Solid Microneedles for Transdermal Delivery of Amantadine Hydrochloride and Pramipexole Dihydrochloride
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Skin Preparation
2.2.2. In Vitro Diffusion Studies
2.2.3. High-Performance Liquid Chromatography–Mass Spectrometry Analysis (HPLC–MS)
2.2.4. Visualization of Microchannels
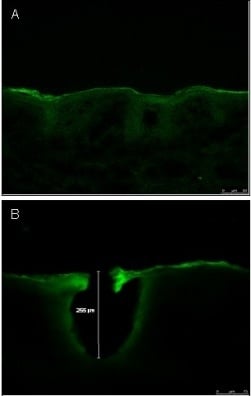
2.2.5. Characterization of Microchannel Depth by Confocal Laser Scanning Microscopy (CLSM)
2.2.6. Burst Strength
2.2.7. Data Analysis
2.2.8. Statistical Analysis
3. Results
3.1. Characterization of Microneedle Array and Microneedle Roller



3.2. Visualization of Microchannels
3.3. Characterization of Microchannel Depth by Confocal Laser Scanning Microscopy (CLSM)
3.4. Burst Strength
| Initial Gradient (N/s) | Mid Gradient (N/s) | Final Gradient (N/s) | Work to Burst Skin (N/s) | Burst Force (N) | Distance to Burst (mm) |
|---|---|---|---|---|---|
| 13.75 ± 2.11 | 43.98 ± 8.17 | 62.14 ± 11.54 | 341.24 ± 42.44 | 187.54 ± 19.38 | 7.77 ± 0.63 |
3.5. In Vitro Diffusion Studies
| Amantadine Qs | Passive (Control) Qs (μg/cm2) | Microneedle Qs (μg/cm2) |
| Mean | 267.65 ± 14.07 | 589.26 ± 23.13 |
| Pramipexole Qs | Passive (Control) Qs (μg/cm2) | Microneedle Qs (μg/cm2) |
| Mean | 1607.86 ± 35.77 | 1583.43 ± 72.99 |
| Amantadine Flux | Passive (Control) Flux (μg/cm2) | Microneedle Flux (μg/cm2) |
| Mean | 22.38 ± 4.73 | 49.04 ± 19.77 |
| Pramipexole Flux | Passive (Control) Flux (μg/cm2) | Microneedle Flux (μg/cm2) |
| Mean | 134.83 ± 13.66 | 134.04 ± 0.98 |


4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Glossary
References
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Hallett, P.J.; Standaert, D.G. Rationale for and use of NMDA receptor antagonists in Parkinson’s disease. Pharmacol. Ther. 2004, 102, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kanthasamy, A.; Ghosh, A.; Anantharam, V.; Kalyanaraman, B.; Kanthasamy, A.G. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim. Biophys. Acta 2014, 1842, 1282–1294. [Google Scholar] [CrossRef]
- Abdullah, R.; Basak, I.; Patil, K.S.; Alves, G.; Larsen, J.P.; Møller, S.G. Parkinson’s disease and age: The obvious but largely unexplored link. Exp. Gerontol. 2015, 68, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Penko, A.L.; Hirsch, J.R.; Voelcker-Rehage, C.; Martin, P.E.; Blackburn, G.; Alberts, J.L. Asymmetrical pedaling patterns in Parkinson’s disease patients. Clin. Biomech. 2014, 29, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.D.; Banga, A.K. Controlled delivery of ropinirole hydrochloride through skin using modulated iontophoresis and microneedles. J. Drug Target. 2013, 21, 354–366. [Google Scholar] [CrossRef]
- Przedborski, S. Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat. Disord. 2005, 11, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Giladi, N.; Boroojerdi, B.; Korczyn, A.D.; Burn, D.J.; Clarke, C.E.; Schapira, A.H. Rotigotine transdermal patch in early Parkinson’s disease: A randomized, double-blind, controlled study versus placebo and ropinirole. Mov. Disord. 2007, 22, 2398–2404. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.H.; Singer, C.; O’Brien, C.; Hauser, R.A.; Lew, M.F.; Marek, K.L.; Dorflinger, E.; Pedder, S.; Deptula, D.; Yoo, K. Randomized, placebo-controlled study of tolcapone in patients with fluctuating Parkinson disease treated with levodopa-carbidopa. Tolcapone Fluctuator Study Group III. Arch. Neurol. 1998, 55, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, K.R.; Schapira, A.H.V. Non-motor symptoms of Parkinson’s disease: Dopaminergic pathophysiology and treatment. Lancet Neurol. 2009, 8, 464–474. [Google Scholar] [CrossRef]
- Guttman, M.; Kish, S.J.; Furukawa, Y. Current concepts in the diagnosis and management of Parkinson’s disease. CMAJ 2003, 168, 293–301. [Google Scholar] [PubMed]
- Horstink, M.; Tolosa, E.; Bonuccelli, U.; Deuschl, G.; Friedman, A.; Kanovsky, P.; Larsen, J.P.; Lees, A.; Oertel, W.; Poewe, W.; et al. Review of the therapeutic management of Parkinson’s disease. Report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES). Part II: Late (complicated) Parkinson’s disease. Eur. J. Neurol. 2006, 13, 1186–1202. [Google Scholar] [PubMed]
- Thanvi, B.; Lo, N.; Robinson, T. Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad. Med. J. 2007, 83, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Chase, T.N.; Oh, J.D. Striatal dopamine- and glutamate-mediated dysregulation in experimental parkinsonism. Trends Neurosci. 2000, 23, S86–S91. [Google Scholar] [CrossRef]
- Kalaria, D.R.; Patel, P.; Merino, V.; Patravale, V.B.; Kalia, Y.N. Controlled iontophoretic delivery of pramipexole: Electrotransport kinetics in vitro and in vivo. Eur. J. Pharm. Biopharm. 2014, 88, 56–63. [Google Scholar] [CrossRef]
- Badran, M.M.; Kuntsche, J.; Fahr, A. Skin penetration enhancement by a microneedle device (Dermaroller) in vitro: Dependency on needle size and applied formulation. Eur. J. Pharm. Sci. 2009, 36, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Ita, K.B. Transdermal drug delivery: Progress and challenges. J. Drug Deliv. Sci. Technol. 2014, 24, 245–250. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.T.; Ita, K.B.; Parikh, S.J.; Popova, I.E.; Bair, D.A. Transdermal Delivery of Captopril and Metoprolol Tartrate with Microneedles. Drug Deliv. Lett. 2014, 4, 236–243. [Google Scholar] [CrossRef]
- Varanese, S.; Birnbaum, Z.; Rossi, R.; Di Rocco, A. Treatment of advanced Parkinson’s disease. Parkinsons Dis. 2011, 2010, 480260. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Ito, R.; Ishiguro, N.; Tamai, I.; Deguchi, Y. Blood–brain barrier transport of pramipexole, a dopamine D2 agonist. Life Sci. 2007, 80, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Pramipexole dihydrochloride molecular weight. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/Pramipexole_dihydrochloride (accessed on 15 July 2015).
- Reichmann, H. Transdermal delivery of dopamine receptor agonists. Parkinsonism Relat. Disord. 2009, 15, S93–S96. [Google Scholar] [CrossRef]
- Amantadine physico-chemical properties. Available online: http://pubchem.ncbi.nlm.nih.gov/compound/Amantadine_hydrochloride#section=Depositor-Supplied-Synonyms (accessed on 15 July 2015).
- Deleu, D.; Northway, M.G.; Hanssens, Y. Clinical pharmacokinetic and pharmacodynamic properties of drugs used in the treatment of Parkinson’s disease. Clin. Pharmacokinet. 2002, 41, 261–309. [Google Scholar] [CrossRef] [PubMed]
- Gannu, R.; Palem, C.R.; Yamsani, S.K.; Yamsani, V.V.; Yamsani, M.R. Enhanced bioavailability of buspirone from reservoir-based transdermal therapeutic system, optimization of formulation employing Box-Behnken statistical design. AAPS PharmSciTech 2010, 11, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Almeida, A.; Sousa, J.; Lamarche, I.; Gobin, P.; Marchand, S.; Couet, W.; Olivier, J.C.; Pais, A. Passive and active strategies for transdermal delivery using co-encapsulating nanostructured lipid carriers: in vitro vs. in vivo studies. Eur. J. Pharm. Biopharm. 2014, 86, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Hayton, W.L.; Chen, T. Correction of perfusate concentration for sample removal. J. Pharm. Sci. 1982, 71, 820–821. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R. Microneedles for transdermal drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, H.; Kolli, C.S.; Banga, A.K. Characterization of microchannels created by metal microneedles: Formation and closure. AAPS J. 2011, 13, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Meyer, W.; Kacza, J.; Zschemisch, N.H.; Godynicki, S.; Seeger, J. Observations on the actual structural conditions in the stratum superficiale dermidis of porcine ear skin, with special reference to its use as model for human skin. Ann. Anat. 2007, 189, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Corr, D.T.; Hart, D.A. Biomechanics of Scar Tissue and Uninjured Skin. Adv. Wound Care 2013, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.; Das, D.B.; Garland, M.J.; Belaid, L.; Donnelly, R.F. Influence of array interspacing on the force required for successful microneedle skin penetration: Theoretical and practical approaches. J. Pharm. Sci. 2013, 102, 1209–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakabayashi, K.; Tanji, K.; Mori, F.; Takahashi, H. The Lewy body in Parkinson’s disease: Molecules implicated in the formation and degradation of a-synuclein aggregates. Neuropathology 2007, 27, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Hurelbrink, C.B.; Lewis, S.J.G. Pathological considerations in the treatment of Parkinson’s disease: More than just a wiring diagram. Clin. Neurol. Neurosurg. 2011, 113, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kulisevsky, J.; Luquin, M.R.; Arbelo, J.M.; Burguera, J.A.; Carrillo, F.; Castro, A.; Chacón, J.; García-Ruiz, P.J.; Lezcano, E.; Mir, P.; et al. Advanced Parkinson’s disease: Clinical characteristics and treatment. Part II. Neurologia 2013, 28, 558–583. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, R.; Tanner, C.M.; Hauser, R.A.; Sethi, K.; Isaacson, S.; Truong, D.; Struck, L.; Ruby, A.E.; McClure, N.L.; Went, G.T.; et al. Amantadine extended release for levodopa-induced dyskinesia in Parkinson's disease (EASED Study). Mov. Disord. 2015, 30, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.J.; Katzenschlager, R.; Bloem, B.R.; Bonuccelli, U.; Burn, D.; Deuschl, G.; Dietrichs, E.; Fabbrini, G.; Friedman, A.; Kanovsky, P.; et al. Summary of the recommendations of the EFNS/MDS-ES review on therapeutic management of Parkinson’s disease. Eur. J. Neurol. 2013, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Cotzias, G.C.; Papavasiliou, P.S.; Gellene, R. Modification of Parkinsonism—Chronic treatment with L-dopa. N. Engl. J. Med. 1969, 280, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Huot, P.; Johnston, T.H.; Koprich, J.B.; Fox, S.H.; Brotchie, J.M. The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol. Rev. 2013, 65, 171–222. [Google Scholar] [CrossRef]
- Thanvi, B.R.; Lo, T.C. Long term motor complications of levodopa: Clinical features, mechanisms, and management strategies. Postgrad. Med. J. 2004, 80, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, A.P. Levodopa-induced hyperactivity in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Mov. Disord. 2007, 22, 99–104. [Google Scholar] [CrossRef]
- Miyasaki, J.M.; Martin, W.; Suchowersky, O.; Weiner, W.J.; Lang, A.E. Practice parameter: initiation of treatment for Parkinson’s disease: An evidence-based review. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2002, 58, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Transdermal drug delivery in Parkinson’s disease. Future Med. 2007, 3, 471–482. [Google Scholar] [CrossRef]
- Tsai, S.T.; Lin, S.H.; Hung, H.Y.; Lin, S.Z.; Chen, S.Y. Long-term comparison of subthalamic nucleus stimulation between patients with young-onset and late-onset Parkinson’s disease. Tzu Chi Med. J. 2012, 24, 65–72. [Google Scholar] [CrossRef]
- Donnelly, R.F.; Singh, T.R.R.; Morrow, D.I.J.; Woolfson, A.D. Transdermal Drug Delivery. In Microneedle-Mediated Transdermal and Intradermal Drug Delivery; John Wiley & Sons, Ltd.: Chichester, UK, 2012. [Google Scholar]
- Saini, N.; Bajaj, A. Recent trend on transdermal drug delivery system ad advancements in drug delivery through skin. Inter. J. Res. Pharm. Biosci. 2014, 4, 5–14. [Google Scholar]
- Ahad, A.; Aquil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M.; Ali, A. Transdermal drug delivery: The inherent challenges and technological advancements. Asian J. Pharm. Sci. 2010, 5, 276–288. [Google Scholar]
- Kolli, C.S.; Banga, A.K. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm. Res. 2008, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, L.; Bajgar, J. Transdermal drug delivery in vitro using diffusion cells. Curr. Med. Chem. 2012, 19, 4671–4677. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Brogden, N.K.; Stinchcomb, A.L. Fluvastatin as a Micropore Lifetime Enhancer for Sustained Delivery Across Microneedle-Treated Skin. J. Pharm. Sci. 2014, 103, 652–660. [Google Scholar] [PubMed]
- Devraj; Bhatt, D.C.; Aqil, M. A Review: Different Generation Approaches of Transdermal drug delivery System. J. Chem. Pharm. Res. 2010, 2, 184–193. [Google Scholar]
- Gandhi, K.; Monika, A.D.; Kaira, T.; Singh, K. Transdermal drug delivery: A Review. Inter. J. Res. Pharm. Sci. 2012, 3, 379–388. [Google Scholar]
- Jhawat, V.C.; Saini, V.; Kamboj, S.; Maggon, N. Transdermal Drug Delivery Systems: Approaches and Advancements in Drug Absorption through Skin. Inter. J. Pharm. Sci. Rev. Res. 2013, 20, 47–56. [Google Scholar]
- Edens, C.; Collins, M.L.; Ayers, J.; Rota, P.A.; Prausnitz, M.R. Measles vaccination using a microneedle patch. Vaccine 2013, 31, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Ita, K.B.; Popova, I.E.; Parikh, S.J.; Bair, D.A. Microneedle-assisted delivery of verapamil hydrochloride and amlodipine besylate. Eur. J. Pharm. Biopharm. 2014, 86, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, C.; Almeida, J.; Gonçalves, L.M.; Almeida, A.J.; Sousa, J.J.; Pais, A.A. Co-encapsulating nanostructured lipid carriers for transdermal application: From experimental design to the molecular detail. J. Control. Release 2013, 167, 301–314. [Google Scholar] [CrossRef]
- Crank, J. The diffusion equations. In The mathematics of diffusion, 2nd ed.; Clareadon Press: Oxford, UK, 1975. [Google Scholar]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Yan, G.; Warner, K.S.; Zhang, J.; Sharma, S.; Gale, B.K. Evaluation needle length and density of microneedle arrays in the pretreatment of skin for transdermal drug delivery. Int. J. Pharm. 2010, 391, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Ita, K.; Hatsakorzian, N.; Tolstikov, V. Microneedle-Mediated Delivery of Atenolol and Bisoprolol Hemifumarate. J. Nanopharm. Drug Deliv. 2013, 1, 38–44. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, M.T.; Ita, K.B.; Bair, D.A. Solid Microneedles for Transdermal Delivery of Amantadine Hydrochloride and Pramipexole Dihydrochloride. Pharmaceutics 2015, 7, 379-396. https://doi.org/10.3390/pharmaceutics7040379
Hoang MT, Ita KB, Bair DA. Solid Microneedles for Transdermal Delivery of Amantadine Hydrochloride and Pramipexole Dihydrochloride. Pharmaceutics. 2015; 7(4):379-396. https://doi.org/10.3390/pharmaceutics7040379
Chicago/Turabian StyleHoang, Mylien T., Kevin B. Ita, and Daniel A. Bair. 2015. "Solid Microneedles for Transdermal Delivery of Amantadine Hydrochloride and Pramipexole Dihydrochloride" Pharmaceutics 7, no. 4: 379-396. https://doi.org/10.3390/pharmaceutics7040379
APA StyleHoang, M. T., Ita, K. B., & Bair, D. A. (2015). Solid Microneedles for Transdermal Delivery of Amantadine Hydrochloride and Pramipexole Dihydrochloride. Pharmaceutics, 7(4), 379-396. https://doi.org/10.3390/pharmaceutics7040379