Multicenter Study of the Effectiveness of Antifungal Stewardship Team Intervention for Candidemia in Japan in 2008–2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Fungal Isolates
2.2. Antimicrobial Stewardship Programs
2.3. Clinical Parameters
2.4. Statistical Analysis
3. Results
3.1. Patient Background and Candida Species
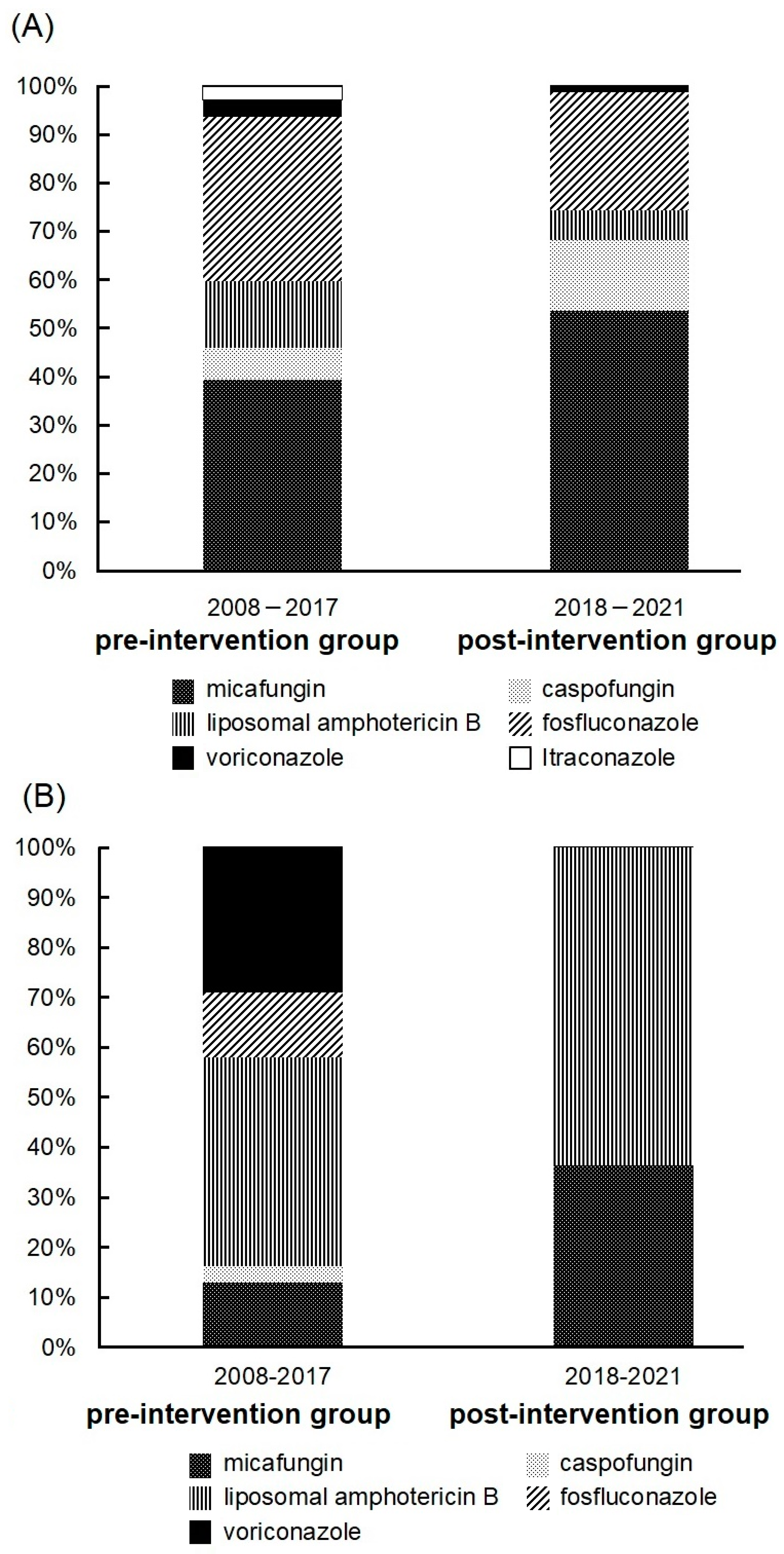
3.2. Antifungal Drugs Received by Patients
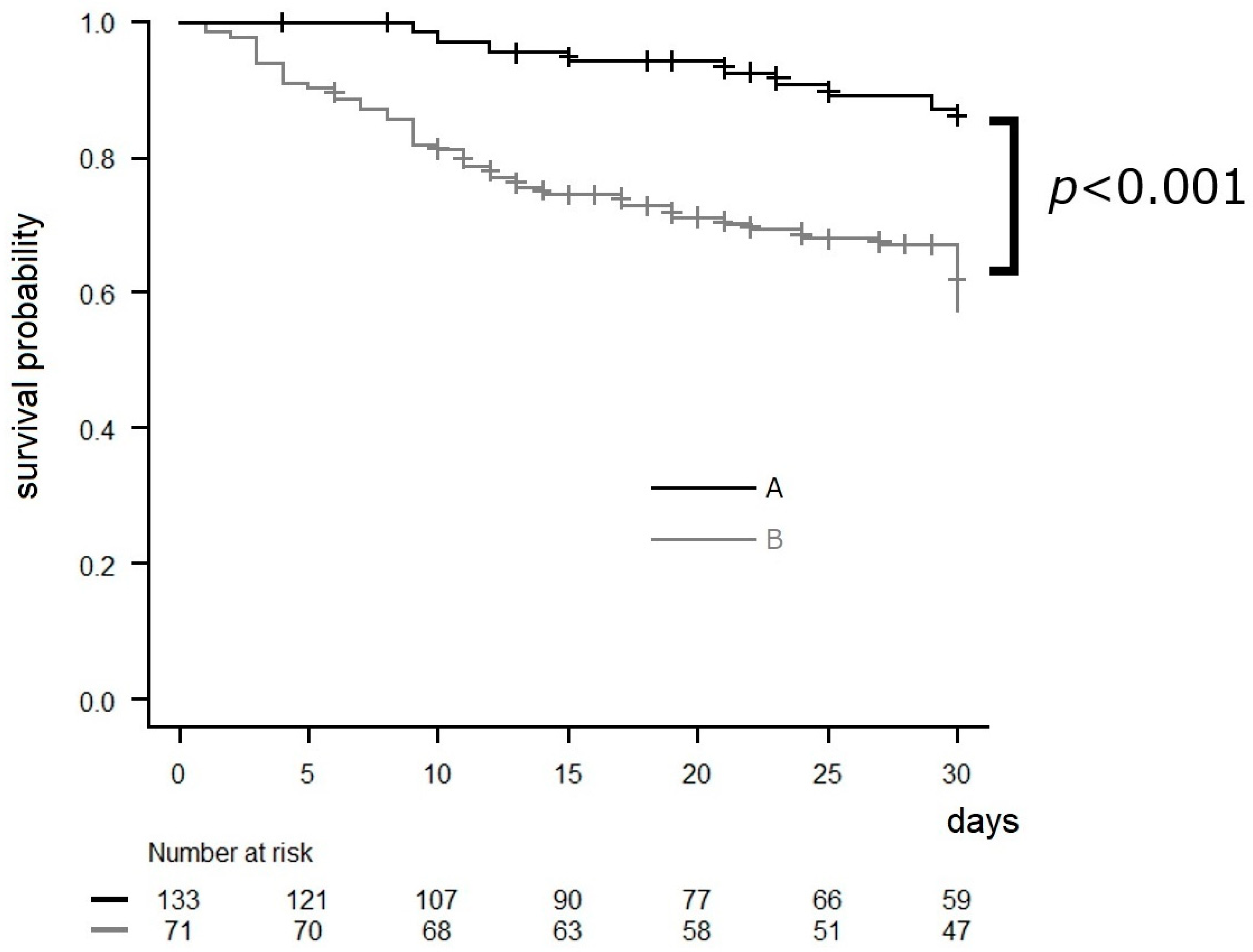
3.3. Effects of AFT Intervention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alobaid, K.; Ahmad, S.; Asadzadeh, M.; Mokaddas, E.; Al-Sweih, N.; Albenwan, K.; Alfouzan, W.; Al-Obaid, I.; Jeragh, A.; Al-Roomi, E.; et al. Epidemiology of Candidemia in Kuwait: A Nationwide, Population-Based Study. J. Fungi 2021, 7, 673. [Google Scholar] [CrossRef] [PubMed]
- Kakeya, H.; Yamada, K.; Kaneko, Y.; Yanagihara, K.; Tateda, K.; Maesaki, S.; Takesue, Y.; Tomono, K.; Kadota, J.-I.; Kaku, M.; et al. National Trends in the Distribution of Candida Species Causing Candidemia in Japan from 2003 to 2014. Med. Mycol. J. 2018, 59, E19–E22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yildirim, M.; Sahin, I.; Kucukbayrak, A.; Ozdemir, D.; Yavuz, M.T.; Oksuz, S.; Cakir, S. Hand carriage of Candida species and risk factors in hospital personnel. Mycoses 2007, 50, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.A.; Slavinski, S.A.; Morgan, J.; Lott, T.; Arthington-Skaggs, B.A.; Brandt, M.E.; Webb, R.M.; Currier, M.; Flowers, R.H.; Fridkin, S.K.; et al. Epidemiologic and molecular characterization of an outbreak of Candida parapsilosis bloodstream infections in a community hospital. J. Clin. Microbiol. 2004, 42, 4468–4472. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumpetis, T.; Manolakaki, D.; Velmahos, G.; Chang, Y.; Alam, H.B.; De Moya, M.M.; Sailhamer, E.A.; Mylonakis, E. Candida infection and colonization among non-trauma emergency surgery patients. Virulence 2010, 1, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Alenazy, H.; Alghamdi, A.; Pinto, R.; Daneman, N. Candida colonization as a predictor of invasive candidiasis in non-neutropenic ICU patients with sepsis: A systematic review and meta-analysis. Int. J. Infect. Dis. 2021, 102, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Madec, Y.; Denoeud-Ndam, L.; Wolff, M.; Fontanet, A.; Bretagne, S.; Dromer, F.; The French Mycosis Study Group. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002–2010). Intensiv. Care Med. 2014, 40, 1303–1312. [Google Scholar] [CrossRef]
- Ueda, T.; Takesue, Y.; Tokimatsu, I.; Miyazaki, T.; Nakada-Motokawa, N.; Nagao, M.; Nakajima, K.; Mikamo, H.; Yamagishi, Y.; Kasahara, K.; et al. The incidence of endophthalmitis or macular involvement and the necessity of a routine ophthalmic examination in patients with candidemia. PLoS ONE 2019, 14, e0216956. [Google Scholar] [CrossRef] [PubMed]
- Takesue, Y.; Ueda, T.; Mikamo, H.; Oda, S.; Takakura, S.; Kitagawa, Y.; Kohno, S.; Masuda, A.; Yoshida, C.; Yasunaga, C.; et al. Management bundles for candidaemia: The impact of compliance on clinical outcomes. J. Antimicrob. Chemother. 2015, 70, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Lewis, R.E.; Ashley, E.S.D.; Ostrosky-Zeichner, L.; Zaoutis, T.; Thompson, G.R.; Andes, D.R.; Walsh, T.J.; Pappas, P.G.; A Cornely, O.; et al. Core Recommendations for Antifungal Stewardship: A Statement of the Mycoses Study Group Education and Research Consortium. J. Infect. Dis. 2020, 222, S175–S198. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, H.; Yamada, K.; Imoto, W.; Yamairi, K.; Shibata, W.; Namikawa, H.; Yoshii, N.; Nakaie, K.; Okada, Y.; Fujita, A.; et al. The effects of antifungal stewardship programs at a tertiary-care teaching hospital in Japan. J. Infect. Chemother. 2019, 25, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Gotoh, K.; Nakamura, S.; Akeda, Y.; Yoshii, T.; Miyaguchi, S.; Inohara, H.; Horii, T.; Oishi, K.; Iida, T.; et al. Fatal sepsis caused by an unusual Klebsiella species that was misidentified by an automated identification system. J. Med. Microbiol. 2013, 62 Pt 5, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Pettit, N.N.; Han, Z.; Nguyen, C.T.; Choksi, A.; Charnot-Katsikas, A.; Beavis, K.G.; Tesic, V.; Pisano, J. Antimicrobial Stewardship Review of Automated Candidemia Alerts Using the Epic Stewardship Module Improves Bundle-of-Care Adherence. Open Forum Infect. Dis. 2019, 6, ofz412. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997–2016. Open Forum Infect. Dis. 2019, 6 (Suppl. S1), S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Rauseo, A.M.; Spec, A. Prevalence of Ocular Complications in Candidemia: Defining the “Battlefield”. Clin. Infect. Dis. 2023, 76, 1750–1752. [Google Scholar] [CrossRef] [PubMed]
- Lashof, A.M.L.O.; Rothova, A.; Sobel, J.D.; Ruhnke, M.; Pappas, P.G.; Viscoli, C.; Schlamm, H.T.; Oborska, I.T.; Rex, J.H.; Kullberg, B.J. Ocular manifestations of candidemia. Clin. Infect. Dis. 2011, 53, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Saito, T.; Doi, S.; Hotta, G.; Yamamoto, M.; Matsumura, Y.; Matsushima, A.; Ito, Y.; Takakura, S.; Ichiyama, S. Clinical characteristics and risk factors of ocular candidiasis. Diagn. Microbiol. Infect. Dis. 2012, 73, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Breazzano, M.P.; Bond, J.B., III; Bearelly, S.; Kim, D.H.; Donahue, S.P.; Lum, F.; Olsen, T.W. American Academy of Ophthalmology Recommendations on Screening for Endogenous Candida Endophthalmitis. Ophthalmology 2022, 129, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Akler, M.E.; Vellend, H.; McNeely, D.M.; Walmsley, S.L.; Gold, W.L. Use of fluconazole in the treatment of candidal endophthalmitis. Clin. Infect. Dis. 1995, 20, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
| (A) | ||||||||
| Total = 204 | Pre-Intervention Group = 120 | Post-Intervention Group = 84 | p Value | |||||
| n | % | n | % | n | % | |||
| Characteristics of patients | ||||||||
| Age, median, years | 72 | 74 | 71 | 0.82 ‡ | ||||
| Male sex | 133 | 65.2 | 79 | 65.8 | 54 | 64.3 | 0.88 † | |
| Gastrointestinal disease | 82 | 40.2 | 51 | 42.5 | 31 | 36.9 | 0.47 † | |
| Diabetes mellitus | 54 | 26.5 | 27 | 22.5 | 27 | 32.1 | 0.15 † | |
| skin disease | 31 | 15.2 | 23 | 19.2 | 8 | 9.5 | 0.07 † | |
| Blood disease | 27 | 13.2 | 18 | 15.0 | 9 | 10.7 | 0.41 † | |
| Urological disease | 20 | 9.8 | 13 | 10.8 | 7 | 8.3 | 0.64 † | |
| Collagen disease | 10 | 4.9 | 5 | 4.2 | 5 | 6.0 | 0.74 † | |
| Febrile neutropenia | 12 | 5.9 | 8 | 6.7 | 4 | 4.8 | 0.77 † | |
| Receiving steroids or immunosuppressive drugs | 65 | 31.9 | 38 | 31.7 | 27 | 32.1 | 1.00 † | |
| Anticancer drug | 41 | 20.1 | 24 | 20.0 | 17 | 20.2 | 1.00 † | |
| Radiation therapy | 13 | 6.4 | 7 | 5.8 | 6 | 7.1 | 0.78 † | |
| Required full assistance with ADLs | 93 | 45.6 | 44 | 36.7 | 49 | 58.3 | <0.01 † | |
| Use of central venous catheter | 161 | 78.9 | 94 | 78.3 | 67 | 79.8 | 0.86 † | |
| CRBSI | 89 | 43.6 | 43 | 35.8 | 46 | 54.8 | <0.01 † | |
| Use of urinary catheters | 162 | 79.4 | 95 | 79.2 | 67 | 79.8 | 1.00 † | |
| Intravital devices | 158 | 77.5 | 95 | 79.2 | 63 | 75.0 | 0.50 † | |
| Administration of high-calorie infusions | 144 | 70.6 | 88 | 73.3 | 56 | 66.7 | 0.35 † | |
| Received surgery | 96 | 47.1 | 57 | 47.5 | 39 | 46.4 | 0.89 † | |
| Number of days until blood culture results are known | 3.3 | 3.4 | 3.2 | 0.42 ‡ | ||||
| Patients who did not receive antifungal drugs | 13 | 6.4 | 11 | 9.2 | 2 | 2.4 | 0.08 † | |
| The average time to change the drug due to inadequate efficacy | 12 | 12.9 | 9.4 | 0.25 ‡ | ||||
| Frequency of candidemia | 0.15 | 0.15 | 0.16 | 0.57 † | ||||
| (number of positive sets/numbers of total sets*100) | ||||||||
| Candidemia patients per 1000 new admissions | 0.47 | 0.4 | 0.62 | <0.01 † | ||||
| Compliance with the action bundle | ||||||||
| Collection of two sets of blood cultures | 121 | 59.3 | 55 | 45.8 | 66 | 78.6 | <0.01 † | |
| Removing intravital devices | 124 | 66.7 (total = 186) | 66 | 59.5 (total = 111) | 58 | 77.3 (total = 75) | 0.01 † | |
| Appropriate antifungal therapy | 179 | 87.7 | 105 | 87.5 | 74 | 88.1 | 1.00 † | |
| Consulting an ophthalmologist | 106 | 52.0 | 42 | 35.0 | 64 | 76.2 | <0.01 † | |
| Ophthalmology re-examination | 37 | 34.9 (total = 106) | 11 | 26.2 (total = 42) | 26 | 40.6 (total = 64) | 0.15 † | |
| Follow-up blood cultures until clearance of candidemia | 147 | 72.1 | 68 | 56.7 | 79 | 94.0 | <0.01 † | |
| Scrutiny of organ involvement when blood cultures are consecutively positive | 33 | 64.7 (total = 51) | 15 | 57.7 (total = 26) | 18 | 72.0 (total = 25) | 0.38 † | |
| (B) | ||||||||
| 30-day mortality rate | 58 | 28.4 | 41 | 34.2 | 17 | 20.2 | 0.04 † | |
| C. albicans | 90 | 44.1 | 56 | 46.7 | 34 | 40.5 | 0.47 † | |
| C. parapsilosis | 54 | 26.5 | 31 | 25.8 | 23 | 27.4 | 0.87 † | |
| C. glabrata | 25 | 12.3 | 12 | 10.0 | 13 | 15.5 | 0.28 † | |
| C. tropicalis | 17 | 8.3 | 9 | 7.5 | 8 | 9.5 | 0.62 † | |
| C. guilliermondii | 7 | 3.4 | 6 | 5.0 | 1 | 1.2 | 0.25 † | |
| C. lustaniae | 4 | 2.0 | 1 | 0.8 | 3 | 3.6 | 0.31 † | |
| C. krusei | 3 | 1.5 | 3 | 2.5 | 0 | 0.0 | 0.27 † | |
| C. dubliniensis | 1 | 0.5 | 1 | 0.8 | 0 | 0.0 | 1.00 † | |
| C. famata | 1 | 0.5 | 1 | 0.8 | 0 | 0.0 | 1.00 † | |
| C. duobshaemulonii | 1 | 0.5 | 0 | 0.0 | 1 | 1.2 | 0.41 † | |
| C. metapsilosis | 1 | 0.5 | 0 | 0.0 | 1 | 1.2 | 0.41 † | |
| Echinocandins Group = 106 | Nonechinocandin Drugs Group = 85 | p Value | |
|---|---|---|---|
| 30-day mortality rate | 28.3 | 27.1 | 0.87 † |
| serum creatinine level (median) | 0.77 | 0.99 | 0.04 ‡ |
| Odds Ratio | p Value | |
|---|---|---|
| Collection of two sets of blood cultures | 0.88 | 0.72 |
| Consulting an ophthalmologist | 0.52 | 0.13 |
| Follow-up blood cultures until clearance of candidemia | 0.23 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokano, M.; Tarumoto, N.; Sakai, J.; Imai, K.; Koizumi, S.; Karaushi, H.; Hatanaka, T.; Kishi, E.; Seki, M.; Mitsutake, K.; et al. Multicenter Study of the Effectiveness of Antifungal Stewardship Team Intervention for Candidemia in Japan in 2008–2021. Infect. Dis. Rep. 2024, 16, 356-366. https://doi.org/10.3390/idr16020027
Tokano M, Tarumoto N, Sakai J, Imai K, Koizumi S, Karaushi H, Hatanaka T, Kishi E, Seki M, Mitsutake K, et al. Multicenter Study of the Effectiveness of Antifungal Stewardship Team Intervention for Candidemia in Japan in 2008–2021. Infectious Disease Reports. 2024; 16(2):356-366. https://doi.org/10.3390/idr16020027
Chicago/Turabian StyleTokano, Mieko, Norihito Tarumoto, Jun Sakai, Kazuo Imai, Sakaru Koizumi, Haruka Karaushi, Tamotsu Hatanaka, Etsuko Kishi, Masafumi Seki, Koutaro Mitsutake, and et al. 2024. "Multicenter Study of the Effectiveness of Antifungal Stewardship Team Intervention for Candidemia in Japan in 2008–2021" Infectious Disease Reports 16, no. 2: 356-366. https://doi.org/10.3390/idr16020027
APA StyleTokano, M., Tarumoto, N., Sakai, J., Imai, K., Koizumi, S., Karaushi, H., Hatanaka, T., Kishi, E., Seki, M., Mitsutake, K., & Maesaki, S. (2024). Multicenter Study of the Effectiveness of Antifungal Stewardship Team Intervention for Candidemia in Japan in 2008–2021. Infectious Disease Reports, 16(2), 356-366. https://doi.org/10.3390/idr16020027






