 ± 3.8 bA | 54.0 ± 3.5 bA | 54.9 ± 3.0 cA | |
| Double emulsion (DE) | Day 0 | Day 21 | |||
| Curcumin | Control DE | 98.5 ± 0.2 bA | 98.2 ± 0.1 cA | ||
| DE-ChNC | 91.1 ± 0.1 aB | 88.6 ± 0.2 bA | |||
| DE-ChNC-C14 | 91.0 ± 0.3 aB | 85.1 ± 1.0 aA | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes-Toledo, J.; Herrera, J.; Morales, J.; Robert, P.; Gómez-Estaca, J.; Giménez, B. Pickering Double Emulsions Stabilized with Chitin Nanocrystals and Myristic Acid-Functionalized Silica Nanoparticles for Curcumin and Chlorogenic Acid Co-Delivery. Pharmaceutics 2025, 17, 521. https://doi.org/10.3390/pharmaceutics17040521
Paredes-Toledo J, Herrera J, Morales J, Robert P, Gómez-Estaca J, Giménez B. Pickering Double Emulsions Stabilized with Chitin Nanocrystals and Myristic Acid-Functionalized Silica Nanoparticles for Curcumin and Chlorogenic Acid Co-Delivery. Pharmaceutics. 2025; 17(4):521. https://doi.org/10.3390/pharmaceutics17040521
Chicago/Turabian StyleParedes-Toledo, Javier, Javier Herrera, Javier Morales, Paz Robert, Joaquín Gómez-Estaca, and Begoña Giménez. 2025. "Pickering Double Emulsions Stabilized with Chitin Nanocrystals and Myristic Acid-Functionalized Silica Nanoparticles for Curcumin and Chlorogenic Acid Co-Delivery" Pharmaceutics 17, no. 4: 521. https://doi.org/10.3390/pharmaceutics17040521
APA StyleParedes-Toledo, J., Herrera, J., Morales, J., Robert, P., Gómez-Estaca, J., & Giménez, B. (2025). Pickering Double Emulsions Stabilized with Chitin Nanocrystals and Myristic Acid-Functionalized Silica Nanoparticles for Curcumin and Chlorogenic Acid Co-Delivery. Pharmaceutics, 17(4), 521. https://doi.org/10.3390/pharmaceutics17040521








