Fremanezumab and Non-High-Dose Galcanezumab for Comorbid Cluster Headache in Patients with Migraine: Three Cases
Abstract
1. Introduction
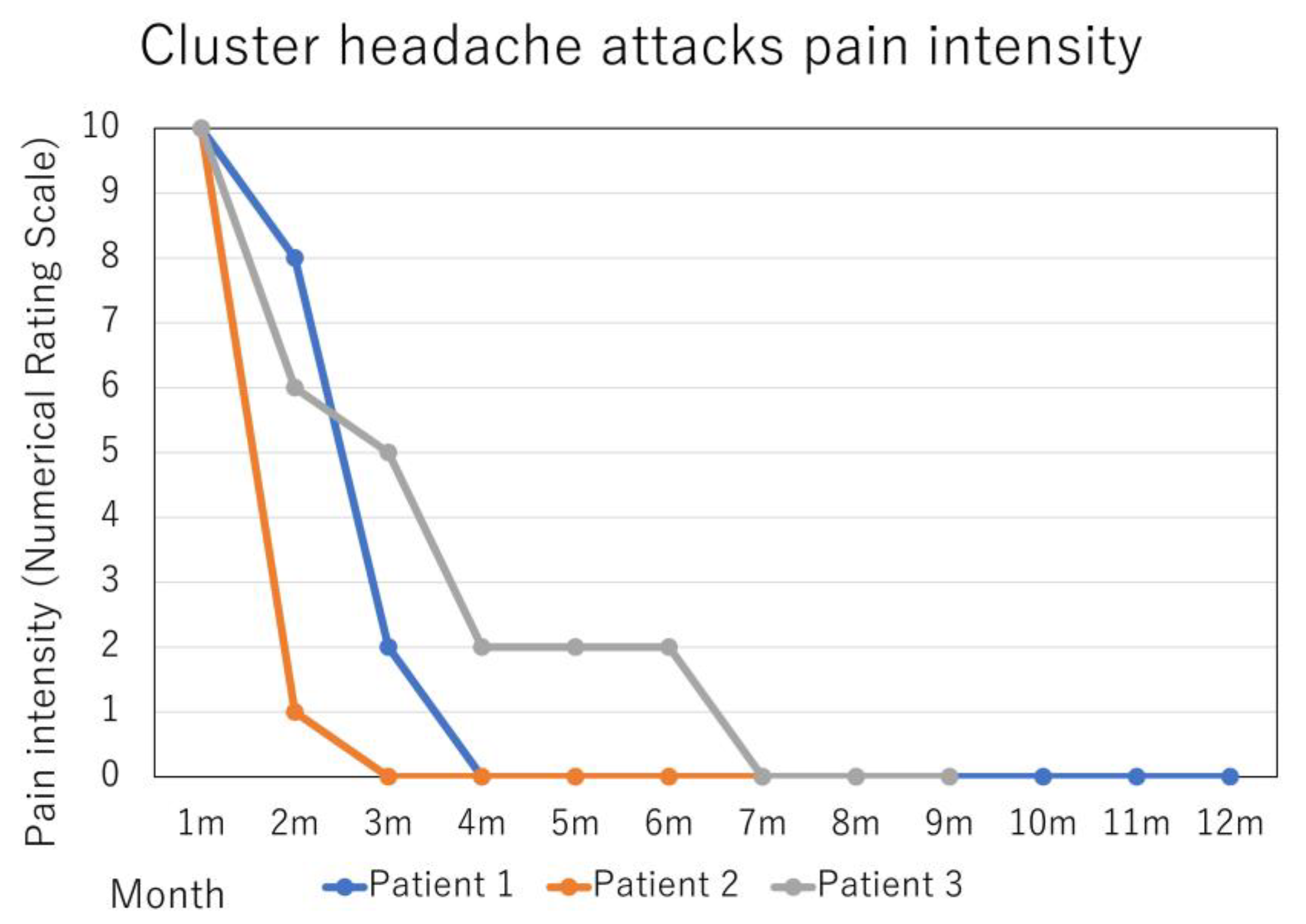
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martelletti, P.; Curto, M. Cluster headache is still lurking in the shadows. Pain Ther. 2021, 10, 777–781. [Google Scholar] [CrossRef]
- Kikui, S.; Sugiyama, H.; Danno, D.; Kashiwaya, Y.; Takeshima, T. Chronic cluster headache with a pediatric onset: The first Japanese case report. Intern. Med. 2020, 59, 2937–2940. [Google Scholar] [CrossRef]
- Imai, N.; Yagi, N.; Kuroda, R.; Konishi, T.; Serizawa, M.; Kobari, M. Clinical profile of cluster headaches in Japan: Low prevalence of chronic cluster headache, and uncoupling of sense and behaviour of restlessness. Cephalalgia 2011, 31, 628–633. [Google Scholar] [CrossRef]
- Danno, D.; Wolf, J.; Ishizaki, K.; Kikui, S.; Yoshikawa, H.; Takeshima, T. Cranial autonomic symptoms of migraine in Japan: Prospective study of 373 migraine patients at a tertiary headache center. Headache 2020, 60, 1592–1600. [Google Scholar] [CrossRef]
- Giani, L.; Proietti Cecchini, A.; Leone, M. Anti-CGRP in cluster headache therapy. Neurol. Sci. 2019, 40, 129–135. [Google Scholar] [CrossRef]
- Negro, A.; Sciattella, P.; Spuntarelli, V.; Martelletti, P.; Mennini, F.S. Direct and indirect costs of cluster headache: A prospective analysis in a tertiary level headache centre. J. Headache Pain 2020, 21, 44. [Google Scholar] [CrossRef]
- Imai, N.; Kitamura, E. Differences in clinical features of cluster headache between drinkers and nondrinkers in Japan. PLoS ONE 2019, 14, e0224407. [Google Scholar] [CrossRef]
- Shimizu, T. New treatments for cluster headache. Rinsho Shinkeigaku 2013, 53, 1131–1133. [Google Scholar] [CrossRef]
- Ito, Y.; Mitsufuji, T.; Asano, Y.; Shimazu, T.; Kato, Y.; Tanahashi, N.; Maruki, Y.; Sakai, F.; Yamamoto, T.; Araki, N. Naratriptan in the prophylactic treatment of cluster headache. Intern. Med. 2017, 56, 2579–2582. [Google Scholar] [CrossRef]
- Kikui, S.; Takeshima, T. Naratriptan may become an alternative prophylactic option for patients with cluster headache. Intern. Med. 2017, 56, 2547–2548. [Google Scholar] [CrossRef]
- Imai, N. A cluster headache responsive to ramelteon, a selective melatonin MT1/MT2 receptor agonist. Intern. Med. 2016, 55, 2483–2485. [Google Scholar] [CrossRef]
- Kawada, S.; Kashihara, K.; Imamura, T.; Ohno, M. High-dose intravenous methylprednisolone for the prophylactic treatment of cluster headache. Springerplus 2013, 2, 156. [Google Scholar] [CrossRef]
- Imai, N. New management of cluster headache. Brain Nerve 2021, 73, 347–355. (In Japanese) [Google Scholar] [CrossRef]
- Katsuki, M.; Kashiwagi, K.; Kawamura, S.; Koh, A. Monoclonal antibodies against the calcitonin gene-related peptide and its receptor in Japanese adolescents with migraines. Cureus 2023, 15, e33689. [Google Scholar] [CrossRef]
- Katsuki, M.; Yamagishi, C.; Matsumori, Y.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Kito, T.; Entani, A.; Yamamoto, T.; Ikeda, T.; et al. Questionnaire-based survey on the prevalence of medication-overuse headache in Japanese one city-Itoigawa study. Neurol. Sci. 2022, 43, 3811–3822. [Google Scholar] [CrossRef]
- Katsuki, M.; Matsumori, Y.; Kawahara, J.; Yamagishi, C.; Koh, A.; Kawamura, S.; Kashiwagi, K.; Kito, T.; Oguri, M.; Mizuno, S.; et al. Headache education by leaflets distribution during COVID-19 vaccination and school-based on-demand E-learning: Itoigawa Geopark Headache Awareness Campaign. Headache 2023, in press. [CrossRef]
- Vollesen, A.L.; on behalf of the School of Advanced Studies of the European Headache Federation (EHF-SAS); Benemei, S.; Cortese, F.; Labastida-Ramírez, A.; Marchese, F.; Pellesi, L.; Romoli, M.; Ashina, M.; Lampl, C. Migraine and cluster headache—The common link. J. Headache Pain 2018, 19, 89. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 1994, 117 Pt 3, 427–434. [Google Scholar] [CrossRef]
- Fanciullacci, M.; Alessandri, M.; Figini, M.; Geppetti, P.; Michelacci, S. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain 1995, 60, 119–123. [Google Scholar] [CrossRef]
- Fanciullacci, M.; Alessandri, M.; Sicuteri, R.; Marabini, S. Responsiveness of the trigeminovascular system to nitroglycerine in cluster headache patients. Brain 1997, 120 Pt 2, 283–288. [Google Scholar] [CrossRef]
- Al-Karagholi, M.A.-M.; Peng, K.-P.; Petersen, A.S.; De Boer, I.; Terwindt, G.M.; Ashina, M. Debate: Are cluster headache and migraine distinct headache disorders? J. Headache Pain 2022, 23, 151. [Google Scholar] [CrossRef]
- Wei, D.Y.; Goadsby, P.J. Cluster headache pathophysiology—Insights from current and emerging treatments. Nat. Rev. Neurol. 2021, 17, 308–324. [Google Scholar] [CrossRef]
- Silvestro, M.; Tessitore, A.; Di Clemente, F.S.; Tedeschi, G.; Russo, A. Erenumab efficacy on comorbid cluster headache in patients with migraine: A real-world case series. Headache 2020, 60, 1187–1195. [Google Scholar] [CrossRef]
- Chen, S.T.; Wu, J.W. CGRP-targeted therapy for episodic and chronic cluster headache. Curr. Pain Headache Rep. 2022, 26, 667–675. [Google Scholar] [CrossRef]
- Lund, N.; Barloese, M.; Petersen, A.; Haddock, B.; Jensen, R. Chronobiology differs between men and women with cluster headache, clinical phenotype does not. Neurology 2017, 88, 1069–1076. [Google Scholar] [CrossRef]
- Diener, H.-C.; Tassorelli, C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. Guidelines of the International Headache Society for controlled trials of acute treatment of migraine attacks in adults: Fourth edition. Cephalalgia 2019, 39, 687–710. [Google Scholar] [CrossRef]
- Nelson, R.F. Cluster migraine—An unrecognized common entity. Can. Med. Assoc. J. 1970, 103, 1026–1030. [Google Scholar]
- Ferraro, S.; Nigri, A.; Demichelis, G.; Pinardi, C.; Chiapparini, L.; Giani, L.; Cecchini, A.P.; Leone, M. Understanding cluster headache using magnetic resonance imaging. Front. Neurol. 2020, 11, 535. [Google Scholar] [CrossRef]
- Katsuki, M.; Tatsumoto, M.; Kimoto, K.; Iiyama, T.; Tajima, M.; Munakata, T.; Miyamoto, T.; Shimazu, T. Investigating the effects of weather on headache occurrence using a smartphone application and artificial intelligence: A retrospective observational cross-sectional study. Headache 2023, in press. [Google Scholar]
- Song, T.-J.; Lee, M.J.; Choi, Y.-J.; Kim, B.-K.; Chung, P.-W.; Park, J.-W.; Chu, M.K.; Sohn, J.-H.; Oh, K.; Kim, D.; et al. Differences in characteristics and comorbidity of cluster headache according to the presence of migraine. J. Clin. Neurol. 2019, 15, 334–338. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Dodick, D.W.; Leone, M.; Bardos, J.N.; Oakes, T.M.; Millen, B.A.; Zhou, C.; Dowsett, S.A.; Aurora, S.K.; Ahn, A.H.; et al. Trial of galcanezumab in prevention of episodic cluster headache. N. Engl. J. Med. 2019, 381, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Z.; Gao, B.; Xuan, H.; Zhu, Y.; Chen, Z.; Wang, Z. Different doses of galcanezumab versus placebo in patients with migraine and cluster headache: A meta-analysis of randomized controlled trials. J. Headache Pain 2020, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Ossipov, M.H.; Raffa, R.B.; Pergolizzi, J.V. Galcanezumab: A humanized monoclonal antibody for the prevention of migraine and cluster headache. Drugs Today 2020, 56, 5–19. [Google Scholar] [CrossRef]
- Mudugal, D.; Monteith, T.S. Drug profile: Galcanezumab for prevention of cluster headache. Expert Rev. Neurother. 2021, 21, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mo, H.; Kim, B.-K.; Moon, H.-S.; Cho, S.-J. Real-world experience with 240 mg of galcanezumab for the preventive treatment of cluster headache. J. Headache Pain 2022, 23, 132. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Broessner, G.; Goßrau, G.; Heinze-Kuhn, K.; Jürgens, T.P.; Kaltseis, K.; Kamm, K.; Peikert, A.; Raffaelli, B.; Rimmele, F.; et al. Effect of calcitonin gene-related peptide (-receptor) antibodies in chronic cluster headache: Results from a retrospective case series support individual treatment attempts. Cephalalgia 2020, 40, 1574–1584. [Google Scholar] [CrossRef]
- Martelletti, P.; Curto, M. Headache: Cluster headache treatment—RCTs versus real-world evidence. Nat. Rev. Neurol. 2016, 12, 557–558. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashiwagi, K.; Katsuki, M.; Kawamura, S.; Tachikawa, S.; Ono, A.; Koh, A. Fremanezumab and Non-High-Dose Galcanezumab for Comorbid Cluster Headache in Patients with Migraine: Three Cases. Neurol. Int. 2023, 15, 318-324. https://doi.org/10.3390/neurolint15010020
Kashiwagi K, Katsuki M, Kawamura S, Tachikawa S, Ono A, Koh A. Fremanezumab and Non-High-Dose Galcanezumab for Comorbid Cluster Headache in Patients with Migraine: Three Cases. Neurology International. 2023; 15(1):318-324. https://doi.org/10.3390/neurolint15010020
Chicago/Turabian StyleKashiwagi, Kenta, Masahito Katsuki, Shin Kawamura, Senju Tachikawa, Atsuko Ono, and Akihito Koh. 2023. "Fremanezumab and Non-High-Dose Galcanezumab for Comorbid Cluster Headache in Patients with Migraine: Three Cases" Neurology International 15, no. 1: 318-324. https://doi.org/10.3390/neurolint15010020
APA StyleKashiwagi, K., Katsuki, M., Kawamura, S., Tachikawa, S., Ono, A., & Koh, A. (2023). Fremanezumab and Non-High-Dose Galcanezumab for Comorbid Cluster Headache in Patients with Migraine: Three Cases. Neurology International, 15(1), 318-324. https://doi.org/10.3390/neurolint15010020






