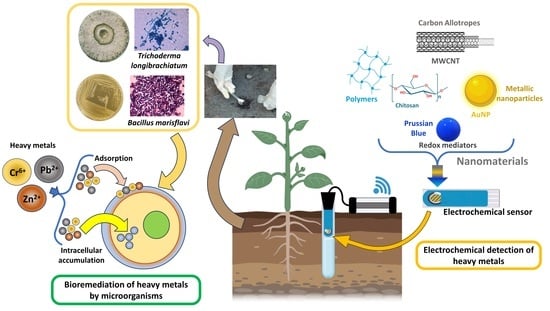
Microbial Removal of Heavy Metals from Contaminated Environments Using Metal-Resistant Indigenous Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. Elemental Analysis of Soil Samples
2.4. Isolation and Identification of Microorganisms from the Contaminated Soil
2.5. Metal Tolerance Assessment and Effect on Microbial Growth
2.6. Removal Efficiency Assessment
2.7. Electrochemical Detection of Heavy Metal Concentration in Solution
2.7.1. Electrochemical Measurements
2.7.2. Preparation of the Nanomaterials Based Electrochemical Sensors
2.8. SEM-EDX and FTIR Analysis
2.9. Data Analysis
3. Results and Discussion
3.1. Elemental Analysis of Contaminated Soil
3.2. Isolation and Identification of the Metal-Tolerant Microorganisms
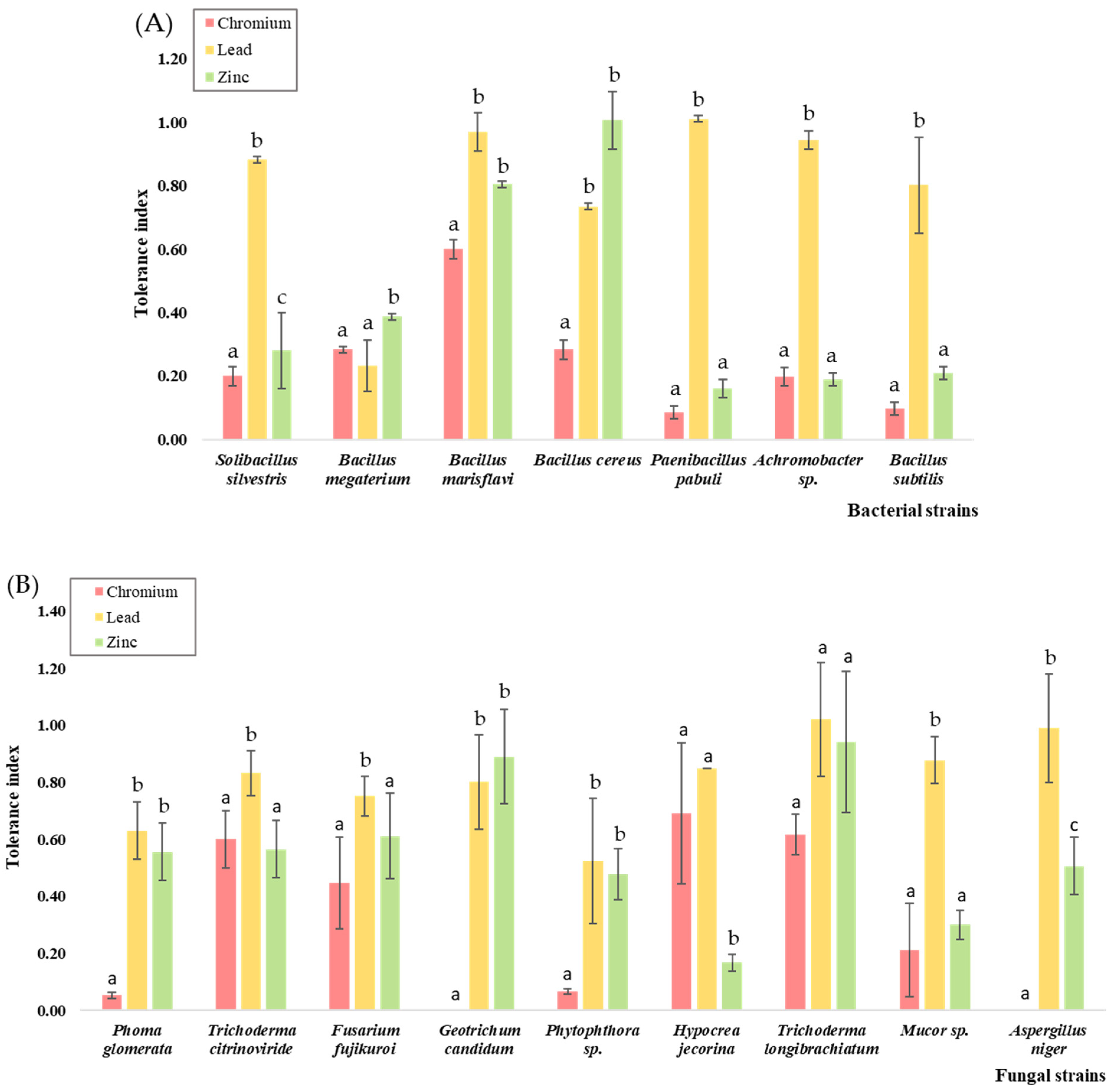
3.3. Metal Tolerance Assessment and Effect on Microbial Growth
3.4. Removal Efficiency Assessment
3.5. Electrochemical Detection of Heavy Metals Concentration
3.5.1. Cyclic Voltammetry Studies
3.5.2. Amperometric Studies
3.6. Electrochemical Detection of Heavy Metals in Treated and Untreated Supernatant
3.7. SEM/EDX Characterization of the Microbial Biomass
3.8. FTIR Characterization of the Microbial Biomass
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ukaogo, P.O.; Ewuzie, U.; Onwuka, C.O. Environmental pollution: Causes, effects, and the remedies. In Microorganisms for Sustainable Environment and Health; Chowdhary, P., Raj, A., Verma, D., Akhter, Y., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Volume 21, pp. 419–429. [Google Scholar] [CrossRef]
- Khomenko, S.; Cirach, M.; Pereira-Barboza, E.; Mueller, N.; Barrera-Gomez, J.; Rojas-Rueda, D.; Hoogh, K.; Hoek, G.; Nieuwenhujsen, M. Premature mortality due to air pollution in European cities: A health impact assessment. Lancet Planet. Health 2021, 5, 121–134. [Google Scholar] [CrossRef]
- Münzel, T.; Hahad, O.; Daiber, A.; Landrigan, P.J. Soil and water pollution and human health: What should cardiologists worry about? Cardiovasc. Res. 2023, 119, 440–449. [Google Scholar] [CrossRef]
- Burkhardt, J.; Bayham, J.; Wilson, A.; Carter, E.; Berman, J.D.; O’Dell, K.; Ford, B.; Fischer, E.V.; Pierce, J.R. The effect of pollution on crime: Evidence from data on particulate matter and ozone. J. Environ. Econ. Manag. 2019, 98, 102267. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, G.; Gupta, A.; Tiwari, R.K. Heavy Metal/Metalloid Contamination: Their Sources in Environment and Accumulation in Food Chain. In Heavy Metal Toxicity: Environmental Concerns, Remediation and Opportunities; Singh, R.P., Singh, P., Srivastava, A., Eds.; Springer Nature: Singapore, 2023; Volume 2, pp. 19–47. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil Sediment Contam. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Major metals demand, supply and environmental impacts to 2100: A critical review. Resour. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- Pouresmaieli, M.; Ataei, M.; Forouzandeh, P.; Azizollahi, P.; Mahmoudifard, M. Recent progress on sustainable phytoremediation of heavy metals from soil. J. Environ. Chem. Eng. 2022, 10, 108482. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Ding, K.; Liu, W.S.; Baker, A.J.M.; Fei, Y.H.; Wang, Y.; Jin, C.; Wang, S.; Tang, Y.T.; et al. Heavy metal contamination affects the core microbiome and assembly processes in metal mine soils across Eastern China. J. Hazard. Mater. 2023, 443, 130241. [Google Scholar] [CrossRef] [PubMed]
- Emenike, C.U.; Jayanthi, B.; Agamuthu, P.; Fauziah, S.H. Biotransformation and removal of heavy metals: A review of phytoremediation and microbial remediation assessment on contaminated soil. Environ. Rev. 2018, 26, 156–168. [Google Scholar] [CrossRef]
- Shen, X.; Chi, Y.; Xiong, K. The effect of heavy metal contamination on humans and animals in the vicinity of a zinc smelting facility. PLoS ONE 2019, 14, e0207423. [Google Scholar] [CrossRef]
- Liang, Y.; Pan, Z.; Zhu, M.; Gao, R.; Wang, Y.; Cheng, Y.; Zhang, N. Exposure to essential and non-essential trace elements and risks of congenital heart defects: A narrative review. Front. Nutr. 2023, 10, 1121826. [Google Scholar] [CrossRef]
- Unitted States Environmental Protection Agency (US EPA). Framework for Metals Risk Assessment, Office of the Science Advisor Risk Assessment Forum, EPA 120/R-07/001, 2007, 1–11. Available online: https://www.epa.gov/risk/framework-metals-risk-assessment (accessed on 1 October 2023).
- Rigueto, V.T.C.; Nazari, M.T.; Pizzutti, I.R.; Chandrasekaran, N.; Dettmer, A.; Piccin, J.S. Toxic Metals An Overview of Main Sources, Exposure Routes, Adverse Effects and Treatment Approaches. In Toxic Metals Contamination Generation, Disposal, Treatment and Valuation; Piccin, J.S., Dettmer, A., Chandrasekaran, N., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–10. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 227. [Google Scholar] [CrossRef]
- Monga, A.; Fulke, A.B.; Dasgupta, D. Recent developments in essentiality of trivalent chromium and toxicity of hexavalent chromium: Implications on human health and remediation strategies. J. Hazard. Mater. Adv. 2022, 7, 100113. [Google Scholar] [CrossRef]
- Colás, R. Ancient Lead, Mercury, and Tin. In Reverse Engineering of Ancient Metals; Carrizo, P.S., Ed.; Springer: Cham, Switzerland, 2022; pp. 29–40. [Google Scholar] [CrossRef]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of Microstructures and Properties of Zinc Alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef]
- Laxmi, V.; Kaushik, G. Toxicity of Hexavalent Chromium in Environment, Health Threats, and Its Bioremediation and Detoxification from Tannery Wastewater for Environmental Safety. In Bioremediation of Industrial Waste for Environmental Safety; Saxena, G., Bharagava, R.N., Eds.; Springer: Singapore, 2019; Volume 11, pp. 223–243. [Google Scholar] [CrossRef]
- Mikhaylina, A.; Ksibe, A.Z.; Scanlan, D.J.; Blindauer, C.A. Bacterial zinc uptake regulator proteins and their regulons. Biochem. Soc. Trans. 2018, 46, 983–1001. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, C.; Zhou, N.; Chi, R. Speciation, toxicity, microbial remediation and phytoremediation of soil chromium contamination. Environ. Chem. Lett. 2021, 19, 1413–1431. [Google Scholar] [CrossRef]
- Sahoo, S.; Goli, D. Bioremediation of Lead by a Halophilic Bacteria Bacillus pumilus Isolated from the Mangrove Regions of Karnataka. Int. J. Sci. Res. 2020, 9, 1337–1343. Available online: https://www.ijsr.net/get_count.php?paper_id=ART20204172 (accessed on 14 November 2023).
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the Underlying Heavy Metal Detoxification Mechanisms of Bacillus Species. Microorganisms 2021, 9, 1628. [Google Scholar] [CrossRef]
- El-Bondkly, A.M.A.; El-Gendy, M.M.A.A. Bioremoval of some heavy metals from aqueous solutions by two different indigenous fungi Aspergillus sp. AHM69 and Penicillium sp. AHM96 isolated from petroleum refining wastewater. Heliyon 2022, 8, 1–16. [Google Scholar] [CrossRef]
- Singh, K.N.; Narzary, D. Diversity and heavy metal tolerance of fungi associated with different coal overburden strata of Tikak Colliery, Assam. Curr. Microbiol. 2023, 80, 72. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Balan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Rehman, Z.; Junair, M.F.; Ijaz, N.; Khalid, U.; Ijaz, Z. Remediation methods of heavy metal contaminated soils from environmental and geotechnical standpoints. Sci. Total Environ. 2023, 867, 161468. [Google Scholar] [CrossRef]
- Liu, L.; Li, W.; Song, W.; Guo, M. Remediation techniques for heavy metal-contaminated soils: Principles and applicability. Sci. Total Environ. 2018, 663, 206–219. [Google Scholar] [CrossRef]
- Kumar, V.; Rout, C.; Singh, J.; Saharan, Y.; Goyat, R.; Umar, A.; Akbar, S.; Baskoutas, S. A review on the clean-up technologies for heavy metal ions contaminated soil samples. Heliyon 2023, 9, e15472. [Google Scholar] [CrossRef]
- Su, R.; Wang, Y.; Huang, S.; Chen, R.; Wang, J. Application for Ecological Restoration of Contaminated Soil: Phytoremediation. Int. J. Environ. Res. Public Health 2022, 19, 13124. [Google Scholar] [CrossRef]
- Al Hasin, A.; Gurman, S.J.; Ashlee, L.M.M.; Smith, T.J.; Gardiner, P.H.E. Remediation of Chromium(VI) by a Methane-Oxidizing Bacterium. Environ. Sci. Technol. 2010, 44, 400–405. [Google Scholar] [CrossRef]
- Peng, W.; Li, X.; Song, J.; Jiang, W.; Liu, Y.; Fan, W. Bioremediation of cadmium- and zinc-contaminated soil using Rhodobacter sphaeroides. Chemosphere 2018, 197, 33–41. [Google Scholar] [CrossRef]
- Strachel, R.; Wyszkowska, J.; Baćmaga, M. Bioaugmentation of Soil Contaminated with Zinc. Water Air Soil Pollut. 2020, 231, 443. [Google Scholar] [CrossRef]
- Wang, R.; Fan, X.W.; Li, Y.Z. Efficient removal of a low concentration of Pb(II), Fe(III) and Cu(II) from simulated drinking water by co-immobilization between low-dosages of metal-resistant/adapted fungus Penicillium janthinillum and graphene oxide and activated carbon. Chemosphere 2022, 286, 131591. [Google Scholar] [CrossRef]
- Xie, Y.; Bu, H.; Feng, Q.; Wassie, M.; Amee, M.; Jiang, Y.; Bi, Y.; Hu, L.; Chen, L. Identification of Cd-resistant microorganisms from heavy metal-contaminated soil and its potential in promoting the growth and Cd accumulation of bermudagrass. Environ. Res. 2021, 200, 111730. [Google Scholar] [CrossRef]
- Nandy, S.; Andraskar, J.; Lanjewar, K.; Kapley, A. Challenges in bioremediation: From lab to land. In Bioremediation for Environmental Sustainability; Sakena, G., Kumar, V., Shah, M.P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2021; Volume 23, pp. 561–583. [Google Scholar] [CrossRef]
- Hassan, A.; Periathamby, A.; Ahmed, A.; Innocent, O.; Hamid, F.S. Effective bioremediation of heavy metal–contaminated landfill soil through bioaugmentation using consortia of fungi. J. Soils Sediments 2020, 20, 66–80. [Google Scholar] [CrossRef]
- Hao, X.; Zhu, J.; Rensing, C.; Liu, Y.; Gao, S.; Chen, W.; Huang, Q.; Liu, Y.R. Recent advances in exploring the heavy metal(loid) resistant microbiome. Comput. Struct. Biotechnol. J. 2021, 19, 94–109. [Google Scholar] [CrossRef]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial Interventions in Bioremediation of Heavy Metal Contaminants in Agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef]
- Blanck, H.; Wänkberg, S.Å.; Molander, S. Pollution- Induced Community Tolerance—A New Ecotoxicological Tool. In Functional Testing of Aquatic Biota for Estimating Hazards of Chemicals; Cairns, J., Ed.; ASTM STP 988: Philadelphia, PA, USA, 1988; pp. 219–231. [Google Scholar]
- Government of Romania. Hotărâre privind aprobarea strategiei naționale și a planului național pentru gestionarea siturilor contaminate din România. Constituția României 2015, 108, 1–23. [Google Scholar]
- Klimek, B.; Niklińska, M. Zinc and Copper Toxicity to Soil Bacteria and Fungi from Zinc Polluted and Unpolluted Soils: A Comparative Study with Different Types of Biolog Plates. Bull. Environ. Contam. Toxicol. 2007, 78, 112–117. [Google Scholar] [CrossRef]
- Santos, I.C.; Hildenbrand, Z.L.; Schug, K.A. Applications of MALDI-TOF MS in environmental microbiology. Analyst 2016, 141, 2827. [Google Scholar] [CrossRef]
- Oladipo, O.G.; Ezeokoli, O.T.; Maboeta, M.S.; Bezuidenhout, J.J.; Tiedt, L.R.; Jordaan, A.; Bezuidenhout, C.C. Tolerance and growth kinetics of bacteria isolated from gold and gemstone mining sites in response to heavy metal concentrations. J. Environ. Manag. 2018, 212, 357–366. [Google Scholar] [CrossRef]
- Oladipo, O.G.; Awotoye, O.O.; Olayinka, A.; Bezuidenhout, C.C.; Maboeta, M.S. Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Braz. J. Microbiol. 2018, 49, 29–37. [Google Scholar] [CrossRef]
- Yaghoubian, Y.; Siadat, S.A.; Telavat, M.R.M.; Pirdashti, H.; Yaghoubian, I. Bio-removal of cadmium from aqueous solutions by filamentous fungi: Trichoderma spp. and Piriformospora indica. Environ. Sci. Pollut. Res. 2019, 26, 7863–7872. [Google Scholar] [CrossRef]
- Khan, I.; Aftab, M.; Shakir, S.; Ali, M.; Qayyum, S.; Rehman, M.U.; Haleem, K.S.; Touseef, I. Mycoremediation of heavy metal (Cd and Cr)–polluted soil through indigenous metallotolerant fungal isolates. Environ. Monit. Assess. 2019, 191, 585. [Google Scholar] [CrossRef]
- Emenike, C.U.; Agamuthu, P.; Fauziah, S.H. Sustainable remediation of heavy metal polluted soil: A biotechnical interaction with selected bacteria species. J. Geochem. Explor. 2017, 182, 275–278. [Google Scholar] [CrossRef]
- Xing, S.; Xu, H.; Shi, G.; Chen, J.; Zeng, L.; Jin, L. A Simple and Sensitive Method for the Amperometric Detection of Trace Chromium(VI) Based on Prussian Blue Modified Glassy Carbon Electrode. Electroanalysis 2009, 21, 1678–1684. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Ji, X.; Yang, W. Preparation of uniform Au@SiO2 particles by direct silica coating on citrate-capped Au nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2011, 392, 220–224. [Google Scholar] [CrossRef]
- Tukur, S.A.; Yusof, N.A.; Hajian, R. Gold Nanoparticles-Modified Screen-Printed Electrode for Determination of Pb(II) Ion Using Linear Sweep Anodic Stripping Voltammetry. IEEE Sens. J. 2015, 15, 2780–2784. [Google Scholar] [CrossRef]
- Ringgit, G.; Siddiquee, S.; Saallah, S.; Mohamad Lal, M.T. A sensitive and rapid determination of zinc ion (Zn(2+)) using electrochemical sensor based on f-MWCNTs/CS/PB/AuE in drinking water. Sci. Rep. 2022, 12, 18582. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Industrial Pollution in Europe. 2019. Available online: https://www.eea.europa.eu/themes/industry/industrial-pollution-in-europe (accessed on 1 July 2023).
- Saxena, A.M.; Kumar, M.; Chakdar, H.; Anuroopa, N.; Bagyaraj, D.J. Bacillus species in soil as a natural resource for plant health and nutrition. J. Appl. Microbiol. 2020, 128, 1583–1594. [Google Scholar] [CrossRef] [PubMed]
- Grady, E.N.; MacDonald, J.; Liu, L.; Richman, A.; Yuan, Z.C. Current knowledge and perspectives of Paenibacillus: A review. Microb. Cell Factories 2016, 15, 203. [Google Scholar] [CrossRef]
- Kaur, M.; Karnwal, A. Screening of plant growth-promoting attributes bearing endogenous bacteria from abiotic stress resisting high altitude plants. J. Agric. Food Res. 2023, 11, 100489. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Asyakina, L.K.; Serazetdinova, Y.R.; Frolova, A.S.; Velichkovich, N.S.; Prosekov, A.Y. Microorganisms for Bioremediation of Soils Contaminated with Heavy Metals. Microorganisms 2023, 11, 864. [Google Scholar] [CrossRef]
- Joo, J.H.; Hussein, K.A. Heavy Metal Tolerance of Fungi Isolated from Contaminated Soil. Korean J. Soil Sci. Fertil. 2012, 45, 565–571. [Google Scholar] [CrossRef]
- Mohammadian, E.; Ahari, A.B.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
- Liaquat, F.; Munis, M.F.H.; Haroon, U.; Arif, S.; Saqib, S.; Zaman, W.; Khan, A.R.; Shi, J.; Che, S.; Liu, Q. Evaluation of Metal Tolerance of Fungal Strains Isolated from Contaminated Mining Soil of Nanjing, China. Biology 2020, 9, 469. [Google Scholar] [CrossRef]
- Imran, M.; Ahmad, I.; Barasubiye, T.; Abulreesh, H.H.; Monjed, M.K.; Elbanna, K. Heavy Metal Tolerance Among Free-living Fungi Isolated from Soil Receiving Long Term Application of Wastewater. J. Pure Appl. Microbiol. 2020, 14, 157–170. [Google Scholar] [CrossRef]
- Finlay, R.D.; Thorn, R.G. The Fungi in Soil. In Modern Soil Microbiology, 3rd ed.; Elsas, J.D., Trevors, J.T., Rosado, A.S., Nannipieri, P., Eds.; CRC Press: London, UK; New York, NY, USA, 2019; Volume 5, pp. 65–91. [Google Scholar]
- Nkongolo, K.K.; Narendrula-Kotha, R. Advances in monitoring soil microbial community dynamic and function. J. Appl. Genet. 2020, 61, 249–263. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, C.; Lin, J.; Wang, X.; Tang, C.; Dai, Z.; Xu, J. Contamination with multiple heavy metals decreases microbial diversity and favors generalists as the keystones in microbial occurrence networks. Environ. Pollut. 2022, 306, 119406. [Google Scholar] [CrossRef]
- Margesin, R.; Plaza, G.A.; Kasenbacher, S. Characterization of bacterial communities at heavy-metal contaminated sites. Chemosphere 2011, 81, 1583–1588. [Google Scholar] [CrossRef]
- Guo, H.; Nasir, M.; Lv, J.; Dai, Y.; Gao, J. Understanding the variation of microbial community in heavy metals contaminated soil using high throughput sequencing. Ecotoxicol. Environ. Saf. 2017, 144, 300–306. [Google Scholar] [CrossRef]
- Ma, S.; Qiao, L.; Liu, X.; Zhang, S.; Zhang, L.; Qiu, Z.; Yu, C. Microbial community succession in soils under long-term heavy metal stress from community diversity-structure to KEGG function pathways. Environ. Res. 2022, 214, 113822. [Google Scholar] [CrossRef]
- Altuğ, G.; Çardak, M.; Türetken, P.S.Ç.; Kalkan, S.; Gürün, S. Antibiotic and Heavy Metal Resistant Bacteria Isolated from Aegean Sea Water and Sediment in Güllük Bay, Turkey. Johns. Matthey Technol. Rev. 2020, 64, 507–525. [Google Scholar] [CrossRef]
- Rajapaksha, R.M.C.P. Heavy metal tolerance of culturable bacteria and fungi in a long-term cultivated tropical ultisol. Eur. J. Soil Biol. 2011, 47, 9–15. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Li, S.W.; Leng, Y.; Kang, X.H. Structural and functional responses of bacterial and fungal communities to multiple heavy metal exposure in arid loess. Sci. Total Environ. 2020, 723, 138081. [Google Scholar] [CrossRef] [PubMed]
- Perelomov, L.V.; Sarkar, B.; Sizova, O.I.; Chilachava, K.B.; Shvikin, A.Y.; Perelomova, I.V.; Atroshchenko, Y.M. Zinc and lead detoxifying abilities of humic substances relevant to environmental bacterial species. Ecotoxicol. Environ. Saf. 2018, 151, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.J.; Ruiz, E.; Abriouel, H.; Gálvez, A.; Ezzouhri, L.; Lairini, K.; Espínola, F. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. Chem. Eng. J. 2012, 210, 325–332. [Google Scholar] [CrossRef]
- Mwandira, W.; Nakashima, K.; Kawasaki, S.; Arabelo, A.; Banda, K.; Nyyambe, I.; Chirwa, M.; Ito, M.; Sato, T.; Igarashi, T.; et al. Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci. Rep. 2020, 10, 21189. [Google Scholar] [CrossRef] [PubMed]
- Danilova, T.A.; Danilina, G.A.; Adzhieva, A.A.; Vostrova, E.I.; Zhukhovitskii, V.G.; Cheknev, S.B. Inhibitory Effect of Copper and Zinc Ions on the Growth of Streptococcus pyogenes and Escherichia coli Biofilms. Immunol. Microbiol. 2020, 169, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Abdalkader, D.; Al-Saedi, F. Antibacterial Effect of Different Concentrations of Zinc Sulfate on Multidrug Resistant Pathogenic Bacteria. Syst. Rev. Pharm. 2020, 11, 282–288. Available online: https://www.sysrevpharm.org/abstract/antibacterial-effect-of-different-concentrations-of-zinc-sulfate-on-multidrug-resistant-pathogenic-bacteria-65702.html (accessed on 14 November 2023).
- Chen, Y.; Mi, H.; Zhang, Y.; Zhang, G.; Li, C.; Ye, L.; Zhang, R.; Shi, J.; Li, Z.; Tian, X.; et al. Impact of ZnSO4 and ZnEDTA applications on wheat Zn biofortification, soil Zn fractions and bacterial community: Significance for public health and agroecological environment. Appl. Soil Ecol. 2022, 176, 104484. [Google Scholar] [CrossRef]
- Kamis, M.; Gouda, G.A.; Nagiub, A.M. Biosynthesis approach of zinc oxide nanoparticles for aqueous phosphorous removal: Physicochemical properties and antibacterial activities. BMC Chem. 2023, 17, 99. [Google Scholar] [CrossRef]
- Sardella, A.; Marieschi, M.; Mercatali, I.; Zanni, C.; Gorbi, G.; Torelly, A. The relationship between sulfur metabolism and tolerance of hexavalent chromium in Scenedesmus acutus (Spheropleales): Role of ATP sulfurylase. Aquat. Toxicol. 2019, 216, 105320. [Google Scholar] [CrossRef]
- Tiquia-Arashiro, S.M. Lead adsorbtion mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 2018, 102, 5437–5444. [Google Scholar] [CrossRef]
- Lensmire, J.M.; Hammer, N.D. Nutrient sulfur acquisition strategies employed by bacterial pathogens. Curr. Opin. Microbiol. 2019, 47, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S.; Prasad, R.; Yadav, P.K. Assessing the Tolerance Impact of Fungal Isolates against Lead and Zinc Heavy Metals under Controlled Conditions. Environ. Ecol. 2023, 41, 1369–1377. [Google Scholar] [CrossRef]
- Chandran, C.S.; Shijith, K.V.; Augusthy, A.R. Study on heavy metal toxicity biomarkers in Aspergillus niger. Int. J. Adv. Pharm. Biol. Chem. 2014, 3, 1–7. [Google Scholar]
- Tian, D.; Jiang, Z.; Jiang, L.; Su, M.; Feng, Z.; Zhang, L.; Wang, S.; Li, Z.; Hu, S. A new insight into lead (II) tolerance of environmental fungi based on a study of Aspergillus niger and Penicillium oxalicum. Environ. Microbiol. 2018, 21, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Long, D.D.; Fu, R.R.; Han, J.R. Tolerance and stress response of sclerotiogenic Aspergillus oryzae G15 to copper and lead. Folia Microbiol. 2017, 62, 295–304. [Google Scholar] [CrossRef]
- Gajewska, J.; Floryszak-Wieczorek, J.; Sobieszczuk-Nowicka, E.; Mattoo, A.; Arasimonwicz-Jelonek, M. Fungal and oomycete pathogens and heavy metals: An inglorious couple in the environment. IMA Fungus 2022, 13, 6. [Google Scholar] [CrossRef]
- Golubović-Ćurguz, V.; Tabaković-Tošić, M.; Veselinović, M.; Rajković, S. The influence of heavy metals on the growth of pathogenic fungi. For. Ideas 2010, 16, 121–125. [Google Scholar]
- Abu-Mejdad, N.M.J.A. Response of some fungal species to the effect of copper, magnesium and zinc under the laboratory condition. Eur. J. Exp. Biol. 2013, 3, 535–540. [Google Scholar] [CrossRef]
- Mwangi, E.S.K.; Gatebe, E.G.; Ndung’s, M.W. Effect of selected metal ions on the mycelial growth of Sclerotinia sclerotiorum isolated from soybean field in Rongai, Kenya. Int. J. Chem. Mater. Res. 2014, 2, 116–125. [Google Scholar]
- Gai, J.P.; Fan, J.Q.; Zhang, S.B.; Mi, N.N.; Christie, P.; Li, X.L.; Feng, G. Direct effects of soil cadmium on the growth and activity of arbuscular mycorrhizal fungi. Rhyzosphere 2018, 7, 43–48. [Google Scholar] [CrossRef]
- Nongmaithem, N.; Roy, A.; Bhattacharya, P.M. Screening of Trichoderma isolates for their potential of biosorption of nickel and cadmium. Braz. J. Microbiol. 2016, 47, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Kim, H. Myco-phytoremediation of arsenic- and lead-contaminated soils by Helianthus annuus and wood rot fungi, Trichoderma sp. isolated from decayed wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Maldaner, J.; Steffen, G.P.K.; Missio, E.L.; Saldanha, C.W.; de Morais, R.M.; Nicoloso, F.T. Tolerance of Trichoderma isolates to increasing concentrations of heavy metals. Int. J. Environ. Stud. 2020, 78, 185–197. [Google Scholar] [CrossRef]
- Zapana-Huarache, S.V.; Romero-Sánchez, C.K.; Gonza, A.P.D.; Torres-Huaco, F.D.; Rivera, A.M.L. Chromium (VI) bioremediation potential of filamentous fungi isolated from Peruvian tannery industry effluents. Braz. J. Microbiol. 2020, 51, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dwivedi, S.K. Hexavalent chromium stress response, reduction capability and bioremediation potential of Trichoderma sp. isolated from electroplating wastewater. Ecotoxicol. Environ. Saf. 2019, 185, 109734. [Google Scholar] [CrossRef] [PubMed]
- Capdevila, D.A.; Wang, J.; Giedroc, D.P. Bacterial Strategies to Maintain Zinc Metallostasis at the Host-Pathogen Interface. Minireviews 2016, 291, 20858–20868. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; González-Feijoo, R.; Arias-Estévez, M.; Fernández-Calviño, D. Influence of soil properties on the development of bacterial community tolerance to Cu, Ni, Pb and Zn. Environ. Res. 2022, 214, 113920. [Google Scholar] [CrossRef] [PubMed]
- Bérard, A.; Capowiez, L.; Mombo, S.; Schreck, E.; Dumat, C.; Deola, F.; Capowiez, Y. Soil microbial respiration and PICT responses to an industrial and historic lead pollution: A field study. Environ. Sci. Pollut. Res. 2016, 23, 4271–4281. [Google Scholar] [CrossRef]
- Renu; Sarim, K.M.; Sahu, U.; Bhoyar, M.S.; Singh, D.P.; Singh, U.B.; Sahu, A.; Gupta, A.; Mandal, A.; Thakur, J.K.; et al. Augmentation of metal-tolerant bacteria elevates growth and reduces metal toxicity in spinach. Bioremediation J. 2020, 25, 108–127. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Rodríguez-González, L.; Arias-Estévez, M.; Fernández-Calviño, F.; Soto-Gómez, D. Influence of Soil Properties and Initial Concentration on the Fractionation of Nickel, Zinc, Copper and Lead in Soils Derived from Different Parent Materials. Agronomy 2021, 11, 301. [Google Scholar] [CrossRef]
- Tansengco, M.; Tejano, J.; Coronado, F.; Gacho, C.; Barcelo, J. Heavy Metal Tolerance and Removal Capacity of Trichoderma species Isolated from Mine Tailings in Itogon, Benguet. Environ. Nat. Resour. J. 2018, 16, 39–57. [Google Scholar] [CrossRef]
- Mardiyono, M.; Sajidan, S.; Masykuri, M.; Setyono, P. Bioremediation of Nickel Heavy Metals in Electroplating Industrial Liquid Waste with Bacillus subtilis. Int. Conf. Sci. Appl. Sci. (ICSAS) AIP Conf. Proc. 2019, 2202, 020084. [Google Scholar] [CrossRef]
- Heidari, P.; Panico, A. Sorption Mechanism and Optimization Study for the Bioremediation of Pb(II) and Cd(II) Contamination by Two Novel Isolated Strains Q3 and Q5 of Bacillus sp. Int. J. Environ. Res. Public Health 2020, 17, 4059. [Google Scholar] [CrossRef]
- Guo, S.; Xiao, C.; Zheng, Y.; Li, Y.; Chi, R. Removal and potential mechanisms of Cr(Ⅵ) contamination in phosphate mining wasteland by isolated Bacillus megatherium PMW-03. J. Clean. Prod. 2021, 322, 129062. [Google Scholar] [CrossRef]
- Khan, M.; Ijaz, M.; Chotana, G.; Murtaza, A.; Malik, G.; Shamim, S. Bacillus altitudinis MT422188: A potential agent for zinc bioremediation. Bioremediation J. 2021, 26, 228–248. [Google Scholar] [CrossRef]
- Arroyo-Herrera, I.; Román-Ponce, B.; Reséndiz-Martínez, A.L.; Estrada-de los Santos, P.; Wang, E.T.; Vásquez-Murrieta, M.S. Heavy-metal resistance mechanisms developed by bacteria from Lerma–Chapala basin. Arch. Microbiol. 2021, 203, 1807–1823. [Google Scholar] [CrossRef]
- Malkoc, S.; Kurt, H.; Ozbayer, C.; Yagci, E. Mycoremediation of Trichoderma harzianum and Penicillium chrysogenum to Pb Exposure: Effect on Metal Bioaccumulation, Oxidative Stress and Antioxidant System. CRPASE Trans. Appl. Sci. 2021, 7, 2345. [Google Scholar] [CrossRef]
- Mohamadhasani, F.; Rahimi, M. Growth response and mycoremediation of heavy metals by fungus Pleurotus sp. Sci. Rep. 2022, 12, 19947. [Google Scholar] [CrossRef] [PubMed]
- Wróbel, M.; Sliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzynski, J. Bioremediation of Heavy Metals by the Genus Bacillus. Int. J. Environ. Res. Public Health 2023, 20, 4974. [Google Scholar] [CrossRef]
- Sevak, P.I.; Pushkar, B.K.; Kapadne, P.N. Lead pollution and bacterial bioremediation: A review. Environ. Chem. Lett. 2021, 19, 4463–4488. [Google Scholar] [CrossRef]
- Utami, U.; Harianie, L.; Dunyana, N.R.; Romaidi. Lead-resistant bacteria isolated from oil wastewater sample for bioremediation of lead. Water Sci. Technol. 2020, 81, 2244–2249. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.L.; Akinyede, O.R.; Obidi, O.F. Microbial Remediation of Heavy Metals Contaminated Media by Bacillus megaterium and Rhizopus stolonier. Sci. Afr. 2020, 10, e00545. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Parhi, P.K.; Pandey, S.; Bindhani, B.K.; Thatoi, H.; Panda, C.R. Active and passive biosorption of Pb(II) using live and dead biomass of marine bacterium Bacillus xiamenensis PbRPSD202: Kinetics and isotherm studies. J. Environ. Manag. 2019, 247, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.R.; Dussan, J. Mathematical Modelling of Toxic Metal Uptake and Efflux Pump in Metal-Resistant Bacterium Bacillus cereus Isolated From Heavy Crude Oil. Water Air Soil Pollut. 2015, 226, 112. [Google Scholar] [CrossRef]
- Nanda, M.; Kumar, V.; Sharma, D.K. Multimetal tolerance mechanisms in bacteria: The resistance strategies acquired by bacteria that can be exploited to ‘clean-up’ heavy metal contaminants from water. Aquat. Toxicol. 2019, 212, 1–10. [Google Scholar] [CrossRef]
- Maynaud, G.; Brunel, B.; Yashiro, E.; Mergeay, M.; Cleyet-Marel, J.C.; Le Quéré, A. CadA of Mesorhizobium metallidurans isolated from a zinc-rich mining soil is a PIB-2-type ATPase involved in cadmium and zinc resistance. Res. Microbiol. 2014, 165, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Doble, M. Novel chromium tolerant microorganisms: Isolation, characterization and their biosorption capacity. Ecotoxicol. Environ. Saf. 2008, 71, 874–879. [Google Scholar] [CrossRef]
- Kayalvizhi, K.; Kathiresan, K. Microbes from wastewater treated mangrove soil and their heavy metal accumulation and Zn solubilization. Biocatal. Agric. Biotechnol. 2019, 22, 101379. [Google Scholar] [CrossRef]
- Kaur, R.; Kumari, A.; Sharma, G.; Singh, D.; Kaur, R. Biodegradation of endocrine disrupting chemicals benzyl butyl phthalate and dimethyl phthalate by Bacillus marisflavi RR014. J. Appl. Microbiol. 2021, 131, 1274–1288. [Google Scholar] [CrossRef]
- Saed, M.; Ilyas, N.; Bibi, F.; Jayachandran, K.; Dattamudi, S.; Elgorban, A.M. Biodegradation of PAHs by Bacillus marsiflavi, genome analysis and its plant growth promoting potential. Environ. Pollut. 2022, 292, 118343. [Google Scholar] [CrossRef]
- Varghese, E.M.; Sivadas, S.; Suresh, C.; Devikrishna, U.; Vidhya, K.; Akhil, K.P.; Jisha, M.S. Biodegradation of chlorpyrifos by an optimized Bacillus consortium isolated from pesticide-contaminated soils of Kerala, India. Int. J. Pest Manag. 2021, 67, 1–9. [Google Scholar] [CrossRef]
- García, R.; Campos, J.; Cruz, J.A.; Calderón, M.E.; Raynal, M.E.; Buitrón, G. Biosorption of Cd, Cr, Mn, and Pb from Aqueous Solutions by Bacillus sp. Strains Isolated from Industrial Waste Activate Sludge. TIP Rev. Espec. En Cienc. Químico-Biológicas 2016, 19, 5–14. [Google Scholar] [CrossRef]
- Rizvi, A.; Ahmed, B.; Zaidi, A.; Khan, M.S. Biosorption of heavy metals by dry biomass of metal tolerant bacterial biosorbents: An efficient metal clean-up strategy. Environ. Monit. Assess. 2020, 192, 801. [Google Scholar] [CrossRef] [PubMed]
- Shylla, L.; Barik, S.K.; Joshi, S.R. Characterization and bioremediation potential of native heavy-metal tolerant bacteria isolated from rat-hole coal mine environment. Arch. Microbiol. 2021, 203, 2379–2392. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Y.; Li, B.; Huang, F.; Sun, C.; Li, X.; Zhao, R.; Wang, Y. Characteristics of a Copper-cadmium Tolerant Strain Screened from Tailings and Its Potential in Remediation of Heavy Metal Contaminated Soil. Water Air Soil Pollut. 2023, 235, 277. [Google Scholar] [CrossRef]
- Chandrangsu, P.; Rensing, C.; Helmann, J.D. Metal homeostasis and resistance in bacteria. Nat. Rev. Microbiol. 2017, 15, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Baran, M.F.; Düz, Z.; Baran, A.; Keskin, C. Fast and Economical Biosorption of Lead (II) Heavy Metal in Aqueous Solutions by Bacillus licheniformis sp. J. Agric. Nat. 2022, 25, 847–858. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, S.; Teng, C.; Song, T.; Dong, L.; Liang, J.; Bai, X.; Xu, X.; Qu, J. Biosorption characteristic of Alcaligenes sp. BAPb.1 for removal of lead(II) from aqueous solution. 3 Biotech 2017, 7, 123. [Google Scholar] [CrossRef]
- Wierzba, S. Biosorption of lead(II), zinc(II) and nickel(II) from industrial wastewater by Stenotrophomonas maltophilia and Bacillus subtilis. Pol. J. Chem. Technol. 2015, 17, 79–87. [Google Scholar] [CrossRef]
- Ren, G.; Jin, Y.; Zhang, C.; Gu, H.; Qu, J. Characteristics of Bacillus sp. PZ-1 and its biosorption to Pb(II). Ecotoxicol. Environ. Saf. 2015, 117, 141–148. [Google Scholar] [CrossRef]
- Qiao, W.; Zhang, Y.; Xia, H.; Luo, Y.; Liu, S.; Wang, S.; Wang, W. Bioimmobilization of lead by Bacillus subtilis X3 biomass isolated from lead mine soil under promotion of multiple adsorption mechanisms. R. Soc. Open Sci. 2019, 6, 181701. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Chinthala, P. Heavy Metal Detoxification by Different Bacillus Species Isolated from Solar Salterns. Scientifica 2015, 2015, 319760. [Google Scholar] [CrossRef]
- Malanovic, N.; Lohner, K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2016, 1858, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.B.; Biju, V.G.; Beeregowda, K.N. Accumulation of lead (Pb II) metal ions by Bacillus toyonensis SCE1 species, innate to industrial-area ground water and nanoparticle synthesis. Appl. Nanosci. 2019, 9, 49–66. [Google Scholar] [CrossRef]
- Sharma, R.; Talukdar, D.; Bhardwaj, S.; Jaglan, S.; Kumar, R.; Kumar, R.; Akhtar, M.S.; Beniwal, V.; Umar, A. Bioremediation potential of novel fungal species isolated from wastewater for the removal of lead from liquid medium. Environ. Technol. Innov. 2020, 18, 100757. [Google Scholar] [CrossRef]
- Palanivel, T.M.; Pracejus, B.; Novo, L.A.B. Bioremediation of copper using indigenous fungi Aspergillus species isolated from an abandoned copper mine soil. Chemosphere 2023, 314, 137688. [Google Scholar] [CrossRef]
- Biswas, D.; Chourasia, A.; Sasmal, A.; Santra, S.; Panigrahi, S.; Kundu, M.; Sarkar, S.; Ghosh, B.; Ghosh, S.; Sarkar, S. Mycoremediation is a Potential Strategy for Environmental Clean-up of Heavy Metal: A Review. J. Surv. Fish. Sci. 2023, 10, 6316–6327. [Google Scholar]
- Tu, C.; Liu, Y.; Wei, J.; Li, L.; Scheckel, K.G.; Luo, Y. Characterization and mechanism of copper biosorption by a highly copper-resistant fungal strain isolated from copper-polluted acidic orchard soil. Environ. Sci. Pollut. Res. 2018, 25, 24965–24974. [Google Scholar] [CrossRef]
- Chen, S.H.; Ng, S.L.; Cheow, Y.L.; Ting, A.S.Y. A novel study based on adaptive metal tolerance behavior in fungi and SEM-EDX analysis. J. Hazard. Mater. 2017, 334, 132–141. [Google Scholar] [CrossRef]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Mechanisms for metal removal established via electron microscopy and spectroscopy: A case study on metal tolerant fungi Penicillium simplicissimum. J. Hazard. Mater. 2019, 362, 394–402. [Google Scholar] [CrossRef]
- Zhang, D.; Yin, C.; Abbas, N.; Mao, Z.; Zhang, Y. Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichoderma brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef]
- Hlihor, R.M.; Roșsca, M.; Drăgoi, E.N.; Simion, I.M.; Favier, L.; Gavrilescu, M. New insights into the application of fungal biomass for chromium(VI) bioremoval from aqueous solutions using Design of Experiments and Differential Evolution based neural network approaches. Chem. Eng. Res. Des. 2023, 190, 233–254. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Aniagor, C.O.; Hashem, A. Isotherms and kinetic modelling of mycoremediation of hexavalent chromium contaminated wastewater. Clean. Eng. Technol. 2021, 4, 100192. [Google Scholar] [CrossRef]
- Chen, S.H.; Cheow, Y.L.; Ng, S.L.; Ting, A.S.Y. Bioaccumulation and Biosorption Activities of Indoor Metal-Tolerant Penicillium simplicissimum for Removal of Toxic Metals. Int. J. Environ. Res. 2020, 14, 235–242. [Google Scholar] [CrossRef]
- Shameer, S. Biosorption of lead, copper and cadmium using the extracellular polysaccharides (EPS) of Bacillus sp., from solar salterns. 3 Biotech 2016, 6, 194. [Google Scholar] [CrossRef] [PubMed]
- Masood, F.; Malik, A. Biosorption of metal ions from aqueous solution and tannery effluent by Bacillus sp. FM1. J. Environ. Sci. Health 2011, 46, 1667–1674. [Google Scholar] [CrossRef] [PubMed]
- Arivalagan, P.; Singaraj, D.; Haridass, V.; Kaliannan, T. Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol. Eng. 2014, 71, 728–735. [Google Scholar] [CrossRef]
- Liaqat, I.; Muhammad, N.; Ara, C.; Hanif, U.; Andleeb, S.; Arshad, M.; Aftab, M.N.; Raza, C.; Mubin, M. Bioremediation of heavy metals polluted environment and decolourization of black liquor using microbial biofilms. Mol. Biol. Rep. 2023, 50, 3985–3997. [Google Scholar] [CrossRef]
- Nandiyanto, A.; Oktiani, R.; Ragadhita, R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Gola, D.; Malik, A.; Namburath, M.; Ahammad, S.Z. Removal of industrial dyes and heavy metals by Beauveria bassiana: FTIR, SEM, TEM and AFM investigations with Pb(II). Environ. Sci. Pollut. Res. 2018, 25, 20486–20496. [Google Scholar] [CrossRef]
- Gupta, B.S.; Jelle, B.J.; Gao, T. In vitro cell composition identification of wood decay fungi by Fourier transform infrared spectroscopy. R. Soc. Open Sci. 2022, 9, 201935. [Google Scholar] [CrossRef] [PubMed]
- Pugazhendhi, A.; Boovaragamoorthy, G.M.; Ranganathan, K.; Naushad, M.; Kaliannan, T. New insight into effective biosorption of lead from aqueous solution using Ralstonia solanacearum: Characterization and mechanism studies. J. Clean. Prod. 2018, 174, 1234–1239. [Google Scholar] [CrossRef]
- Ramaswamy, R.; Ahn, J.; Balasubramaniam, V.M.; Saona, L.R.; Yousef, A.E. Food safety engineering. In Handbook of Farm and Food Machinery Engineering; Kutz, M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 91–113. [Google Scholar] [CrossRef]





| Metals | 542 F—D1 | 543 F—D2 | 544 F—D3 | 545 F—D4 |
|---|---|---|---|---|
| Cr (mg/kg dw) | 170 ± 0.01 | 81.1 ± 0.02 | 80.7 ± 0.02 | 75.5 ± 0.01 |
| Pb (mg/kg dw) | <4.0 * ± 0.01 | 41.7 ± 0.09 | 211 ± 0.05 | <4.0 * ± 0.01 |
| Zn (mg/kg dw) | 129 ± 0.05 | 403 ± 0.07 | 235 ± 0.05 | 108 ± 0.03 |
| Microbial Strain | Cr (mg/L) | Pb (mg/L) | Zn (mg/L) |
|---|---|---|---|
| Solibacillus silvestris | 600 | 800 | 200 |
| Bacillus megaterium | 200 | 200 | 200 |
| Bacillus marisflavi | 800 | 200 | 200 |
| Bacillus cereus | 200 | 200 | 200 |
| Paenibacillus pabuli | 200 | 200 | 400 |
| Achromobacter sp. | 600 | 200 | 600 |
| Bacillus subtilis | 200 | 200 | 800 |
| Phoma glomerata | 200 | 400 | 600 |
| Trichoderma citrinoviride | 600 | 400 | 1000 |
| Fusarium fujikuroi | 400 | 600 | 600 |
| Geotrichum candidum | 400 | 600 | 800 |
| Phytophthora sp. | 600 | 400 | 1000 |
| Hypocrea jecorina | 800 | 1000 | 1000 |
| Trichoderma longibrachiatum | 800 | 800 | 800 |
| Mucor sp. | 800 | 600 | 1000 |
| Aspergillus niger | 600 | 600 | 1000 |
| B. marisflavi | T. longibrachiatum | |||||
|---|---|---|---|---|---|---|
| Metal Concentration (mM) | ||||||
| Chromium | Lead | Zinc | Chromium | Lead | Zinc | |
| Before bioremediation | 42.2 | 1.716 | 4.308 | 37.5 | 2.544 | 9.762 |
| After bioremediation | 39.31 | 0.228 | 3.584 | 4.95 | 1.320 | 3.224 |
| Decrease in metal concentration (%) | 6.85 | 86.71 | 16.8 | 87.5 | 48.11 | 66.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firincă, C.; Zamfir, L.-G.; Constantin, M.; Răut, I.; Capră, L.; Popa, D.; Jinga, M.-L.; Baroi, A.M.; Fierăscu, R.C.; Corneli, N.O.; et al. Microbial Removal of Heavy Metals from Contaminated Environments Using Metal-Resistant Indigenous Strains. J. Xenobiot. 2024, 14, 51-78. https://doi.org/10.3390/jox14010004
Firincă C, Zamfir L-G, Constantin M, Răut I, Capră L, Popa D, Jinga M-L, Baroi AM, Fierăscu RC, Corneli NO, et al. Microbial Removal of Heavy Metals from Contaminated Environments Using Metal-Resistant Indigenous Strains. Journal of Xenobiotics. 2024; 14(1):51-78. https://doi.org/10.3390/jox14010004
Chicago/Turabian StyleFirincă, Cristina, Lucian-Gabriel Zamfir, Mariana Constantin, Iuliana Răut, Luiza Capră, Diana Popa, Maria-Lorena Jinga, Anda Maria Baroi, Radu Claudiu Fierăscu, Nicoleta Olguța Corneli, and et al. 2024. "Microbial Removal of Heavy Metals from Contaminated Environments Using Metal-Resistant Indigenous Strains" Journal of Xenobiotics 14, no. 1: 51-78. https://doi.org/10.3390/jox14010004
APA StyleFirincă, C., Zamfir, L.-G., Constantin, M., Răut, I., Capră, L., Popa, D., Jinga, M.-L., Baroi, A. M., Fierăscu, R. C., Corneli, N. O., Postolache, C., Doni, M., Gurban, A.-M., Jecu, L., & Șesan, T. E. (2024). Microbial Removal of Heavy Metals from Contaminated Environments Using Metal-Resistant Indigenous Strains. Journal of Xenobiotics, 14(1), 51-78. https://doi.org/10.3390/jox14010004