[125I]IPC-Lecanemab: Synthesis and Evaluation of Aβ-Plaque-Binding Antibody and Comparison with Small-Molecule [18F]Flotaza and [125I]IBETA in Postmortem Human Alzheimer’s Disease
Abstract
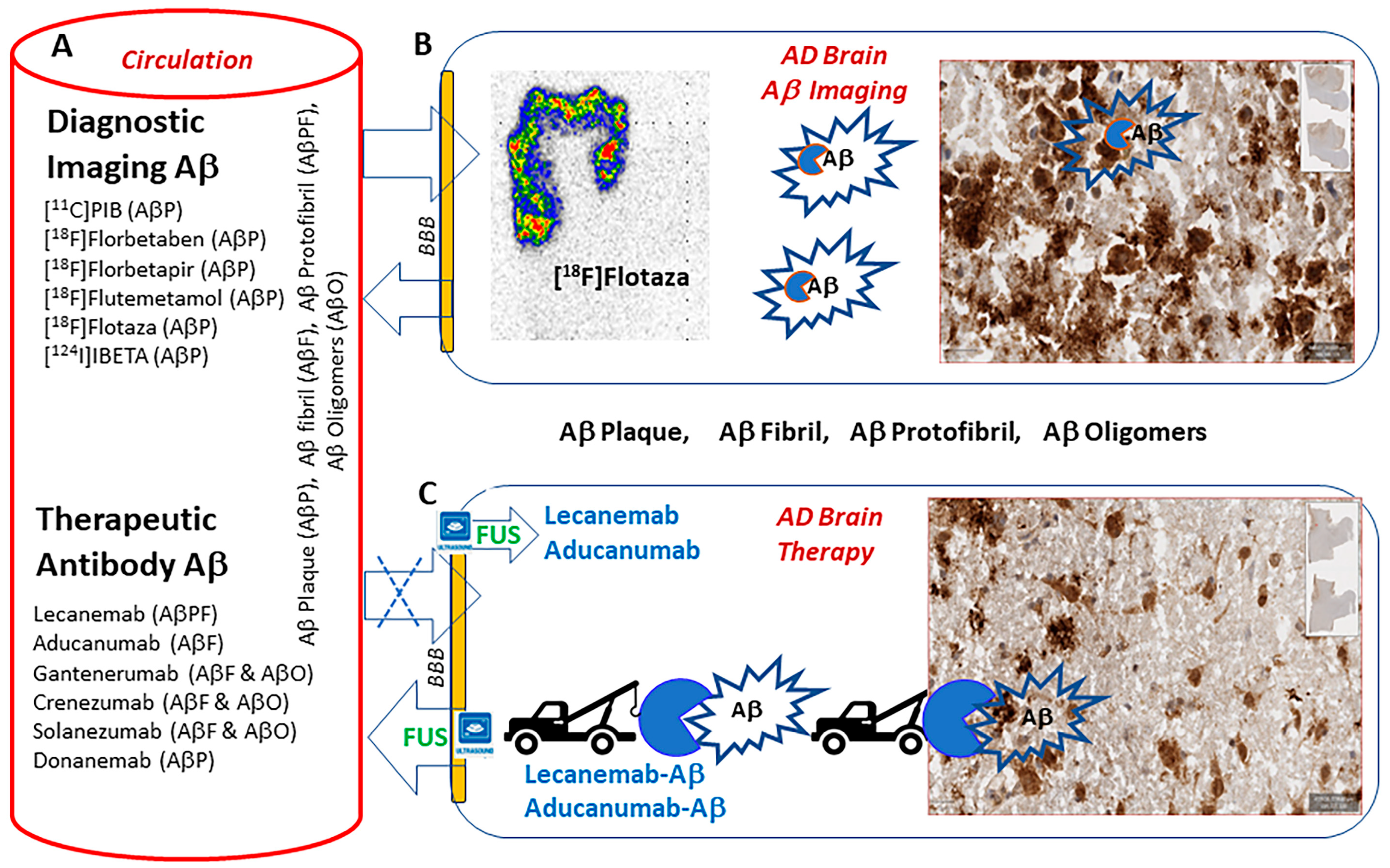
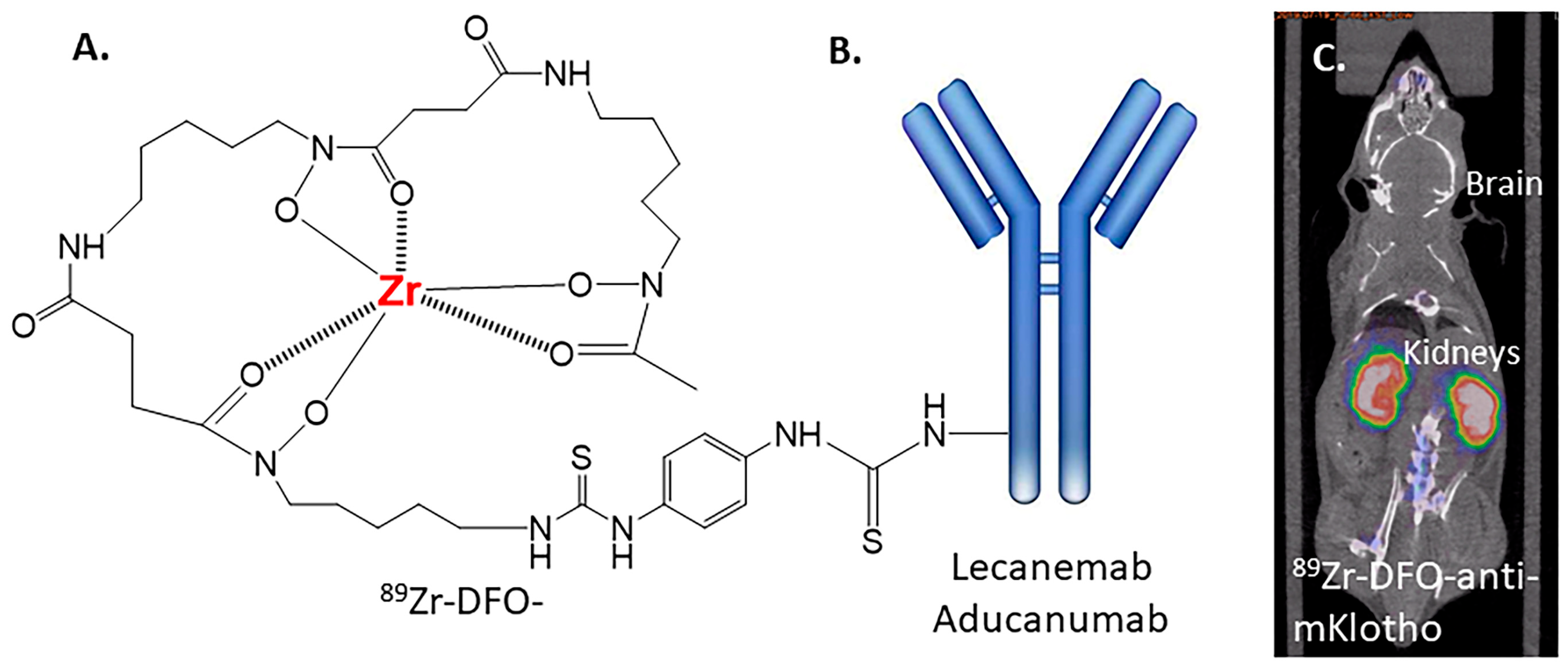
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Radiosynthesis of [125I]IPC-Lecanemab
2.3. Human Tissue
2.4. Postmortem Human Brain Autoradiography
2.4.1. [125I]IPC-Lecanemab
2.4.2. [18F]Flotaza
2.4.3. [125I]IBETA and [124I]IBETA
2.5. Immunohistochemistry
2.6. Image Analysis
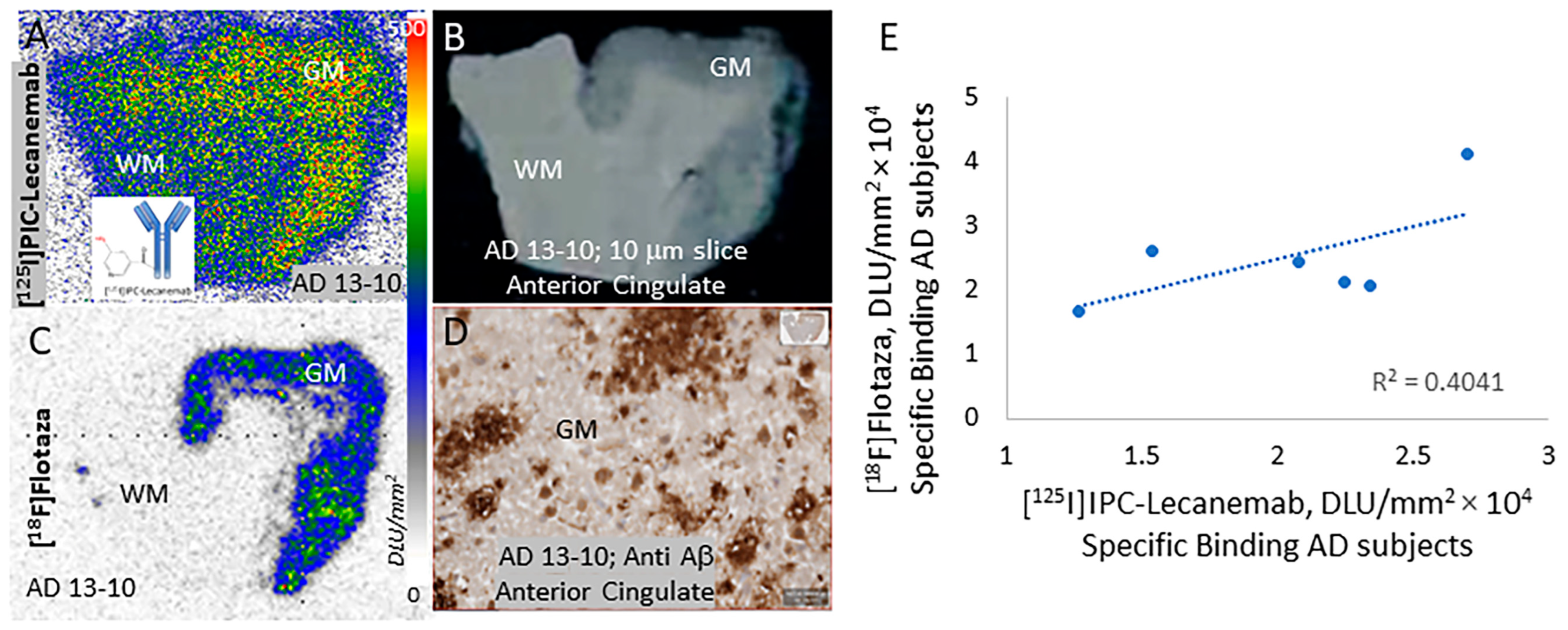
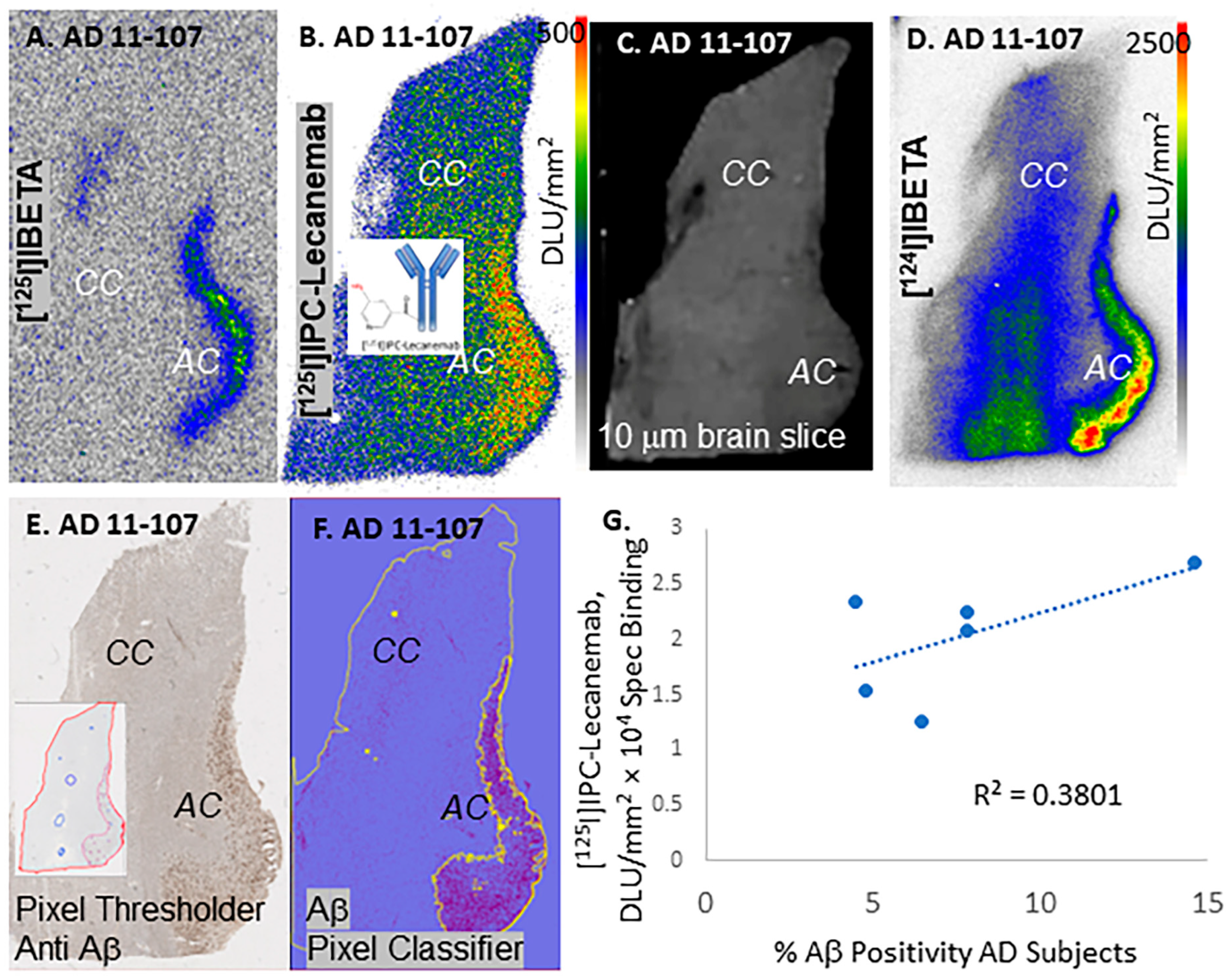
3. Results
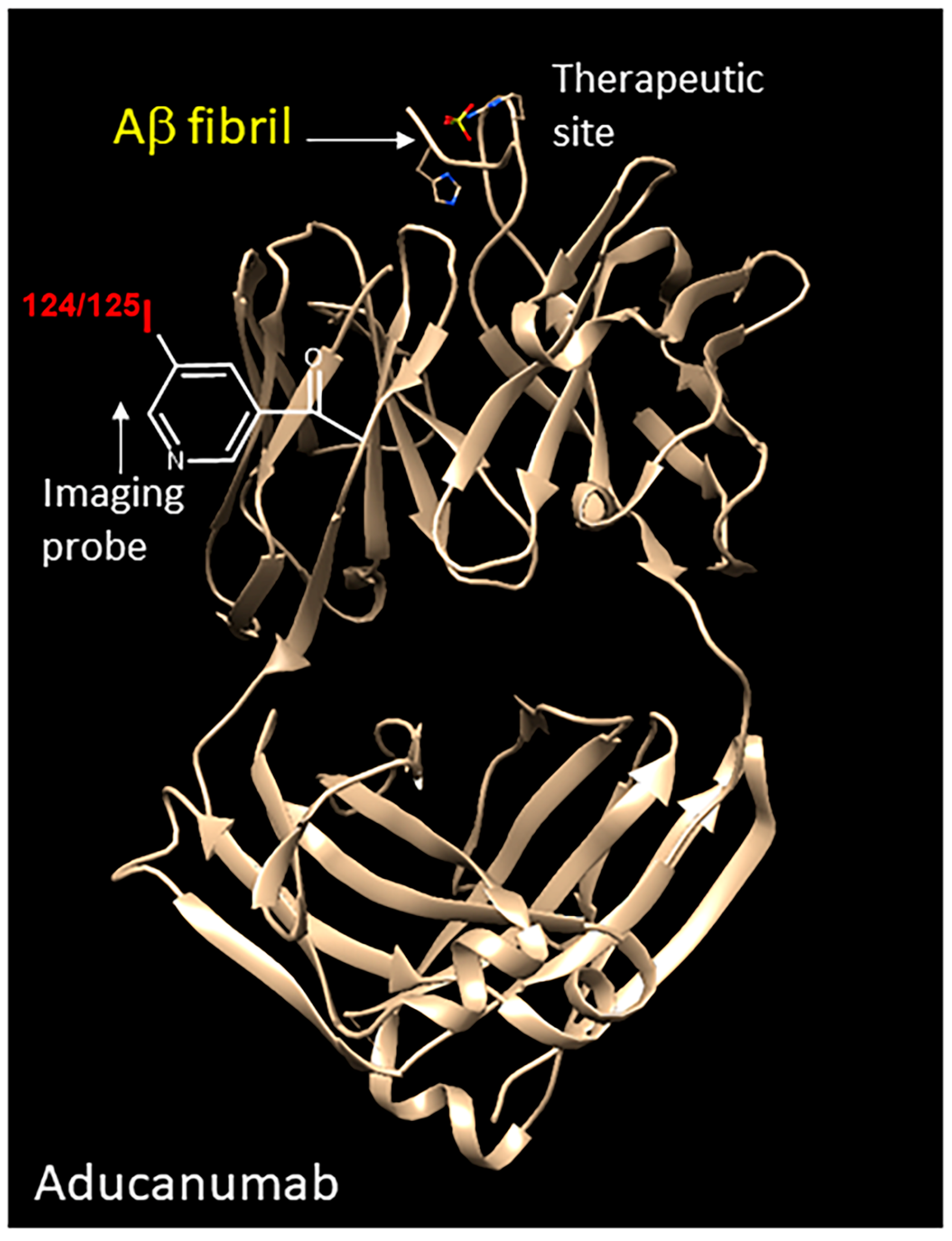
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marucci, G.; Buccioni, M.; Ben, D.D.; Lambertucci, C.; Volpini, R.; Amenta, F. Efficacy of acetylcholinesterase inhibitors in Alzheimers disease. Neuropharmacology 2021, 190, 108352. [Google Scholar] [CrossRef] [PubMed]
- Siemers, E.R.; Sundell, K.L.; Carlson, C.; Case, M.; Sethuraman, G.; Liu-Seifert, H.; Dowsett, S.A.; Pontecorvo, M.J.; Dean, R.A.; Demattos, R.; et al. Phase 3 solanezumab trials: Secondary outcomes in mild Alzheimer’ disease patients. Alzheimers Dement. 2015, 12, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Soderberg, L.; Johannesson, M.; Nygren, P.; Laudon, H.; Eriksson, F.; Osswald, G.; Moller, C.; Lannfelt, L. Lecanemab, Aducanumab and Gantenerumab-Binding profiles to different forms of amyloid-beta might explain efficacy and side effects in clinical trials for Alzheimer’s disease. Neurotherapeutics 2023, 20, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Mummery, C. An anti-amyloid therapy works for Alzheimer’s disease: Why has it taken so long and what is next. Brain 2023, 146, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Vassar, R. BACE1 inhibitor drugs in clinical trials for Alzheimer’s disease. Alzheimer’s Res. Ther. 2014, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Samra, G.K.; Dang, K.; Ho, H.; Baranwal, A.; Mukherjee, J. Dual targeting agents for Aβ plaque/P-glycoprotein and Aβ plaque/nicotinic acetylcholine α4β2* receptors—Potential approaches to facilitate Aβ Plaque removal in Alzheimer’s disease brain. Med. Chem. Res. 2018, 27, 1634–1646. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-glycoprotein: A role in the export of amyloid-b in Alzheimer’s disease. FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef] [PubMed]
- McCormick, J.W.; Ammerman Chen, G.; Vogel, P.D.; Wise, J.G. Transport of Alzheimer’s associated amyloid-b catalyzed by P-glycoprotein. PLoS ONE 2021, 16, e0250371. [Google Scholar] [CrossRef]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship between amyloid-b deposition and blood brain barrier dysfunction in Alzheimer’s disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Dore, V.; Burnham, S.C.; Masters, C.L.; Rowe, C. Imaging tau and amyloid-b proteinopathies in Alzheimer’s disease and other conditions. Nat. Rev. 2018, 14, 225–236. [Google Scholar]
- Cools, R.; Kerkhofs, K.; Leitao, R.C.F.; Bormans, G. Preclinical evaluation of novel PET probes for dementia. Sem. Nucl. Med. 2023, 53, 599–629. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The amyloid-b pathway in Alzheimer’s disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Felix, M.R.; Liang, C.; Mukherjee, J. Development and evaluation [18F]Flotaza for Aβ plaque imaging in post-mortem Alzheimer’s disease brain. Bioorg. Med. Chem. Lett. 2021, 46, 128164. [Google Scholar] [CrossRef]
- Nguyen, G.A.H.; Liang, C.; Mukherjee, J. [124I]IBETA, a new Ab amyloid plaque PET imaging agent for Alzheimer’s disease. Molecules 2022, 27, 4552. [Google Scholar] [CrossRef]
- Mondal, R.; Sandhu, Y.K.; Kamalia, V.M.; Delaney, B.A.; Syed, A.U.; Nguyen, G.A.H.; Moran, T.R.; Limpengco, R.R.; Liang, C.; Mukherjee, J. Measurement of Ab amyloid and Tau in postmortem human Alzheimer’s disease brain by immunohistochemistry analysis using QuPath and autoradiography using [18F]flotaza, [125I]IBETA and [124/125I]IPPI. Biomedicines 2023, 11, 1033. [Google Scholar] [PubMed]
- Loeffler, D.A. Antibody-mediated clearance of brain amyloid-b: Mechanisms of action, effects of natural and monoclonal anti-Ab antibodies, and downstream effects. J. Alz. Dis. Reports 2023, 7, 873–899. [Google Scholar] [CrossRef]
- Tatulian, S.A. Challenges and hopes for Alzheimer’s disease. Drug Discoery Today 2022, 27, 1027–1043. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.A.; Bernardes, C.; Bernardo-Castro, S.; Lino, M.; Albino, I.; Ferreira, L.; Bras, J.; Guerreiro, R.; Tabuuas-Pereira, M.; Baldeiras, I.; et al. Reconsidering the role of blood-brain barrier in Alzheimer’s disease: From delivery to target. Front. Aging Neurosci. 2023, 15, 1102809. [Google Scholar] [CrossRef]
- Leinenga, G.; Koh, W.K.; Gotz, J. A comparative study of the effects of Aducanumab and scanning ultrasound on amyloid plaques and behavior in the APP23 mouse model of Alzheimer’s disease. Alz. Res. Ther. 2021, 13, 76. [Google Scholar] [CrossRef]
- Rezai, A.R.; D’Haese, P.-F.; Finomore, V.; Carpenter, J.; Ranjan, M.; Wilhelmsen, K.; Mehta, R.I.; Wang, P.; Najib, U.; Teixeira, C.V.L.; et al. Ultrasound blood-brain barrier opening and Aducanumab in Alzheimer’s disease. N. Engl. J. Med. 2024, 390, 55–62. [Google Scholar] [CrossRef]
- Shi, M.; Chu, F.; Zhu, F.; Zhu, J. Ipact of anti-amyloid-b monoclonal antibodies on the pathology and clinical profile of Alzheimer’s disease: A focus on Aducanumab and Lecanemab. Front. Aging Neurosci. 2022, 14, 870517. [Google Scholar] [CrossRef]
- Golde, T.E.; Levey, A.I. Immunotherapies for Alzheimer’s disease. Science 2023, 382, 1242–1244. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, N.; Zan, L.; Liao, J.; Jin, J. Radioiodination of insulin using N-succinimidyl-5-(tributylstannyl)-3-pyridinecarboxylate (SPC) as a bifunctional linker: Synthesis and biodistribution in mice. J. Radioanal. Nucl. Chem. 2006, 268, 205–210. [Google Scholar] [CrossRef]
- Beach, T.G.; Adler, C.H.; Sue, L.I.; Serrano, G.; Shill, H.A.; Walker, D.G.; Lue, L.; Roher, A.E.; Dugger, B.N.; Maarouf, C.; et al. Arizona study of aging and neurodegenerative disorders and brain and body donation program. Neuropathology 2015, 35, 354–389. [Google Scholar] [CrossRef]
- Syed, A.U.; Liang, C.; Patel, K.K.; Mondal, R.; Kamalia, V.M.; Moran, T.R.; Ahmed, S.T.; Mukherjee, J. Comparison of Monoamine oxidase-A, Ab plaques, Tau and Translocator protein in postmortem human Alzheimer’s disease brain. Int. J. Mol. Sci. 2023, 24, 10808. [Google Scholar] [CrossRef]
- Mukherjee, J.; Liang, C.; Patel, K.K.; Lam, P.Q.; Mondal, R. Development and evaluation [125I]IPPI for tau imaging in post-mortem human Alzheimer’s disease brain. Synapse 2021, 74, e22183. [Google Scholar] [CrossRef]
- Pandey, S.K.; Venugopal, A.; Kant, R.; Coleman, R.A.; Mukherjee, J. 124I-Epidepride: A high affinity and selective PET radiotracer with potential for extended imaging of dopamine D2/D3 receptors. Nucl. Med. Biol. 2014, 41, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.T.; Iguban, M.H.; Melkonyan, L.; Shergill, J.; Liang, C.; Mukherjee, J. Development and evaluation of [124/125I]IAZA as a new proteinopathy imaging agent for Alzheimer’s disease. Molecules 2023, 28, 865. [Google Scholar] [CrossRef] [PubMed]
- Sehlin, D.; Fang, X.T.; Cato, L.; Antoni, G.; Lannfelt, L.; Syvanen, S. Antibody-based PET imaging of amyloid beta in mouse models of Alzheimer’s disease. Nat. Commun. 2016, 7, 10759. [Google Scholar] [CrossRef]
- Gustavsson, T.; Syvanen, S.; O’Callaghan, P.; Sehlin, D. SPECT imaging of distribution and retention of a brain-penetrating bispecific amyloid b antibody in a mouse model of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussiere, T.; Harmann, S.; et al. Structural and kinetic basis for the selectivity of Aducanumab for aggregated forms of amyloid-b. Sci. Rep. 2018, 8, 6412. [Google Scholar] [CrossRef]
- Cummings, J.; Aisen, P.; Lemere, C.; Atri, A.; Sabbagh, M.; Salloway, S. Aducanumab produced a clinically meaningful benefit in association with amyloid lowering. Alzheimer’s Res. Ther. 2021, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Thambisetty, M.; Howard, R. Lecanemab trial in AD brings hope but requires greater clarity. Nature Revs Neurology 2023, 19, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.-C.S.; Jennings, G.T.; Mohsen, M.O.; Vogel, M.; Bachman, M.F. Alzheimer’s disease: A brief history of immunotherapies targeting amyloid b. Int. J. Mol. Sci. 2023, 24, 3895. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussière, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody Aducanumab reduces Ab plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; de Backer, M.E.; Rio, J.; Nabuurs, R.J.A.; Gaillard, P.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. Enhances glutathion PEGylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for Alzheimer’s disease. J. Control. Release 2015, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Banka, V.; Kelleher, A.; Sehlin, D.; Hulqvist, G.; Sigurdsson, E.M.; Syvanen, S.; Ding, Y.S. Development of brain-penetrable antibody radioligands for in vivo PET imaging of amyloid b and tau. Front. Nucl. Med. 2023, 3, 1173693. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.A.; de Veer, M.; Miles, L.A.; Kelderman, C.A.; McLean, C.A.; Masters, C.L.; Barnham, K.J.; White, J.M.; Paterson, B.M.; Donnelly, P.S.; et al. Pre-targeting amyloid b with antibodies for potential molecular imaging of Alzheimer’s disease. Chem. Commun. 2023, 59, 2243–2246. [Google Scholar] [CrossRef]
- Lau, W.L.; Liang, C.; Liu, H.; Singh, K.; Mukherjee, J. Development of Zirconium-89 PET for in vivo imaging of alpha-Klotho. Am. J. Nucl. Med. Mol. Imaging 2020, 10, 95–105. [Google Scholar]
- Stergiou, N.; Wuensche, T.E.; Schreurs, M.; Mes, I.; Verlaan, M.; Koojiman, E.J.M.; Windhorst, A.D.; Helboe, L.; Vergo, S.; Christensen, S.; et al. Application of 89Zr-DFO*-immuno-PET to assess improved target engagement of a bispecific anti-amyloid-ß monoclonal antibody. Eur. J. Nucl. Med. Mol. Imag. 2023, 50, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Hur, Y.; Hwang, J.; Park, J. Biological effects of blood-brain barrier disruption using a focused ultrasound. Biomed. Eng. Lett. 2017, 7, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Baseri, B.; Choi, J.J.; Tung, Y.-S.; Konofagouu, E.E. Multi-modality safety assessment of blood brain barrier opening using focused ultrasound and definity microbubbles: A short-term study. Ultrasound Med. Biol. 2010, 36, 1445–1459. [Google Scholar] [CrossRef]
- Gandhi, K.; Barzegar-Fallah, A.; Banstola, A.; Rizwan, S.B.; Reynolds, J.N.J. Ultrasound-mediated blood brain barrier disruption for drug delivery: A systematic review of protocols, efficacy, and safety outcomes from preclinical and clinical studies. Pharmaceutics 2022, 14, 833. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.; Paclibar, C.G.; Gonzaga, N.L.; Sison, S.A.; Bath, H.S.; Biju, A.P.; Mukherjee, J. [125I]IPC-Lecanemab: Synthesis and Evaluation of Aβ-Plaque-Binding Antibody and Comparison with Small-Molecule [18F]Flotaza and [125I]IBETA in Postmortem Human Alzheimer’s Disease. Neurol. Int. 2024, 16, 419-431. https://doi.org/10.3390/neurolint16020031
Liang C, Paclibar CG, Gonzaga NL, Sison SA, Bath HS, Biju AP, Mukherjee J. [125I]IPC-Lecanemab: Synthesis and Evaluation of Aβ-Plaque-Binding Antibody and Comparison with Small-Molecule [18F]Flotaza and [125I]IBETA in Postmortem Human Alzheimer’s Disease. Neurology International. 2024; 16(2):419-431. https://doi.org/10.3390/neurolint16020031
Chicago/Turabian StyleLiang, Christopher, Cayz G. Paclibar, Noresa L. Gonzaga, Stephanie A. Sison, Harman S. Bath, Agnes P. Biju, and Jogeshwar Mukherjee. 2024. "[125I]IPC-Lecanemab: Synthesis and Evaluation of Aβ-Plaque-Binding Antibody and Comparison with Small-Molecule [18F]Flotaza and [125I]IBETA in Postmortem Human Alzheimer’s Disease" Neurology International 16, no. 2: 419-431. https://doi.org/10.3390/neurolint16020031






