Establishing In Vitro Screening Protocols Based on Phenotypic Plasticity of Amaranthus dubius and Galinsoga parviflora Seeds for Drought, Salinity, and Heat Tolerance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
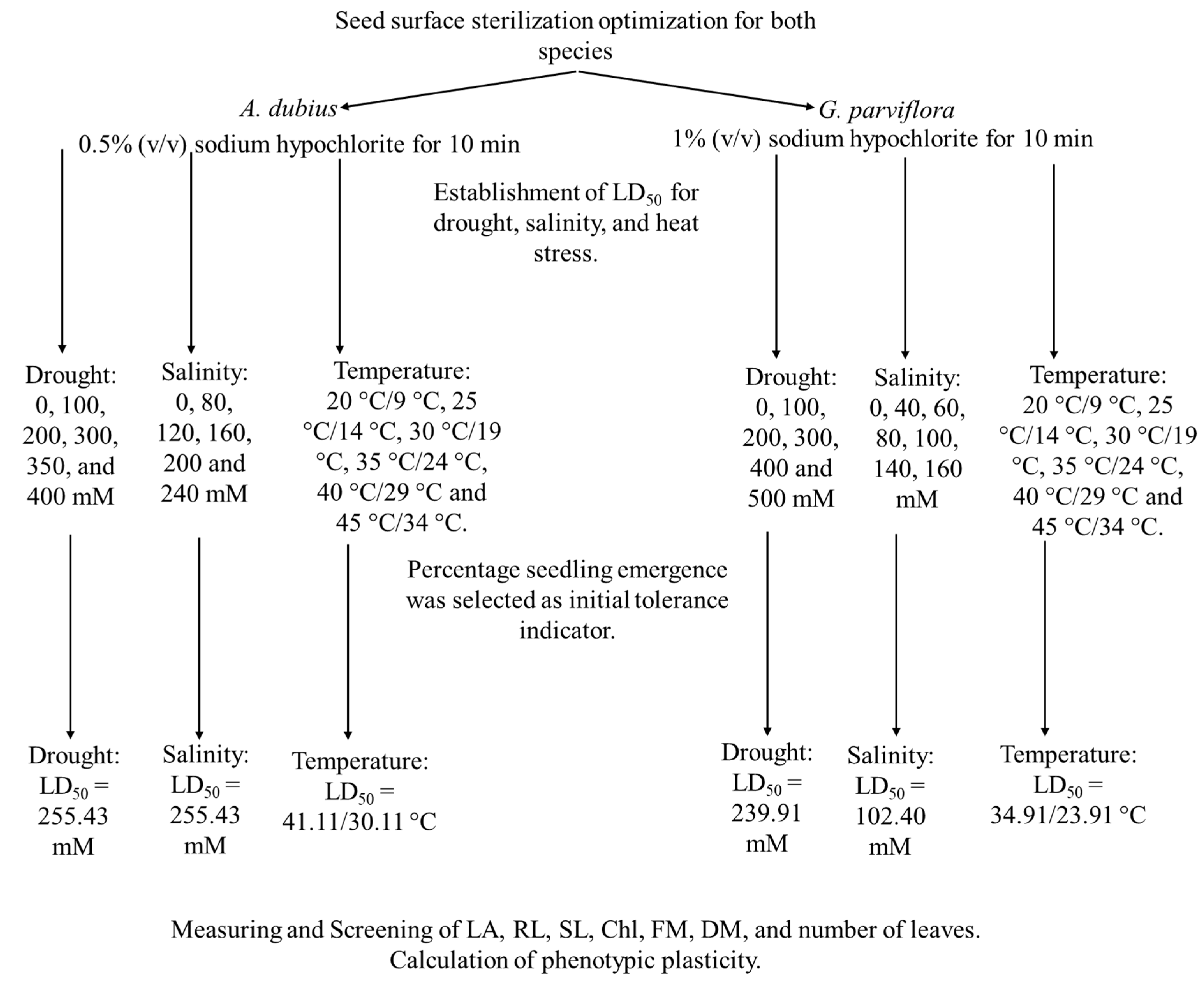
2.2. Seed Surface Sterilization and In Vitro Culture
2.3. Establishment of LD50 for Drought, Salinity, and LT50 Heat Stress
2.4. In Vitro Screening for Phenotypic Plasticity at LD/T50
2.5. Statistical Analyses
3. Results
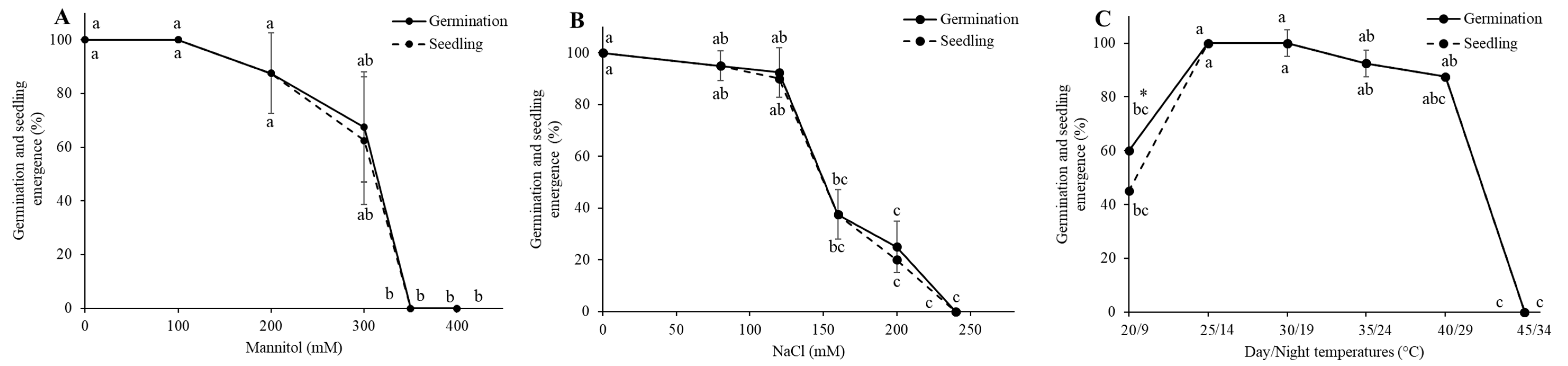
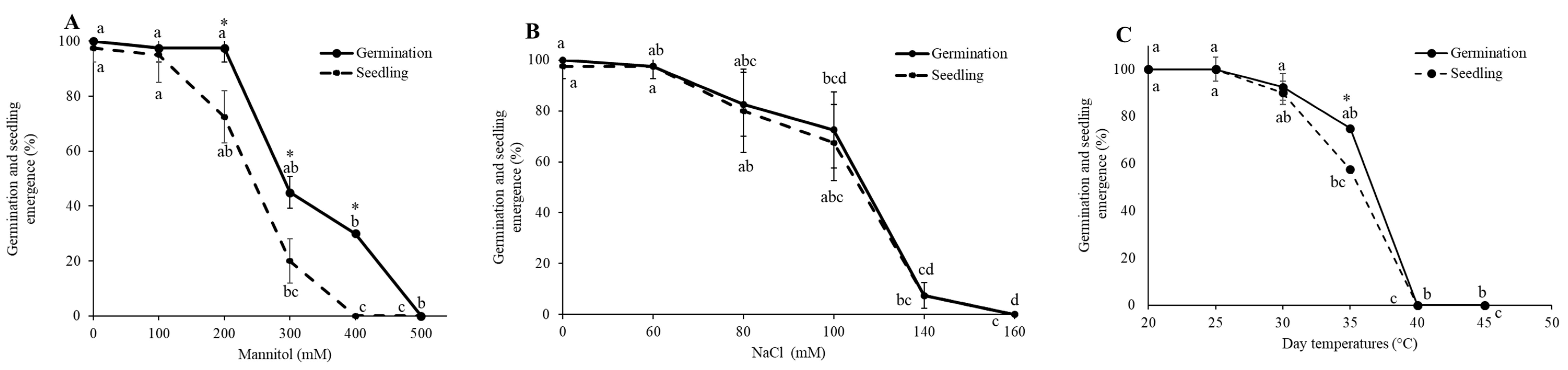
3.1. Establishment of LD50 for Drought, Salinity, and LT50 Heat Stress
3.2. In Vitro Screening for Phenotypic Plasticity at LD/T50
4. Discussion
5. Concluding Remarks and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Odhav, B.; Beekrum, S.; Akula, U.S.; Baijnath, H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal. S. Afr. J. Food Compos. Anal. 2007, 20, 430–435. [Google Scholar] [CrossRef]
- Achigan-Dako, E.G.; Sogbohossou, O.E.D.; Maundu, P. Current knowledge on Amaranthus spp.: Research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 2014, 197, 303–317. [Google Scholar] [CrossRef]
- Mabhaudhi, T.; Chibarabada, T.P.; Chimonyo, V.G.P.; Murugani, V.G.; Pereira, L.M.; Sobratee, N.; Govender, L.; Slotow, R.; Modi, A.T. Mainstreaming underutilized indigenous and traditional crops into food systems: A South African perspective. Sustainability 2019, 11, 172. [Google Scholar] [CrossRef]
- Amma, C.S.; Rajalakshmi, R. Morphological and Physiological Responses in Red Amaranthus (Amaranthus tricolor L.) and Green Amaranthus (Amaranthus dubius Mart. ex Thell.) Under Progressive Water Stress. Asian J. Biol. Life Sci. 2023, 12, 61. [Google Scholar]
- Joshi, D.C.; Sood, S.S.; Hosahatti, R.; Kant, L.; Pattanayak, A.; Kumar, A.; Yadav, D.; Stetter, M.G. From zero to hero: The past, present and future of grain amaranth breeding. Theor. Appl. Genet. 2018, 131, 1807–1823. [Google Scholar] [CrossRef]
- Ripanda, A.; Luanda, A.; Sule, K.S.; Mtabazi, G.S.; Makangara, J.J. Galinsoga parviflora (Cav.): A comprehensive review on ethnomedicinal, phytochemical and pharmacological studies. Heliyon 2023, 9, e13517. [Google Scholar] [CrossRef]
- Sattar, M.; Saeed, F.; Afzaal, M.; Rasheed, A.; Asif, A.; Sharif, S.; Hussain, M.; Asad Ur Rehman, H.; Raza, M.A.; Munir, H.; et al. An overview of the nutritional and therapeutic properties of amaranth. Int. J. Food Prop. 2024, 27, 263–272. [Google Scholar] [CrossRef]
- Damalas, C.A. Distribution, biology, and agricultural importance of Galinsoga parviflora (Asteraceae). Weed Biol. Manag. 2008, 8, 147–153. [Google Scholar] [CrossRef]
- Benhin, J.K.A. South African crop farming and climate change: An economic assessment of impacts. Glob. Environ. Chang. 2008, 18, 666–678. [Google Scholar] [CrossRef]
- Songoni, A.; Jimoh, M.O.; Kambizi, L.; Laubscher, C.P. The impact of salt stress on plant growth, mineral composition, and antioxidant activity in Tetragonia decumbens Mill: An underutilized edible halophyte in South Africa. Horticulturae 2021, 7, 140. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant Productivity and Environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.E.; Holzapfel, C.M. Evolutionary response to rapid climate change. Science 2006, 312, 1477–1478. [Google Scholar] [CrossRef] [PubMed]
- Nicotra, A.B.; Atkin, O.K.; Bonser, S.P.; Davidson, A.M.; Finnegan, E.J.; Mathesius, U.; Poot, P.; Purugganan, M.D.; Richards, C.L.; Valladares, F.; et al. Plant phenotypic plasticity in a changing climate. Trends Plant Sci. 2010, 15, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, S.; Milla, R. Differential plasticity to water and nutrients between crops and their wild progenitors. Environ. Exp. Bot. 2018, 145, 54–63. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetic research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Carrascosa, A.; Silvestre, M.; Morgado, L.; Azcárate, F.M. Diversity and mean specific leaf area of Mediterranean woody vegetation changes in response to summer drought across a double stress gradient: The role of phenotypic plasticity. J. Veg. Sci. 2023, 34, 13180. [Google Scholar] [CrossRef]
- Dupont, L.; Thierry, M.; Zinger, L.; Legrand, D.; Jacob, S. Beyond reaction norms: The temporal dynamics of phenotypic plasticity. Trends Ecol. Evol. 2024, 39, 41–51. [Google Scholar] [CrossRef]
- Valladares, F.; Wright, S.J.; Lasso, E.; Kitajima, K.; Pearcy, R.W. Plastic phenotypic response to light of 16 congeneric shrubs from a Panamanian rainforest. Ecology 2000, 81, 1925–1936. [Google Scholar] [CrossRef]
- Khanam UK, S.; Oba, S. Phenotypic plasticity of vegetable Amaranth, Amaranthus tricolor L. under a natural climate. Plant Prod. Sci. 2014, 17, 166–172. [Google Scholar] [CrossRef]
- Enoki, T.; Takagi, M.; Ugawa, S.; Nabeshima, E.; Ishii, H. Regional and topographic growth variation among 45-year-old clonal plantations of Cryptomeria japonica: Effects of genotypes and phenotypic plasticity. J. For. Res. 2020, 25, 329–338. [Google Scholar] [CrossRef]
- Duvick, D.N. Plant breeding, an evolutionary concept. Crop Sci. 1996, 36, 539–548. [Google Scholar] [CrossRef]
- Duncan, R.R. Tissue culture-induced variation and crop improvement. Adv. Agron. 1997, 58, 201–240. [Google Scholar] [CrossRef]
- Bradshaw, J.E. Plant breeding: Past, present and future. Euphytica 2017, 213, 60. [Google Scholar] [CrossRef]
- Borlaug, N.E. Contributions of conventional plant breeding to food production. Science 1983, 219, 689–693. [Google Scholar] [CrossRef]
- Roy, S.J.; Tucker, E.J.; Tester, M. Genetic analysis of abiotic stress tolerance in crops. Curr. Opin. Plant Biol. 2011, 14, 232–239. [Google Scholar] [CrossRef]
- Maleki, M.; Ghorbanpour, M.; Nikabadi, S.; Wani, S.H. In vitro screening of crop plants for abiotic stress tolerance. In Recent Approaches in Omics for Plant Resilience to Climate Change; Wani, S.H., Ed.; Springer: Cham, Switzerland, 2019; pp. 75–91. [Google Scholar] [CrossRef]
- Charlesworth, D.; Charlesworth, B. Quantitative genetics in plants: The effects of the breeding system on genetic variability. Evolution 1995, 49, 911–920. [Google Scholar] [CrossRef]
- Jain, S.M. Tissue culture-derived variation in crop improvement. Euphytica 2001, 118, 153–166. [Google Scholar] [CrossRef]
- Ceccarelli, S. Efficiency of plant breeding. Crop Sci. 2015, 55, 87–97. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Slabbert, R.; Spreeth, M.; Krüger, G.H.J. Drought tolerance, traditional crops and biotechnology: Breeding towards sustainable development. S. Afr. J. Bot. 2004, 70, 116–123. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, K.; Jat, S.L.; Srivastava, S.; Tiwari, T.; Kumar, S.; Meenakshi Pradhan, H.R.; Kumar, B.; Chaturvedi, G.; Jha, A.K.; et al. Rapid method of screening for drought stress tolerance in maize (Zea mays L.). Indian J. Genet. Plant Breed. 2020, 80, 16–25. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A. Screening methods for salinity tolerance: A case study with tetraploid wheat. Plant Soil 2003, 253, 201–218. [Google Scholar] [CrossRef]
- Lin, L.; Liao, M.; Ren, Y.; Luo, L.; Zhang, X.; Yang, D. Effects of mulching tolerant plant straw on soil surface on growth and cadmium accumulation of Galinsoga parviflora. PLoS ONE 2014, 9, e114957. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Catalase, superoxide dismutase and ascorbate-glutathione cycle enzymes confer drought tolerance of Amaranthus tricolor. Sci. Rep. 2018, 8, 16496. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iori, V.; Cheremisina, A.; Shevyakova, N.I.; Radyukina, N.; Kuznetsov, V.V.; Zacchini, M. Evaluation of nickel tolerance in Amaranthus paniculatus L. plants by measuring photosynthesis, oxidative status, antioxidative response and metal-binding molecule content. Environ. Sci. Pollut. Res. 2015, 22, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Maseko, I.; Ncube, B.; Mabhaudhi, T.; Tesfay, S.; Chimanyo, V.G.P.; Araya, H.T.; Fessehazian, M.; Du Plooy, C.P. Moisture stress on physiology and yield of some indigenous leafy vegetables under field conditions. S. Afr. J. Bot. 2019, 126, 85–91. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, K.; Liu, T.; Tang, L.; Khan, A.A.; Yang, T.; Zheng, P.; Wang, T.; Han, J.; Shi, F. Response in phenotypic plasticity of Amaranthus palmeri and Polygonum orientale to soil factors under different habitats. Clean-Soil Air Water 2020, 48, 1900203. [Google Scholar] [CrossRef]
- Lin, L.; Jin, Q.; Liu, Y.; Ning, B.; Liao, M.; Luo, L. Screening of new cadmium hyperaccumulator, Galinsoga parviflora, from winter farmland weeds using the artificially high soil cadmium concentration method. Environ. Toxicol. Chem. 2014, 33, 2422–2428. [Google Scholar] [CrossRef]
- Hayat, S.; Mir, B.A.; Wani, A.S.; Hasan, S.A.; Irfan, M.; Ahmad, A. Screening of salt-tolerant genotypes of Brassica juncea based on photosynthetic attributes. J. Plant Interact. 2011, 6, 53–60. [Google Scholar] [CrossRef]
- Bibi, A.; Sadaqat, H.A.; Tahir MH, N.; Akram, H.M. Screening of sorghum (Sorghum bicolor Var Moench) for drought tolerance at seedling stage in polyethylene glycol. J. Anim. Plant Sci. 2012, 22, 671–678. [Google Scholar]
- Qadir, M.; Bibi, A.; Tahir, M.H.N.; Saleem, M.; Sadaqat, H.A. Screening of sorghum (Sorghum bicolor L.) genotypes under various levels of drought stress. Maydica 2015, 60, M35. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol. Plant. 1962, 15, 473–479. [Google Scholar] [CrossRef]
- Coste, S.; Baraloto, C.; Leroy, C.; Macron, E.; Renaud, A.; Richardson, A.D.; Roggy, J.C.; Schimann, H.; Uddling, J.; Herault, B. Assessing foliar chlorophyll contents with the SPAD-502 chlorophyll meter: A calibration test with thirteen tree species of tropical rainforest in French Guiana. Ann. For. Sci. 2010, 67, 607. [Google Scholar] [CrossRef]
- dos Santos, J.C.C.; Costa, R.N.; Silva, D.M.R.; de Souza, A.A.; de Barros Prado Moura, F.; da Silva Junior, J.M.; Silva, J.V. Use of allometric models to estimate leaf area in Hymenaea courbaril L. Theor. Exp. Plant Physiol. 2016, 28, 357–369. [Google Scholar] [CrossRef]
- Yadav, P.; Mina, U.; Bhatia, A. Screening of forty Indian Amanrthus hypochondriacus cultivars for tolerance and susceptibility to tropospheric ozone stress. Nucleus 2020, 63, 281–291. [Google Scholar] [CrossRef]
- Areington, C.A.; Neto, M.C.L.; Watt, P.M. Assessing the utility of selected photosynthetic and related traits in screening Amaranthus dubius Mart. ex Thell. and Galinsoga parviflora Cav. 1796 seedlings for elevated temperature stress tolerance. S. Afr. J. Bot. 2022, 145, 444–457. [Google Scholar] [CrossRef]
- Kausar, A.; Ashraf, M.Y.; Niaz, M. Some physiological and genetic determinants of salt tolerance in sorghum (Sorghum bicolor (L.) Moench): Biomass production and nitrogen metabolism. Pak. J. Bot. 2014, 44, 47–52. [Google Scholar]
- Carvalho, M.; Matos, M.; Castro, I.; Monteiro, E.; Rosa, E.; Lino-Neto, T.; Carnide, V. Screening of worldwide cowpea collection to drought tolerant at a germination stage. Sci. Hortic. 2019, 247, 107–115. [Google Scholar] [CrossRef]
- Liu, S.; Zenda, T.; Dong, A.; Yang, Y.; Liu, X.; Wang, Y.; Li, J.; Tao, Y.; Duan, H. Comparative proteomic and morpho-physiological analyses of maize wild-type Vp16 and mutant vp16 germinating seed responses to PEG-induced drought stress. Int. J. Mol. Sci. 2019, 20, 5586. [Google Scholar] [CrossRef]
- Sun, J.; He, L.; Li, T. Response of seedling growth and physiology of Sorghum bicolor (L.) Moench to saline-alkali stress. PLoS ONE 2019, 14, e0220340. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.; Wang, C.; Li, K.; Zhang, X.; Yang, Z.; Li, M.; Wang, B. An effective screening method and reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 2019, 10, 530. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.Q.; Liao, X.L.; Qian, Y.E.; Zhang, W.; Li, Y.K.; Bhat, J.A.; Kan, G.Z.; Yu, D.Y. Linkage and association mapping of wild soybean (Glycine soja) seeds germinating under salt stress. J. Integr. Agric. 2022, 21, 2833–2847. [Google Scholar] [CrossRef]
- Masondo, N.A.; Kulkarni, M.G.; Finnie, J.F.; Van Staden, J. Influence of biostimulants-seed-priming on Ceratotheca triloba germination and seedling growth under low temperatures, low osmotic potential and salinity stress. Ecotoxicol. Environ. Saf. 2018, 147, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.U.; Hussain, A.; Lee, I.J.; Yun, B.W. Drought-induced AtbZIP62 transcription factors regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Goatley, M.; Hensler, K.; Askew, S. Cool-season turfgrass germination and morphological development comparisons at adjusted osmotic potentials. Crop Sci. 2017, 57, 201–208. [Google Scholar] [CrossRef]
- Mahlanza, T.; Rutherford, R.S.; Snyman, S.J.; Watt, M.P. In vitro generation of somaclonal variant plants of sugarcane for tolerance to Fusarium sacchari. Plant Cell Rep. 2013, 32, 249–262. [Google Scholar] [CrossRef]
- Synman, S.J.; Mhlanga, P.; Watt, M.P. Rapid screening of sugarcane plantlets for in vitro mannitol-induced stress. Sugar Tech 2016, 18, 437–440. [Google Scholar] [CrossRef]
- Saraswathi, M.S.; Kannan, G.; Uma, S.; Thangavelu, R.; Backiyarani, S. Improvement of banana cv. Rasthali (Silk, AAB) against Fusarium oxysporum f.sp. cubense (VCG 0124/5) through induced mutagenesis: Determination of LD50 specific to mutagen, explants, toxins and in vitro and in vivo screening for Fusarium wilt resistance. Indian J. Exp. Biol. 2016, 54, 345–353. [Google Scholar]
- Koch, A.C.; Ramgareed, S.; Rutherford, R.S.; Snyman, S.J.; Watt, M.P. An in vitro mutagenesis protocol for the production of sugarcane tolerant to herbicide imazapyr. Vitr. Cell. Dev. Biol.-Plant 2012, 48, 417–427. [Google Scholar] [CrossRef]
- Peel, A.D.; Waldon, B.L.; Jensen, K.B.; Chatterton, N.J.; Horton, H.; Dudley, L.M. Screening for salinity tolerance in alfalfa: A repeatable method. Crop Sci. 2004, 44, 2049–2053. [Google Scholar] [CrossRef]
- Liu, F.; Stützel, H. Biomass partitioning, specific leaf area, and water use efficiency of vegetable amaranth (Amaranthus spp.) in response to drought stress. Sci. Hortic. 2004, 102, 15–27. [Google Scholar] [CrossRef]
- Veerappa, R.; Slocum, R.D.; Siegenthaler, A.; Wang, J.; Clark, G.; Roux, S.J. Ectopic expression of a pea apyrase enhances root system architecture and drought survival in Arabidopsis and soybean. Plant Cell Environ. 2019, 42, 337–353. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef]
- Hoang, L.H.; De Guzman, C.C.; Cadiz, N.M.; Tran, D.H. Physiological and phytochemical responses of red amaranth (Amaranthus tricolor L.) and green amaranth (Amaranthus dubius L.) to different salinity levels. Legume Res. 2020, 43, 206–211. [Google Scholar] [CrossRef]
- Hossain, M.N.; Sarker, U.; Raihan, M.S.; Al-Huqail, A.A.; Siddiqui, M.H.; Oba, S. Influence of salinity stress on color parameters, leaf pigmentation, polyphenol and flavonoid contents and antioxidant activity of Amaranthus lividus leafy vegetables. Molecules 2022, 27, 1821. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.G.; Chao, H.C.; Lin, H.L. Differential responses of Pak Choi and edible amaranth to elevated temperature. HortScience 2018, 53, 195–199. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Gautam, M. Biochemical parameters of plants as indicators of air pollution. J. Environ. Biol. 2007, 28, 127–132. [Google Scholar] [PubMed]
- Areington, C.A.; Varghese, B.; Ramdhani, S.; Sershen. An assessment of morphological, physiological and biochemical biomarkers of industrial air pollution in the leaves of Brachylaena discolor. Water Air Soil Pollut. 2015, 226, 291. [Google Scholar] [CrossRef]
- Areington, C.A.; Varghese, B. The utility of biochemical, physiological and morphological biomarkers of leaf sulfate levels in establishing Brachylaena discolor leaves as bioindicator of SO2 pollution. Plant Physiol. Biochem. 2017, 118, 295–305. [Google Scholar] [CrossRef]
- Bahrami, F.; Arzani, A.; Rahimmalek, M. Photosynthetic yield performance of wild barley (Hordeum vulgare ssp. spontaneum) under terminal heat stress. Photosynthetica 2019, 57, 9–17. [Google Scholar] [CrossRef]
- Hamilton, E.W., III; Heckathorn, S.A.; Joshi, P.; Wang, D.; Barua, D. Interactive effects of elevated CO2 and growth temperatures on the tolerance of photosynthesis to acute heat stress in C3 and C4 species. J. Integr. Plant Biol. 2008, 50, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fu, Y.; Hu, D.; Yu, J. Effect of green, yellow and purple radiation on biomass, photosynthesis, morphology and soluble sugar content of leafy lettuce via spectral wavebands “knock out”. Sci. Hortic. 2018, 236, 10–17. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, Y.; Zhao, X.; Han, L.N.; Yang, S.J.; Li, W.F.; Mao, J.; Ma, Z.H.; Chen, B.H. Effects of CO2 on transplantation of grape plantlets cultured in vitro by promoting photosynthesis. Sci. Hortic. 2021, 287, 110286. [Google Scholar] [CrossRef]
- Smethurst, C.F.; Shabala, S. Screening methods for waterlogging tolerance in lucerne: Comparative analysis of waterlogging effects on chlorophyll fluorescence, photosynthesis, biomass and chlorophyll content. Funct. Plant Biol. 2003, 30, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Olorunwa, O.; Adhikari, B.; Shi, A.; Barickman, T.C. Screening of cowpea (Vigna unguiculata (L.) Walp.) genotypes for waterlogging tolerance using morpho-physiological traits at early growth stage. Plant Sci. 2022, 315, 111136. [Google Scholar] [CrossRef]
- Valladares, F.; Sanchez-Gomez, D.; Zavala, M.A. Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications. J. Ecol. 2006, 94, 1103–1116. [Google Scholar] [CrossRef]
| Stressor | Leaf Area (cm2) | Root Length (cm) | Shoot Length (cm) | Chlorophyll Content (SPAD Units) | Fresh Mass (g) | Dry Mass (g) | Number of Leaves |
|---|---|---|---|---|---|---|---|
| Control | 0.56 ± 0.29 a | 3.67 ± 0.84 b | 1.65 ± 0.51 b | 12.28 ± 2.98 b | 0.0619 ± 0.0371 a | 0.0022 ± 0.0013 a | 5.83 ± 0.68 a |
| Drought (255.43 mM) | 0.03 ± 0.01 c | 0.79 ± 0.26 c | 0.40 ± 0.08 c | 2.31 ± 0.67 c | 0.0051 ± 0.0013 c | 0.0004 ± 0.0002 b | 2.00 ± 0.00 c |
| Salinity (148.65 mM) | 0.07 ± 0.03 b | 0.47 ± 0.27 c | 0.59 ± 0.25 c | 2.26 ± 1.53 c | 0.0067 ± 0.0030 b | 0.0004 ± 0.0002 b | 2.00 ± 0.00 c |
| Heat (41.11/30.11 °C) | 0.65 ± 0.15 a | 6.14 ± 1.59 a | 4.17 ± 0.30 a | 19.70 ± 3.37 a | 0.0797 ± 0.0274 a | 0.0029 ± 0.0007 a | 4.63 ± 0.49 b |
| Stressor | Leaf Area (cm2) | Root Length (cm) | Shoot Length (cm) | Chlorophyll Content (SPAD Units) | Fresh Mass (g) | Dry Mass (g) | Number of Leaves |
|---|---|---|---|---|---|---|---|
| Control | 0.49 ± 0.40 a | 3.28 ± 1.47 a | 0.36 ± 0.24 c | 17.23 ± 9.29 a | 0.0769 ± 0.0513 a | 0.0026 ± 0.0015 a | 6.25 ± 1.56 a |
| Drought (239.91 mM) | 0.04 ± 0.02 b | 1.54 ± 0.64 bc | 0.43 ± 0.08 b | 4.90 ± 3.64 b | 0.0075 ± 0.0073 b | 0.0006 ± 0.0006 b | 2.38 ± 0.77 b |
| Salinity (102.40 mM) | 0.06 ± 0.04 b | 1.17 ± 0.87 c | 0.31 ± 0.12 c | 5.51 ± 3.84 b | 0.0071 ± 0.0064 b | 0.0006 ± 0.0006 b | 2.63 ± 1.33 b |
| Heat (34.91/23.91 °C) | 0.06 ± 0.03 b | 1.64 ± 0.62 b | 0.91 ± 0.17 a | 2.90 ± 0.92 b | 0.0077 ± 0.0038 b | 0.0002 ± 0.0001 c | 2.50 ± 0.85 b |
| Stressor | Leaf Area | Root Length | Shoot Length | Chlorophyll Content | Fresh Mass | Dry Mass | Number of Leaves | MPI |
|---|---|---|---|---|---|---|---|---|
| Control | 0.91 | 0.58 | 0.60 | 0.63 | 0.94 | 0.92 | 0.29 | 0.70 ± 0.24 |
| Drought (255.43 mM) | 0.55 | 0.71 | 0.46 | 0.71 | 0.62 | 0.86 | 0.00 | 0.56 ± 0.28 |
| Salinity (148.65 mM) | 0.90 | 0.92 | 0.84 | 0.95 | 0.83 | 0.88 | 0.00 | 0.76 ± 0.34 |
| Heat (41.11/30.11 °C) | 0.55 | 0.66 | 0.22 | 0.45 | 0.69 | 0.98 | 0.20 | 0.54 ± 0.28 |
| MPI | 0.73 ± 0.21 | 0.72 ± 0.15 | 0.53 ± 0.26 | 0.69 ± 0.21 | 0.77 ± 0.14 | 0.91 ± 0.05 | 0.12 ± 0.15 |
| Stressor | Leaf Area | Root Length | Shoot Length | Chlorophyll Content | Fresh Mass | Dry Mass | Number of Leaves | MPI |
|---|---|---|---|---|---|---|---|---|
| Control | 0.94 | 0.81 | 0.90 | 0.93 | 0.97 | 0.98 | 0.50 | 0.86 ± 0.17 |
| Drought (239.41 mM) | 0.83 | 0.81 | 0.50 | 0.97 | 0.95 | 0.95 | 0.50 | 0.79 ± 0.21 |
| Salinity (102.40 mM) | 0.97 | 0.97 | 0.83 | 0.97 | 0.72 | 0.96 | 0.83 | 0.89 ± 0.10 |
| Heat (34.91/23.91 °C) | 0.91 | 0.76 | 0.50 | 0.67 | 0.90 | 0.75 | 0.50 | 0.71 ± 0.17 |
| MPI | 0.91 ± 0.06 | 0.84 ± 0.09 | 0.68 ± 0.21 | 0.89 ± 0.14 | 0.89 ± 0.11 | 0.91 ± 0.11 | 0.58 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Areington, C.A.; O’Kennedy, M.M.; Sershen. Establishing In Vitro Screening Protocols Based on Phenotypic Plasticity of Amaranthus dubius and Galinsoga parviflora Seeds for Drought, Salinity, and Heat Tolerance. Int. J. Plant Biol. 2024, 15, 878-894. https://doi.org/10.3390/ijpb15030063
Areington CA, O’Kennedy MM, Sershen. Establishing In Vitro Screening Protocols Based on Phenotypic Plasticity of Amaranthus dubius and Galinsoga parviflora Seeds for Drought, Salinity, and Heat Tolerance. International Journal of Plant Biology. 2024; 15(3):878-894. https://doi.org/10.3390/ijpb15030063
Chicago/Turabian StyleAreington, Candyce Ann, Martha M. O’Kennedy, and Sershen. 2024. "Establishing In Vitro Screening Protocols Based on Phenotypic Plasticity of Amaranthus dubius and Galinsoga parviflora Seeds for Drought, Salinity, and Heat Tolerance" International Journal of Plant Biology 15, no. 3: 878-894. https://doi.org/10.3390/ijpb15030063
APA StyleAreington, C. A., O’Kennedy, M. M., & Sershen. (2024). Establishing In Vitro Screening Protocols Based on Phenotypic Plasticity of Amaranthus dubius and Galinsoga parviflora Seeds for Drought, Salinity, and Heat Tolerance. International Journal of Plant Biology, 15(3), 878-894. https://doi.org/10.3390/ijpb15030063







