Abstract
Exopolysaccharide (EPS)-producing bacteria were isolated from the intestines of freshwater fish as prebiotics. Among the isolates, Bacillus sp. P1 was the potent EPS producer, with a high EPS production, and was then identified as Bacillus subtilis P1 based on 16S rRNA and biochemical characteristics. The produced microbial EPS was characterized by its functional groups by FTIR, showing a 90.20% correlation to inulin, while the EPS molecular weight was approximately 105 Da with a high PDI (>1.5). Moreover, the EPS from B. subtilis P1 was assessed for prebiotic properties by growing probiotic bacteria, and significant cell growth occurred in sugar-free TSB with 0.5% EPS (p < 0.05). EPS exhibited approximately 55.37% DPPH radical scavenging at 20 mg/mL and inhibited certain fish pathogens at 400 μg/mL (10–13 mm inhibition zone). Low EPS cytotoxicity was confirmed (<1% hemolysis) prior to use as immunobiotics in Nile Tilapia (Oreochromis niloticus) diet supplementation. Nile tilapia growth and immune response were monitored after feeding with control (C), basal diet (BD), and treatment (T): BD + EPS 5 g/kg. In the T-group, the weight gain (WG), % specific growth rate (SGR), and average daily gain (ADG) significantly increased compared to the control (p < 0.05) by week 4, with no negative effects on blood chemistry parameters. Lysozyme activity and respiratory burst activity in T-groups were significantly higher than the control (p < 0.05), with a 50% RLP survival rate of Nile Tilapia infected by Aeromonas veronii in the T-group after a two-week challenge. On the other hand, the proximate analysis revealed higher protein content in the T-group. These findings suggest that EPS from B. subtilis P1 in fish diets efficiently supports fish growth and stimulates innate immune response in Nile Tilapia, thus showing potential as the immunobiotics for aquaculture.
1. Introduction
Tilapia farming, particularly Nile tilapia (Oreochromis niloticus), plays a vital role in Thailand’s aquaculture sector. Nile tilapia stands out as the most economically crucial freshwater fish, boasting an annual production exceeding 210,000 metric tons and a market value over USD 300 million [1]. However, the intensive culture system has raised concerns about potential negative impacts on fish health and water quality, particularly disease outbreaks in the aquaculture industry. The most common bacterial disease outbreaks are Motile Aeromonas septicemia (AMS) [2,3,4]. At present, a range of methods such as synthetic chemicals and antibiotics are applied for disease control. However, the use of these chemotherapeutic agents has been associated with negative effects including residue accumulation and the emergence of drug resistance. Hence, the adoption of prebiotics presents an alternative strategy for sustaining healthy aquaculture systems. Prebiotics demonstrate immunomodulatory and/or immunostimulatory properties, contributing to the production of high-quality and safe aquatic products [3,4,5,6,7]. Prebiotics are non-digestible food ingredients that exhibit favorable effect on the host by stimulating growth activity and/or activity of beneficial bacteria in the gastrointestinal (GI) tract or a limited number of bacteria in the colon, thus improving host health [8]. It was reported that prebiotics can modify the GI tract microbial community, in turn enhancing non-specific immune responses, increasing fermentation products, improving mineral uptake, enhancing livestock performance indices such as protein efficiency ratio and feed conversion ratio, and improving disease resistance [2,3,4,5]. Microbial exopolysaccharide (EPS) is one of the potent prebiotics secreted by microorganisms with a variety of functional groups that cannot be found in animals and plant polysaccharides and are also regarded as being affordable, safe, and biodegradable [9,10,11]. Moreover, microbial EPS can be formulated under precise constraints with enduring stability in the GI tract to enrich the colonization of useful microorganisms [12], robust operability, high reproductive efficiency, and superior performance [13]. Notably, although EPS possesses biological activities [10,11,14,15] including antibacterial activities [13,16,17], antioxidant activities [15,18,19,20], and immunomodulatory activities [10,13], the application of microbial EPS as fish immunomodulatory and/or immunostimulatory agents has been little reported [13]. A study by Mahdhi et al. (2020) showed that supplementing fish diets with EPS from probiotics such as Lactobacillus plantarum and Bacillus strain (HM117830) enhanced antibacterial, anti-biofilm, and antioxidant activities. This significantly increased the expression of immune-related genes in the head kidney and liver of European sea bass (Dicentrarchus labrax L.) [17]. In Nile tilapia (O. niloticus), EPS from Bacillus tequilensis PS21 was newly found to have antioxidant and antimicrobial effects, improving survival rates after Streptococcus agalactiae infection [13]. Additionally, EPS from Bacillus licheniformis Dahb1 boosted antioxidant responses and enhanced disease resistance in Oreochromis mossambicus [12]. However, at present, the commercial use of microbial EPS is limited by low production yields during fermentation. The challenges are to improve productivity and produce EPS that possesses the desired structure and size, positively affecting its functionality [9,21,22,23,24]. In this study, we examined the potential of microbial EPS produced from the new bacterial isolates from freshwater fish intestines by identification of a strain that produces the highest amount of EPS. We also determined the EPS optimal medium compositions, and then the physiochemical properties of the produced EPS, such as its molecular weight and its functional groups, were characterized. The aim of this study was to identify and optimize EPS production from the new bacterial isolate, characterize the EPS physiochemical properties, and evaluate its antibacterial, antioxidant, and prebiotic activities, as well as its cytotoxicity, before it can be included in the diet of Nile tilapia (O. niloticus) as an immunobiotic agent to promote fish growth and enhance their innate immune response. We hypothesized that this microbial EPS is able to enhance growth performance, boost immune responses, and provide disease resistance in fish when used as a dietary supplement.
2. Materials and Methods
2.1. Screening and Isolation of EPS-Producing Bacteria from Freshwater Scaled Fish Intestine
Five local freshwater scaled fish species—Oreochromis niloticus (Linnaeus, 1758), Notopterus notopterus (Pallas, 1769), Oxyeleotris marmorata (Bleeker, 1852), Pristolepis fasciata (Bleeker, 1851), and Hampala macrolepidota (Kuhl & Van Hasselt, 1823)—were obtained from a natural freshwater lake in Khuan Khanun District, Phatthalung, Southern Thailand (coordinates: 7°47′20.0″ N 100°09′13.4″ E) by collecting five samples of each fish. This collection area has been designated as a Ramsar site since 1998 due to its ecological biodiversity, including various species of fish. The environmental conditions consist of freshwater with a depth of about 1.2 m and an abundance of plankton, and the area is considered an ecological niche. The fish intestines were then aseptically dissected according to the different fish species, followed by dilution of the samples with 0.85% (w/v) NaCl. Then, the diluted samples were plated on de Man, Rogosa, Sharpe (MRS) agar, and Trypticase soy agar (TSA) supplemented with amphotericin B for the isolation of lactic acid bacteria (LAB) and Bacillus spp., respectively. The cultures were then incubated at 28 ± 2 °C for 24–48 h under anaerobic conditions for LAB, while Bacillus spp. was aerobically incubated. The obtained isolates were subjected to Gram staining and biochemical examination based on Bergey’s manual. Notably, LAB exhibited catalase-negative and non-spore-forming characteristics [25]. In addition, the quantitative analysis of EPS formation was measured using crystal violet staining of the attached cells [26]. To initially determine the EPS production, an overnight bacterial culture with an approximate OD600 of 1.0 was inoculated with a 10% (v/v) inoculum into MRS broth or Trypticase soy broth (TSB) supplemented with 2% (w/v) glucose in sterilized glass tubes. The tubes were then incubated at 30 °C for 3 days, and the bacterial attachment was measured using crystal violet staining. In brief, the attached cells were stained with 1% (w/v) crystal violet for 20 min and then de-stained with 95% (v/v) ethanol followed by measurement of the optical density at 595 nm [27,28]. The EPS formation of all isolates was compared according to the intensity of crystal violet staining (method modified from Todhanakasem et al. [28]).
2.2. Exopolysaccharide Production, Purification, and Quantification
The bacterial isolates were cultivated in MRS broth or TSB, and we then adjusted the concentration to 1 × 108 CFU/mL before transferring 5 mL of inoculum into 50 mL EPS-optimum medium supplemented with 20 g/L sucrose (modified from Liu et al. [29]). The bacterial culture was incubated at 30 °C for 72 h before harvesting by high-speed centrifugation (15,000× g for 30 min at 4 °C). The cell-free supernatant was then collected and further precipitated in three volumes of ice-cold 96% ethanol before allowing the mixture to stand at 4 °C overnight. The precipitated EPS was then separated by centrifugation again. The resulting EPS pellet was suspended in ultrapure water and dialyzed against ultrapure water using a 10 kDa cut-off cellulose membrane for 48 h under stirring. The retentate was freeze-dried using a freeze-drier (Christ Delta 2-24 LSC plus, Osterode am Harz, Germany) [9]. The yield (%) of EPS can be calculated by the weight of EPS produced divided by dry cell weight and expressed as a percentage [9,30].
2.3. EPS Characterization of the Purified EPS
2.3.1. Molecular Mass Determination
The average molecular mass of the purified EPS was analyzed by gel permeation chromatography (GPC) with TSKgel SuperHM-N columns (Tosoh Bioscience LLC, Grove City, OH, USA). The EPS was eluted with 0.1 M sodium nitrate (NaNO3) at a flow rate of 0.35 mL/min at 40 °C. Detection was carried out using a refractive index detector (RI) and a multi-angle laser light scattering detector [31]. The linear regression was calibrated using high-molecular-weight polystyrene standards (Sigma-Aldrich, Inc., St. Louis, MO, USA).
2.3.2. Functional Groups Determination
The chemical structural characterization of the bacterial EPS was examined using Fourier transform infrared (FTIR) spectroscopy to determine the distribution of functional groups. The pellets were prepared by mixing and compressing the freeze-dried EPS samples with KBr powder (in a ratio of 5:100 w/w). FTIR spectra were recorded in transmittance mode over a spectral range from 4000 to 400 cm−1, with an accumulation of 15 scans and a resolution of 4 cm−1 with a Fourier transform infrared spectrometer (VERTEX 70, Bruker, Ettlingen, Germany). Band identification was compared with known standard compounds such as inulin from chicory, dextran from Leuconostoc spp., and levan from Erwinia herbicola (Sigma-Aldrich) [13,31].
2.3.3. Monosaccharide and Oligosaccharide Determination
Monosaccharide composition analysis was performed according to the method described by Sutthi et al. [13]. A sealed tube containing 5 mL of 2 M trifluoroacetic acid (TFA) was employed to hydrolyze 100 mg of microbial EPS at 100 °C for 6 h followed by neutralization with 1 N NaOH. The hydrolysate was then filtered using a 0.22 μm syringe filter. Subsequently, the high-performance liquid chromatography (HPLC) was then analyzed for the EPS compositions by LC-20 AD system (a RID-10 A refractive index detector, Shimadzu, Tokyo, Japan) with an Aminex HPX-87H column (Bio-Rad Laboratories, Inc., CA, USA). The column was maintained at 65 °C, and the mobile phase used was 0.005 M H2SO4 with a flow rate of 0.5 mL/min for 40 min. The monosaccharide and oligosaccharide were finally identified using a UV detector, referencing sugar standards.
2.3.4. Scanning Electron Microscopy (SEM) Analysis
About 5 mg of lyophilized EPS sample obtained from the cell-free supernatant culture of Bacillus sp. P1 was affixed to an SEM stub and then coated with a gold layer approximately 10 nm thick on both sides. An SEM image was taken at 3.0 kV using a scanning electron microscope (FEI Quanta 450 FEG).
2.4. Optimization Condition of EPS Production from the Selected Strain
The EPS production efficiency of five EPS-producing media formulations, as mentioned in previous studies [21,22,23,24,29], containing various types and different amounts of carbon and nitrogen and/or supplementation with amino acids, minerals, and vitamins, was compared to obtain the suitable and optimum formulation. Moreover, to achieve a maximum EPS yield (the response value (Y)), medium composition, e.g., carbon content and nitrogen content sucrose (X1: 20–40 g/L), yeast extract (X2: 0.5–1.5 g/L), and (NH4)2SO4 (X3: 5–15 g/L), were optimized using the response surface methodology (RSM) with Box–Behnken design (BBD) [22]. Three factors, each at three levels, were used to create a second-order response surface with 17 experimental runs. Data analysis was conducted using Design Expert software (Version 12). An ANOVA was used to assess the statistical significance of the model, and the terms were accepted or rejected based on the p-value, with a 95% confidence level.
2.5. Bacterial Identification
Identification of the bacterial isolate was conducted based on nucleotide sequencing of the 16S rRNA gene and biochemical characteristics using the API-50 CHB/API-20NE kit (bioMérieux, Craponne, France). For 16S rRNA gene identification, the genomic DNA from Bacillus sp. P1 was extracted by the DNA Extraction Kit (Vivantis Technologies Sdn. Bhd, Selangor, Malaysia) followed by PCR amplification. In short, the 16S rRNA gene was amplified using universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′). The PCR cycle comprised an initial denaturation step at 95 °C for 5 min, followed by 30 cycles at 95 °C for 20 s, 53 °C for 30 s, and 72 °C for 1.5 min, with a final extension at 72 °C for 5 min. Finally, the obtained sequence alignment was conducted using MEGA-X and cross-referencing by BLAST against sequences in the GenBank database to identify its species. Phylogenetic trees were then constructed using the neighbor-joining method [26,29]. Furthermore, analysis of the biochemical characteristics, e.g., carbohydrate utilization and certain enzyme production, was conducted to confirm species identification by API-50 CHB/API-20NE test kit (bioMérieux, Craponne, France). The method for assay followed the protocol provided by the manufacturer. The data were interpreted using ApiwebTM (bioMérieux), available at https://apiweb.biomerieux.com/ (accessed on 1 June 2024).
2.6. In Vitro Biological Activity
2.6.1. Prebiotic Properties
The prebiotic properties of the produced EPS were determined by examining its effect on the growth of the probiotic strain Bacillus amyloliquefaciens NL1.2, which was isolated and its probiotic properties confirmed by Khongkool and co-workers [32]. For this purpose, the strain was cultivated at 35 °C under aerobic conditions in TSB for 24 h. To evaluate the prebiotic effect of EPS, sugar-free TSB (pH 7.0) was prepared, and 0.5% (w/v) EPS from Bacillus sp. P1 was used as a carbon source. The pre-culture of the probiotic strain B. amyloliquefaciens NL1.2 was adjusted to a concentration of 108 CFU/mL beforehand and then inoculated into TSB supplemented with the EPS, while sugar-free TSB served as the negative control. Changes in cell density were monitored in a multi-plate reader at 600 nm for 24 h [26,33].
2.6.2. EPS Antioxidant Studies
Various concentrations (0.625, 1.25, 2.5, 5.0, 10, and 20 mg/mL) of the purified EPS sample were prepared for DPPH radical scavenging activity. To elaborate, 2 mL of deionized water and 2.0 mL of DPPH solution (0.16 mM) were added to 1.0 mL of the different concentrated EPS samples as above. The mixture was then incubated at 37 °C in the dark for 30 min, followed by measurement of an absorbance at 517 nm [19,23]. The radical scavenging activity of EPS was performed using the equation given below.
where A0 is the DPPH solution without sample, and A1 refers to the solution containing samples in different concentrations (0.625, 1.25, 2.5, 5.0, 10, and 20 mg/mL).
DPPH radical scavenging (%) = [(A0 − A1)/A0] × 100
2.6.3. Antibacterial Activity Studies
The agar well diffusion assay was performed to assess the antibacterial activity of the bacterial EPS. This activity was tested against a panel of fish pathogens, including Aeromonas veronii, Aeromonas hydrophila, Streptococcus agalactiae, and Edwardsiella ictaluri. Each bacterial culture was adjusted to 108 CFU/mL and then swabbed on TSA plates. The wells with a diameter of 6 mm were corked and filled with 50 μL of various EPS concentrations ranging from 50 to 400 μg/mL. All tests were conducted in triplicate, and the plates were then incubated at 35 °C for 24–48 h. DMSO was used as a negative control. After incubation, the diameters of growth inhibition were measured in millimeters (mm). Data were presented as mean ± SD. Four levels of inhibition intensity were categorized based on the inhibition zone: weak (<5 mm), moderate (5–10 mm), strong (10–20 mm), and very strong (>20 mm) [34,35].
2.6.4. Cytotoxicity of EPS by Hemolytic Test
Hemolytic activity of the EPS from Bacillus sp. P1 was evaluated as described by Abinaya et al. [36]. An amount of 100 μL of 3.8% (w/v) sodium citrate was gently mixed with 900 μL of fish blood to prevent blood coagulation. The sample was then centrifuged at 3000× g for 10 min, and the resulting pellet containing red blood cells (RBCs) was washed three times with 10 mL of PBS (pH 7.4), followed by uniform suspension in PBS. A mixture of 2 mL of erythrocyte suspension with EPS at varying concentrations (0.25, 0.50, 1.0, 2.5, and 5.0 mg/mL) was added and gently inverted. All tests were conducted in triplicate, and, subsequently, the mixtures were incubated at 37 °C for 1 h, with PBS used as a control. After incubation, the samples were centrifuged again to pellet the RBC. The supernatant was then collected and measured an absorbance at OD540. The percentage of hemolysis was calculated as follows:
Hemolysis (%) = [(Asample − Ablank)/Acontrol] × 100
2.7. Experimental Animal
2.7.1. Acclimatization of Oreochromis niloticus Linn.
Oreochromis niloticus Linn. (Nile tilapia) with an average weight of 20 ± 1.5 g was obtained from Phatthalung Inland Fisheries Research and Development Center, Phatthalung, Thailand, and was prior acclimatized for 2 weeks before the feeding trial. According to the method of Ibrahem et al. (2010) [2] and Gobi et al. (2018) [18], the fish were sustained in a 100 L FRP tank and provided with a commercial feed (ad libitum) twice a day. Water temperature was maintained at 28 ± 2 °C, pH at 7.0, and DO at 6.63 ± 7.78 mg/L.
2.7.2. Experimental Fish Diet Formulation
The basal fish diet formulation used in this study as a control was modified from the formulation described by Figueiredo-Silva et al. [37] containing about 30% protein content, and the ingredients were as follows (g/kg): fish meal (30); soybean meal (420); ground poultry by-product (70); wheat gluten (20); DL-methionine (3.6); L-lysine (3.8); L-threonine (0.6); rice flour (202.6); rice bran (155); rice hull (2.4); soybean oil (23.5); vitamin premix (5); mineral premix (5); Ca2HPO4 (35); and vitamin C (0.6). All the ingredients were ground and sieved through a fine mesh sieve (60 mesh) prior to being mixed thoroughly by 20 L stand food mixer (EM20, Spring Green Evolution) while in the treatment formulation, the basal diet was mixed homogeneously with EPS from B. subtilis P1 at a concentration of 5.0 g/kg, as recommended by Ibrahem et al. [2]. The mixtures were then pelleted by using an animal feed pellet machine (model 150, SIF machinery) with 3 mm diameter of the pellet mold. The fish diet pellets were then dried at 55–60 °C for 16–18 h before being stored at 4 °C.
2.7.3. Experimental Design and Sample Collection
Fish were randomly selected and divided into two groups: a control group (C) and a treatment group (T), each with three replicate tanks. Each tank housed 25 fish and was equipped with aeration and filtration systems, with daily monitoring of water parameters (temperature, dissolved oxygen (DO), and pH). Both groups were fed twice daily (ad libitum) for a duration of 6 weeks. Growth performance was examined at the 2nd and 6th weeks. Additionally, blood and serum samples were collected to analyze the humoral immune parameters and antioxidant activities [33]. Briefly, approximately 1 mL of fish blood was drawn from a caudal puncture and was then transferred to a heparinized vacutainer tube. The tube was then centrifuged at 5000× g for 10 min, and the resulting serum was stored at −20 °C for further analysis [36].
2.8. Growth Performance Parameters
Growth performance parameters including weight gain (WG; g), average daily weight gain (ADG; g/day), specific growth rate (SGR; %/day), and feed conversion ratio (FCR) were determined at 2, 4, and 6 weeks and calculated as follows:
WG (g) = final weight (FW) − initial weight (IW0)
ADG (g/day) = [final weight (FW) − initial weight (IW0)]/days
SGR (%/day) = 100 × [Ln (FW) − Ln (IW0)]/days
FCR (g) = [feed intake (g)/weight gain (g)
All the calculations were an average per fish tank and an average per fish for WG and ADG.
2.9. Measurement of Immune Response Parameters
2.9.1. Lysozyme Activity
Serum lysozyme activity was measured using a colorimetric method [38]. In a cuvette, 3 mL of Micrococcus luteus suspension in phosphate buffer (with an absorbance at 450 nm ranging from 0.5 to 0.7) was prepared, into which 50 μL of diluted serum sample was added. The suspension was thoroughly mixed for 15 s, and readings were taken using a spectrophotometer at 450 nm. The bacterial lysis readings were recorded immediately at 2 min intervals for 20 min. A unit of lysozyme activity was defined as the amount of sample resulting in a reduction of an absorbance of 0.001 per min, and lysozyme activity is expressed as units of enzyme per min.
2.9.2. Respiratory Burst Activity
A nitroblue tetrazolium (NBT) assay was performed as mentioned by [39,40,41]. Briefly, 100 μL of blood was added into the wells of ‘U’-bottom micro-titer plates and incubated at 37 °C for 1 h to facilitate cell adhesion. After incubation, the supernatant was discarded, and the loaded wells were washed three times with PBS. Following washing, 100 μL of 0.2% (w/v) NBT was added, and the plate was incubated for another 1 h. Subsequently, the cells were treated with 100% methanol for 2–3 min and washed with 70% methanol three times. The plates were then air-dried. After that, a solution containing 60 μL of 2 N KOH and 70 μL of DMSO was added to each well to produce the formazan blue precipitate. The OD of the turquoise-blue solution was measured at 630 nm. This notable color change can be easily quantified with a standard plate reader, and since the absorbance of the solution correlates directly with the amount of superoxide produced, absorbance can be used to estimate respiratory burst activity [40].
2.9.3. Total Immunoglobulin
Total immunoglobulin (Ig) was estimated by the modifications described by Sewaka et al. [42]. Using this method, immunoglobulin was precipitated out of the plasma with polyethylene glycol, and the remaining protein of the plasma was determined. The total immunoglobulin was calculated by subtracting the total plasma protein concentration from the remaining protein in the plasma concentration.
2.10. Measurement of Serum Biochemical Parameters
The obtained serum samples were analyzed by using an automate chemistry analyzer (AU400, Olympus, Tokyo, Japan). The following parameters were measured: glucose, cholesterol, total protein, albumin, blood urea nitrogen (BUN), total bilirubin (T-bilirubin), direct bilirubin (D-bilirubin), serum alanine transaminase (ALT), and serum aspartate aminotransferase (AST).
2.11. Challenge Test
Six-week-old fish from each group (totaling ten individuals) were randomly transferred to a FRP tank for 6 h. Subsequently, they were exposed to a challenge with a reference fish pathogenic strain of A. veronii, which had been previously assessed for its pathogenicity with a lethal dose (LD50) of 108 CFU/mL. A bacterial suspension was prepared by culturing the strain in TSA for 24 h, followed by suspension in sterile saline solution (0.85%) and adjustment to a concentration of 108 CFU/mL that was equivalent to 0.5 McFarland standard bacterial suspension turbidity using the Densichek instrument. The fish were then artificially infected via intra-peritoneal injection with 0.25 mL of the microbial suspension of A. veronii (modified method according to Ibrahem et al. [2]). The relative level of protection (RLP) among the challenged fish was determined after two weeks using the following equation:
RLP % = 1 − (% of mortality in treated groups/% of mortality in control group) × 100
Generally, two weeks is a reasonable timeframe to observe initial responses, such as changes in survival rates, immune responses, and disease progression [2,13].
2.12. Statistical Analysis
All experiments were performed in triplicate and were reported as means ± standard error (SE). Differences between the treatments were determined through an independent sample t-test for two-group comparison (Control vs. Treatment) and a one-way analysis of variance (ANOVA) followed by Tukey’s test for multiple conditions, in which p-values less than 0.05 were considered statistically significant by using SPSS software, Version 29 (SPSS Inc., Armonk, NY, USA).
3. Results and Discussion
3.1. Screening and Isolation of EPS-Producing Bacteria
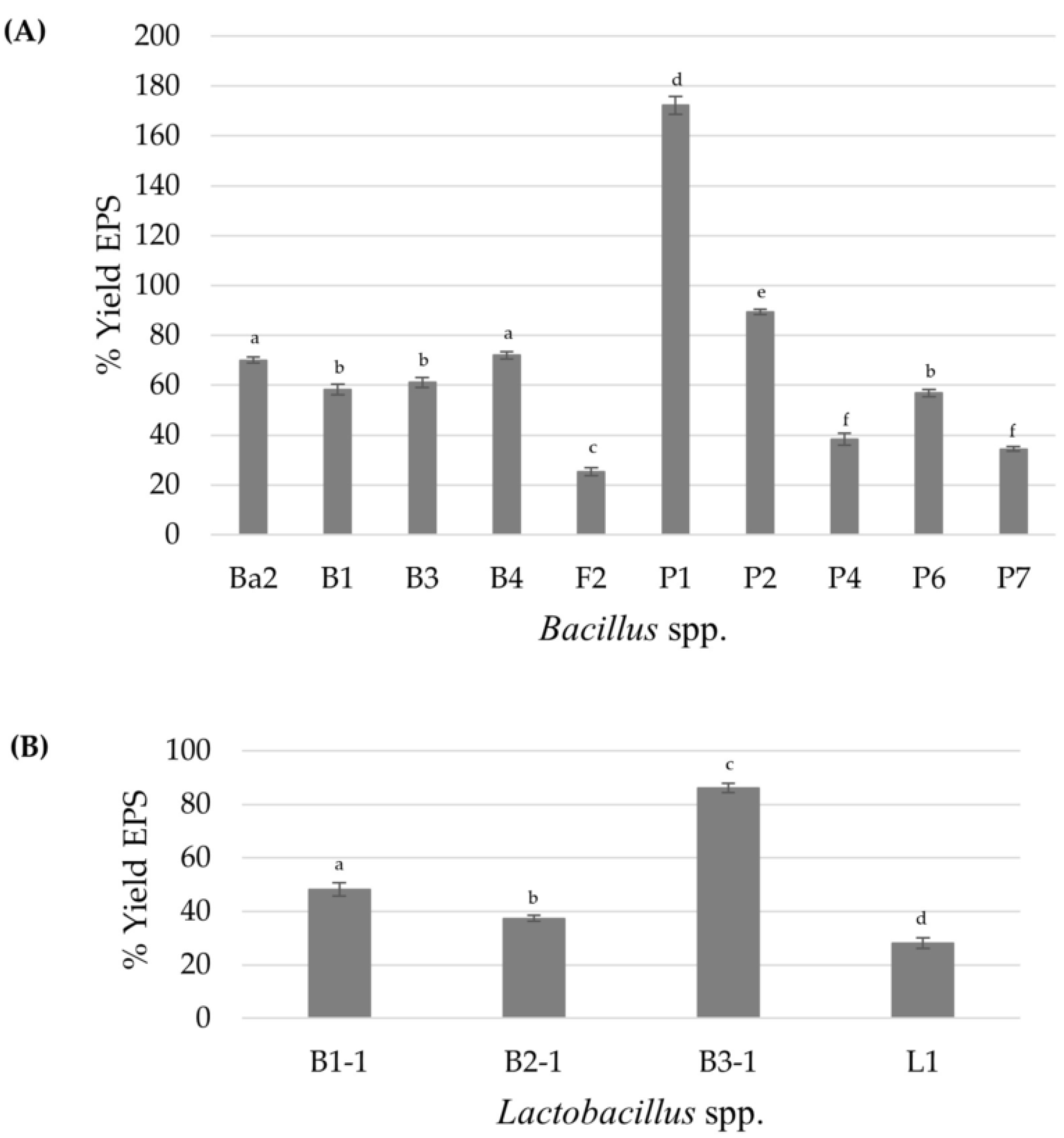
A total of 66 bacterial isolates were obtained from the intestines of local freshwater scaled fish. Out of these, only 30 isolates exhibited distinct mucoid colonies on MRS or TSA supplemented with 2.0% (w/v) sucrose and were consequently classified as Lactobacillus spp. for 7 isolates, while the remaining 23 isolates were identified as Bacillus spp. based on morphological and biochemical tests, such as the catalase test. Then, the EPS formation of all isolates was compared based on the intensity of crystal violet staining [27,28,43]. The results demonstrated that only 4 from 7 isolates of Lactobacillus spp. and 10 from 23 isolates of Bacillus spp. produced a relatively high EPS amount, with an OD595 above 2.0, in the range from 2.21 ± 0.08 to 2.85 ± 0.34. These results agreed with reports of Bacillus subtilis J1 and Bifidobacterium animalis J28 as strong EPS producers with a production ability from 1.71 ± 0.21 to 1.80 ± 0.68 [44], while Acinetobacter and Bacillus isolated by Lim et al. (2023) [27] exhibited an absorbance range of 0.02–4.16 and 0.03–12.77, respectively, when grown in BHI at 25 °C for 24 h. These can be explained by the fact that the higher absorbance seems to produce higher amounts of EPS owing to crystal violet, a cationic dye that can bind to negatively charged bacteria and polysaccharides of the EPS. The amount of dye solubilized by the solvent, such as ethanol, is directly proportional to EPS production [28,43]. Figure 1 shows the highest percentage EPS yield of 86.19 produced from Lactobacillus sp. B3-1, isolated from the intestine of O. marmorata, while in Bacillus spp., the isolate of Bacillus sp. P1, isolated from the intestine of O. niloticus, was able to synthesize a maximum EPS of 1.72 gEPS/gDCW among the other isolates when we grew the cells in EPS medium supplemented with 20 g/L sucrose. Therefore, the two isolates were further selected for EPS characterization due to their high EPS production. However, the amount of EPS varies considerably depending on the variation of species and strains; this variation could also depend on the EPS isolation method employed, growth conditions (pH, temperature, and incubation time), and medium composition (carbon, nitrogen sources, and other nutrients), which can directly affect the polymer yield and the sugar composition [10,11,14,31,45].
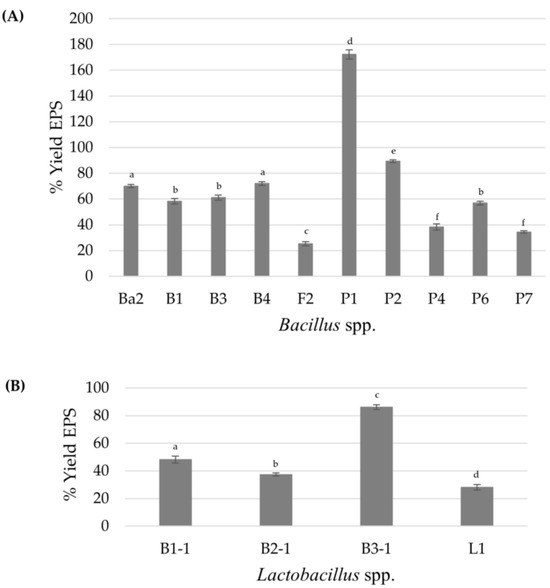
Figure 1.
The percentage yield of EPS when the bacterial cells are grown in liquid EPS-production medium for 48 h: Bacillus spp. (A) and Lactobacillus spp. (B). Different letters (a–f) in a bar mean they are statistically significantly different (p < 0.05). In this case, a one-way ANOVA followed by Tukey HSD test (p < 0.05) was analyzed; the bar with the same letter indicates no significant difference. Error bars represent mean ± SE.
3.2. Characterization of the Purified EPS
3.2.1. Molecular Mass
After the average molecular mass of the purified EPS was detected by GPC analysis, the results showed that the two EPS-producing strains—Lactobacillus sp. B3-1 and Bacillus sp. P1—had a different EPS molecular mass (Table 1). The molar mass of the EPS produced by each microorganism varied according to the strain, polymer type, and cultivation conditions [9,10,31,44,45]. For example, the molecular mass of the EPS produced by Lb. plantarum C7 was about 33 kDa, which was similar to the EPS produced from Lb. plantarum EP56 (about 44 kDa) [46] but lower than that of Lb. plantarum C88, which was up to 1150 kDa of the EPS of Lb. plantarum C88 [47]. The polydispersity index (Mw/Mn) indicates the extent of heterogeneity within polymer chain lengths. The higher PDI observed in this study signifies a diverse range of polysaccharide chain sizes, suggesting that the EPS may consist of multiple polymers exhibiting variations in monomer composition and molecular mass ratios [9,11,31,48]. It is reported that the average molar mass for hetero-polysaccharides ranges from 4 × 104 to 6 × 106 Da. In this case, the produced EPS from Lactobacillus sp. B3-1 and Bacillus sp. P1 presented an average molar mass in comparison with most of the reports [14,45,49,50,51].

Table 1.
Molecular weight (Mw), molecular number (Mn), and polydispersity index (PDI) of the purified EPS.
3.2.2. Functional Groups of EPS and Its Monosaccharide Compositions
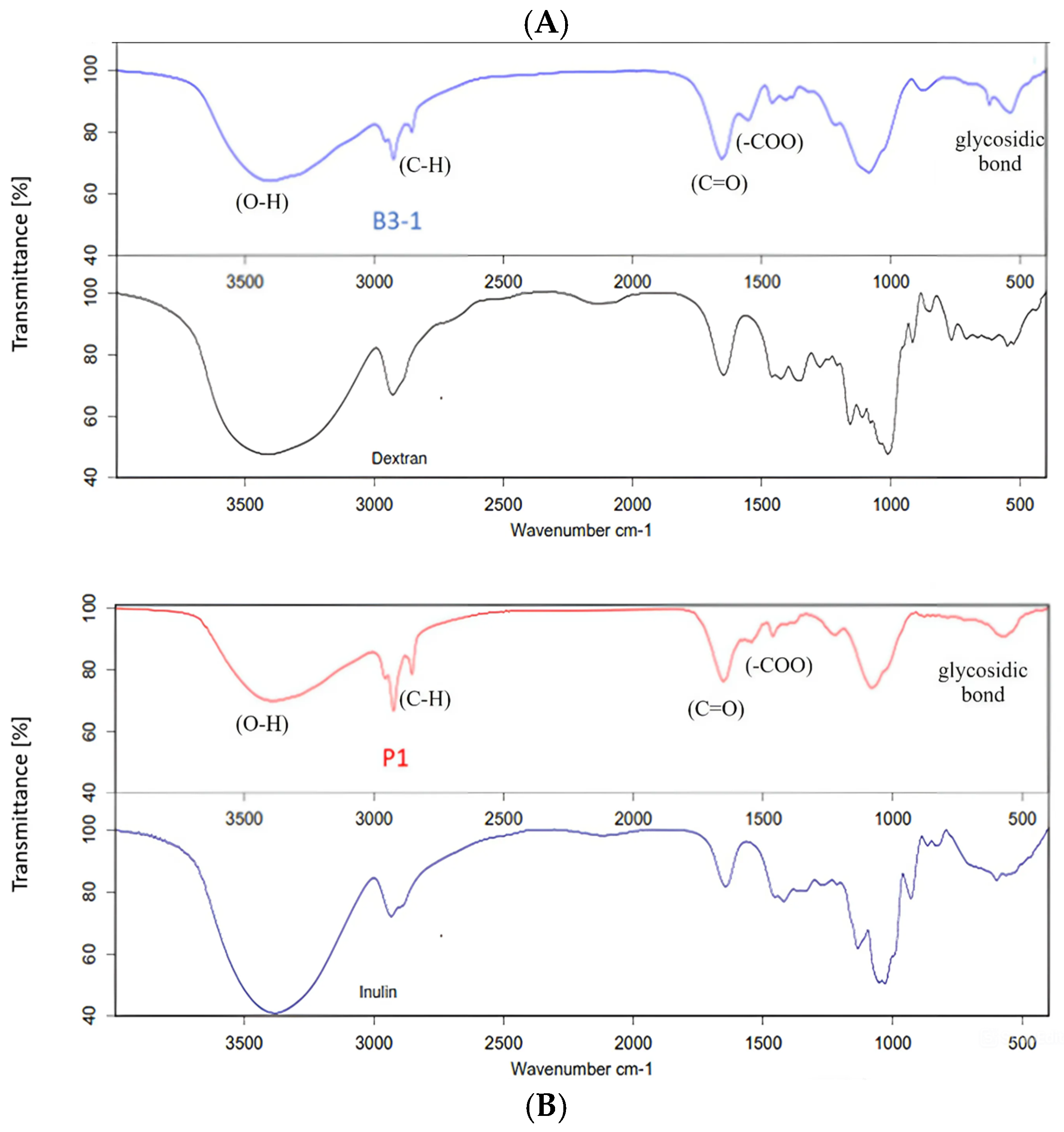
FTIR spectroscopy is a powerful analytical method to investigate the nature of the functional groups of the EPS in terms of monomeric units and their linkages [29,31,44]. Figure 2 presents the FTIR spectra of EPS fractions from Lactobacillus sp. B3-1 and Bacillus sp. P1. The EPS spectrum was identified within the range from 4000 to 400 cm−1 and exhibited numerous peaks. For instance, a broad absorption peak observed at approximately 3420–3434 cm−1 indicated the presence of intense hydroxyl groups (O-H) stretching frequency, confirming the polysaccharide nature. Signals at around 2850 and 2928 cm−1 were attributed to C-H stretching. Additionally, the stretch vibration of the carboxyl group (C=O) was observed as an absorption peak at approximately 1600 cm−1, while the symmetric stretching of -COO was noted at about 1400 cm−1. Furthermore, a weak adoption band around 500 cm−1 suggested the presence of a glycosidic linkage peak for polysaccharides [13,17,29,31]. The EPS produced from Lactobacillus sp. B3-1 exhibited functional groups highly similar to dextran, with 92.25% correlation (Figure 2A), while Bacillus sp. P1 generated EPS that showed a percentage of correlation of about 90.20 to inulin (Figure 2B). In this case, the percentage correlation could be converted into an identical match between the sample and standard spectra.
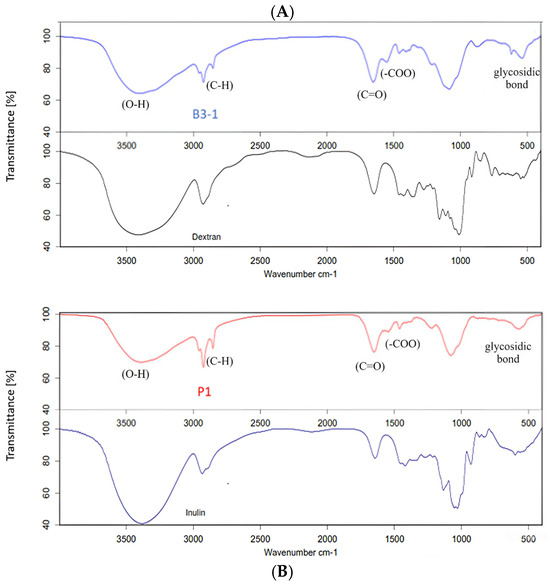
Figure 2.
FTIR spectra of EPS fractions from Lactobacillus sp. B3-1 (A) and Bacillus sp. P1 (B) compared with the standard dextran and standard inulin.
However, both EPS from Lactobacillus sp. B3-1 and Bacillus sp. P1 demonstrated only about 66% correlation to levan. Dextran is a glucose-based polysaccharide synthesized by LAB such as Lb. sakei MN1, composed of a linear chain of D-glucoses linked by α-(1→6) bonds, with possible branches of D-glucose [14,26,52,53]. On the other hand, inulin belongs to fructose-based polymers and is composed mainly of β-D-fructosyl subgroups linked by (2→1) glycosidic bonds [9,16,51]. Bacillus sp. and strains produce a variety of EPS such as levan, β-1,3-glucan, and also heteropolymers [7,10,54]. These findings are unlike the previous studies that found that most Bacillus spp. tended to produce levan-type EPS, e.g., B. subtilis, B. polymyxa, B. licheniformis, and B. megaterium [7,29,30,50]. However, in this study, the sugar and its derivative compositions in the EPS produced by Bacillus sp. P1 were further confirmed, and we found that it was composed of fructose (1.51% w/v) and glucose (0.35% w/v) as major monosaccharides in a ratio of 4:1. In addition, fructooligosaccharides (FOSs) such as kestose (GF2), nestose (GF3), and fructofuranosyl-D-nystose (GF4) were also detected. Thus, it can be indicated that the EPS produced from Bacillus sp. P1 is classified as an inulin–fructan type. The characteristics of EPS in terms of molecular weight and branches depend on the producing strain; thus, there is a great variety in its properties [50,51]. However, it has been reported that certain lactic acid bacteria can produce homo-polysaccharides (HoPSs), which include α-D-glucans such as dextran, alternan, and reuteran, as well as β-D-fructans such as inulin and levan. Additionally, some hetero-polysaccharides (HePSs) can also be synthesized [26,52]. Bacillus spp. has been revealed to produce complex homo-and/or heteropolysaccharides, a variety of EPS such as levan and β(1→3) glucan, and hetero-polymers composed mainly of neutral sugar, uronic acid, uncommon sugar, or sugar−protein conjugates [54]. It is noteworthy that these variations in functional groups lead to diverse bioactive functions, encompassing immunomodulatory properties, antioxidant activity, antibacterial activity, and various other biological properties [10,11,14,15,50]. In this study, sucrose is utilized as a specific substrate by Bacillus sp. P1, which is cleaved into glucose and fructose and polymerized into glucan and fructan by glucan sucrase and fructan sucrase. These polymerized HoPSs are directly released to the extracellular environment [16].
3.3. Optimization Conditions for EPS Production
Due to the fact that the highest EPS yield (up to 172%) was produced by Bacillus sp. P1 when cultivated in EPS production medium, as described by Liu et al. [29], an optimization of EPS biosynthesis was then performed by using this isolate for larger-scale production. Among five formulations of EPS-producing media described by previous studies [21,22,23,24,29], the medium consisting of sucrose 20 g, yeast extract 1 g, K2HPO4 8 g, KH2PO4 2 g, MgSO4 · 7H2O 0.5 g, and (NH4)2SO4 10 g per liter, as mentioned by Berekaa [22], provided a maximum EPS concentration of about 4.13 ± 0.12 g/L, which accounted for about 474% EPS yield. The carbon and nitrogen content in the medium composition were then optimized by using RSM with a BBD model. The BBD experiment (Table S1) was specifically designed to determine the optimal EPS medium composition for maximizing EPS production from the isolate Bacillus sp. P1. The following quadratic polynomial equation was used to evaluate the EPS yield by multiple regression analysis: % yield EPS = −2902.54875 + 93.33953X1 + 1485.44450X2 + 246.05420X3 − 9.32200X1X2 − 0.621550X1X3 − 19.24500X2X3 − 1.18917X12 − 441.76600X22 − 11.24076X32. The regression model was analyzed by variance analysis (ANOVA), and statistical tests were performed with the F-test shown in Table 2. The F-value of 20.23 (p < 0.0003) was statistically significant, indicating that the model had good simulation, and R2 and the correction coefficient R2Adj were 0.9630 and 0.9154, respectively, indicating that the established regression equation had a good degree of fit and could successfully predict the response value. The model data showed the primary term C and the interaction terms A2, B2, and C2 had a very significant impact on the EPS yield (p < 0.01), and the primary terms A and B had a significant impact on the EPS yield (p < 0.05). In summary, the order of the significant differences in the influence of the three factors was the concentration of (NH4)2SO4 > yeast extract > sucrose.

Table 2.
ANOVA for the fitted quadratic polynomial regression model using BBD.
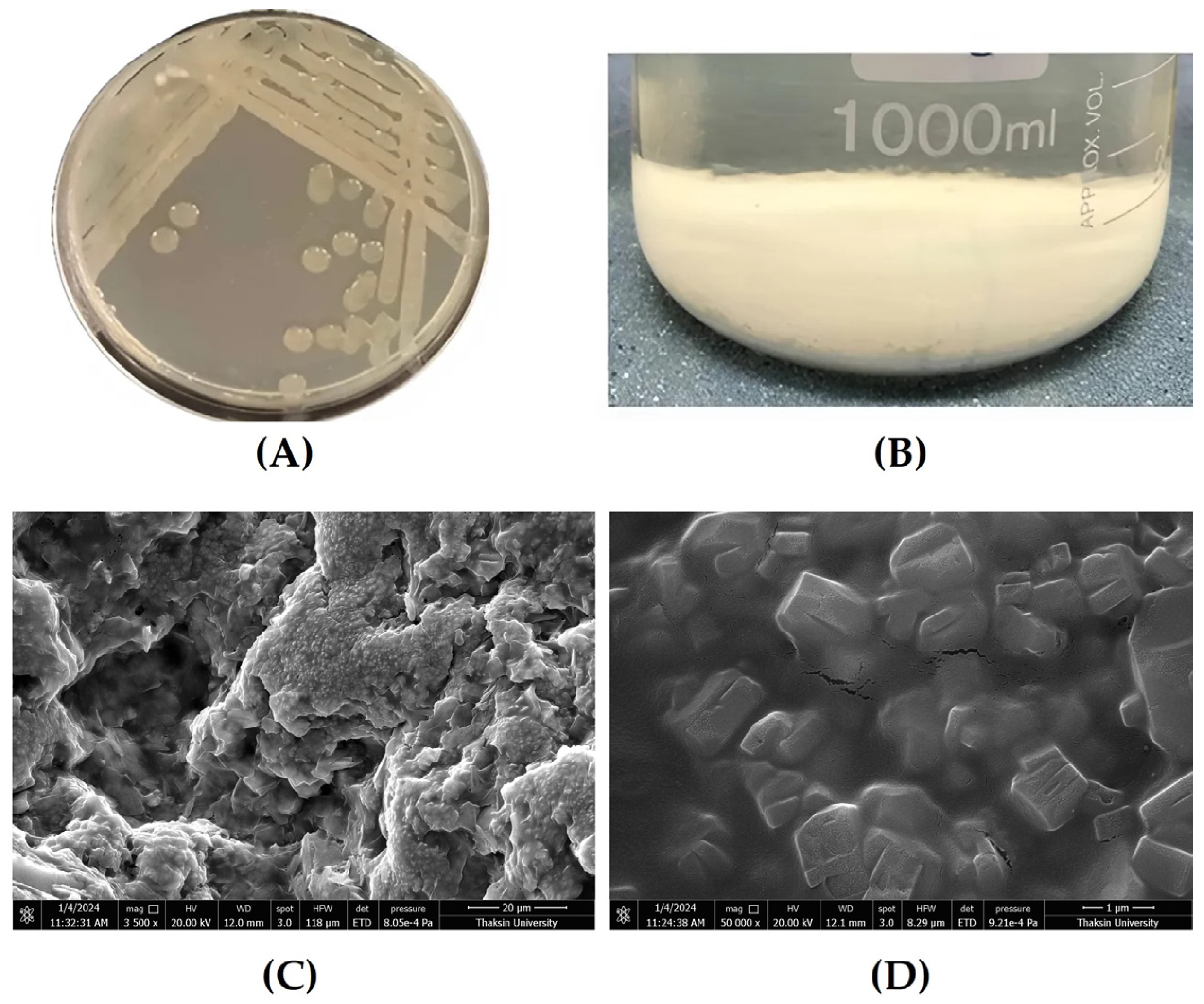
To visualize the effect of an interaction among those three factors on the EPS yield, the contour plots between each factor and EPS yield were drawn (Figure S1). Finally, the optimal EPS medium composition, predicted to obtain the highest EPS yield (about 570%), was as follows: sucrose, yeast extract, and (NH4)2SO4 were 31.75, 1.13, and 9.14 g/L. To validate the effectiveness of the RSM, the conditions were set to 30 g/L sucrose, 1 g/L yeast extract, and 10 g/L (NH4)2SO4. Under these conditions, Bacillus sp. P1 produced an EPS yield of 552%, which was close to the predicted range of 522.22–571.43%. The five parallel experiments at the response surface center point showed that the experimental results closely matched the predicted values, confirming the accuracy of the mathematical model for simulating EPS production by Bacillus sp. P1. To sum up, after optimization, the EPS medium composition, i.e., sucrose concentration, increased from 20 to 30 g/L, and following verification of the RSM model, it was found that the EPS yield had increased from 474% to 552%, accounting for a 16.5% increase. These obtained results were similar to those for the EPS from B. licheniformis 14580, which had greater EPS yields with increased concentrations of sucrose [55]. According to the literature, the quantity of EPS produced by different bacterial strains varies significantly, with LAB typically producing the lowest yields, ranging from 0.01 to 2.0 g/L. Lactobacilli, in particular, are among the least prolific EPS producers, typically yielding less than 1.0 g/L, and this production may further decrease under unfavorable culture conditions [25,30,50,53]. The low yields in LAB are due to the acidification of the culture medium to below pH 5.0, which activates glycosyl-hydrolase, leading to enzymatic digestion or hydrolysis of the EPS, reducing final yields [25,38,42,43]. In contrast, most Bacillus species produce higher EPS concentrations. For instance, B. aerophilus rk1 and B. licheniformis QS5 synthesized about 3.73 g/L and 3.78 g/L of EPS, respectively, [22,23] while B. subtilis produced 4.86 g/L using cane molasses [10], and B. licheniformis 14580 yielded 3.5 g/L in a mineral medium with yeast extract [42]. In this study, Bacillus sp. P1 produced 4.96 g/L of EPS when cultivated in 30 g/L sucrose. In this study, after cultivation of Bacillus sp. P1 under the optimal medium, it could synthesize about 4.96 g/L of EPS when cultivated in 30 g/L sucrose. In general, bacterial EPSs are formed in an amount from 0.29 to 65.27–100 g/L depending on their type, the type of microorganism, the cultivation conditions, and bioreactor type [9,24,50,52]. Moreover, the source of carbon and C/N ratio have a significant effect on EPS biosynthesis. The synthesis of bacterial EPS typically demands a high concentration of carbon sources in the culture medium and restricted nitrogen availability. Glucose or sucrose are commonly employed as primary carbon sources due to their ability to provide a substantial carbon yield per molecule. This ensures a considerable amount of carbon is accessible for bacterial growth and EPS production. Additionally, these sugars may serve as inducers or amplify the expression of genes responsible for EPS synthesis in certain bacterial strains [5,28,33,42]. Sucrose in particular has been known to be the best substrate to stimulate EPS production [13,42]. Notably, the use of EPS optimum medium for the growth of Bacillus sp. P1 proposed to produce heteropolymers with a higher MW of about 105 Da (as shown in Table 1), similar to the EPS from B. licheniformis 14580 that had a high MW in the range of 30–100 kDa and was confirmed to produce heteropolymers [55]. The heterogeneity in the EPS produced by Bacillus spp. is well documented, but the constitutive monomeric units are highly variable in nature and number depending on the strain and culture conditions [31,45,55]. The morphology of mucoid colonies of Bacillus sp. P1 and its purified EPS, which was examined under SEM, are presented in Figure 3.
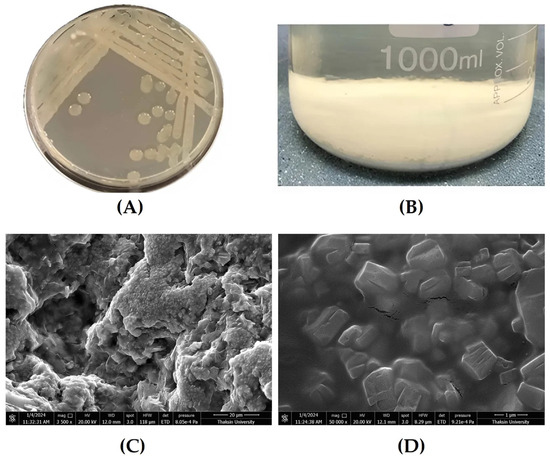
Figure 3.
Morphology of EPS-producing bacteria Bacillus sp. P1 and its purified EPS (A) mucoid colony of Bacillus sp. P1 on TSA, (B) EPS precipitation in ice-cold ethanol, (C) SEM morphological analysis of EPS at 3500X magnification (scale bar = 20 μm), and (D) A matrix of EPS at 50,000X magnification (scale bar = 1 μm).
3.4. Bacterial Identification
Species of the EPS-producer of Bacillus sp. P1 were then identified by 16S rRNA gene analysis. The nucleotide sequence revealed that this strain showed 99.27% homology with Bacillus subtilis NCIB 3610T (ATCC 6051) by phylogenetic analysis; thus, the isolate was identified as Bacillus subtillis P1 (GenBank accession number PP124894) (Figure S2). Additionally, an analysis of the carbohydrate utilization patterns of B. subtilis P1 using an API-50 CHB/API-20NE kit (bioMérieux, Craponne, France) further confirmed the bacterial species and is presented in Table S2. The biochemical characteristics of B. subtilis P1 showed about 98% homology with Bacillus subtilis. Finally, the pure culture of Bacillus subtilis P1 was then deposited in the culture collection of the Thailand Bioresource Research Center (TBRC) under the accession number TBRC 18356. Furthermore, Bacillus subtilis P1 was primarily screened for biosafety by hemolysis assay, and the results demonstrated that B. subtilis P1 displayed α-hemolytic activity on sheep blood agar. However, most B. subtilis species are non-pathogenic and are not associated with infections. γ-hemolytic and α-hemolytic strains are remarkable as safe, which means the Bacillus species did not show any risk to the host [56]. In addition, Bacillus subtilis is a probiotic that is very well researched and is one of the most widely used probiotics in aquaculture [57].
3.5. In Vitro Biological Activity
3.5.1. Prebiotic Properties of the Produced EPS
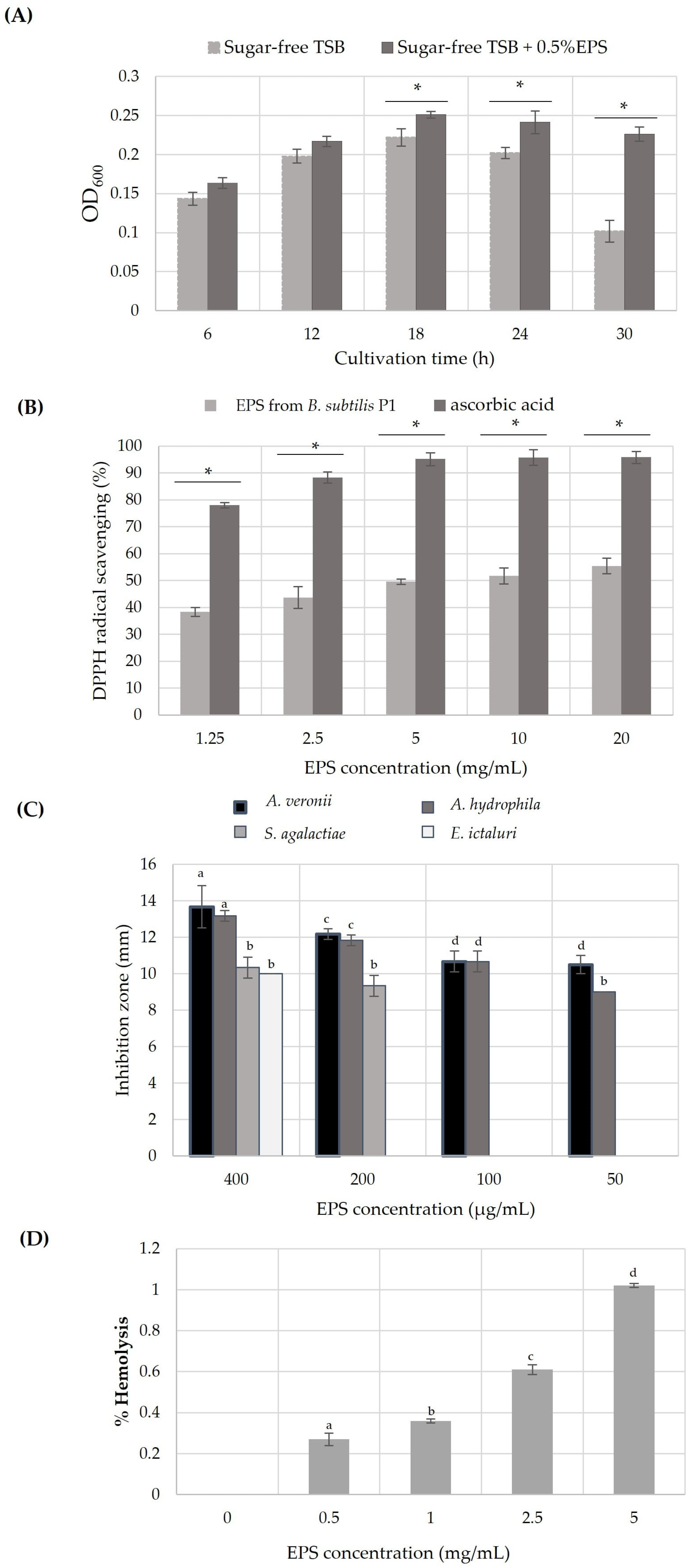
After 6 h of incubation, the growth of probiotic Bacillus amyloliquefaciens NL1.2 was significantly achieved (p < 0.05) when EPS produced from B. subtilis P1 was used as a carbon source rather than in sugar-free TSB. It can be indicated that B. amyloliquefaciens NL1.2 was able to utilize EPS as an energy source. However, the cell density gradually increased and reached a maximum at 18 h of cultivation. Then, the cells were maintained at a certain level and were likely to decrease along the stationary phase, while in the cells growing in sugar-free TSB, there was a distinct drop after 24 h (Figure 4A). These results may explain that probiotic B. amyloliquefaciens NL1.2 enters sporulation due to the depletion of the carbon source, which is the main stimulus for sporulation by Bacillus spp. [58]. The findings indicate a positive prebiotic impact of the EPS derived from B. subtilis P1, as evidenced by its ability to enhance the growth of the evaluated probiotic. This suggests that bacterial EPS comprises complex structures capable of stimulating the growth and metabolic functions of beneficial bacteria. Furthermore, certain bacteria can ferment EPS and generate valuable metabolites, particularly short-chain fatty acids (SCFAs), in the presence of probiotics. SCFAs are known to play crucial roles in supporting the growth of probiotics by fostering a favorable intestinal environment and reducing intestinal pH [8,14,26,33,50,51]. According to a report by Angelin and Kavitha (2020), Bacillus subtilis produced levan containing homopolysaccharides of fructose with β-2,6 glycosidic bonds showing prebiotic properties [8,16]. Nevertheless, the prebiotic impact of inulin hinges largely on its degree of polymerization, which dictates its degradation site, hydrolysis rate, and fermentation by-products. Additionally, inulin aids in suppressing endotoxin secretion by elevating the proportion of probiotics, thus exerting anti-inflammatory effects. Intriguingly, long-chain inulin predominantly fosters the proliferation of gut microbiota together with diverse enzymes capable of breaking down complex polysaccharides into oligosaccharides and monosaccharides [8,59].
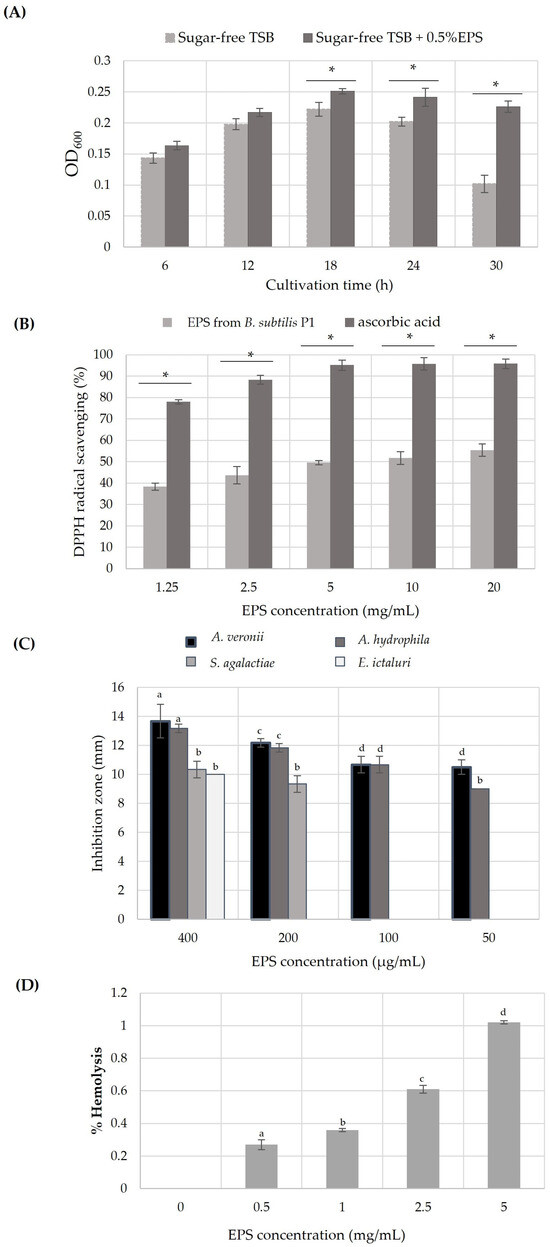
Figure 4.
In vitro biological activities of EPS from B. subtilis P1: (A) the growth of probiotic B. amyloliquefaciens with 0.5% (w/v) EPS and without EPS supplementation; (B) DPPH radical scavenging activity of different concentrations of the EPS. Error bars represent mean ± SE, and the symbol * indicates a statistically significant difference between the two groups (p < 0.05) analyzed by independent t-test. (C) The antibacterial activity profile of EPS against fish pathogens (expressed as diameter of inhibition zone in mm), and (D) cytotoxicity test (% hemolysis) of the produced EPS from B. subtilis P1. In this case, a one-way ANOVA followed by Tukey HSD test (p < 0.05) was performed; the bar with same letter indicates no significant difference.
3.5.2. Anti-Oxidant Activity
The DPPH free radical, being stable, can be rendered inert upon encountering a proton radical scavenger, which transfers either an electron or a hydrogen atom to the DPPH molecule. As shown in Figure 4B, the percentage of scavenging activity of the EPS from B. subtilis P1 on a DPPH radical increased with an EPS concentration increase, and it had maintained about 50% from 5–20 mg/mL, while the standard ascorbic acid showed almost 96% DPPH scavenging. Similarly, EPS produced by Bacillus tequilensis PS21 and Bacillus subtilis AF17 displayed the strongest DPPH radical scavenging activity of 43.93–58.54% with an increase in EPS concentration [30,60]. The EPS of B. amyloquefaciens RT7 showed the highest scavenging activity of 67% at 7.5 mg/mL of EPS, while the EPS produced from B. amyloliquefaciens GSBa-1 had a DPPH scavenging activity of 76.7% at 5 mg/mL [19,48]. In addition, the EPS from Bacillus coagulans RK-02 with Mw of 30 kDa and Bacillus anthracis showed an antioxidant activity when used at concentrations of 0.05–0.5 mg/mL and 0.2–5 mg/mL, respectively [10]. Correspondingly, the EPS from Bacillus licheniformis Dahb1 reached about 83% scavenging capability when tested at 100 μg/mL of EPS [36]. In comparison, the relatively low molecular mass of EPS (<30 kDa) exhibited potential DPPH radical scavenging activity [29]. Reports suggest that a lower molecular weight of the polysaccharide could contribute to its heightened antioxidant activities. This is attributed to increased solubility and reduced steric hindrance, enabling more efficient interaction with reactive oxygen species (ROS), facilitating faster reactions [8,48,59]. However, in this study, the produced EPS from B. subtilis P1 had a relative high MW (>105 Da), which may reduce the antioxidant activity. Furthermore, the antioxidant capacity of microbial EPS is related to its diverse functional groups, including hydroxyl, carboxyl, sulfate, or acetyl groups. These functional groups either donate electron pairs, release a proton, or aid in metal binding processes. This remarkable scavenging ability leads to the conversion of free radicals into stable substances. Additionally, the presence of negatively charged functional groups may facilitate EPS hydrolysis, thereby increasing the exposure of hemiacetal hydroxyl groups and enhancing antioxidant activity [10,15].
3.5.3. Anti-Bacterial Activity
Microbial EPS demonstrates significant inhibitory effects on a range of pathogenic bacteria by competing for adherence and colonization. Bacteria belonging to LAB, such as Lactobacillus or Bacillus genera, produce EPS that exhibits either bacteriostatic or bactericidal activity. This microbial EPS exhibits either broad-spectrum or specific activity [16]. However, there are few studies on the use of microbial EPS against fish pathogenic bacteria. In this study, freshwater fish pathogenic bacteria, including A. veronii, A. hydrophila, S. agalactiae, and E. ictalurid, were tested for growth inhibition by EPS from B. subtilis P1 using an agar well diffusion method. The results demonstrated that the produced EPS seems to inhibit Aeromonas spp. with a strong intensity (>13 mm), while in S. agalactiae and E. ictaluri, it showed a moderate inhibition, with a diameter of only 10 mm at 400 μg/mL of EPS (Figure 4C). Conversely, EPS from B. licheniformis Dahb1, at a little amount of EPS (75 μg/mL), showed strong antibacterial activity against numerous pathogenic bacteria [36], while B. tequilensis PS22 showed a maximum inhibition on S. agalactiae (>12 mm) among the other tested Bacillus spp. In addition, B. amyloliquefaciens KW8 revealed a moderate inhibition (<10 mm) on S. aureus that mostly causes diseases in Nile tilapia [60]. On the other hand, Mahdhi et al. (2020) [17] reported that EPS from probiotic Lb. plantarum and Bacillus strain HM117830 showed an inhibition diameter between 13 and 15 mm against S. aureus, V. alginolyticus, and P. aeruginosa at concentration of 2 mg/mL. The antibacterial mechanisms of microbial EPSs may involve disrupting the structure of bacterial cell membranes, cell walls, or respiratory chains, thereby affecting the cell division machinery of fish pathogens [4,10,11]. Additionally, microbial EPSs contain a variety of functional groups, such as hydroxyl, phosphate, and carbonyl groups, which play a role in the interaction of the EPS with the cell membranes or cell walls of bacterial pathogens [4,10,14,34].
3.5.4. Cytotoxicity Test
EPS cytotoxicity was assessed using a hemolytic activity test. Although increasing EPS concentration slightly raised hemolysis, the overall hemolytic activity remained very low (≤1%) (Figure 4D). Similarly, Abinaya et al. (2018) detected hemolysis activity at low levels of cytotoxicity, even at 5 mg/mL of EPS. In contrast, the cytotoxicity activities of EPS from Physarum polycephalum microplasmodia were investigated against various cell lines and revealed different effects. These discrepancies were attributed to distinct chemical properties, including carbohydrate, protein, and total sulfate group contents, as well as monosaccharide composition and molecular weights. Numerous evidence suggests that some polysaccharides isolated from cultivable sources have low cytotoxicity due to their remarkable functional properties [10,11,12,36].
3.6. In Vivo Study of the Effect of EPS Supplementation in Fish Diet on Growth Performance and Immune Response
The body weight gain (WG), specific growth rates (SGR), average daily gain (ADG), and survival rate were significantly higher (p < 0.05) in fishes supplemented with 0.5% EPS for 4 weeks than those on a control basal diet. However, FCR showed no significant difference between the two groups (Table 3). Interestingly, the SGR in this study was slightly higher than previous works that fed the Oreochromis tilapia and, with a similar concentration of inulin-based EPS, had an average SGR of about 2.10–2.39% [2,36,61]. In addition, there was for all fish tanks during the whole experiment.

Table 3.
Growth performances and feed utilization of Nile tilapia for 6 weeks feeding.
Additionally, immunological parameters such as NBT and lysozyme increase significantly (p < 0.05) in the EPS-supplemented group compared to the control group (Table 4). Conversely, total immunoglobulins (Ig) that are referred to as antibodies are generated by B cells and released into both the bloodstream and mucosal surfaces. Their primary function is to neutralize pathogens such as bacteria, viruses, and other foreign substances [42]. Results obtained from the T-group showed no statistical difference from the control group.

Table 4.
Immunological parameters and relative level of protection of Nile tilapia for 6 weeks feeding with EPS.
The short feeding period of 4–6 weeks was not able to efficiently trigger a strong immune response, as seen in previous studies, which supplemented Lactobacillus rhamnosus in rainbow trout (Oncorhynchus mykiss) and red tilapia (Oreochromis spp.) [42]. However, Ibrahem et al. [2] reported that feeding Nile tilapia with 0.5% commercial inulin (Sigma-Aldrich) for 2 months significantly improved lysozyme activity from 9.29 ± 0.16 to 9.34 ± 0.13 U/min, with NBT reaching a peak value of 0.297 ± 0.04. Furthermore, Cerezuela et al. (2013) utilized inulin-type EPS as a prebiotic in Gilthead seabream (Sparus aurata) and observed an augmentation in intraepithelial leukocytes, as well as an upregulation in IL-8 and IL-6 and the expression of Casp-1 and COX-2 genes, implicated in various cellular processes associated with inflammation, immune responses, and cell death. Additionally, they noted a significant enhancement in complement activity and IgM levels in fish fed with EPS. Correspondingly, previous studies by Reyes-Becerril et al. (2014) reported that lysozyme activity, IgM level, and myeloperoxidase activity elevated significantly in leopard grouper (Mycteroperca rosacea) fed with a diet supplemented with 5 g/kg inulin for eight weeks, while hybrid surubim (Pseudoplatystoma sp.) fed with a similar dose of inulin showed an increase in total immunoglobulin [4,62]. Additionally, Ghafarifarsan et al. (2020) evaluated the effects of dietary inulin on growth performance and immune factors as well as innate immune response in Oncorhynchus mykiss (rainbow trout) fry challenged with A. hydrophila. They found that inulin supplementation of the diet significantly increased WG, FCR, SGR, and protein content, as well as components of the humoral immune response such as lysozyme, complement activities, and total IgM [63]. The relative level of protection (RLP) (about 50%) in the six-week-old fish fed EPS after challenge infection with A. veronii was higher than the RLP in fish fed commercial inulin (33.33–35.71%) [2]. Furthermore, the EPS produced from B. megaterium 1 showed immunostimulant and immunomodulatory effects for Cyprinus carpio juveniles and C. carpio fry, respectively [7]. Lysozyme activity is an important marker of innate immunity in fish. Pathogen recognition, facilitated by pattern recognition receptors (PRRs), is instrumental in triggering innate immune responses through various signaling pathways. Elevated lysozyme levels aid in pathogen inhibition by targeting the peptidoglycan within bacterial cell walls [4,10]. The innate immune response is characterized by its swift reaction to pathogen presence, activating cellular defense mechanisms and networks of action including the production of antimicrobial substances and proteins, non-classical complement activation, release of cytokines, inflammation, and phagocytosis. Ultimately, the innate immune response paves the way for the development of an adaptive response [36,38]. On the other hand, NBT analysis is an assay to evaluate the respiratory burst activity of the immune cells, such as neutrophils and macrophages, especially in the context of phagocytosis, which generates ROS and free radicals, e.g., superoxide anion, playing a significant role in killing and destroying engulfed pathogens during the immune responses [4,13,41,61]. Also, the blood chemistry parameters were investigated as presented in Table 5. The results strongly proved that ALT, AST, T-bilirubin, and D-bilirubin in the T-group were not statistically different from the control group. These indicated that the supplementation of the fish diet with microbial EPS from B. subtilis P1 had no negative effect on the kidney and liver of Nile Tilapia [64]. Furthermore, blood urea nitrogen, total cholesterol, total protein, and albumin content in the T-group showed no significant difference compared to the control group; thus, the EPS feeding had no adverse effect on fish health [2,42]. The glucose content in the T-group was slightly higher than in the control group (p < 0.05), leading to an increased absorptive area in the fish intestine and a positive effect on serum glucose levels [42,64].

Table 5.
Blood chemical parameters and proximate chemical analysis.
Finally, the proximate analysis based on AOAC (2000) revealed higher protein content in the T-group. These findings suggest that EPS from B. subtilis P1 in fish diets efficiently supports fish growth and stimulates innate immune responses in Nile Tilapia, thus showing potential as the immunobiotics for aquaculture. How microbial inulin interacts with the fish body remains intricate and not fully elucidated. Inulin potentially influences the intestinal microbiota by promoting the rapid growth of beneficial bacteria while suppressing the proliferation of harmful microbes. Additionally, it may impact the immune system and systemic metabolism. Moreover, the efficacy of this prebiotic microbial EPS in aquatic cultures significantly relies on factors such as type, dosage, and duration of administration [2,4,51,59]. The present findings indicate that fish dietary administration of EPS-based inulin produced from B. subtilis P1 is able to promote the growth and enhance the serum immune responses of Nile tilapia; therefore, this produced EPS is promising for immunobiotics with immune-stimulatory and/or immune-modulatory activities in aquaculture. Most previous studies on microbial EPS in aquaculture focused on dextran, levan, or glucan, with few reports on microbial inulin use [3,4,6,51]. In contrast, plant-based inulin from chicory roots (Cichorium intybus L.) and Jerusalem artichoke tubers (Helianthus tuberosus L.) is more commonly used in feed additives [2,4,5]. This study introduces the use of bacterial inulin in Nile tilapia, which may offer advantages over plant-based sources, such as higher purity, consistent polymerization levels, and customizable functional properties such as prebiotic effectiveness, solubility, and fermentability [12,51,54]. Additionally, microbial inulin production can be continuous and is not limited by seasonal or geographical factors.
4. Conclusions
This study isolated and produced a substantial amount of microbial EPS from Bacillus subtilis P1. The EPS, composed of inulin–fructan molecules, was added to the diet of Nile tilapia (O. niloticus) due to its benefits in promoting probiotic growth, inhibiting certain pathogens, enhancing antioxidant activity, and showing non-cytotoxic effects. Fish in the EPS-supplemented group demonstrated improved immune responses, especially in innate immunity, and had higher survival rates and increased protein content. These findings suggest that EPS from B. subtilis P1 is a promising immunobiotic for aquaculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microbiolres15040148/s1, Figure S1: response surface plots of three variables in medium composition on EPS production; Figure S2: phylogenetic tree of Bacillus sp. P1 based on the sequence of 16S rDNA gene; Table S1: experimental design and the response values of % yield EPS produced by B. subtilis P1; Table S2: carbohydrate substrate utilization and enzyme activity of Bacillus sp. P1.
Author Contributions
Conceptualization, M.S., M.L. and W.C.; methodology, A.P., S.K., M.S., M.L. and W.C.; investigation, K.B., A.Y., A.P., S.K. and W.C.; data curation, K.B., A.Y., S.K., M.S. and W.C.; writing—original draft preparation, K.B. and W.C.; writing—review and editing, K.B., A.P., S.K., M.S., M.L. and W.C.; supervision, A.P., S.K., M.S., M.L. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by National Higher Education, Science, Research and Innovation Policy Council, Thaksin University (Research grant no. TSU-66A105000004) for the fiscal year 2023, as well as by Thaksin University Research Fund (Research grant no. TSU-66LGS003) for the fiscal year 2023.
Institutional Review Board Statement
The fishes were anesthetized with an excess dose of tricaine methane sulfonate MS-222 at 200 mg/L to reduce stress and suffering. The standard operation procedure as stipulated in Thaksin University guide for the use of animals for experiment was followed. This research received approval from the Animal Ethics Screening Committee, Thaksin University (Permit number: COA No. TSU 2023-009 and date of approval: 25 March 2023), for studies involving animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access these datasets should be directed to wankuson.c@tsu.ac.th.
Conflicts of Interest
The authors have no financial conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Lertwanakarn, T.; Purimayata, T.; Luengyosluechakul, T.; Grimalt, P.B.; Pedrazzani, A.S.; Quintiliano, M.H.; Surachetpong, W. Assessment of Tilapia (Oreochromis spp.) Welfare in the Semi-Intensive and Intensive Culture Systems in Thailand. Animals 2023, 13, 2498. [Google Scholar] [CrossRef] [PubMed]
- Ibrahem, M.D.; Fathi, M.; Mesalhy, S.; El-Aty, A.A. Effect of dietary supplementation of inulin and vitamin C on the growth, hematology, innate immunity, and resistance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2010, 29, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.; Faggio, C. Importance of prebiotics in aquaculture as immunostimulants. Effects on immune system of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol. 2016, 54, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Esteban, M.Á.; Cuesta, A.; Sun, Y.-Z. Prebiotics and fish immune response: A review of current knowledge and future perspectives. Rev. Fish. Sci. Aquac. 2015, 23, 315–328. [Google Scholar] [CrossRef]
- Yousefian, M.; Amiri, M.S. A review of the use of prebiotic in aquaculture for fish and shrimp. Afr. J. Biotechnol. 2009, 8, 1–6. [Google Scholar]
- Camacho-Chab, J.C.; Lango-Reynoso, F.; Castañeda-Chávez, M.D.R.; Galaviz-Villa, I.; Hinojosa-Garro, D.; Ortega-Morales, B.O. Implications of extracellular polymeric substance matrices of microbial habitats associated with coastal aquaculture systems. Water 2016, 8, 369. [Google Scholar] [CrossRef]
- Gupta, S.; Das, P.; Singh, S.; Akhtar, M.; Meena, D.; Mandal, S. Microbial levari, an ideal prebiotic and immunonutrient in aquaculture. World Aquac. 2011, 42, 61. [Google Scholar]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 1000517. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Liyaskina, E.V.; Kurgaeva, I.V.; Liyaskina, A.U.; Yang, G.; Revin, V.V. Exopolysaccharides Producing Bacteria: A Review. Microorganisms 2023, 11, 1541. [Google Scholar] [CrossRef]
- Salimi, F.; Farrokh, P. Recent advances in the biological activities of microbial exopolysaccharides. World J. Microbiol. Biotechnol. 2023, 39, 213. [Google Scholar] [CrossRef]
- Wang, W.; Ju, Y.; Liu, N.; Shi, S.; Hao, L. Structural characteristics of microbial exopolysaccharides in association with their biological activities: A review. Chem. Biol. Technol. Agric. 2023, 10, 137. [Google Scholar] [CrossRef]
- Abinaya, M.; Shanthi, S.; Palmy, J.; Al-Ghanim, K.A.; Govindarajan, M.; Vaseeharan, B. Exopolysaccharides-mediated ZnO nanoparticles for the treatment of aquatic diseases in freshwater fish. Oreochromis mossambicus. Toxics 2023, 11, 313. [Google Scholar] [CrossRef]
- Sutthi, N.; Wangkahart, E.; Panase, P.; Karirat, T.; Deeseenthum, S.; Ma, N.L.; Luang-In, V. Dietary Administration Effects of Exopolysaccharide Produced by Bacillus tequilensis PS21 Using Riceberry Broken Rice, and Soybean Meal on Growth Performance, Immunity, and Resistance to Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Animals 2023, 13, 3262. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Hugenholtz, J.; Zoon, P. An overview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int. Dairy J. 2002, 12, 163–171. [Google Scholar] [CrossRef]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2019, 487, 107881. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Mahdhi, A.; Chakroun, I.; Espinosa-Ruiz, C.; Messina, C.M.; Arena, R.; Majdoub, H.; Santulli, A.; Mzoughi, R.; Esteban, M.A. Dietary administration effects of exopolysaccharide from potential probiotic strains on immune and antioxidant status and nutritional value of European sea bass (Dicentrarchus labrax L.). Res. Vet. Sci. 2020, 131, 51–58. [Google Scholar] [CrossRef]
- Gobi, N.; Vaseeharan, B.; Chen, J.-C.; Rekha, R.; Vijayakumar, S.; Anjugam, M.; Iswarya, A. Dietary supplementation of probiotic Bacillus licheniformis Dahb1 improves growth performance, mucus and serum immune parameters, antioxidant enzyme activity as well as resistance against Aeromonas hydrophila in tilapia Oreochromis mossambicus. Fish Shellfish Immunol. 2018, 74, 501–508. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Jiang, Y.-Y.; Zhao, X.; Na Hao, X.; Li, L.; Yang, Z.-N. Characterization and antioxidant activity of the exopolysaccharide produced by Bacillus amyloliquefaciens GSBa-1. J. Microbiol. Biotechnol. 2018, 28, 1282–1292. [Google Scholar] [CrossRef]
- Petrova, P.; Arsov, A.; Ivanov, I.; Tsigoriyna, L.; Petrov, K. New Exopolysaccharides produced by Bacillus licheniformis 24 display substrate-dependent content and antioxidant activity. Microorganisms 2021, 9, 2127. [Google Scholar] [CrossRef]
- Moghannem, S.A.; Farag, M.M.; Shehab, A.M.; Azab, M.S. Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Braz. J. Microbiol. 2018, 49, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Berekaa, M.M. Improved exopolysaccharide production by Bacillus licheniformis strain-QS5 and application of statistical experimental design. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 876–886. [Google Scholar]
- Gangalla, R.; Sampath, G.; Beduru, S.; Sarika, K.; Govindarajan, R.K.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and characterization of exopolysaccharide produced by Bacillus aerophilus rk1 and its in vitro antioxidant activities. J. King Saud Univ. Sci. 2021, 33, 101470. [Google Scholar] [CrossRef]
- Razack, S.A.; Velayutham, V.; Thangavelu, V. Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes. Turk. J. Biol. 2013, 37, 280–288. [Google Scholar] [CrossRef]
- Mgomi, F.C.; Yang, Y.-R.; Cheng, G.; Yang, Z.-Q. Lactic acid bacteria biofilms and their antimicrobial potential against pathogenic microorganisms. Biofilm 2023, 5, 100118. [Google Scholar] [CrossRef]
- Lee, M.-G.; Joeng, H.; Shin, J.; Kim, S.; Lee, C.; Song, Y.; Lee, B.-H.; Park, H.-G.; Lee, T.-H.; Jiang, H.-H.; et al. Potential probiotic properties of exopolysaccharide-producing Lacticaseibacillus paracasei EPS DA-BACS and prebiotic activity of its exopolysaccharide. Microorganisms 2022, 10, 2431. [Google Scholar] [CrossRef]
- Lim, E.S.; Nam, S.J.; Koo, O.K.; Kim, J.-S. Protective role of Acinetobacter and Bacillus for Escherichia coli O157:H7 in biofilms against sodium hypochlorite and extracellular matrix-degrading enzymes. Food Microbiol. 2023, 109, 104125. [Google Scholar] [CrossRef]
- Todhanakasem, T.; Sangsutthiseree, A.; Areerat, K.; Young, G.M.; Thanonkeo, P. Biofilm production by Zymomonas mobilis enhances ethanol production and tolerance to toxic inhibitors from rice bran hydrolysate. New Biotechnol. 2014, 31, 451–459. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Lu, L.; Liu, Y.; Wang, F.; Xiao, M. Isolation, structural characterization and immunological activity of an exopolysaccharide produced by Bacillus licheniformis 8-37-0-1. Bioresour. Technol. 2010, 101, 5528–5533. [Google Scholar] [CrossRef]
- Lin, T.; Chen, C.; Chen, B.; Shaw, J.; Chen, Y. Optimal economic productivity of exopolysaccharides from lactic acid bacteria with production possibility curves. Food Sci. Nutr. 2019, 7, 2336–2344. [Google Scholar] [CrossRef]
- Ziadi, M.; Bouzaiene, T.; M’hIr, S.; Zaafouri, K.; Mokhtar, F.; Hamdi, M.; Boisset-Helbert, C. Evaluation of the efficiency of ethanol precipitation and ultrafiltration on the purification and characteristics of exopolysaccharides produced by three lactic acid bacteria. BioMed Res. Int. 2018, 2018, 1896240. [Google Scholar] [CrossRef] [PubMed]
- Khongkool, K.; Prakit, B.; Chiyod, R.; Suttibul, T.; Lertworapreecha, M. Qualitative analysis of fibre-degrading enzymes production by Bacillus isolated from native swine manures. Burapha Sci. J. 2023, 28, 647–659. [Google Scholar]
- Yılmaz, T.; Şimşek, Ö. Potential health benefits of ropy exopolysaccharides produced by Lactobacillus plantarum. Molecules 2020, 25, 3293. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, E.H.; Ibrahim, M.I.A.; Zaghloul, H.A.H. Antibacterial activity of exopolysaccharide produced by bee gut-resident Enterococcus sp. BE11 against marine fish pathogens. BMC Microbiol. 2023, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Wanja, D.W.; Mbuthia, P.G.; Waruiru, R.M.; Bebora, L.C.; Ngowi, H.A.; Nyaga, P.N. Antibiotic and disinfectant susceptibility patterns of bacteria isolated from farmed fish in Kirinyaga County, Kenya. Int. J. Microbiol. 2020, 2020, 8897338. [Google Scholar] [CrossRef]
- Abinaya, M.; Vaseeharan, B.; Divya, M.; Vijayakumar, S.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-Anbr, M.N.; Benelli, G. Structural characterization of Bacillus licheniformis Dahb1 exopolysaccharide—Antimicrobial potential and larvicidal activity on malaria and Zika virus mosquito vectors. Environ. Sci. Pollut. Res. 2018, 25, 18604–18619. [Google Scholar] [CrossRef]
- Figueiredo-Silva, C.; Lemme, A.; Sangsue, D.; Kiriratnikom, S. Effect of DL-methionine supplementation on the success of almost total replacement of fish meal with soybean meal in diets for hybrid tilapia (Oreochromis niloticus × Oreochromis mossambicus). Aquac. Nutr. 2015, 21, 234–241. [Google Scholar] [CrossRef]
- Biller, J.D.; Polycarpo, G.D.V.; Moromizato, B.S.; Sidekerskis, A.P.D.; da Silva, T.D.; dos Reis, I.C.; Fierro-Castro, C. Lysozyme activity as an indicator of innate immunity of tilapia (Oreochromis niloticus) when challenged with LPS and Streptococcus agalactiae. Rev. Bras. Zootec. 2021, 50, e20210053. [Google Scholar] [CrossRef]
- Stasiak, S.A.; Baumann, P.C. Neutrophil activity as a potential bioindicator for contaminant analysis. Fish Shellfish Immunol. 1996, 6, 537–539. [Google Scholar] [CrossRef]
- Hampton, L.M.T.; Jeffries, M.K.S.; Venables, B.J. A practical guide for assessing respiratory burst and phagocytic cell activity in the fathead minnow, an emerging model for immunotoxicity. MethodsX 2020, 7, 100992. [Google Scholar] [CrossRef]
- Biller, J.D.; Takahashi, L.S. Oxidative stress and fish immune system: Phagocytosis and leukocyte respiratory burst activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef] [PubMed]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Nuryastuti, T. Current in vitro assay to determine bacterial biofilm formation of clinical isolates. J. Med. Sci. Berk. Ilmu Kedokt. 2014, 46, 142–152. [Google Scholar] [CrossRef]
- Amao, J.A.; Omojasola, P.F.; Barooah, M. Isolation and characterization of some exopolysaccharide producing bacteria from cassava peel heaps. Sci. Afr. 2019, 4, e00093. [Google Scholar] [CrossRef]
- Marvasi, M.; Visscher, P.T.; Martinez, L.C. Exopolymeric substances (EPS) from Bacillus subtilis: Polymers and genes encoding their synthesis. FEMS Microbiol. Lett. 2010, 313, 1–9. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M.C. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Li, D.; Zhao, Y.; Zhang, X.; Zeng, X.; Yang, Z.; Li, S. Antioxidant activity of an exopolysaccharide isolated from Lactobacillus plantarum C88. Int. J. Biol. Macromol. 2013, 54, 270–275. [Google Scholar] [CrossRef]
- Sánchez-León, E.; Huang-Lin, E.; Amils, R.; Abrusci, C. Production and characterisation of an exopolysaccharide by Bacillus amyloliquefaciens: Biotechnological applications. Polymers 2023, 15, 1550. [Google Scholar] [CrossRef]
- Huang-Lin, E.; Sánchez-León, E.; Amils, R.; Abrusci, C. Potential applications of an exopolysaccharide produced by Bacillus xiamenensis RT6 isolated from an acidic environment. Polymers 2022, 14, 3918. [Google Scholar] [CrossRef]
- Jurášková, D.; Ribeiro, S.C.; Silva, C.C.G. Exopolysaccharides produced by lactic acid bacteria: From biosynthesis to health-promoting properties. Foods 2022, 11, 156. [Google Scholar] [CrossRef]
- Dănăilă-Guidea, S.M.; Tamba-Berehoiu, R.M.; Popa, C.-N.; Toma, R.; Vișan, L.; Simion, V. Inulin: Unique among the polyglucides with significant functional properties and biotechnological perspectives. Rom. Biotechnol. Lett. 2020, 25, 1387–1395. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from lactic acid bacteria: Techno-functional application in the food industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic acid bacteria exopolysaccharides producers: A sustainable tool for functional foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.A.M.; Hassan, A.I.; Mahmoud, M.G.; Asker, M.S. Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complement. Altern. Med. 2016, 16, 112. [Google Scholar] [CrossRef]
- Malick, A.; Khodaei, N.; Benkerroum, N.; Karboune, S. Production of exopolysaccharides by selected Bacillus strains: Optimization of media composition to maximize the yield and structural characterization. Int. J. Biol. Macromol. 2017, 102, 539–549. [Google Scholar] [CrossRef]
- Daneshazari, R.; Khorasgani, M.R.; Hosseini-Abari, A.; Kim, J.-H. Bacillus subtilis isolates from camel milk as probiotic candidates. Sci. Rep. 2023, 13, 3387. [Google Scholar] [CrossRef]
- Nayak, S.K. Multifaceted applications of probiotic Bacillus species in aquaculture with special reference to Bacillus subtilis. Rev. Aquac. 2020, 13, 862–906. [Google Scholar] [CrossRef]
- Elisashvili, V.; Kachlishvili, E.; Chikindas, M.L. Recent advances in the physiology of spore formation for bacillus probiotic production. Probiotics Antimicrob. Proteins 2018, 11, 731–747. [Google Scholar] [CrossRef]
- Zhu, L.; Qin, S.; Zhai, S.; Gao, Y.; Li, L. Inulin with different degrees of polymerization modulates composition of intestinal microbiota in mice. FEMS Microbiol. Lett. 2017, 364, fnx075. [Google Scholar] [CrossRef]
- Karirat, T.; Saengha, W.; Deeseenthum, S.; Ma, N.L.; Sutthi, N.; Wangkahart, E.; Luang-In, V. Data on exopolysaccharides produced by Bacillus spp. from cassava pulp with antioxidant and antimicrobial properties. Data Brief 2023, 50, 109474. [Google Scholar] [CrossRef]
- Yones, A.-M.A.S. Effects of dietary inulin as prebiotic on growth performance, immuno-haematological indices and ectoparasitic infection of fingerlings Nile tilapia, Oreochromis niloticus. Egypt. J. Histol. 2019, 43, 88–103. [Google Scholar] [CrossRef]
- Reyes-Becerril, M.; Ascencio, F.; Gracia-Lopez, V.; Macias, M.E.; Roa, M.C.; Esteban, M. Single or combined effects of Lactobacillus sakei and inulin on growth, non-specific immunity and IgM expression in leopard grouper (Mycteroperca rosacea). Fish Physiol. Biochem. 2014, 40, 1169–1180. [Google Scholar] [CrossRef] [PubMed]
- Ghafarifarsani, H.; Rashidian, G.; Bagheri, T.; Hoseinifar, S.H.; Van Doan, H. Study on growth enhancement and the protective effects of dietary prebiotic inulin on immunity responses of rainbow trout (Oncorhynchus mykiss) fry infected with Aeromonas hydrophila. Ann. Anim. Sci. 2021, 21, 543–559. [Google Scholar] [CrossRef]
- Kumar, V.; Makkar, H.; Becker, K. Nutritional, physiological and haematological responses in rainbow trout (Oncorhynchus mykiss) juveniles fed detoxified Jatropha curcas kernel meal. Aquac. Nutr. 2010, 17, 451–467. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).