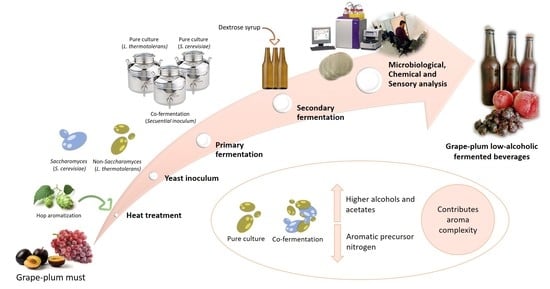
Use of Mixed Cultures for the Production of Grape–Plum Low-Alcohol Fermented Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
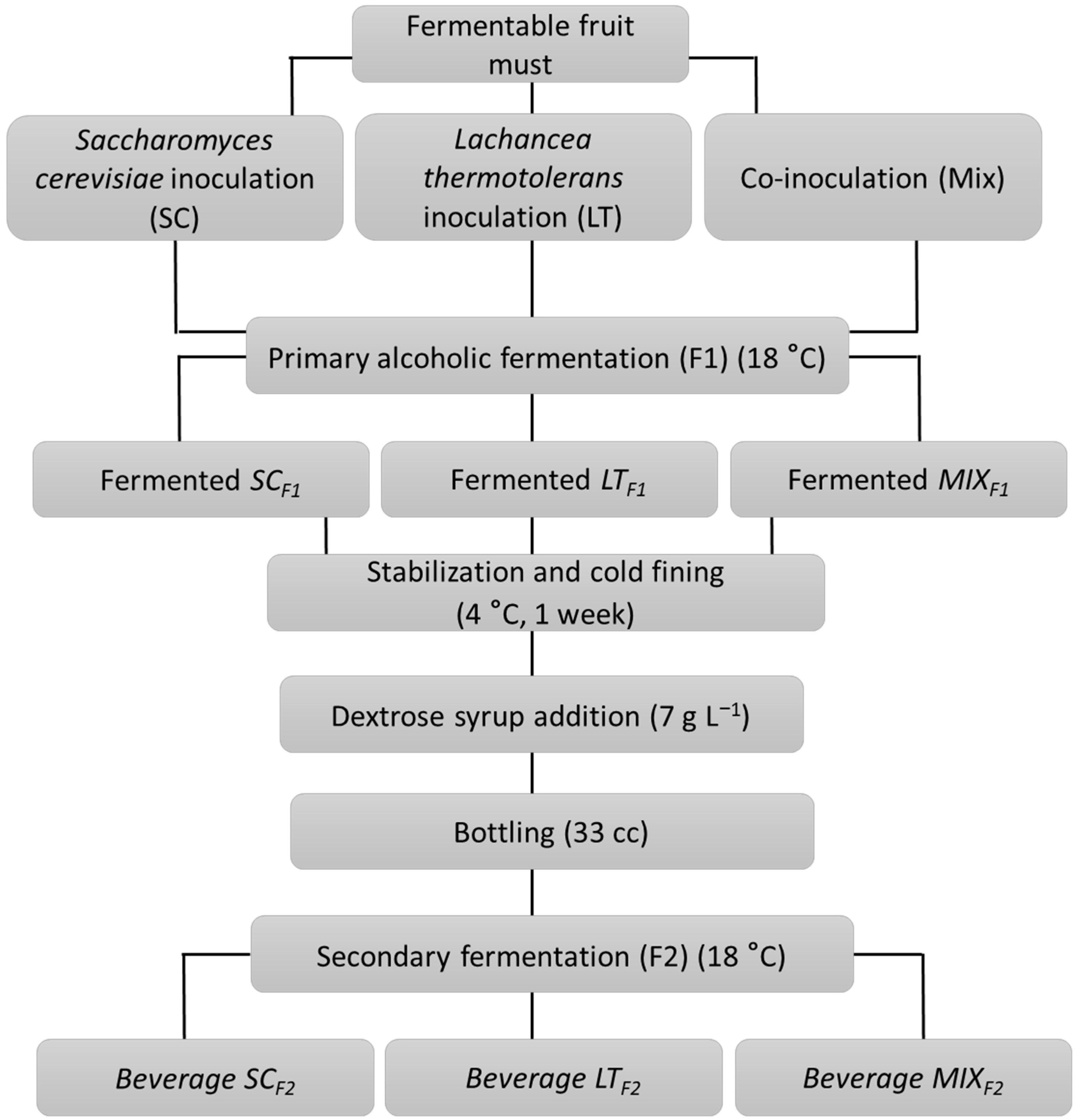
2.2. Preparation of Samples and Pure and Co-Inoculated Fermentation Process
2.3. Microbiological Analysis
2.4. Must and Fermented Beverages Chemical Analysis
2.4.1. General Parameters
2.4.2. Phenolic Content and Chromatic Characteristics
2.4.3. Total Antioxidant Activity
2.5. Determination of Amino Acids in Musts and Fermented Beverages
- −
- Total amino content (TAC, mg L−1).
- −
- Free amino nitrogen (FAN, mgN L−1): calculated as the sum of the nitrogen concentration of all quantified free amino acids.
- −
- Yeast assimilable nitrogen (YAN, mgN L−1): calculated as the exclusion of the concentration of nitrogen contributed by hydroxyproline and proline amino acids from the FAN value and the sum of the concentration of nitrogen contributed by the ammonium ion.
- −
- Aromatic precursor nitrogen (APN, mgN L−1): calculated as the sum of the nitrogen concentration contributed by the amino acids’ aspartic acid (Asp), isoleucine (Ille), leucine (Leu), threonine (Thr), tyrosine (Tyr), and valine (Val).
- −
- Sulfur amino nitrogen (SAN, mgN L−1): calculated as the sum of the nitrogen concentration contributed by the amino acids’ taurine (Tau), cysteine (Cys), and methionine (Met).
- −
- Amines (AM, mgN L−1): calculated as the nitrogen concentration contributed by the amino acid ethanolamine (Ethan).
2.6. Analysis of the Volatile Compounds by GC-MS
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Fermentation and Microbiological Parameters
Fermentation Dynamics Control
3.2. Chemical Analysis of Processed Beverages
3.2.1. General Oenological Parameters
3.2.2. Total Concentration of Phenols, Chromatic Characteristics, and Antioxidative Properties
3.3. Results of the Detailed Amino Acid Profile
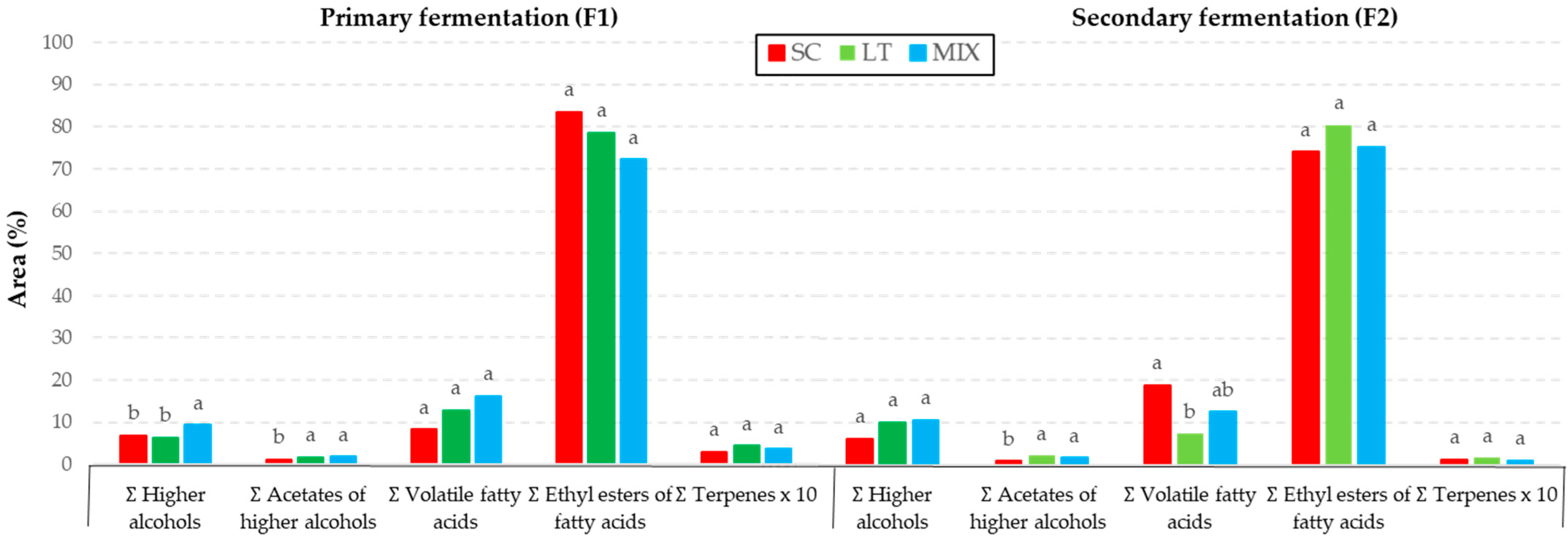
3.4. Volatile Beverage Compounds
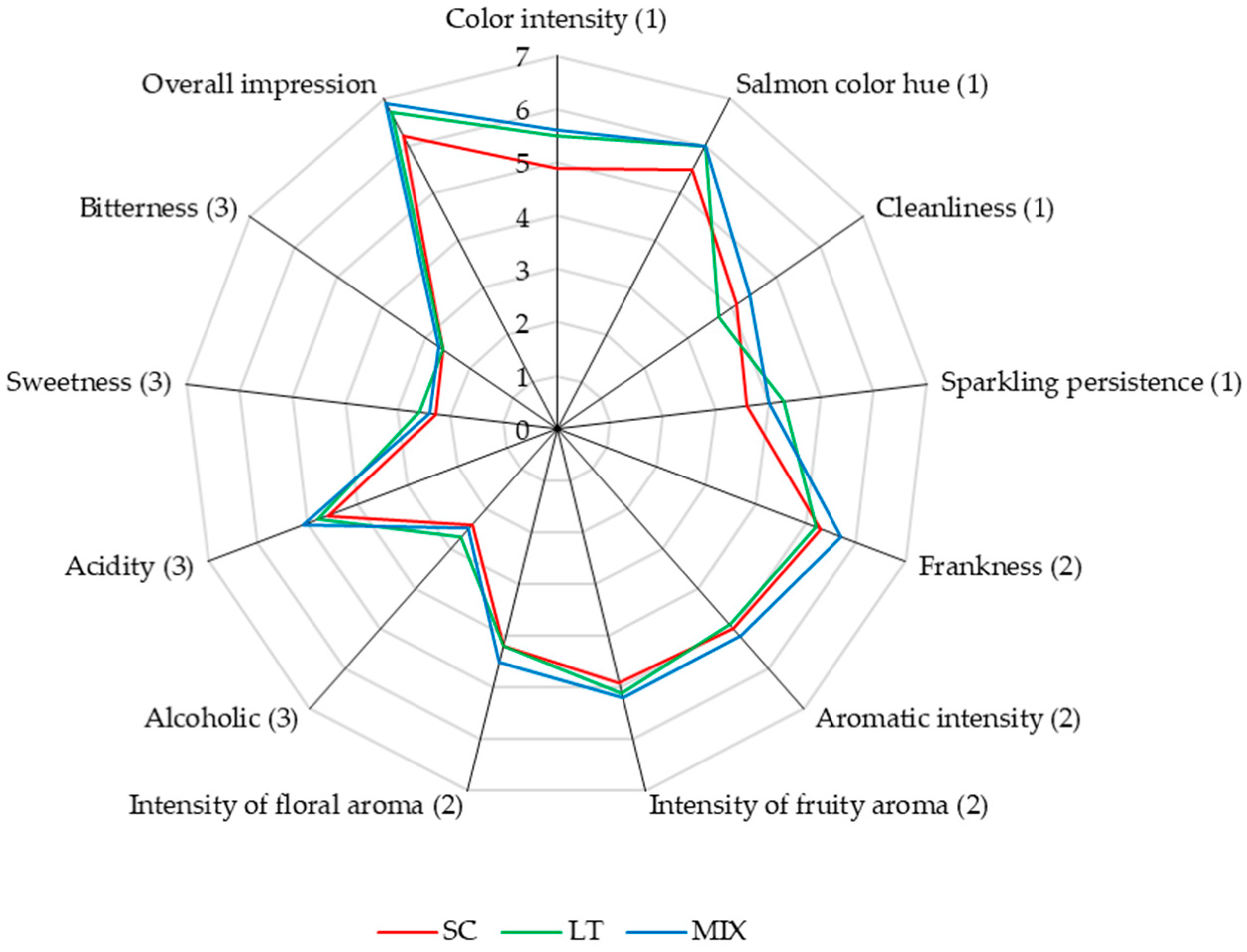
3.5. Sensory Analysis
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podstawski, R.; Wesołowska, E.; Choszcz, D.; Markowski, P.; Klimczak, J. Drinking behaviours and alcohol preferences of female and male students at a Polish university in 2000–2016. DrugsEduc. Prev. Policy 2017, 26, 280–286. [Google Scholar] [CrossRef]
- Muggah, E.M.; McSweeney, M.B. Females’ attitude and preference for beer: A conjoint analysis study. Int. J. Food Sci. 2017, 52, 808–816. [Google Scholar] [CrossRef]
- Gschaedler, A. Contribution of Non-Conventional Yeasts in Alcoholic Beverages. Curr. Opin. Food Sci. 2017, 13, 73–77. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Ciani, M.; Maccarelli, F. Oenological properties of non-Saccharomyces yeasts associated with wine-making. World J. Microbiol. Biotechnol. 1997, 14, 199–203. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef]
- Mora, J.; Barbas, J.I.; Ramis, B.; Mulet, A. Yeast microflora associated with some Majorcan musts and wines. Am. J. Enol. Vitic. 1988, 39, 344–346. [Google Scholar]
- Torija, M.J.; Rozes, N.; Poblet, M.; Guillamón, J.M.; Mas, A. Yeast population dynamics in spontaneous fermentations: Comparison between two different wine-producing areas over a period of three years. Antonie Van Leeuwenhoek Anton. Leeuw Int. J. G. 2001, 79, 345–352. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and fermentation characteristics of a strain of the wine yeast Kluyveromyces thermotolerans isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Nurgel, C.; Erten, H.; Canbas, A.; Cabaroglu, T.; Selli, S. Yeast flor during the fermentation of wines made from Vitis vinifera L. cv. Emir and Kalecik Karasi grown in Anatolia. World J. Microbiol. Biotechnol. 2005, 21, 1187–1194, Erratum in World J. Microbiol. Biotechnol. 2006, 22, 95–96. [Google Scholar] [CrossRef]
- Morata, A.; Bañuelos, M.A.; Vaquero, C.; Loira, I.; Cuerda, R.; Palomero, F.; González, C.; Suárez-Lepe, J.A.; Wang, J.; Han, S.; et al. Lachancea thermotolerans as a tool to improve pH in red wines from warm regions. Eur. Food Res. Technol. 2019, 245, 885–894. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, M.; Comitini, F.; Domizio, C.; Lecioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 3, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kapsopoulou, K.; Mourtzini, A.; Anthoulas, M.; Nerantzis, E. Biological acidifcation during grape must fermentation using mixed cultures of Kluyveromyces thermotolerans and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2007, 23, 735–739. [Google Scholar] [CrossRef]
- Benito, N.; Calderón, F.; Palomero, F.; Benito, S. Quality and Composition of Airén Wines Fermented by Sequential Inoculation of Lachancea thermotolerans and Saccharomyces cerevisiae. Food Technol. Biotechnol. 2016, 54, 135. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Palomero, F.; Benito, S. Combine use of selected Schizosaccharomyces pombe and Lachancea thermotolerans yeast strains as an alternative to the traditional malolactic fermentation in red wine production. Molecules 2015, 20, 9510–9523. [Google Scholar] [CrossRef]
- Sgouros, G.; Mallouchos, A.; Filippousi, M.E.; Banilas, G.; Nisiotou, A. Molecular characterization and enological potential of a high lactic acid-producing Lachancea thermotolerans vineyard strain. Foods 2020, 9, 595. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine (OIV). Recueil des Methodes Internationales d’Analyse des Vins et des Moûts; Organisation Internationale de la Vigne et du Vin: Paris, France, 1990. [Google Scholar]
- Zoecklein, B.W.; Fugelsang, K.C.; Gump, B.H.; Nury, F.S. Wine Analysis and Production, 1st ed.; Van Nostrand and Reinhold: New York, NY, USA, 1990. [Google Scholar]
- International Organization of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis Vol. 1 & 2; OIV: Paris, France, 2010. [Google Scholar]
- International Organization of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis Vol. 1 & 2; OIV: Paris, France, 2016. [Google Scholar]
- Singleton, V.L.; Rossi, J.A., Jr. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 30, 144–158. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Glories, Y. La maturitá fenólica delle uve: Primo parámetro da controllare peruna corretta vinificazione in rosso. Vignevini 1999, 3, 46–50. [Google Scholar]
- Ayala, F.; Echávarri, J.F.; Negueruela, A.I. A new simplified method for measuring the color of wines. I. Red and rose wines. Am. J. Enol. Vitic. 1997, 48, 357–363. [Google Scholar] [CrossRef]
- Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Buxaderas, S.; de la Torre-Boronat, M.D.C. Influence of variety and aging on foaming properties of cava (sparkling wine). 2. J. Agric. Food Chem. 1997, 45, 2520–2525. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Valdés, E.; Vilanova, M.; Sabio, E.; Benalte, M.J. Clarifying agents effect on the nitrogen composition in must and wine during fermentation. Food chem. 2011, 125, 430–437. [Google Scholar] [CrossRef]
- Valdés, M.E.; Talaverano, M.I.; Moreno, D.; Prieto, M.H.; Mancha, L.A.; Uriarte, D.; Vilanova, M. Effect of the timing of water deficit on the must amino acid profile of Tempranillo grapes grown under the semiarid conditions of SW Spain. Food Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Rodrigues, C.; Portugal, F.C.M.; Nogueira, J.M.F. Static headspace analysis using polyurethane phases-Application to roasted coffee volatiles characterization. Talanta 2012, 89, 521–525. [Google Scholar] [CrossRef]
- UNE-EN ISO 8589:2010; Análisis sensorial. Guía general para el diseño de una sala de cata. ISO: Geneva, Switzerland, 2007.
- Porter, T.J.; Divol, B.; Setati, M.E. Investigating the biochemical and fermentation attributes of Lachancea species and strains: Deciphering the potential contribution to wine chemical composition. Int. J. Food Microbiol. 2019, 290, 273–287. [Google Scholar] [CrossRef]
- Balikci, E.K.; Tanguler, H.; Jolly, N.P.; Erten, H. Influence of Lachancea thermotolerans on cv. Emir wine fermentation. Yeast 2016, 33, 313–321. [Google Scholar] [CrossRef]
- Alexandre, H. Autolysis of Yeasts. Compr. Biotechnol. 2011, 2, 641–649. [Google Scholar] [CrossRef]
- Villacreces, S.; Blanco, C.A.; Caballero, I. Developments and characteristics of craft beer production processes. Food Biosci. 2022, 45, 101495. [Google Scholar] [CrossRef]
- Zdaniewicz, M.; Satora, P.; Pater, A.; Bogacz, S. Low lactic acid-producing strain of Lachancea thermotolerans as a new starter for beer production. Biomolecules 2020, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.G.; Rodríguez, M.E.; Oteiza, J.M.; Barbagelata, R.J.; Lopes, C.A. Physiological characterization of Saccharomyces uvarum and Saccharomyces eubayanus from Patagonia and their potential for cidermaking. Int. J. Food Microbiol. 2017, 249, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Lopes, D.; Delgado, T.; Canas, S.; Anjos, O. Development of blueberry liquor: Influence of distillate, sweetener and fruit quantity. J. Sci. Food Agric. 2018, 98, 1088–1094. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Varela, C.; Henschke, P.A.; Chambers, P.J.; Stanley, G.A. Microbiological approaches to lowering ethanol concentration in wine. Trends Food Sci. Technol. 2010, 21, 293–302. [Google Scholar] [CrossRef]
- Varela, C.; Dry, P.R.; Kutyna, D.R.; Francis, I.L.; Henschke, P.A.; Curtin, C.D.; Chambers, P.J. Strategies for reducing alcohol concentration in wine. Aust. J. Grape Wine Res. 2015, 21, 670–679. [Google Scholar] [CrossRef]
- Mora, J.; Barbas, J.I.; Mulet, A. Growth of yeast species during the fermentation of musts inoculated with Kluyveromyces thermotolerans and Saccharomyces cerevisiae. Am. J. Enol. Vitic. 1990, 41, 156–159. [Google Scholar]
- Vaquero, C.; Izquierdo-Cañas, P.M.; Mena-Morales, A.; Marchante-Cuevas, L.; Heras, J.M.; Morata, A. Use of lachancea thermotolerans for biological vs. Chemical acidification at pilot-scale in white wines from warm areas. Fermentation 2021, 7, 193. [Google Scholar] [CrossRef]
- Benito, A.; Calderon, F.; Benito, S. The combined use of Schizosaccharomyces pombe and Lachancea thermotolerans-effect on the anthocyanin wine composition. Molecules 2017, 22, 739. [Google Scholar] [CrossRef]
- Chen, K.; Escott, C.; Loira, I.; del Fresno, J.M.; Morata, A.; Tesfaye, W.; Calderon, F.; Suárez-Lepe, J.A.; Han, S.; Benito, S. Use of non-Saccharomyces yeasts and oenological tannin in red winemaking: Influence on colour, aroma and sensorial properties of young wines. Food Microbiol. 2018, 69, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2017, 24, 166–180. [Google Scholar] [CrossRef]
- Blouin, J.; Peynaud, E. Enología práctica. In Conocimiento y Elaboración del Vino, 4th ed.; Ed.Mundi-Prensa: Barcelona, Spain, 2004. [Google Scholar]
- Suárez, C.; Palacios, A.; Santiago, L.; Santamaría, P.; López, R.; Gutiérrez, A.R. Efecto del uso de levaduras inactivas en las paradas de fermentación. Influencia de los ácidos grasos en el desarrollo de la fermentación alcohólica. SEVI 2003, 2990, 4134–4140. [Google Scholar]
- Roca-mesa, H.; Sendra, S.; Mas, A.; Beltran, G.; Torija, M.J. Nitrogen preferences during alcoholic fermentation of different non-saccharomyces yeasts of oenological interest. Microorganisms 2020, 8, 157. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; De Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile composition of Merlot red wine and its contribution to the aroma: Optimization and validation of analytical method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Synos, K.; Reynolds, A.G.; Bowen, A.J. Effect of yeast strain on aroma compounds in Cabernet franc ice wines. LWT-Food Sci. Technol. 2015, 64, 227–235. [Google Scholar] [CrossRef]
- Hranilovic, A.; Albertin, W.; Capone, D.L.; Gallo, A.; Grbin, P.R.; Danner, L.; Bastian, S.E.P.; Masneuf-Pomarede, I.; Coulon, J.; Bely, M.; et al. Impact of Lachancea thermotolerans on chemical composition and sensory profiles of Merlot wines. Food Chem. 2021, 349, 129015. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Escribano, R.; González-Arenzana, L.; Portu, J.; Garijo, P.; López-Alfaro, I.; López, R.; Santamaría, P.; Gutiérrez, A.R. Aromatic compound production and fermentative behavior within different non- Saccharomyces species and clones. J. Appl. Microbiol. 2018, 124, 1521–1531. [Google Scholar] [CrossRef]
- Wang, X.J.; Tao, Y.S.; Wu, Y.; An, R.Y.; Yue, Z.Y. Aroma compounds and characteristics of noble-rot wines of Chardonnay grapes artificially botrytized in the vineyard. Food Chem. 2017, 226, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, M.; Genisheva, Z.; Masa, A.; Oliveira, J.M. Correlation between volatile composition and sensory properties in Spanish Albariño wines. Microchem. J. 2010, 95, 240–246. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Jiang, X.M.; Zhao, Y.P. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae on alcoholic fermentation behaviour and wine aroma of cherry wines. Food Microbiol. 2014, 44, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Gong, H.S.; Zhao, Y.P.; Liu, W.L.; Jin, C. Sequential culture with Torulaspora delbrueckii and Saccharomyces cerevisiae and management of fermentation temperature to improve cherry wine quality. J. Sci. Food Agric. 2016, 96, 1880–1887. [Google Scholar] [CrossRef]



| General Oenological Parameters | ||||||
| Density (g mL−1) | Total soluble solids (°Brix) | pH | Titratable acidity (g malic acid L−1) | Malic acid (g L−1) | Tartaric acid (g L−1) | |
| 1.042 | 10.76 | 3.53 | 5.18 | 5.03 | 0.29 | |
| Nitrogen parameters | ||||||
| Total amino content (TAC, mg L−1) | Free amino nitrogen (FAN, mgN L−1) | Yeast assimilable nitrogen (YAN, mgN L−1) | Ammonium (mgN L−1) | Aromatic precursor nitrogen (APN, mgN L−1) | ||
| 616.04 | 111.77 | 110.13 | 15.74 | 7.83 | ||
| Phenolic parameters | ||||||
| Total phenols (mg L−1) | Total anthocyanins (mg L−1) | TAADPPH (mmol TE L−1) | TAAABTS (mmol TE L−1) | |||
| 507.47 | 17.64 | 4.54 | 2.78 | |||
| Chromatic parameters | ||||||
| Red− greenness (a*) | Yellow− blueness (b*) | Lightness (L*) | Chroma (C*ab) | Hue angle (hab) | Color intensity (CI, u.a) | Color hue (CT) |
| 1.16 | 5.19 | 97.6 | 5.33 | 78.31 | 0.13 | 2.15 |
| Variable | Primary Fermentation (F1) | Secondary Fermentation (F2) | ||||
|---|---|---|---|---|---|---|
| Treatment | Treatment | |||||
| General oenological parameters | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Ethanol (% v/v) | 5.32 a | 5.27 a | 5.34 a | 5.71 a | 5.70 a | 5.71 a |
| pH | 3.57 a | 3.52 b | 3.54 ab | 3.57 a | 3.55 a | 3.55 a |
| Titratable acidity (g malic acid L−1) | 5.43 b | 5.64 ab | 5.76 a | 6.42 a | 6.20 b | 6.15 b |
| Volatile acidity (g acetic acid L−1) | 0.20 a | 0.21 a | 0.14 a | 0.21 a | 0.30 a | 0.29 a |
| Tartaric acid (g L−1) | 0.36 a | 0.24 a | 0.29 a | 0.38 a | 0.30 ab | 0.17 b |
| Malic acid (g L−1) | 4.31 b | 4.50 a | 4.34 ab | 5.08 a | 5.06 a | 5.20 a |
| Lactic acid (g L−1) | 0.04 a | 0.06 a | 0.04 a | 0.02 a | 0.04 a | 0.03 a |
| Phenolic parameters | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Total phenols (mg L−1) | 306.20 b | 340.79 ab | 375.37 a | 313.90 a | 331.60 a | 306.22 a |
| Total anthocyanins (mg L−1) | 6.97 a | 9.57 a | 6.17 a | 8.72 a | 11.56 a | 8.55 a |
| Total hydroxycinnamic acid index (u.a) | 4.92 a | 5.18 a | 5.05 a | 4.80 a | 4.84 a | 4.77 a |
| Total flavonoid compounds index (u.a) | 5.38 a | 4.77 a | 5.12 a | 5.31 a | 4.19 a | 4.56 a |
| Chromatic parameters | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Red−greenness (a*) | 8.40 a | 9.43 a | 9.34 a | 5.45 a | 5.80 a | 6.92 a |
| Yellow−blueness (b*) | 25.73 a | 28.55 a | 27.18 a | 20.57 a | 22.02 a | 21.92 a |
| Lightness (L*) | 84.63 a | 83.40 a | 83.60 a | 89.37 a | 88.67 a | 88.00 a |
| Chroma (C*ab) | 27.07 a | 30.07 a | 28.74 a | 21.29 a | 22.77 a | 22.99 a |
| Hue angle (hab) | 71.95 a | 71.72 a | 71.04 a | 75.20 a | 75.36 a | 72.49 b |
| Color intensity (CI, u.a) | 0.83 a | 0.92 a | 0.90 a | 0.59 a | 0.63 a | 0.65 a |
| Color hue (CT) | 1.92 b | 1.98 a | 1.93 b | 2.09 ab | 2.13 a | 1.99 b |
| Total antioxidant activity (TAA) | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| TAADPPH (mmol TE L−1) | 3.34 a | 3.69 a | 3.48 a | 3.05 a | 3.31 a | 3.31 a |
| TAAABTS (mmol TE L−1) | 2.63 a | 2.65 a | 2.79 a | 2.48 a | 2.63 a | 2.37 a |
| Variable | Primary Fermentation (F1) | Secondary Fermentation (F2) | ||||
|---|---|---|---|---|---|---|
| Treatment | Treatment | |||||
| SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 | |
| Total amino content (TAC, mg L−1) | 431.47 a | 624.82 a | 382.59 a | 450.34 a | 463.99 a | 357.80 a |
| Free amino nitrogen (FAN, mg N L−1) | 63.53 a | 105.52 a | 60.64 a | 65.43 a | 78.23 a | 56.02 a |
| Yeast assimilable nitrogen (YAN, mg N L−1) | 43.40 a | 88.64 a | 46.58 a | 51.20 a | 69.51 a | 44.98 a |
| Aromatic precursor nitrogen (APN, mg N L−1) | 4.97 a | 2.27 a | 2.82 a | 7.36 a | 2.64 b | 2.89 b |
| Sulfur amino nitrogen (SAN, mg N L−1) | 2.05 a | 0.80 b | 0.88 b | 1.94 a | 0.88 b | 1.03 b |
| Amines (AM, mg N L−1) | 1.47 a | 1.42 a | 1.30 a | 1.34 ab | 1.14 b | 1.52 a |
| Histidine (mg N L−1) | 0.57 a | 0.25 a | 0.45 a | 0.39 a | 0.10 b | 0.03 b |
| Aspartic acid (mg N L−1) | 0.68 a | 0.42 a | 0.51 a | 0.90 a | 0.47 ab | 0.39 b |
| Alanine (mg N L−1) | 5.06 a | 4.38 a | 3.56 a | 5.48 a | 4.10 a | 3.10 a |
| Arginine (mg N L−1) | 4.59 a | 8.42 a | 11.48 a | 3.30 b | 8.31 ab | 11.80 a |
| Asparagine (mg N L−1) | 15.37 a | 45.03 a | 19.39 a | 17.38 a | 32.13 a | 11.68 a |
| Phenylalanine (mg N L−1) | 1.10 a | 0.35 a | 0.49 a | 1.50 a | 0.54 b | 0.70 b |
| Glycine (mg N L−1) | 2.81 a | 3.09 a | 2.77 a | 2.69 a | 2.48 a | 2.87 a |
| Isoleucine (mg N L−1) | 0.60 a | 0.15 a | 0.26 a | 0.90 a | 0.24 b | 0.24 b |
| Lysine (mg N L−1) | 1.45 a | 0.72 a | 1.12 a | 1.65 a | 0.34 b | 0.36 b |
| Leucine (mg N L−1) | 1.61 a | 0.60 a | 0.84 a | 2.29 a | 0.71 b | 0.81 b |
| Ornithine (mg N L−1) | 0.95 a | 0.89 a | 0.64 a | 0.88 a | 0.84 a | 0.87 a |
| Methionine (mg N L−1) | 0.52 a | 0.14 a | 0.21 a | 0.56 a | 0.13 b | 0.12 b |
| Serine (mg N L−1) | 0.73 a | 0.70 a | 0.57 a | 0.89 a | 0.42 ab | 0.26 b |
| Tyrosine (mg N L−1) | 0.48 a | 0.36 a | 0.34 a | 0.94 a | 0.37 b | 0.56 ab |
| Threonine (mg N L−1) | 0.64 a | 0.45 a | 0.39 a | 0.81 a | 0.30 b | 0.19 b |
| Valine (mg N L−1) | 0.96 a | 0.29 a | 0.48 a | 1.53 a | 0.56 b | 0.70 b |
| Taurine (mg N L−1) | 0.63 a | 0.49 a | 0.49 a | 0.7 a | 0.5 b | 0.6 ab |
| Cysteine (mg N L−1) | 0.91 a | 0.17 a | 0.18 a | 0.67 a | 0.52 b | 0.65 b |
| Proline (mg N L−1) | 21.67 a | 18.45 ab | 15.50 b | 14.96 a | 9.91 c | 12.65 a |
| Hydroxyproline (mg N L−1) | 0.24 a | 0.21 a | 0.26 a | 0.08 a | 0.00 a | 0.11 a |
| Ethanolamine (mg N L−1) | 1.47 a | 1.42 a | 1.30 a | 1.34 ab | 1.14 b | 1.52 a |
| Ammonium (mg N L−1) | 0.91 a | 1.10 a | 0.95 a | 0.99 a | 1.19 a | 1.21 a |
| Compound (%) | Primary Fermentation (F1) | Secondary Fermentation (F2) | ||||
|---|---|---|---|---|---|---|
| Treatment | Treatment | |||||
| Higher alcohols | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| 3-methyl-1-butanol | 3.68 b | 3.27 b | 5.14 a | 3.50 a | 5.08 a | 5.96 a |
| 2-methyl-1-butanol | 1.12 b | 0.97 b | 1.82 a | 1.03 a | 1.75 a | 1.48 a |
| 2-phenylethanol | 2.00 a | 2.17 a | 2.38 a | 1.64 a | 3.23 a | 3.16 a |
| Acetates of higher alcohols | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Isobutyl acetate | 0.07 a | 0.07 a | 0.07 a | 0.05 b | 0.08 a | 0.05 b |
| Isoamyl acetate | 1.06 b | 1.59 a | 1.74 a | 0.78 b | 2.01 a | 1.58 a |
| Volatile fatty acids | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Octanoic acid | 3.20 a | 9.58 a | 11.38 a | 13.29 a | 4.65 b | 8.43 ab |
| Butyl caprate | 0.28 a | 0.12 b | 0.12 b | 0.16 a | 0.08 b | 0.08 b |
| n-decanoic acid | 1.31 a | 0.00 a | 1.14 a | 2.73 a | 0.70 b | 1.36 b |
| 9-decanoic acid | 3.41 a | 3.01 a | 3.38 a | 2.52 a | 1.69 a | 2.44 a |
| Acetic acid | 0.08 b | 0.21 ab | 0.23 a | 0.14 a | 0.15 a | 0.14 a |
| Ethyl esters of fatty acids | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Ethyl laurate | 35.96 a | 28.72 a | 29.23 a | 29.89 a | 30.89 a | 25.08 a |
| Ethyl palmitate | 1.02 a | 0.71 a | 0.93 a | 2.15 a | 1.44 a | 1.34 a |
| Ethyl 9-hexadecenoate | 0.50 a | 0.33 a | 0.37 a | 0.32 a | 0.10 b | 0.16 b |
| Isoamyl octanoate | 1.25 a | 1.30 a | 1.19 a | 0.84 a | 0.83 a | 0.90 a |
| Isopentyl decanoate | 4.36 a | 2.62 b | 3.39 ab | 2.92 a | 2.20 b | 2.33 ab |
| Isopentyl laurate | 0.85 a | 0.31 b | 0.47 ab | 0.69 a | 0.44 a | 0.31 a |
| Ethyl myristate | 3.91 a | 1.69 b | 1.94 b | 3.85 a | 2.87 b | 1.97 b |
| Ethyl octanoate | 6.73 b | 9.59 a | 9.76 a | 6.09 b | 9.41 a | 9.21 a |
| Ethyl hexanoate | 2.28 a | 2.65 a | 2.71 a | 1.72 a | 2.11 a | 1.64 a |
| Ethyl decanoate | 26.64 a | 30.67 a | 22.22 a | 25.55 a | 30.12 a | 32.28 a |
| Terpenes | SCF1 | LTF1 | MIXF1 | SCF2 | LTF2 | MIXF2 |
| Humulene | 0.30 a | 0.44 a | 0.36 a | 0.12 a | 0.16 a | 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno, D.; Redondo, P.; Lozano, E.; Valdés, M.E.; Pérez-Nevado, F. Use of Mixed Cultures for the Production of Grape–Plum Low-Alcohol Fermented Beverages. Fermentation 2023, 9, 29. https://doi.org/10.3390/fermentation9010029
Moreno D, Redondo P, Lozano E, Valdés ME, Pérez-Nevado F. Use of Mixed Cultures for the Production of Grape–Plum Low-Alcohol Fermented Beverages. Fermentation. 2023; 9(1):29. https://doi.org/10.3390/fermentation9010029
Chicago/Turabian StyleMoreno, Daniel, Patricia Redondo, Eduardo Lozano, M. Esperanza Valdés, and Francisco Pérez-Nevado. 2023. "Use of Mixed Cultures for the Production of Grape–Plum Low-Alcohol Fermented Beverages" Fermentation 9, no. 1: 29. https://doi.org/10.3390/fermentation9010029
APA StyleMoreno, D., Redondo, P., Lozano, E., Valdés, M. E., & Pérez-Nevado, F. (2023). Use of Mixed Cultures for the Production of Grape–Plum Low-Alcohol Fermented Beverages. Fermentation, 9(1), 29. https://doi.org/10.3390/fermentation9010029