Volume of Amygdala Subregions and Plasma Levels of Brain-Derived Neurotrophic Factor and Cortisol in Patients with s/s Genotype of Serotonin Transporter Gene Polymorphism of First-Episode and Drug-Naive Major Depressive Disorder: An Exploratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment and Blood Sampling
2.3. Genotyping
2.4. MRI Acquisition
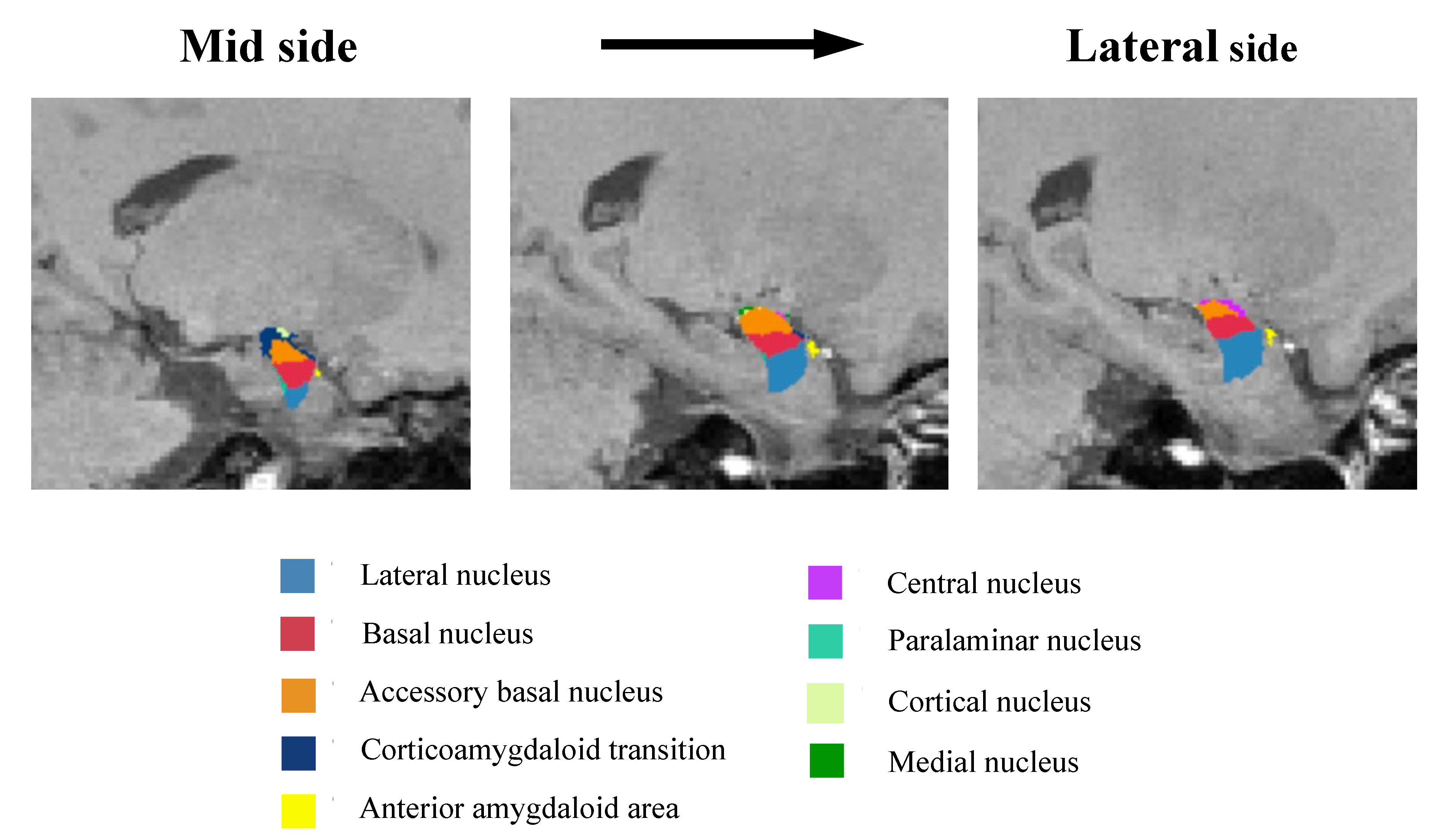
2.5. Amygdala Subregion Volume
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. Estimated Total Intracranial Volume and Plasma Levels of BDNF and Cortisol among Both Groups
3.3. Amygdala Volume
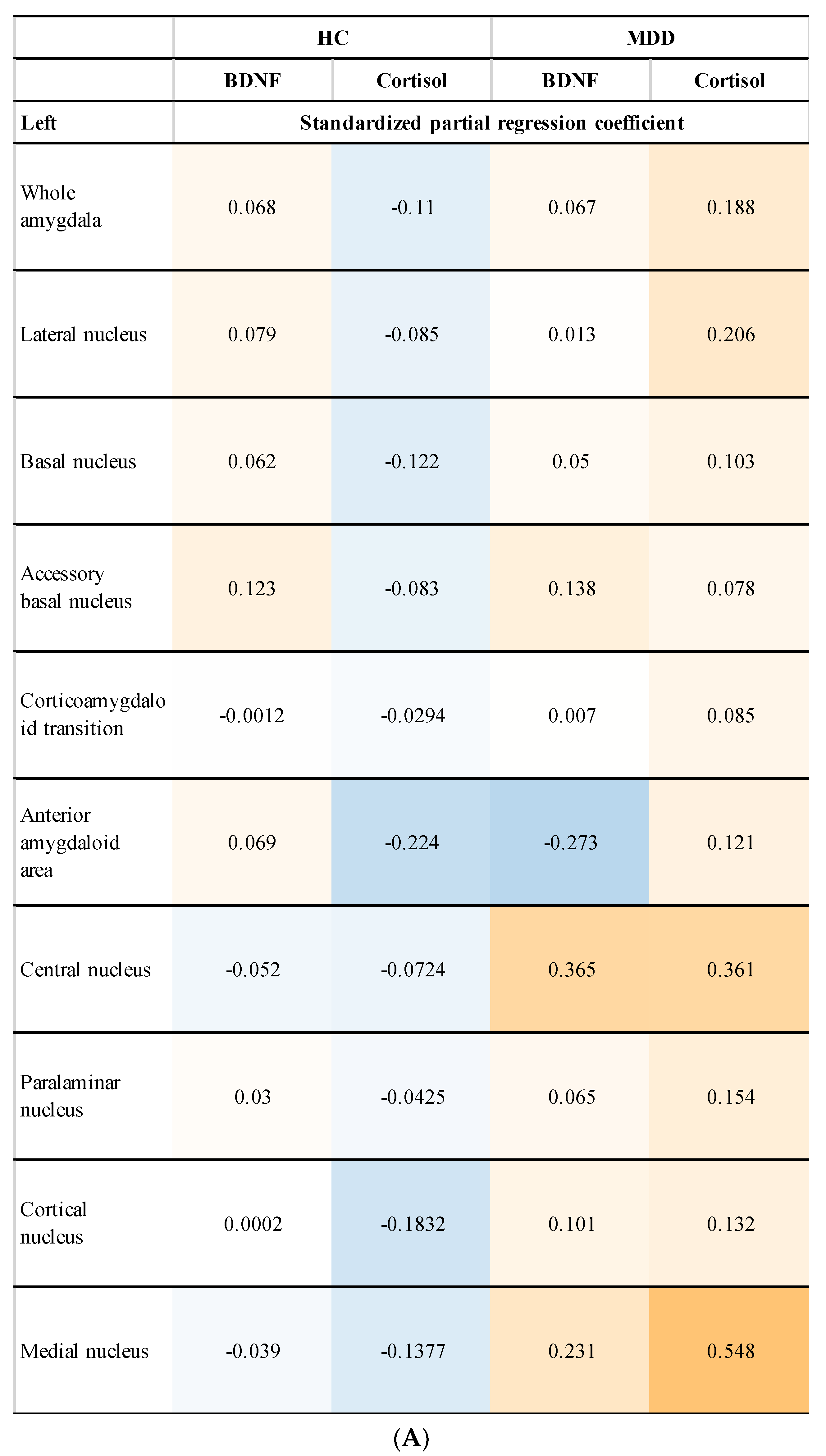
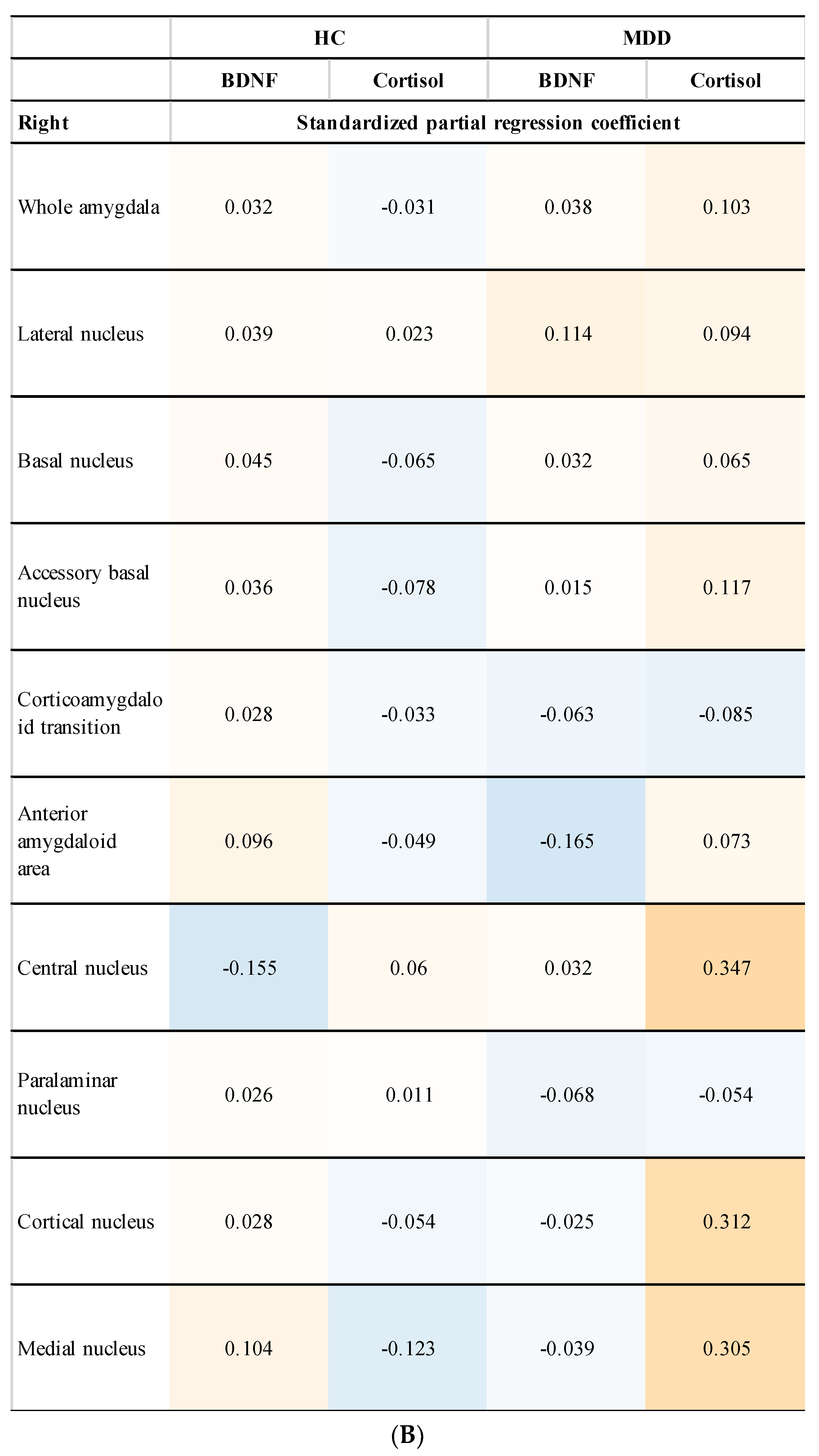
3.4. Relationship between Amygdala Volume and Plasma Levels of BDNF and Cortisol (Subgroup Analysis)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BDNF | brain-derived neurotrophic factor |
| CRH | corticotropin-releasing hormone |
| DSM-5 | Diagnostic and Statistical Manual for Mental Disorders-5 |
| HAMD | Hamilton Depression Scale |
| HC | healthy control |
| HPA | hypothalamic-pituitary-adrenal |
| LCSPT | limbic-cortico-striatal-pallidal-thalamic |
| MDD | major depressive disorder |
| MRI | magnetic resonance imaging |
| PCR | polymerase chain reaction |
| pgACC | part of the anterior cingulate cortex |
| SNP | single-nucleotide polymorphism |
| TrkB | tyrosine kinase B receptor |
| 3D-FSPGR | three-dimensional fast-spoiled gradient-recalled acquisition |
| 5-HT | serotonin |
| 5-HTT | serotonin transporter |
| 5-HTTLPR | serotonin transporter gene polymorphism |
References
- Schuhmacher, A.; Mössner, R.; Jessen, F.; Scheef, L.; Block, W.; Belloche, A.C.; Lennertz, L.; Welper, H.; Höfels, S.; Pfeiffer, U.; et al. Association of amygdala volumes with cortisol secretion in unipolar depressed patients. Psychiatry Res. 2012, 202, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.; Eisendrath, S.J.; Fryer, S.L.; Gillung, E.; Roach, B.J.; Mathalon, D.H. Blunted amygdala activity is associated with depression severity in treatment-resistant depression. Cogn. Affect. Behav. Neurosci. 2017, 17, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Zavorotnyy, M.; Zöllner, R.; Schulte-Güstenberg, L.R.; Wulff, L.; Schöning, S.; Dannlowski, U.; Kugel, H.; Arolt, V.; Konrad, C. Low left amygdala volume is associated with a longer duration of unipolar depression. J. Neural. Transm. 2018, 125, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.G.; Lambert, H.K.; Rodman, A.M.; Peverill, M.; Sheridan, M.A.; McLaughlin, K.A. Reduced hippocampal and amygdala volume as a mechanism underlying stress sensitization to depression following childhood trauma. Depress. Anxiety 2020, 37, 916–925. [Google Scholar] [CrossRef]
- Roddy, D.; Kelly, J.R.; Farrell, C.; Doolin, K.; Roman, E.; Nasa, A.; Frodl, T.; Harkin, A.; O’Mara, S.; O’Hanlon, E.; et al. Amygdala substructure volumes in Major Depressive Disorder. NeuroImage Clin. 2021, 31, 102781. [Google Scholar] [CrossRef]
- Sears, R.M.; Schiff, H.C.; LeDoux, J.E. Molecular mechanisms of threat learning in the lateral nucleus of the amygdala. Prog. Mol. Biol. Transl. Sci. 2014, 122, 263–304. [Google Scholar] [CrossRef]
- Bora, E.; Fornito, A.; Pantelis, C.; Yucel, M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012, 138, 9–18. [Google Scholar] [CrossRef]
- Drevets, W.C.; Price, J.L.; Furey, M.L. Brain structural and functional abnormalities in mood disorders: Implications for neurocircuitry models of depression. Brain Struct. Funct. 2008, 213, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Sheline, Y.I. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical comorbidity. Biol. Psychiatry 2000, 48, 791–800. [Google Scholar] [CrossRef]
- Maras, P.M.; Petrulis, A. Lesions that functionally disconnect the anterior and posterodorsal sub-regions of the medial amygdala eliminate opposite-sex odor preference in male Syrian hamsters (Mesocricetus auratus). Neuroscience 2010, 165, 1052–1062. [Google Scholar] [CrossRef] [Green Version]
- Alarcón, G.; Cservenka, A.; Rudolph, M.D.; Fair, D.A.; Nagel, B.J. Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage 2015, 115, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, J.; Tao, S.; Wang, X.; Shi, J.; Chen, Y.; Tian, S.; Yao, Z.; Lu, Q. Brain functional abnormalities in the amygdala subregions is associated with anxious depression. J. Affect. Disord. 2020, 276, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Igata, N.; Kakeda, S.; Watanabe, K.; Ide, S.; Kishi, T.; Abe, O.; Igata, R.; Katsuki, A.; Iwata, N.; Yoshimura, R.; et al. Voxel-based morphometric brain comparison between healthy subjects and major depressive disorder patients in Japanese with the s/s genotype of 5-HTTLPR. Sci. Rep. 2017, 7, 3931. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Stöber, G.; Li, T.; Heils, A.; Catalano, M.; Di Bella, D.; Arranz, M.J.; Murray, R.M.; Vallada, H.P.; Bengel, D.; et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: Possible role in susceptibility to affective disorders. Mol. Psychiatry 1996, 1, 453–460. [Google Scholar] [PubMed]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Bradley, S.L.; Dodelzon, K.; Sandhu, H.K.; Philibert, R.A. Relationship of serotonin transporter gene polymorphisms and haplotypes to mRNA transcription. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2005, 136, 58–61. [Google Scholar] [CrossRef]
- Carroll, J.C.; Boyce-Rustay, J.M.; Millstein, R.; Yang, R.; Wiedholz, L.M.; Murphy, D.L.; Holmes, A. Effects of mild early life stress on abnormal emotion-related behaviors in 5-HTT knockout mice. Behav. Genet. 2007, 37, 214–222. [Google Scholar] [CrossRef]
- Heiming, R.S.; Jansen, F.; Lewejohann, L.; Kaiser, S.; Schmitt, A.; Lesch, K.P.; Sachser, N. Living in a dangerous world: The shaping of behavioral profile by early environment and 5-HTT genotype. Front. Behav. Neurosci. 2009, 3, 26. [Google Scholar] [CrossRef]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef]
- Gotlib, I.H.; Joormann, J.; Minor, K.L.; Hallmayer, J.; Axis, H.P.A. HPA axis Reactivity: A mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol. Psychiatry 2008, 63, 847–851. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.H.; Kakeda, S.; Watanabe, K.; Katsuki, A.; Sugimoto, K.; Igata, N.; Shinkai, T.; Abe, O.; Korogi, Y.; Ikenouchi, A.; et al. Brain structural network alterations related to serum cortisol levels in drug-naïve, first-episode major depressive disorder patients: A source-based morphometric study. Sci. Rep. 2020, 10, 22096. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Kishi, T.; Atake, K.; Katsuki, A.; Iwata, N. Serum brain-derived neurotrophic factor, and plasma catecholamine metabolites in people with major depression: Preliminary cross-sectional study. Front. Psychiatry 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishi, T.; Yoshimura, R.; Ikuta, T.; Iwata, N. Brain-derived neurotrophic factor and major depressive disorder: Evidence from meta-analyses. Front. Psychiatry 2017, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Caviedes, A.; Lafourcade, C.; Soto, C.; Wyneken, U. BDNF/NF-κB signaling in the neurobiology of depression. Curr. Pharm. Des. 2017, 23, 3154–3163. [Google Scholar] [CrossRef]
- Hamon, M.; Blier, P. Monoamine neurocircuitry in depression and strategies for new treatments. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 45, 54–63. [Google Scholar] [CrossRef] [PubMed]
- de Assis, G.G.; Gasanov, E.V. BDNF and Cortisol integrative system—Plasticity vs. degeneration: Implications of the Val66Met polymorphism. Front. Neuroendocrinol. 2019, 55, 100784. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.J.; Tian, J.S.; Gao, X.X.; Zhou, Y.Z.; Qin, X.M. Research on the Pathological Mechanism and Drug Treatment Mechanism of Depression. Curr. Neuropharmacol. 2015, 13, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Tesen, H.; Watanabe, K.; Okamoto, N.; Ikenouchi, A.; Igata, R.; Konishi, Y.; Kakeda, S.; Yoshimura, R. Volume of amygdala subregions and clinical manifestations in patients With first-episode, drug-naïve major depression. Front. Hum. Neurosci. 2022, 15, 780884. [Google Scholar] [CrossRef]
- Jovicich, J.; Czanner, S.; Greve, D.; Haley, E.; Van Der Kouwe, A.; Gollub, R.; Kennedy, D.; Schmitt, F.; Brown, G.; MacFall, J.; et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage 2006, 30, 436–443. [Google Scholar] [CrossRef]
- Fischl, B. Free. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saygin, Z.M.; Kliemann, D.; Iglesias, J.E.; van der Kouwe, A.J.W.; Boyd, E.; Reuter, M.; Stevens, A.; Van Leemput, K.; McKee, A.; Frosch, M.P.; et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: Manual segmentation to automatic atlas. Neuroimage 2017, 155, 370–382. [Google Scholar] [CrossRef] [PubMed]
- Pittard, W.S.; Li, S. The essential toolbox of data science: Python, R, Git, and Docker. Methods Mol. Biol. 2020, 2104, 265–311. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, D. Incorporating the empirical null hypothesis into the Benjamini-Hochberg procedure. Stat. Appl. Genet. Mol. Biol. 2012, 11. [Google Scholar] [CrossRef]
- Knorr, U.; Vinberg, M.; Kessing, L.V.; Wetterslev, J. Salivary cortisol in depressed patients versus control persons: A systematic review and meta-analysis. Psychoneuroendocrinology 2010, 35, 1275–1286. [Google Scholar] [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.M., Jr.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef]
- Egeland, M.; Zunszain, P.A.; Pariante, C.M. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat. Rev. Neurosci. 2015, 16, 189–200. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Spinhoven, P.; Polak, M.; Bus, B.A.A.; Penninx, B.W.J.H.; Elzinga, B.M. Serum BDNF concentrations as peripheral manifestations of depression: Evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 2014, 19, 791–800. [Google Scholar] [CrossRef]
- Hamilton, J.P.; Siemer, M.; Gotlib, I.H. Amygdala volume in major depressive disorder: A meta-analysis of magnetic resonance imaging studies. Mol. Psychiatry 2008, 13, 993–1000. [Google Scholar] [CrossRef] [Green Version]
- Espinoza Oyarce, D.A.; Shaw, M.E.; Alateeq, K.; Cherbuin, N. Volumetric brain differences in clinical depression in association with anxiety: A systematic review with meta-analysis. J. Psychiatry Neurosci. 2020, 45, 406–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Verweij, E.W.E.; Krugers, H.J.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct. Funct. 2014, 219, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the hypothalamic-pituitary-adrenocortical stress response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakamata, Y.; Komi, S.; Moriguchi, Y.; Izawa, S.; Motomura, Y.; Sato, E.; Mizukami, S.; Kim, Y.; Hanakawa, T.; Inoue, Y.; et al. Amygdala-centred functional connectivity affects daily cortisol concentrations: A putative link with anxiety. Sci. Rep. 2017, 7, 8313. [Google Scholar] [CrossRef] [Green Version]
- Hakamata, Y.; Mizukami, S.; Izawa, S.; Okamura, H.; Mihara, K.; Marusak, H.; Moriguchi, Y.; Hori, H.; Hanakawa, T.; Inoue, Y.; et al. Implicit and explicit emotional memory recall in anxiety and depression: Role of basolateral amygdala and cortisol-norepinephrine interaction. Psychoneuroendocrinology 2022, 136, 105598. [Google Scholar] [CrossRef]
- Meis, S.; Endres, T.; Lessmann, V. Neurotrophin signalling in amygdala-dependent cued fear learning. Cell Tissue Res. 2020, 382, 161–172. [Google Scholar] [CrossRef]
- Chhatwal, J.P.; Stanek-Rattiner, L.; Davis, M.; Ressler, K.J. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat. Neurosci. 2006, 9, 870–872. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, D.; Li, Y.; Chu, H.; Zhang, L.; Hou, M.; Jiang, X.; Chen, Z.; Su, B.; Sun, T. Pre-synaptic TrkB in basolateral amygdala neurons mediates BDNF signaling transmission in memory extinction. Cell Death Dis. 2017, 8, e2959. [Google Scholar] [CrossRef]
- Wurst, C.; Schiele, M.A.; Stonawski, S.; Weiß, C.; Nitschke, F.; Hommers, L.; Domschke, K.; Herrmann, M.J.; Pauli, P.; Deckert, J.; et al. Impaired fear learning and extinction, but not generalization, in anxious and non-anxious depression. J. Psychiatr. Res. 2021, 135, 294–301. [Google Scholar] [CrossRef]
- Wheeler, A.L.; Felsky, D.; Vivino, J.D.; Stojanovski, S.; Ameis, S.H.; Szatmari, P.; Lerch, J.P.; Chakravarty, M.M.; Voineskos, A.N. BDNF-Dependent Effects on Amygdala-Cortical Circuitry and Depression Risk in Children and Youth. Cereb. Cortex 2018, 28, 1760–1770. [Google Scholar] [CrossRef]
| HC | MDD | |
|---|---|---|
| (n = 46) | (n = 25) | |
| Demographic data | ||
| Age, years | 39 (32–49.5) | 42 (33–54) |
| Sex, male/female | 34/12 | 14/11 |
| Dominant hand, right/left | 46/0 | 23/2 |
| Smoking, smoking/non-smoking | 22/24 | 12/13 |
| Education, years | 16 (2.8) | 13 (2.5) |
| Clinical characteristics | ||
| Duration of the disease, month | - | 4.5 (5.6) |
| HAMD total score | - | 22 (6.0) |
| HC | MDD | z-Value | p-Value | |
|---|---|---|---|---|
| Estimated total intracranial volume, mm3 | 1,600,482 (146,824) | 1,555,121 (136,717) | 1.35 | 0.18 |
| Plasma metabolites levels | ||||
| BDNF, ng/mL | 4.60 (2.63–7.60) | 3.55 (1.45–7.20) | 1.33 | 0.19 |
| Cortisol, ug/dl | 8.90 (6.55–12.3) | 11.8 (10.2–12.9) | −1.84 | 0.067 |
| (A) | |||
| HC | MDD | Adjusted p-Value | |
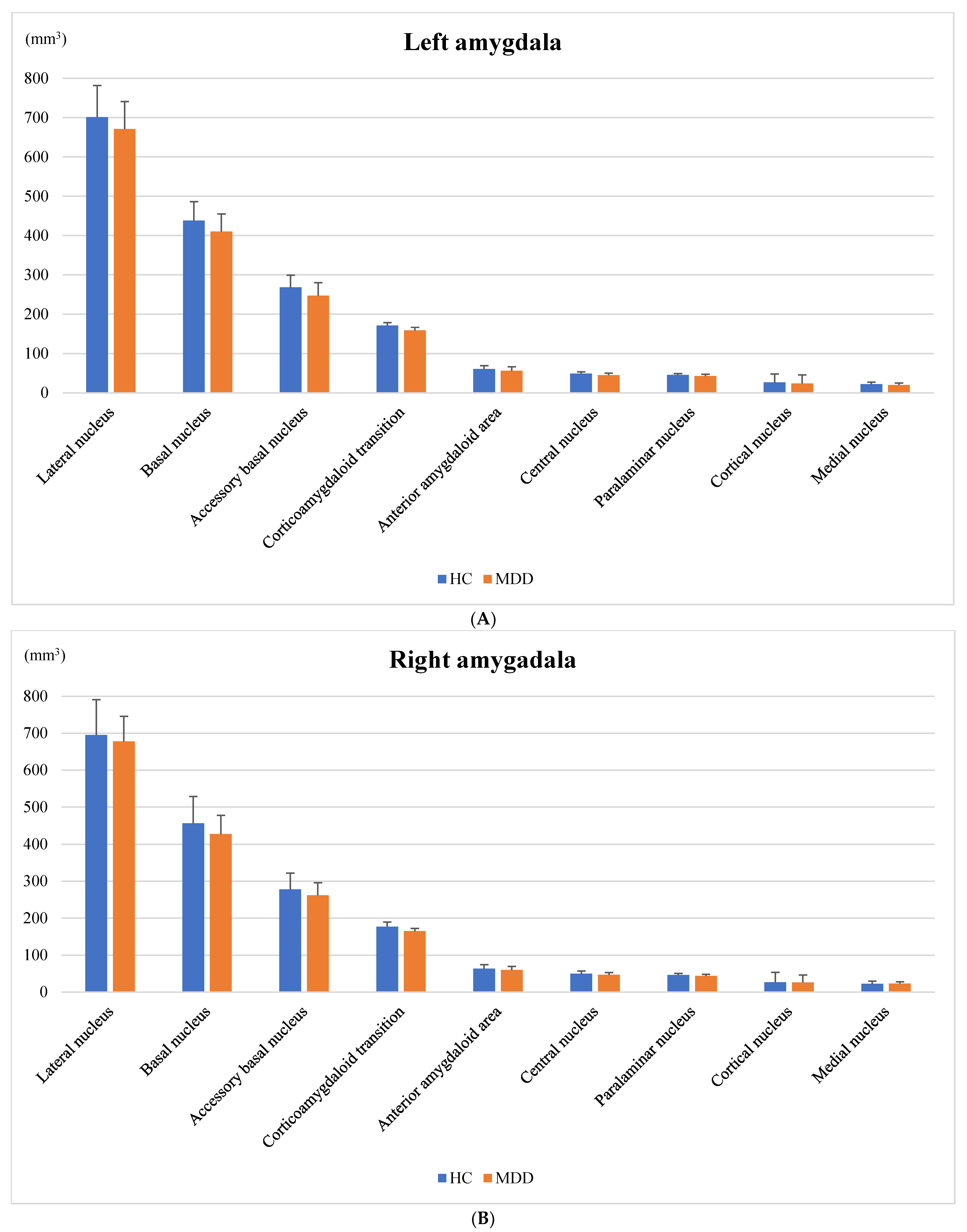
| Whole amygdala | 1783 (188) | 1677 (172) | 0.14 |
| Lateral nucleus | 701 (81) | 671 (70) | 0.65 |
| Basal nucleus | 438 (48) | 410 (45) | 0.11 |
| Accessory basal nucleus | 268 (31) | 247 (33) | 0.052 |
| Corticoamygdaloid transition | 171 (21.8) | 159 (21.9) | 0.15 |
| Anterior amygdaloid area | 61.0 (7.62) | 56.3 (7.49) | 0.071 |
| Central nucleus | 48.9 (8.73) | 45.2 (10.1) | 0.42 |
| Paralaminar nucleus | 45.5 (5.38) | 43.1 (4.87) | 0.38 |
| Cortical nucleus | 26.5 (3.63) | 24.2 (4.64) | 0.17 |
| Medial nucleus | 22.3 (5.02) | 20.3 (5.31) | 0.43 |
| (B) | |||
| HC | MDD | Adjusted p-value | |
| Whole amygdala | 1813 (259) | 1734 (182) | 0.77 |
| Lateral nucleus | 695 (96) | 678 (68) | 0.64 |
| Basal nucleus | 456 (73) | 427 (51) | 0.43 |
| Accessory Basal nucleus | 278 (44) | 262 (34) | 0.59 |
| Corticoamygdaloid transition | 177 (26.9) | 165 (20.4) | 0.28 |
| Anterior amygdaloid area | 63.3 (12.5) | 59.9 (7.22) | 0.62 |
| Central nucleus | 49.6 (11.1) | 46.6 (9.79) | 0.27 |
| Paralaminar nucleus | 46.3 (7.53) | 43.7 (5.25) | 0.56 |
| Cortical nucleus | 26.7 (4.52) | 26.3 (4.36) | 0.47 |
| Medial nucleus | 22.4 (7.74) | 23.1 (6.31) | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okamoto, N.; Watanabe, K.; Tesen, H.; Ikenouchi, A.; Igata, R.; Konishi, Y.; Natsuyama, T.; Fujii, R.; Kakeda, S.; Kishi, T.; et al. Volume of Amygdala Subregions and Plasma Levels of Brain-Derived Neurotrophic Factor and Cortisol in Patients with s/s Genotype of Serotonin Transporter Gene Polymorphism of First-Episode and Drug-Naive Major Depressive Disorder: An Exploratory Study. Neurol. Int. 2022, 14, 378-390. https://doi.org/10.3390/neurolint14020031
Okamoto N, Watanabe K, Tesen H, Ikenouchi A, Igata R, Konishi Y, Natsuyama T, Fujii R, Kakeda S, Kishi T, et al. Volume of Amygdala Subregions and Plasma Levels of Brain-Derived Neurotrophic Factor and Cortisol in Patients with s/s Genotype of Serotonin Transporter Gene Polymorphism of First-Episode and Drug-Naive Major Depressive Disorder: An Exploratory Study. Neurology International. 2022; 14(2):378-390. https://doi.org/10.3390/neurolint14020031
Chicago/Turabian StyleOkamoto, Naomichi, Keita Watanabe, Hirofumi Tesen, Atsuko Ikenouchi, Ryohei Igata, Yuki Konishi, Tomoya Natsuyama, Rintaro Fujii, Shingo Kakeda, Taro Kishi, and et al. 2022. "Volume of Amygdala Subregions and Plasma Levels of Brain-Derived Neurotrophic Factor and Cortisol in Patients with s/s Genotype of Serotonin Transporter Gene Polymorphism of First-Episode and Drug-Naive Major Depressive Disorder: An Exploratory Study" Neurology International 14, no. 2: 378-390. https://doi.org/10.3390/neurolint14020031
APA StyleOkamoto, N., Watanabe, K., Tesen, H., Ikenouchi, A., Igata, R., Konishi, Y., Natsuyama, T., Fujii, R., Kakeda, S., Kishi, T., Iwata, N., & Yoshimura, R. (2022). Volume of Amygdala Subregions and Plasma Levels of Brain-Derived Neurotrophic Factor and Cortisol in Patients with s/s Genotype of Serotonin Transporter Gene Polymorphism of First-Episode and Drug-Naive Major Depressive Disorder: An Exploratory Study. Neurology International, 14(2), 378-390. https://doi.org/10.3390/neurolint14020031







