Chitosan-Stabilized Lipid Vesicles with Indomethacin for Modified Release with Prolonged Analgesic Effect: Biocompatibility, Pharmacokinetics and Organ Protection Efficacy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substances
2.2. Preparation of Lipid Vesicles Encapsulating IND
2.3. Animals
2.4. Ethical Aspects
2.5. Biocompatibility Evaluation
2.5.1. Blood Analysis
2.5.2. Histopathological Examination
2.6. The In Vivo Kinetics Release Profile Assessment
2.7. The Nociceptive Reactivity Testing
2.8. Data Analysis
3. Results
3.1. Hematological and Biochemical Results
3.2. Histological Aspects
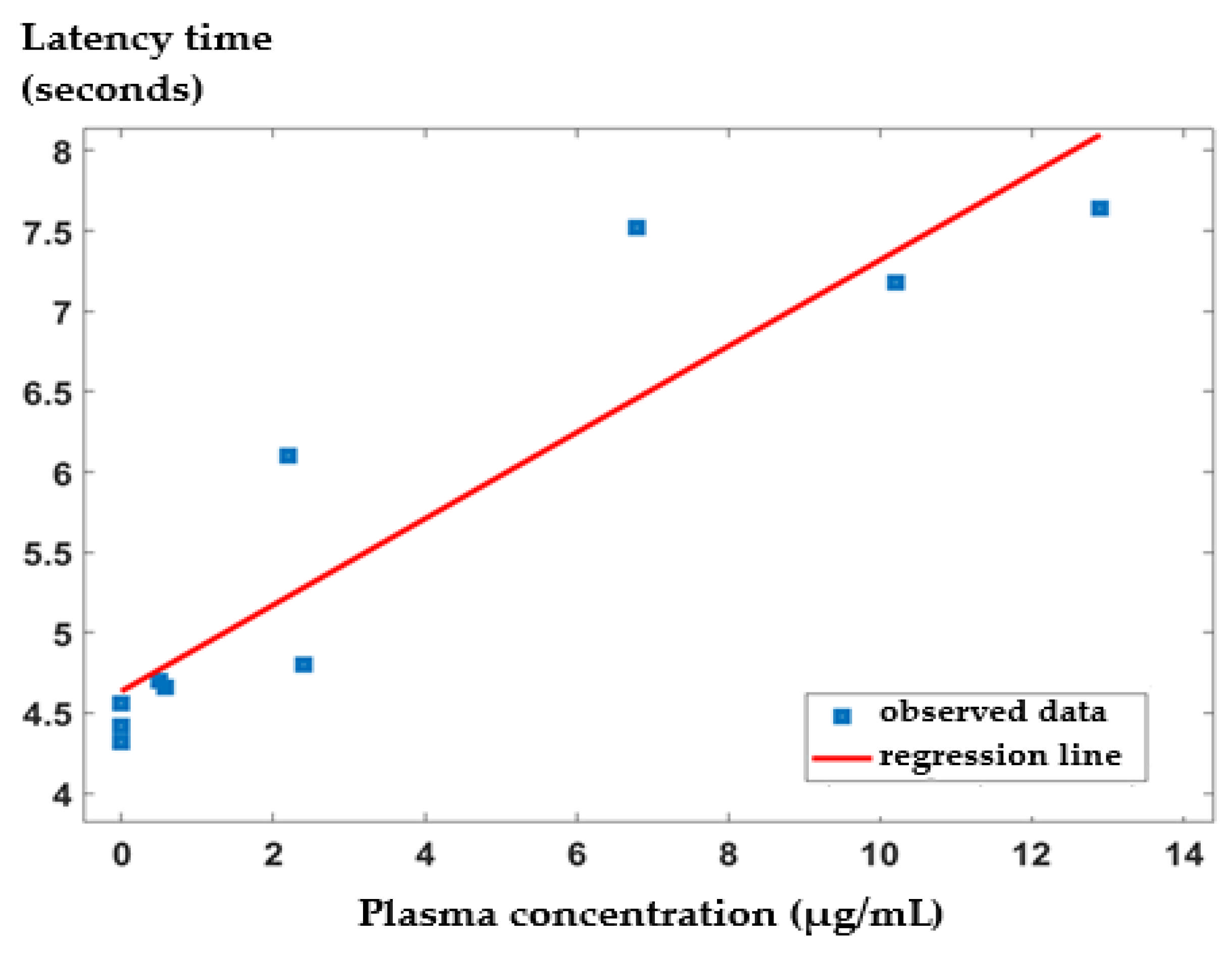
3.3. In Vivo Release Kinetics of IND from the IND-ves
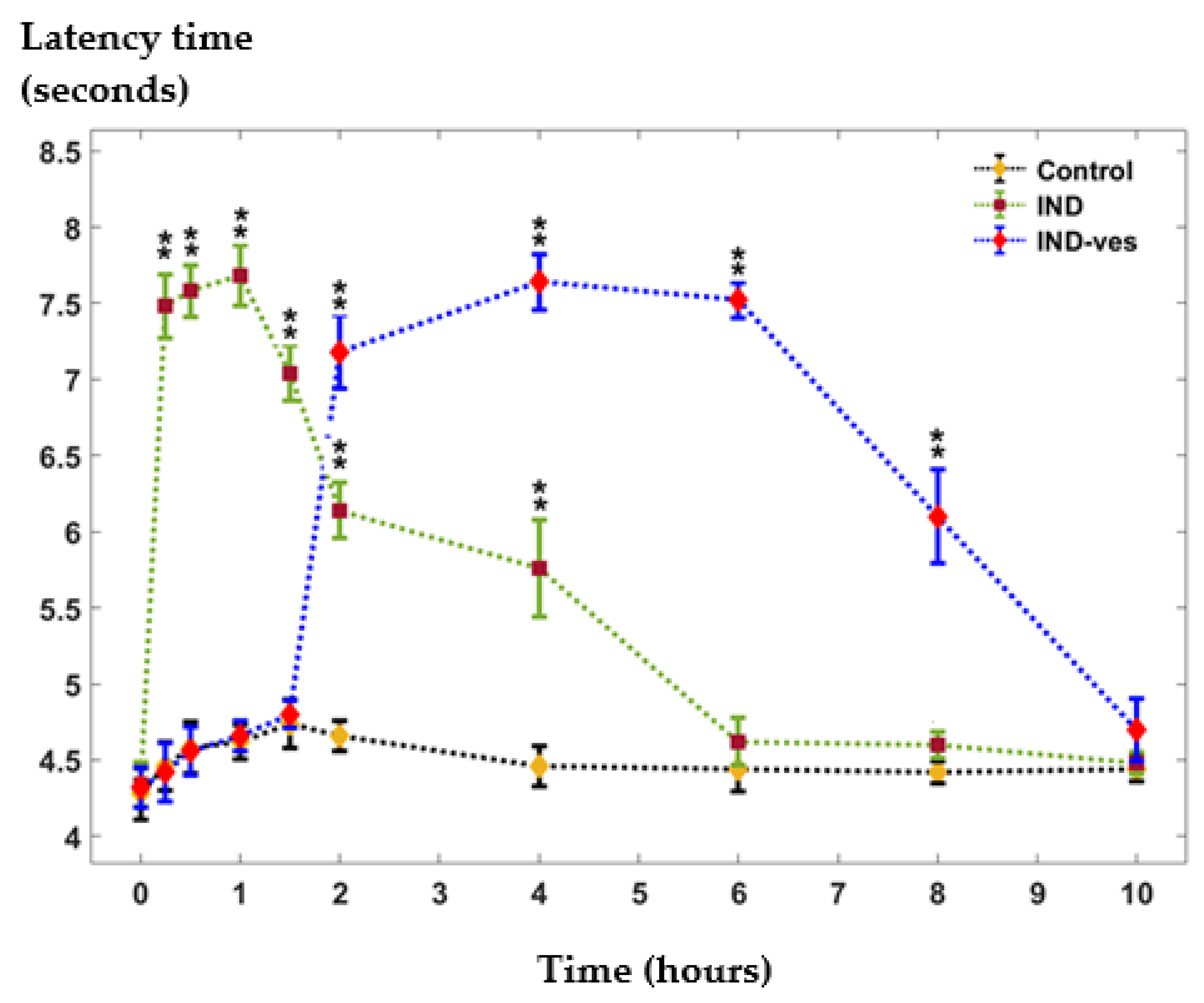
3.4. The Somatic Nociceptive Reactivity
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ewii, U.E.; Onugwu, A.L.; Nwokpor, V.C.; Akpaso, I.; Ogbulie, T.E.; Aharanwa, B.; Chijioke, C.; Verla, N.; Iheme, C.; Ujowundu, C.; et al. Novel drug delivery systems: Insight into self-powered and nano-enabled drug delivery systems. Nano TransMed 2024, 3, 100042. [Google Scholar] [CrossRef]
- Alfutaimani, A.S.; Alharbi, N.K.; Alahmari, A.S.; Alqabbani, A.A.; Aldayel, A.M. Exploring the landscape of Lipid Nanoparticles (LNPs): A comprehensive review of LNPs types and biological sources of lipids. Int. J. Pharm. X 2024, 8, 100305. [Google Scholar] [CrossRef]
- Mustafa, M.A.; Rasheed, N.; Islam, M.; Irshad, I.; Noor-ul-Ain; Batool, S.; Naveed, M.; Bibi, G.; Nazeer, J.; Wajid, F.; et al. Advancements in novel drug delivery systems: Techniques for pre and post formulation analysis. J. Pharm. Res. Int. 2024, 36, 142–153. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric nanoparticles for drug delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for delivery of natural bioactive agents: Recent advances and challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Sreena, R.; Nathanael, A.J. Biodegradable biopolymeric nanoparticles for biomedical applications-challenges and future outlook. Materials 2023, 16, 2364. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, Z.; Liu, H.; Man, J.; Oladejo, A.O.; Ibrahim, S.; Wang, S.; Hao, B. Novel drug delivery systems: An important direction for drug innovation research and development. Pharmaceutics 2024, 16, 674. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Biodegradable polymeric nanoparticle-based drug delivery systems: Comprehensive overview, perspectives and challenges. Polymers 2024, 16, 2536. [Google Scholar] [CrossRef]
- Patel, D.; Solanki, J.; Kher, M.M.; Azagury, A. A Review: Surface engineering of lipid-based drug delivery systems. Small 2024, 20, e2401990. [Google Scholar] [CrossRef]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-based nanoformulations for drug delivery: An ongoing perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Gatto, M.S.; Johnson, M.P.; Najahi-Missaoui, W. Targeted liposomal drug delivery: Overview of the current applications and challenges. Life 2024, 14, 672. [Google Scholar] [CrossRef]
- Rampado, R.; Peer, D. Design of experiments in the optimization of nanoparticle-based drug delivery systems. J. Control. Release 2023, 358, 398–419. [Google Scholar] [CrossRef]
- Kesharwani, R.; Jaiswal, P.; Patel, D.K.; Yadav, P.K. Lipid-based drug delivery system (LBDDS): An emerging paradigm to enhance oral bioavailability of poorly soluble drugs. Biomed. Mater. Devices 2023, 1, 648–663. [Google Scholar] [CrossRef]
- Senjab, R.M.; AlSawaftah, N.; Abuwatfa, W.H.; Husseini, G.A. Advances in liposomal nanotechnology: From concept to clinics. RSC Pharm. 2024, 1, 928–948. [Google Scholar] [CrossRef]
- Izadiyan, Z.; Misran, M.; Kalantari, K.; Webster, T.J.; Kia, P.; Basrowi, N.A.; Rasouli, E.; Shameli, K. Advancements in liposomal nanomedicines: Innovative formulations, therapeutic applications, and future directions in precision medicine. Int. J. Nanomed. 2025, 20, 1213–1262. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Ravikumar, C.; Jagadeesh, S.S.; Shridhar, N.B.; Sg, R. An overview of NSAID loaded nanomaterials. J. Pharm. Innov. 2023, 12, 43–58. [Google Scholar]
- Shalini, C.M.; Anila, S.; Rao, T.R. Strategies in the delivery of nonsteroidal antiinflammatory drugs. Trends Drug Deliv. 2024, 11, 44–61. [Google Scholar]
- Ghlichloo, I.; Gerriets, V. Nonsteroidal anti-inflammatory drugs (NSAIDs). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chelly, J.E.; Goel, S.K.; Kearns, J.; Kopac, O.; Sadhasivam, S. Nanotechnology for pain management. J. Clin. Med. 2024, 13, 2611. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Bhansali, D.; Teng, S.L.; Lee, C.S.; Schmidt, B.L.; Bunnett, N.W.; Leong, K.W. Nanotechnology for pain management: Current and future therapeutic interventions. Nano Today 2021, 39, 101223. [Google Scholar] [CrossRef]
- Mazaleuskaya, L.L.; Muzykantov, V.R.; FitzGerald, G.A. Nanotherapeutic-directed approaches to analgesia. Trends Pharmacol. Sci. 2021, 42, 527–550. [Google Scholar] [CrossRef]
- Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Indomethacin (accessed on 30 January 2025).
- Zulbeari, N.; Hansen, S.M.; Holm, R. Two in one: Size characterization and accelerated short-term physical stability of dual-drug suspensions with two acidic compounds (indomethacin and naproxen). Pharmaceutics 2024, 16, 1495. [Google Scholar] [CrossRef]
- Available online: https://cdn.caymanchem.com/cdn/insert/70270.pdf (accessed on 30 January 2025).
- Benmore, C.J.; Yarger, J.L.; Davidowski, S.K.; Shrader, C.D.; Smith, P.A.; Byrn, S.R. Hydrogen bonding in amorphous indomethacin. Pharmaceutics 2024, 16, 1002. [Google Scholar] [CrossRef]
- Munjal, A.; Allam, A.E. Indomethacin. [Updated 2024 May 28]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK555936/ (accessed on 2 March 2025).
- Krzyzanski, W.; Stockard, B.; Gaedigk, A.; Scott, A.; Nolte, W.; Gibson, K.; Leeder, J.S.; Lewis, T. Developmental pharmacokinetics of indomethacin in preterm neonates: Severely decreased drug clearance in the first week of life. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 110–121. [Google Scholar] [CrossRef]
- Dandić, A.; Rajkovača, K.; Jozanović, M.; Pukleš, I.; Széchenyi, A.; Budetić, M.; Samardžić, M. Review of characteristics and analytical methods for determination of indomethacin. Rev. Anal. Chem. 2022, 41, 34–62. [Google Scholar] [CrossRef]
- Abu Koush, A.; Popa, E.G.; Pricop, D.A.; Nita, L.; Foia, C.-I.; Pauna, A.-M.R.; Buca, B.R.; Pavel, L.L.; Mititelu-Tartau, L. Enhanced stability and in vitro biocompatibility of chitosan-coated lipid vesicles for indomethacin delivery. Pharmaceutics 2024, 16, 1574. [Google Scholar] [CrossRef]
- Pacifici, G.M. Clinical pharmacology of indomethacin. World J. Pharm. Med. Res. 2024, 10, 54–63, ISSN 2455-3301. [Google Scholar]
- Lucas, S. The pharmacology of indomethacin. Headache 2016, 56, 436–446. [Google Scholar] [CrossRef]
- Villar-Martínez, M.D.; Moreno-Ajona, D.; Chan, C.; Goadsby, P.J. Indomethacin-responsive headaches-A narrative review. Headache 2021, 61, 700–714. [Google Scholar] [CrossRef]
- Onel, K.B.; Horton, D.B.; Lovell, D.J.; Shenoi, S.; Cuello, C.A.; Angeles-Han, S.T.; Becker, M.L.; Cron, R.Q.; Feldman, B.M.; Ferguson, P.J.; et al. 2021 American College of Rheumatology Guideline for the treatment of juvenile idiopathic arthritis: Therapeutic approaches for oligoarthritis, temporomandibular joint arthritis, and systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2022, 74, 553–569. [Google Scholar] [CrossRef]
- FitzGerald, J.D.; Dalbeth, N.; Mikuls, T.; Brignardello-Petersen, R.; Guyatt, G.; Abeles, A.M.; Gelber, A.C.; Harrold, L.R.; Khanna, D.; King, C.; et al. 2020 American College of Rheumatology guideline for the management of gout. Arthritis Care Res. 2020, 72, 744–760. [Google Scholar] [CrossRef]
- Evans, P.; O’Reilly, D.; Flyer, J.N.; Soll, R.; Mitra, S. Indomethacin for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst. Rev. 2021, 1, CD013133. [Google Scholar] [CrossRef]
- Sohail, R.; Mathew, M.; Patel, K.K.; Reddy, S.A.; Haider, Z.; Naria, M.; Habib, A.; Abdin, Z.U.; Razzaq, C.W.; Akbar, A. Effects of non-steroidal anti-inflammatory drugs (nsaids) and gastroprotective nsaids on the gastrointestinal tract: A narrative review. Cureus 2023, 15, e37080. [Google Scholar] [CrossRef]
- Klomjit, N.; Ungprasert, P. Acute kidney injury associated with non-steroidal anti-inflammatory drug. Eur. J. Int. Med. 2022, 101, 21–28. [Google Scholar] [CrossRef]
- Kim, J.-W. Are nonsteroidal anti-inflammatory drugs safe for the kidney in ankylosing spondylitis? J. Rheum. Dis. 2023, 30, 139–140. [Google Scholar] [CrossRef]
- Ikdahl, E.; Kerola, A.; Sollerud, E.; Semb, A.G. Cardiovascular implications of non-steroidal anti-inflammatory drugs: A comprehensive review, with emphasis on patients with rheumatoid arthritis. Eur. Cardiol. 2024, 19, e27. [Google Scholar] [CrossRef]
- Liang, S.; Wang, X.; Zhu, X. Insights from pharmacovigilance and pharmacodynamics on cardiovascular safety signals of NSAIDs. Front. Pharmacol. 2024, 15, 1455212. [Google Scholar] [CrossRef]
- Wang, B.; Liao, L.; Liang, H.; Chen, J.; Qiu, Y. Preparation and in vitro/in vivo characterization of mixed-micelles-loaded dissolving microneedles for sustained release of indomethacin. Pharmaceutics 2024, 16, 1505. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Badea, G.; Mihaila, M.; Ott, C.; Stan, R.; Meghea, A. Advanced bioactive lipid nanocarriers loaded with natural and synthetic anti-inflammatory actives. Chem. Eng. Sci. 2019, 200, 113–126. [Google Scholar] [CrossRef]
- Baig, M.S.; Karade, S.K.; Ahmad, A.; Khan, M.A.; Haque, A.; Webster, T.J.; Faiyazuddin, M.; Al-Qahtani, N.H. Lipid-based nanoparticles: Innovations in ocular drug delivery. Front. Mol. Biosci. 2024, 11, 1421959. [Google Scholar] [CrossRef]
- Thiruchenthooran, V.; Świtalska, M.; Maciejewska, G.; Palko-Łabuz, A.; Bonilla-Vidal, L.; Wietrzyk, J.; Souto, E.B.; Sánchez-López, E.; Gliszczyńska, A. Multifunctional indomethacin conjugates for the development of nanosystems targeting cancer treatment. Int. J. Nanomed. 2024, 19, 12695–12718. [Google Scholar] [CrossRef]
- Available online: https://legislatie.just.ro/Public/DetaliiDocument/52457 (accessed on 6 March 2025).
- Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj (accessed on 6 March 2025).
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood sample collection in small laboratory animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Hilițanu, L.N.; Mititelu-Tarțău, L.; Popa, G.E.; Buca, B.R.; Pavel, L.L.; Pelin, A.M.; Meca, A.D.; Bogdan, M.; Pricop, D.A. The analysis of chitosan-coated nanovesicles containing erythromycin-characterization and biocompatibility in mice. Antibiotics 2021, 10, 1471. [Google Scholar] [CrossRef]
- Gholami, A.; Golbabaei, F.; Teimori, G.; Kianmehr, M.; Yaseri, M. Investigation of blood and urine malondialdehyde levels in mice exposed to silica dust. Open Biochem. J. 2019, 13, 32–36. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Margaritelis, N.V.; Kyparos, A.; Paschalis, V.; Nikolaidis, M.G. Spectrophotometric assays for measuring redox biomarkers in blood and tissues: The NADPH network. Redox Rep. 2018, 23, 47–56. [Google Scholar] [CrossRef]
- Jomova, K.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Several lines of antioxidant defense against oxidative stress: Antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024, 98, 1323–1367. [Google Scholar] [CrossRef]
- Larson, C.M.; Wilcox, G.L.; Fairbanks, C.A. The study of pain in rats and mice. Comp. Med. 2019, 69, 555–570. [Google Scholar] [CrossRef]
- Muhammad, N. In-vivo models for management of pain. Pharmacol. Pharm. 2014, 5, 92–96. [Google Scholar] [CrossRef]
- Mirgorodskaya, A.B.; Koroleva, M.Y.; Kushnazarova, R.A.; Mishchenko, E.V.; Petrov, K.A.; Lenina, O.A.; Vyshtakalyuk, A.B.; Voloshina, A.D.; Zakharova, L.Y. Microemulsions and nanoemulsions modified with cationic surfactants for improving the solubility and therapeutic efficacy of loaded drug indomethacin. Nanotechnology 2022, 33, 155103. [Google Scholar] [CrossRef]
- Kalvodová, A.; Zbytovská, J. Lipid nanocapsules enhance the transdermal delivery of drugs regardless of their physico-chemical properties. Int. J. Pharm. 2022, 628, 122264. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Nowaczyk, M. Lipid formulations and bioconjugation strategies for indomethacin therapeutic advances. Molecules 2021, 26, 1576. [Google Scholar] [CrossRef]
- Kalepu, S.; Manthina, M.; Padavala, V. Oral lipid-based drug delivery systems–an overview. Acta Pharm. Sin. B 2013, 3, 361–372. [Google Scholar] [CrossRef]
- Nihala, N.; Mathan, S.; Rajalekshmi, V.R.; Bineesha, K.B. Development of formulation and in vitro evaluation of sterically stabilized (stealth) liposomes containing selected anti-arthritic drug. J. Pharm. Sci. Res. 2019, 11, 3526–3535. [Google Scholar]
- Babu, V.S.; Yachendhra, P.G.; Raju, K.N.V.S.; Rao, K.A. Preparation and characterization of indomethacinloaded chitosan nanoparticles. Indo Am. J. Pharm. Sci. 2022, 9, 85–94. [Google Scholar] [CrossRef]
- Wu, H.-T.; Yang, C.-P.; Huang, S.-C. Dissolution enhancement of indomethacin-chitosan hydrochloride composite particles produced using supercritical assisted atomization. J. Taiwan Inst. Chem. Eng. 2016, 67, 98–105. [Google Scholar] [CrossRef]
- Shan, R.M.; Eldridge, D.S.; Palombo, E.A.; Harding, I.H. Stability mechanisms for microwave-produced solid lipid nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128774. [Google Scholar] [CrossRef]
- Liu, H.; Bolleddula, J.; Nichols, A.; Tang, L.; Zhao, Z.; Prakash, C. Metabolism of bioconjugate therapeutics: Why, when, and how? Drug Metab. Rev. 2020, 52, 66–124. [Google Scholar] [CrossRef]
- Badri, W.; Miladi, K.; Robin, S.; Viennet, C.; Nazari, O.; Agusti, G.; Fessi, H.; Elaissari, A. Polycaprolactone based nanoparticles loaded with indomethacin for anti-inflammatory therapy: From preparation to ex vivo study. Pharm. Res. 2017, 34, 1773–1783. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Nistor, M.T.; Tartau, L. Evaluation of the controlled release ability from the poly(2-hydroxyethyl methacrylate-co-3,9-divinyl-2,4,8,10-tetraoxaspiro[5.5]-undecane) polymer network synthesized in the presence of β-cyclodextrin. J. Mater. Sci. Mater. Med. 2012, 23, 1211–1223. [Google Scholar] [CrossRef]
- Damiati, S.A.; Damiati, S. Microfluidic synthesis of indomethacin-loaded PLGA microparticles optimized by machine learning. Front. Mol. Biosci. 2021, 8, 677547. [Google Scholar] [CrossRef]
- Arabia, M.; Maretti, E.; Sedighidarijani, A.; Rustichelli, C.; Leo, E. Optimizing formulation conditions of PLGA microparticles to enhance indomethacin encapsulation. Part. Part. Syst. Charact. 2024, 42, 2400135. [Google Scholar] [CrossRef]
- Chiriac, A.P.; Nita, L.E.; Diaconu, A.; Bercea, M.; Tudorachi, N.; Pamfil, D.; Mititelu-Tartau, L. Hybrid gels by conjugation of hyaluronic acid with poly(itaconic, anhydride-co-3,9-divinyl-2,4,8,10-tetraoxaspiro (5.5)undecane) copolymers. Int. J. Biol. Macromol. 2017, 98, 407–418. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Nistor, M.T.; Tartau, L. Indomethacin-loaded polymer nanocarriers based on poly(2-hydroxyethyl methacrylate-co-3,9-divinyl-2,4,8,10-tetraoxaspiro (5.5) undecane): Preparation, in vitro and in vivo evaluation. J. Biomed. Mater. Res. Part B-Appl. Biomater. 2012, 100, 1121–1133. [Google Scholar] [CrossRef]
- Dupeyrón, D.; Kawakami, M.; Ferreira, A.M.; Rocio Caceres-Velez, P.; Rieumont, J.; Bentes Azevedo, R.; Carvalho, J.C.T. Design of indomethacin-loaded nanoparticles: Effect of polymer matrix and surfactant. Int. J. Nanomed. 2013, 8, 3467–3477. [Google Scholar] [CrossRef]
- Mahajan, A.G.; Jagtab, L.S.; Chaudhari, A.L.; Swami, S.P.; Mali, P. Formulation and evaluation of microsponge drug delivery system using indomethacin. Int. Res. J. Pharm. 2011, 2, 64–69, ISSN 2230-8407. [Google Scholar]
- Keßler, L.; Mishra, R.; Hietala, S.; Lammens, M.; Peltonen, L.; Rades, T.; van Veen, B.; Juppo, A.; Laaksonen, T.; Strachan, C.; et al. Amorphous solid dispersions of amphiphilic polymer excipients and indomethacin prepared by hot melt extrusion. Eur. J. Pharm. Sci. 2025, 204, 106960. [Google Scholar] [CrossRef]
- Alizadeh, N.; Dianatdar, P. Characterization of behavior of the inclusion complex between Sulfobutylether-β-cyclodextrin loaded alginate/chitosan nanoparticles and indomethacin as a potential approach for drug delivery. J. Mol. Liq. 2024, 407, 125222. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S. Cyclodextrin based nanoparticles for drug delivery and theranostics. Adv. Pharm. Bull. 2020, 10, 166–183. [Google Scholar] [CrossRef]
- Alopaeus, J.F.; Göbel, A.; Breitkreutz, J.; Sande, S.A.; Tho, I. Investigation of hydroxypropyl-β-cyclodextrin inclusion complexation of two poorly soluble model drugs and their taste-sensation-Effect of electrolytes, freeze-drying and incorporation into oral film formulations. J. Drug Deliv. Sci. Technol. 2021, 61, 102245. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, J.; Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Electrohydrodynamic atomization: A two-decade effort to produce and process micro-/nanoparticulate materials. Chem. Eng. Sci. 2015, 125, 32–57. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Li, X.; Cao, Y.; Zhang, W.; Yu, Q.; Hu, L. Self-assembled micelles of indomethacin functionalized linear polyglycidol: Surface property and antitumor activity study. Colloids Surf. A Physicochem. Eng. Asp. 2024, 701, 134915. [Google Scholar] [CrossRef]
- Martínez-Borrajo, R.; Rouco, H.; Virzì, N.F.; Diaz-Rodriguez, P.; Landin, M. Modulation of IFN-γ induced macrophage inflammatory responses via indomethacin-loaded NLCs for OA management. Int. J. Pharm. 2024, 666, 124823. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Ahmad, K.; Zhang, Y.; Chen, P.; Yang, X.; Hou, H. Chitosan interaction with stomach mucin layer to enhances gastric retention and mucoadhesive properties. Carbohydr. Polym. 2024, 333, 121926. [Google Scholar] [CrossRef]
- Mura, P.; Maestrelli, F.; Cirri, M.; Mennini, N. Multiple roles of chitosan in mucosal drug delivery: An updated review. Mar. Drugs 2022, 20, 335. [Google Scholar] [CrossRef]
- Subramanian, D.A.; Langer, R.; Traverso, G. Mucus interaction to improve gastrointestinal retention and pharmacokinetics of orally administered nano-drug delivery systems. J. Nanobiotechnol. 2022, 20, 362. [Google Scholar] [CrossRef]
- Collado-González, M.; González Espinosa, Y.; Goycoolea, F.M. Interaction between chitosan and mucin: Fundamentals and applications. Biomimetics 2019, 4, 32. [Google Scholar] [CrossRef]

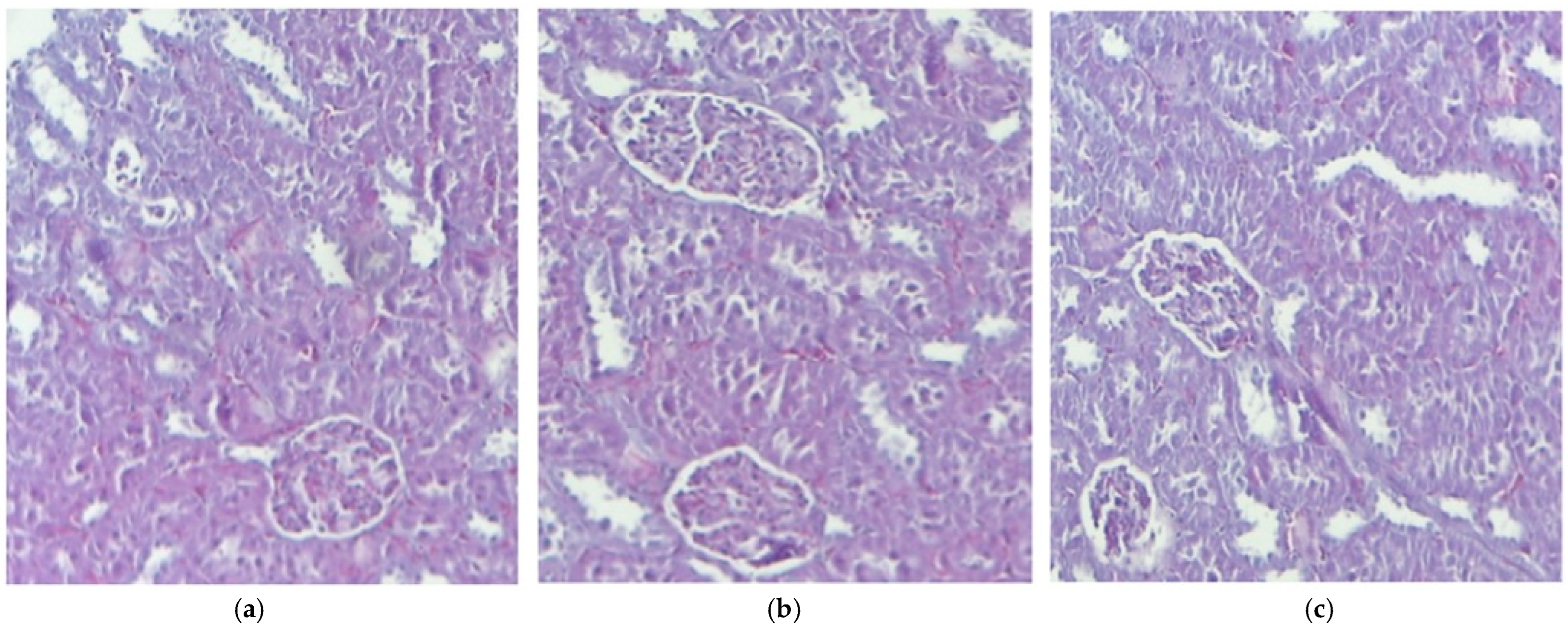




| Evaluation Points | GR (mil/μL) | Leukocyte Formula Elements (%) | |||||
|---|---|---|---|---|---|---|---|
| PMN | Ly | E | M | B | |||
| Control | 24 h | 8.2 ± 1.3 | 28.4 ± 1.9 | 65.9 ± 5.9 | 0.1 ± 0.03 | 5.4 ± 0.3 | 0.2 ± 0.01 |
| 7 days | 8.4 ± 1.1 | 29.1 ± 1.5 | 65.2 ± 5.5 | 0.2 ± 0.01 | 5.3 ± 0.1 | 0.2 ± 0.01 | |
| IND | 24 h | 8.3 ± 0.5 | 28.9 ± 2.3 | 65.4 ± 6.3 | 0.1 ± 0.01 | 5.4 ± 0.5 | 0.2 ± 0.01 |
| 7 days | 8.6 ± 1.1 | 29.3 ± 2.7 | 65,1 ± 5.9 | 0.1 ± 0.01 | 5.3 ± 0.3 | 0.2 ± 0.01 | |
| IND-ves | 24 h | 8.4 ± 0.9 | 28.6 ± 2.3 | 65.8 ± 6.3 | 0.1 ± 0.03 | 5.5 ± 0.3 | 0.2 ± 0.01 |
| 7 days | 8.7 ± 0.3 | 28.5 ± 2.5 | 65.5 ± 5.7 | 0.2 ± 0.03 | 5.6 ± 0.1 | 0.2 ± 0.01 | |
| Evaluation Points | ALT (U/mL) | AST (U/mL) | LDH (U/L) | Urea (mg/dL) | Creatinine (mg/dL) | |
|---|---|---|---|---|---|---|
| Control | 24 h | 39.5 ± 3.9 | 153.7 ± 12.7 | 322.3 ± 19.7 | 27.1 ± 3.1 | 0.4 ± 0.03 |
| 7 days | 39.3 ± 4.5 | 156.1 ± 14.3 | 325.5 ± 18.9 | 27.5 ± 3.3 | 0.5 ± 0.01 | |
| IND | 24 h | 38.3 ± 5.1 | 155.7 ± 13.5 | 325.5 ± 19.3 | 26.5 ± 2.9 | 0.5 ± 0.01 |
| 7 days | 38.5 ± 4.7 | 157.5 ± 13.9 | 326.9 ± 19.5 | 26.3 ± 3.7 | 0.4 ± 0.03 | |
| IND-ves | 24 h | 38.7 ± 4.3 | 155.9 ± 14.5 | 323.3 ± 18.7 | 27.3 ± 2.5 | 0.4 ± 0.03 |
| 7 days | 39.1 ± 4.9 | 158.1 ± 14.3 | 327.7 ± 20.3 | 27.7 ± 2.9 | 0.4 ± 0.01 |
| Evaluation Points | OC (Colonies/mL) | BC (Colonies/mL) | PC (Colonies/mL) | |
|---|---|---|---|---|
| Control | 7 days | 757.9 ± 31.7 | 705.7 ± 30.5 | 525.5 ± 20.9 |
| IND | 7 days | 760.1 ± 34.5 | 708.8 ± 31.3 | 528.5 ± 23.3 |
| IND-ves | 7 days | 759.5 ± 33.7 | 706.1 ± 30.7 | 523.3 ± 24.1 |
| Evaluation Points | SOD (U/mg Protein) | GPx (µm/mg Protein) | MDA (nMol/mg Protein) | |
|---|---|---|---|---|
| Control | 24 h | 27.5 ± 3.3 | 0.4 ± 0.01 | 16.7 ± 2.1 |
| 7 days | 27.7 ± 2.9 | 0.5 ± 0.03 | 16.9 ± 1.5 | |
| IND | 24 h | 28.3 ± 2.9 | 0.5 ± 0.03 | 17.1 ± 1.7 |
| 7 days | 28.1 ± 3.1 | 0.6 ± 0.01 | 17.3 ± 2.3 | |
| IND-ves | 24 h | 27.5 ± 3.3 | 0.4 ± 0.01 | 17.5 ± 1.5 |
| 7 days | 28.3 ± 3.1 | 0.5 ± 0.01 | 16.7 ± 2.1 |
| (µg/mL) | (hours) | (hours) | AUC (µg·h/mL) | |
|---|---|---|---|---|
| Free IND | 13.8 | 0.5 | 1.9 | 28.375 |
| IND-ves | 13.5 | 3 | 4.5 | 58.735 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koush, A.A.; Popa, E.G.; Buca, B.R.; Tartau, C.G.; Stoleriu, I.; Pauna, A.-M.R.; Pavel, L.L.; Fotache, P.A.; Tartau, L.M. Chitosan-Stabilized Lipid Vesicles with Indomethacin for Modified Release with Prolonged Analgesic Effect: Biocompatibility, Pharmacokinetics and Organ Protection Efficacy. Pharmaceutics 2025, 17, 523. https://doi.org/10.3390/pharmaceutics17040523
Koush AA, Popa EG, Buca BR, Tartau CG, Stoleriu I, Pauna A-MR, Pavel LL, Fotache PA, Tartau LM. Chitosan-Stabilized Lipid Vesicles with Indomethacin for Modified Release with Prolonged Analgesic Effect: Biocompatibility, Pharmacokinetics and Organ Protection Efficacy. Pharmaceutics. 2025; 17(4):523. https://doi.org/10.3390/pharmaceutics17040523
Chicago/Turabian StyleKoush, Angy Abu, Eliza Gratiela Popa, Beatrice Rozalina Buca, Cosmin Gabriel Tartau, Iulian Stoleriu, Ana-Maria Raluca Pauna, Liliana Lacramioara Pavel, Paula Alina Fotache, and Liliana Mititelu Tartau. 2025. "Chitosan-Stabilized Lipid Vesicles with Indomethacin for Modified Release with Prolonged Analgesic Effect: Biocompatibility, Pharmacokinetics and Organ Protection Efficacy" Pharmaceutics 17, no. 4: 523. https://doi.org/10.3390/pharmaceutics17040523
APA StyleKoush, A. A., Popa, E. G., Buca, B. R., Tartau, C. G., Stoleriu, I., Pauna, A.-M. R., Pavel, L. L., Fotache, P. A., & Tartau, L. M. (2025). Chitosan-Stabilized Lipid Vesicles with Indomethacin for Modified Release with Prolonged Analgesic Effect: Biocompatibility, Pharmacokinetics and Organ Protection Efficacy. Pharmaceutics, 17(4), 523. https://doi.org/10.3390/pharmaceutics17040523











