Comparison of Drying Techniques to Produce Stable and Bioavailable Encapsulated ACE-2 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ACE-2 Nanoparticle Preparation
2.3. ACE-2 NP Dehydration
2.4. Characterization of Nanoparticles
2.5. Fourier Transform Infrared–Attenuated Total Reflectance (FTIR-ATR) Spectroscopy
2.6. Reconstitution Test
2.7. In Vitro Release Profile of ACE-2 Loaded NPs
2.8. In Vitro Cellular Uptake Study
2.9. In Vitro Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Chitosan–TPP Cross-Linked ACE-2 NPs
3.2. Morphological Analysis of ACE-2 NPs Dehydrated by Different Methods
3.3. FTIR–ATR Spectroscopy Analysis
3.4. Yield Rate, Reconstitution, and Stability Analysis
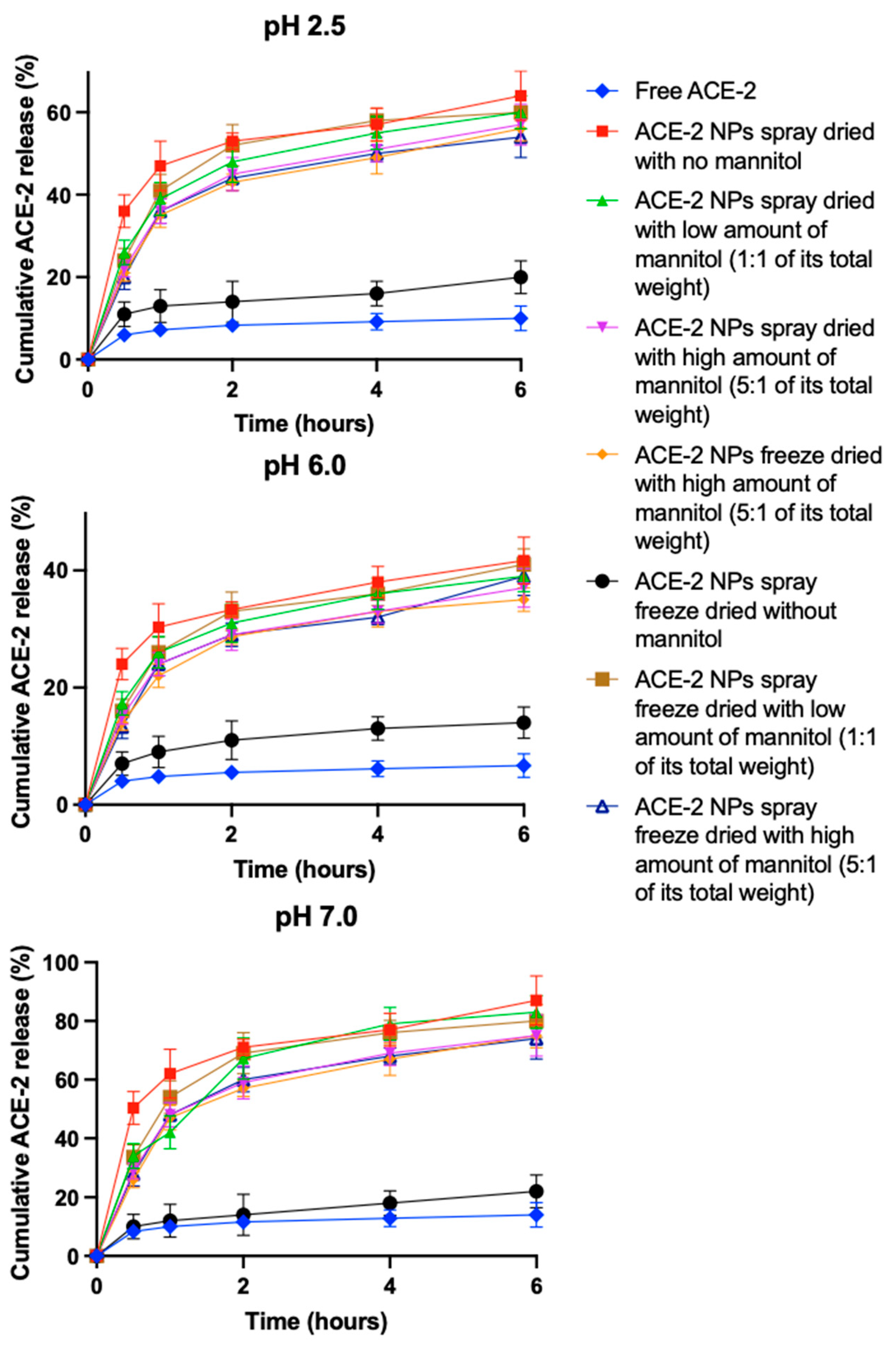
3.5. In Vitro Release of ACE-2 from NPs at Different pHs
3.6. In Vitro Cellular (Cacp-2) Uptake of Dehydrated ACE-2 NPs
3.7. In Vitro Toxicity Evaluation of Dehydrated ACE-2 NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Hu, S.; Popowski, K.D.; Liu, S.; Zhu, D.; Mei, X.; Li, J.; Hu, Y.; Dinh, P.-U.C.; Wang, X.; et al. Inhalation of ACE2-expressing lung exosomes provides prophylactic protection against SARS-CoV-2. Nat. Commun. 2024, 15, 2236. [Google Scholar] [CrossRef]
- Saito, A.; Irie, T.; Suzuki, R.; Maemura, T.; Nasser, H.; Uriu, K.; Kosugi, Y.; Shirakawa, K.; Sadamasu, K.; Kimura, I.; et al. Enhanced fusogenicity and pathogenicity of SARS-CoV-2 Delta P681R mutation. Nature 2022, 602, 300–306. [Google Scholar] [CrossRef]
- Baldelli, A.; Wong, C.Y.J.; Oguzlu, H.; Gholizadeh, H.; Guo, Y.; Ong, H.X.; Singh, A.; Traini, D.; Pratap-Singh, A. Nasal delivery of encapsulated recombinant ACE2 as a prophylactic drug for SARS-CoV-2. Int. J. Pharm. 2024, 655, 124009. [Google Scholar] [CrossRef] [PubMed]
- Hathout, R.M.; Abdelhamid, S.G.; Metwally, A.A. Chloroquine and hydroxychloroquine for combating COVID-19: Investigating efficacy and hypothesizing new formulations using Bio/chemoinformatics tools. Inform. Med. Unlocked 2020, 21, 100446. [Google Scholar] [CrossRef] [PubMed]
- Mannar, D.; Saville, J.W.; Zhu, X.; Srivastava, S.S.; Berezuk, A.M.; Zhou, S.; Tuttle, K.S.; Kim, A.; Li, W.; Dimitrov, D.S.; et al. Structural analysis of receptor binding domain mutations in SARS-CoV-2 variants of concern that modulate ACE2 and antibody binding. Cell Rep. 2021, 37, 110156. [Google Scholar] [CrossRef] [PubMed]
- Zoufaly, A.; Poglitsch, M.; Aberle, J.H.; Hoepler, W.; Seitz, T.; Traugott, M.; Grieb, A.; Pawelka, E.; Laferl, H.; Wenisch, C.; et al. Human recombinant soluble ACE2 in severe COVID-19. Lancet Respir. Med. 2020, 8, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Diao, X.; Jia, R.; Wang, Y.; Liu, G.; Chen, X.; Liu, D.; Guan, H. The physicochemical properties, microstructure, and stability of diacylglycerol-loaded multilayer emulsion based on protein and polysaccharides. LWT 2024, 196, 115879. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.; Wasan, K.M.; Wasan, E.K. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Rampino, A.; Borgogna, M.; Blasi, P.; Bellich, B.; Cesàro, A. Chitosan nanoparticles: Preparation, size evolution and stability. Int. J. Pharm. 2013, 455, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Balde, A.; Kim, S.-K.; Benjakul, S.; Nazeer, R.A. Pulmonary drug delivery applications of natural polysaccharide polymer derived nano/micro-carrier systems: A review. Int. J. Biol. Macromol. 2022, 220, 1464–1479. [Google Scholar] [CrossRef] [PubMed]
- Rezvankhah, A.; Emam-Djomeh, Z.; Askari, G. Encapsulation and delivery of bioactive compounds using spray and freeze-drying techniques: A review. Dry. Technol. 2020, 38, 235–258. [Google Scholar] [CrossRef]
- Guo, Y.; Baldelli, A.; Singh, A.; Fathordoobady, F.; Kitts, D.; Pratap-Singh, A. Production of high loading insulin nanoparticles suitable for oral delivery by spray drying and freeze drying techniques. Sci. Rep. 2022, 12, 9949. [Google Scholar] [CrossRef] [PubMed]
- Vishali, D.; Monisha, J.; Sivakamasundari, S.; Moses, J.; Anandharamakrishnan, C. Spray freeze drying: Emerging applications in drug delivery. J. Control Release 2019, 300, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Eggerstedt, S.N.; Dietzel, M.; Sommerfeld, M.; Süverkrüp, R.; Lamprecht, A. Protein spheres prepared by drop jet freeze drying. Int. J. Pharm. 2012, 438, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Guo, Y.; Kitts, D.D.; Dolati, D.; Pratap-Singh, A.; Singh, A. Enhancing resveratrol bioavailability and intestinal uptake using an oil-based blueberry extract formulated with chitosan/PEG containing nanoparticles. Food Hydrocoll. 2024, 156, 110373. [Google Scholar] [CrossRef]
- Gan, Q.; Wang, T.; Cochrane, C.; McCarron, P. Modulation of surface charge, particle size and morphological properties of chitosan–TPP nanoparticles intended for gene delivery. Colloids Surf. B Biointerfaces 2005, 44, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.-M.; Wang, L.-J.; Li, D.; Adhikari, B. The effect of annealing and cryoprotectants on the properties of vacuum-freeze dried starch nanoparticles. Carbohydr. Polym. 2012, 88, 1334–1341. [Google Scholar] [CrossRef]
- Kumar, S.; Gokhale, R.; Burgess, D.J. Sugars as bulking agents to prevent nano-crystal aggregation during spray or freeze-drying. Int. J. Pharm. 2014, 471, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef] [PubMed]




| Freshly Prepared | FD ++ | SD − | SD + | SD ++ | SFD − | SFD + | SFD − | |
|---|---|---|---|---|---|---|---|---|
| PS (nm) | 303 ± 12 a | 674 ± 47 b | 343 ± 33 c | 535 ± 24 d | 613 ± 37 b | 366 ± 29 c | 548 ± 30 d | 664 ± 42 b |
| PDI | 0.19 ± 0.02 a | 0.24 ± 0.03 a | 0.22 ± 0.02 a | 0.23 ± 0.03 a | 0.25 ± 0.05 a | 0.22 ± 0.04 a | 0.21 ± 0.03 a | 0.20 ± 0.04 a |
| EE (%) | 98.40± 0.32 a | 98.01 ± 0.43 a | 97.63 ± 0.29 a | 99.01 ± 0.51 a | 98.23 ± 0.43 a | 99.03 ± 0.39 a | 98.93 ± 0.36 a | 99.02 ± 0.22 a |
| LC (%) | 28.42 ± 0.21 a | 4.71 ± 0.13 b | 18.14 ± 0.44 c | 3.92 ± 0.10 d | 2.02 ± 0.06 e | 27.84 ± 0.30 a | 14.22 ± 0.32 f | 4.69 ± 0.36 b |
| ZP (mV) | 6.8 ± 0.2 a | 6.5 ± 0.3 a | 6.6 ± 0.2 a | 7.1 ± 0.3 a | 6.5 ± 0.2 a | 6.7 ± 0.3 a | 6.7 ± 0.4 a | 6.8 ± 0.1 a |
| YR (%) | NA | 99.83 ± 0.13 a | 47.87 ± 2.13 b | 53.32 ± 3.13 c | 55.31 ± 2.13 c | 99.67 ± 0.13 a | 98.98 ± 0.13 a | 99.32 ± 0.13 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Baldelli, A.; Shi, D.; Kitts, D.D.; Pratap-Singh, A.; Singh, A. Comparison of Drying Techniques to Produce Stable and Bioavailable Encapsulated ACE-2 Nanoparticles. Pharmaceutics 2025, 17, 537. https://doi.org/10.3390/pharmaceutics17040537
Guo Y, Baldelli A, Shi D, Kitts DD, Pratap-Singh A, Singh A. Comparison of Drying Techniques to Produce Stable and Bioavailable Encapsulated ACE-2 Nanoparticles. Pharmaceutics. 2025; 17(4):537. https://doi.org/10.3390/pharmaceutics17040537
Chicago/Turabian StyleGuo, Yigong, Alberto Baldelli, Dai Shi, David D. Kitts, Anubhav Pratap-Singh, and Anika Singh. 2025. "Comparison of Drying Techniques to Produce Stable and Bioavailable Encapsulated ACE-2 Nanoparticles" Pharmaceutics 17, no. 4: 537. https://doi.org/10.3390/pharmaceutics17040537
APA StyleGuo, Y., Baldelli, A., Shi, D., Kitts, D. D., Pratap-Singh, A., & Singh, A. (2025). Comparison of Drying Techniques to Produce Stable and Bioavailable Encapsulated ACE-2 Nanoparticles. Pharmaceutics, 17(4), 537. https://doi.org/10.3390/pharmaceutics17040537








