Beeswax Alcohol and Fermented Black Rice Bran Synergistically Ameliorated Hepatic Injury and Dyslipidemia to Exert Antioxidant and Anti-Inflammatory Activity in Ethanol-Supplemented Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
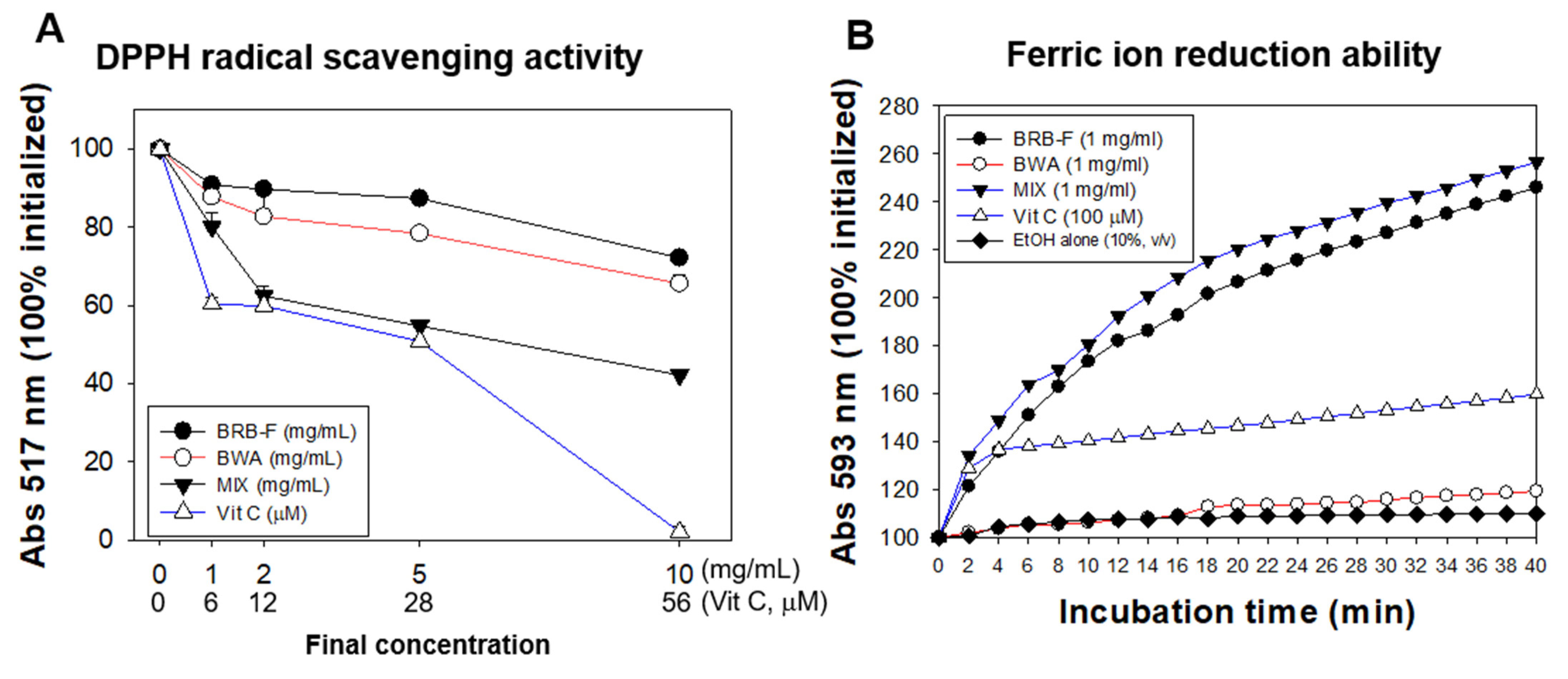
2.2. DPPH-Radical-Scavenging Assay and Ferric-Ion-Reducing Ability
2.3. Zebrafish Maintenance
2.4. Plasma Lipid Profile
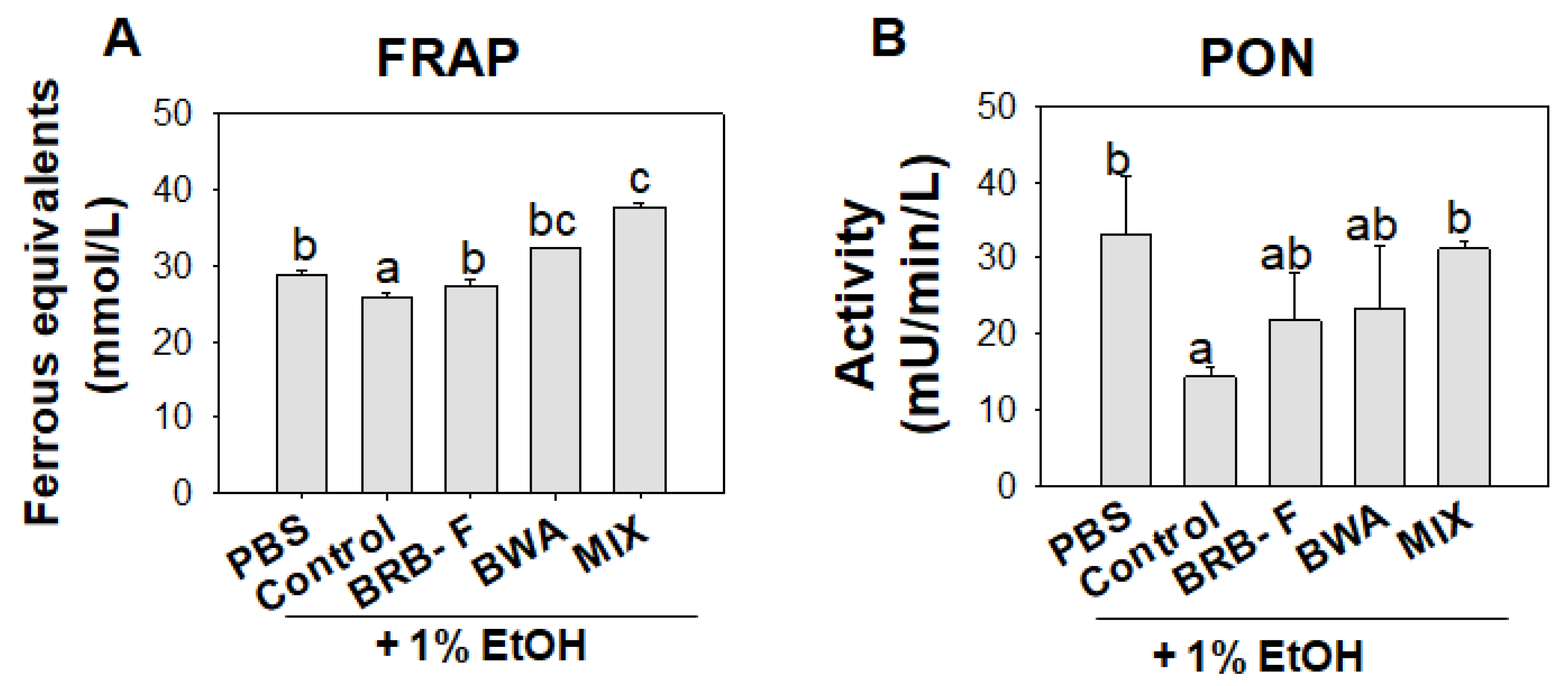
2.5. Ferric-Ion-Reducing Ability of Plasma
2.6. Paraoxonase Activity
2.7. Histological and Immunohistochemical Analysis
2.8. Imaging of Reactive Oxygen Species
2.9. Statistical Analysis
3. Results
3.1. Mixture of BWA and BRB-F Showed the Strongest Antioxidant Activities in a Synergistic Manner
3.2. Mixture of BWA and BRB-F Increases the Survival of Zebrafish under Supplementation with 1% EtOH
3.3. Mixture of BWA and BRB-F Improves the Alcohol-Induced Liver Injury in Zebrafish under Supplementation with 1% EtOH
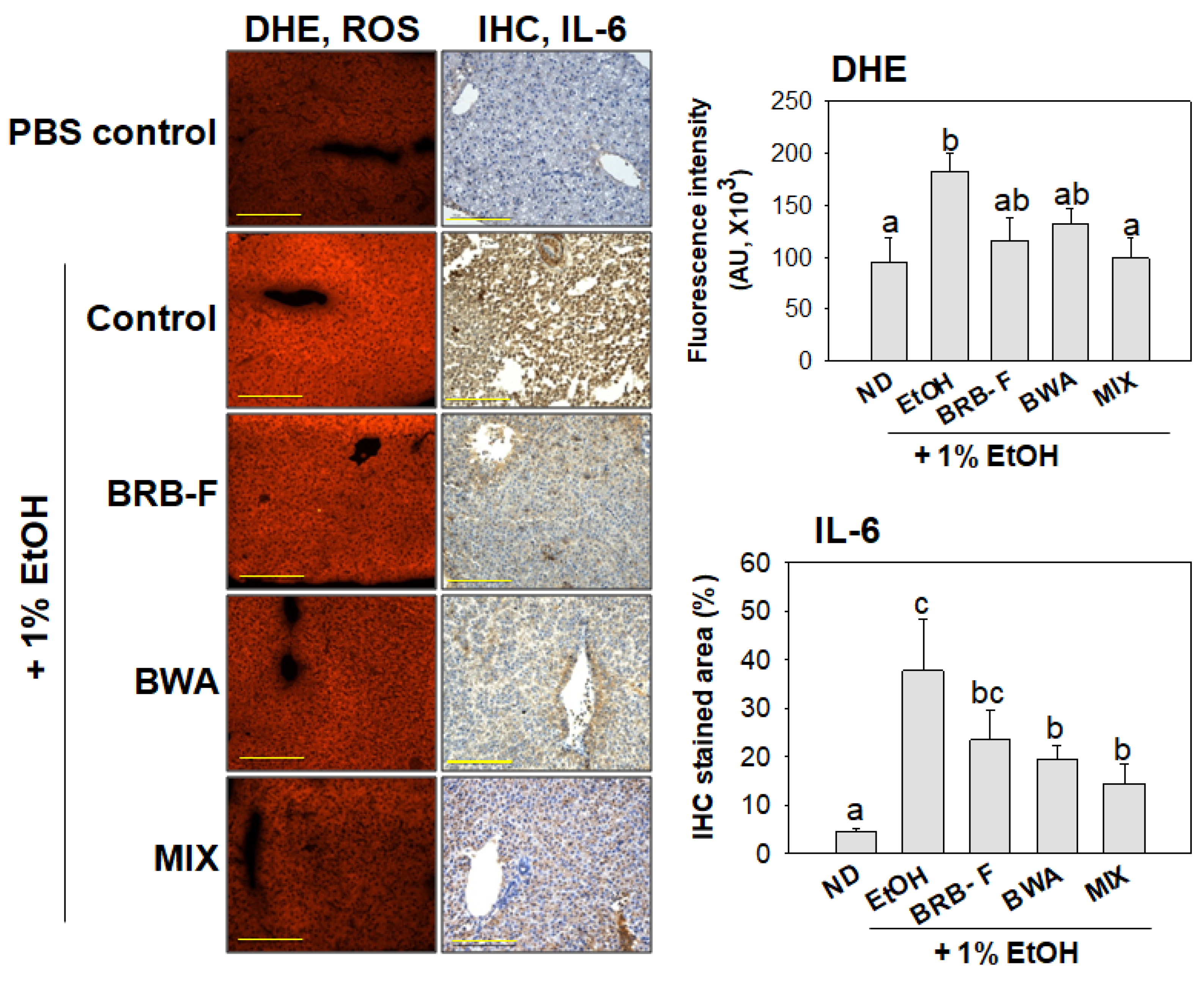
3.4. Mixture of BWA and BRB-F Suppressed ROS Production with Anti-Inflammatory Properties in Zebrafish under Supplementation with 1% EtOH
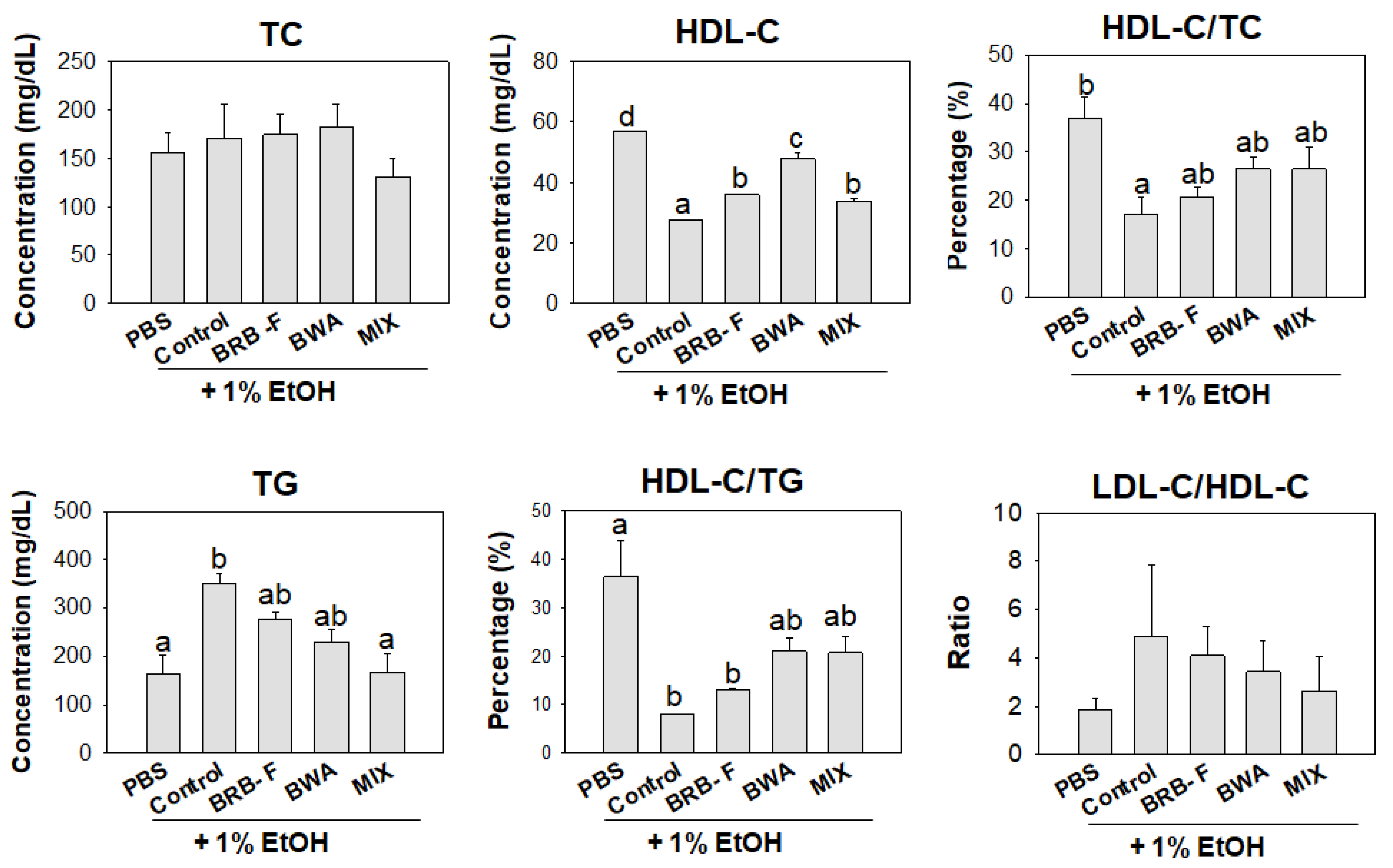
3.5. Mixture of BWA and BRB-F Attenuates the Plasma Lipid Profiles in Zebrafish under Supplementation with 1% EtOH
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V.; Gao, B.; Song, B.J. Molecular mechanisms of alcoholic fatty liver. Alcohol. Clin. Exp. Res. 2009, 33, 191–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Status Report on Alcohol and Health 2018; World Health Organization: Geneva, Switzerland, 2019.
- Park, S.H.; Kim, D.J. Global and regional impacts of alcohol use on public health: Emphasis on alcohol policies. Clin. Mol. Hepatol. 2020, 26, 652. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, J.; Lee, S.; Park, M.S.; Park, S.; Huh, S. Cause-of-death statistics in 2018 in the Republic of Korea. J. Korean Med. Assoc. 2020, 63, 286–297. [Google Scholar]
- Ravelo, Y.; Molina, V.; Carbajal, D.; Fernández, L.; Fernández, J.C.; Arruzazabala, M.L.; Más, R. Evaluation of anti-inflammatory and antinociceptive effects of D-002 (beeswax alcohols). J. Nat. Med. 2011, 65, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Carbajal, D.; Molina, V.; Valdés, S.; Arruzazabala, M.d.L.; Más, R.; Magraner, J. Anti-inflammatory activity of D-002: An active product isolated from beeswax. Prostagland. Leukot. Essent. Fat. Acids 1998, 59, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Pérez, Y.; Oyárzabal, A.; Mas, R.; Molina, V.; Jiménez, S. Protective effect of D-002, a mixture of beeswax alcohols, against indomethacin-induced gastric ulcers and mechanism of action. J. Nat. Med. 2013, 67, 182–189. [Google Scholar] [CrossRef]
- Puig, M.N.; Castaño, S.M.; Ferreiro, R.M.; Clara, M.V.; Hernansez, N.M. Effects of Oral Administration of D-002 (Beeswax Alcohols) on Histological and Functional Outcomes in a Rat Model of Antigen-Induced Arthritis: Preliminary Study. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 5, 60–68. [Google Scholar]
- Zhang, M.W.; Zhang, R.F.; Zhang, F.X.; Liu, R.H. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J. Agric. Food Chem. 2010, 58, 7580–7587. [Google Scholar] [CrossRef]
- Nam, S.; Choi, S.; Kang, M.; Koh, H.; Kozukue, N.; Friedman, M. Bran extracts from pigmented rice seeds inhibit tumor promotion in lymphoblastoid B cells by phorbol ester. Food Chem. Toxicol. 2005, 43, 741–745. [Google Scholar] [CrossRef]
- Nam, S.H.; Choi, S.P.; Kang, M.Y.; Koh, H.J.; Kozukue, N.; Friedman, M. Antioxidative activities of bran extracts from twenty one pigmented rice cultivars. Food Chem. 2006, 94, 613–620. [Google Scholar] [CrossRef]
- Kim, S.P.; Lee, J.R.; Kwon, K.S.; Jang, Y.J.; Kim, J.; Yu, K.H.; Lee, S.Y.; Friedman, M. A bioprocessed black rice bran glutathione-enriched yeast extract protects rats and mice against alcohol-induced hangovers. Food Nutr. Sci. 2021, 12, 223. [Google Scholar] [CrossRef]
- Kim, S.P.; Park, S.O.; Lee, S.J.; Nam, S.H.; Friedman, M. A polysaccharide isolated from the liquid culture of Lentinus edodes (Shiitake) mushroom mycelia containing black rice bran protects mice against a Salmonella lipopolysaccharide-induced endotoxemia. J. Agric. Food Chem. 2013, 61, 10987–10994. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Y.; Zhang, Y.-J.; Zhou, Y.; Li, S.; Li, H.-B. Natural products for the prevention and treatment of hangover and alcohol use disorder. Molecules 2016, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Ji-Hye Lee, S.-H.; Cho, K.-H. Culinary Cinnamon and Clove Powder Ameliorate Fatty Liver Formation Induced by Ethanol Supplementation in Zebrafish. J. Exp. Biomed. Sci. 2010, 16, 33–38. [Google Scholar]
- Park, K.-H.; Kim, S.-H. Adult zebrafish as an in vivo drug testing model for ethanol induced acute hepatic injury. Biomed. Pharmacother. 2020, 132, 110836. [Google Scholar] [CrossRef]
- Song, L.; Li, M.; Feng, C.; Sa, R.; Hu, X.; Wang, J.; Yin, X.; Qi, C.; Dong, W.; Yang, J. Protective effect of curcumin on zebrafish liver under ethanol-induced oxidative stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 258, 109360. [Google Scholar] [CrossRef]
- Schneider, A.C.R.; Gregório, C.; Uribe-Cruz, C.; Guizzo, R.; Malysz, T.; Faccioni-Heuser, M.C.; Longo, L.; da Silveira, T.R. Chronic exposure to ethanol causes steatosis and inflammation in zebrafish liver. World J. Hepatol. 2017, 9, 418. [Google Scholar] [CrossRef]
- Molina, V.; Mas, R.; Carbajal, D. D-002 (beeswax alcohols): Concurrent joint health benefits and gastroprotection. Indian J. Pharm. Sci. 2015, 77, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Akowuah, G.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Jin, S.; Cho, K.-H. Water extracts of cinnamon and clove exhibits potent inhibition of protein glycation and anti-atherosclerotic activity in vitro and in vivo hypolipidemic activity in zebrafish. Food Chem. Toxicol. 2011, 49, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.I.; Arrol, S.; Durrington, P.N. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991, 286, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, prot4986. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghamdi, T.H.; Atta, I.S. Efficacy of interleukin-6 in the induction of liver cell proliferation after hemi-hepatectomy: Histopathologic and immunohistochemical study. Int. J. Clin. Exp. Pathol. 2020, 13, 1540. [Google Scholar]
- Owusu-Ansah, E.; Yavari, A.; Mandal, S.; Banerjee, U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008, 40, 356–361. [Google Scholar] [CrossRef]
- Araujo-Silva, H.; Pinheiro-da-Silva, J.; Silva, P.F.; Luchiari, A.C. Individual differences in response to alcohol exposure in zebrafish (Danio rerio). PLoS ONE 2018, 13, e0198856. [Google Scholar] [CrossRef] [Green Version]
- Passeri, M.J.; Cinaroglu, A.; Gao, C.; Sadler, K.C. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology 2009, 49, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.L.; Yin, C. Histological analyses of acute alcoholic liver injury in zebrafish. JoVE J. Vis. Exp. 2017, 123, e55630. [Google Scholar] [CrossRef] [Green Version]
- Beier, J.I.; Arteel, G.E.; McClain, C.J. Advances in alcoholic liver disease. Curr. Gastroenterol. Rep. 2011, 13, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Theise, N.D. Histopathology of alcoholic liver disease. Clin. Liver Dis. 2013, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.; Marsano, L.; Mendez, C.; Bentley, F.; McClain, C.J.J.B.P.; Gastroenterology, R.C. Advances in alcoholic liver disease. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 625–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, E.; Illnait, J.; Molina, V.; Oyarzabal, A.; Fernandez, L.; Perez, Y.; Mas, R.; Mesa, M.; Fernandez, J.; Mendoza, S. Effects of D-002 (beeswax alcohols) on lipid peroxidation in middle-aged and older subjects. Lat. Am. J. Pharm. 2008, 27, 695–703. [Google Scholar]
- Hill, D.; Marsano, L.; Cohen, D.; Allen, J.; Shedlofsky, S.; McClain, C. Increased plasma interleukin-6 concentrations in alcoholic hepatitis. J. Lab. Clin. Med. 1992, 119, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Luna, J.M.; Moon, Y.P.; Liu, K.M.; Spitalnik, S.; Paik, M.C.; Cheung, K.; Sacco, R.L.; Elkind, M.S. High-sensitivity C-reactive protein and interleukin-6-dominant inflammation and ischemic stroke risk: The northern Manhattan study. Stroke 2014, 45, 979–987. [Google Scholar] [CrossRef]
- Karatayli, E.; Hall, R.A.; Weber, S.N.; Dooley, S.; Lammert, F. Effect of alcohol on the interleukin 6-mediated inflammatory response in a new mouse model of acute-on-chronic liver injury. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2019, 1865, 298–307. [Google Scholar] [CrossRef]
- Heberlein, A.; Käser, M.; Lichtinghagen, R.; Rhein, M.; Lenz, B.; Kornhuber, J.; Bleich, S.; Hillemacher, T. TNF-α and IL-6 serum levels: Neurobiological markers of alcohol consumption in alcohol-dependent patients? Alcohol 2014, 48, 671–676. [Google Scholar] [CrossRef]
- You, M.; Arteel, G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019, 70, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Capurso, N.A.; Petrakis, I. Dyslipidemia associated with heavy alcohol use. Am. J. Addict. 2016, 25, 188–190. [Google Scholar] [CrossRef]
- Wakabayashi, I. Associations between heavy alcohol drinking and lipid-related indices in middle-aged men. Alcohol 2013, 47, 637–642. [Google Scholar] [CrossRef]
- Cho, K.-H.; Nam, H.-S.; Kang, D.-J.; Park, M.-H.; Kim, J.-H. Long-Term Alcohol Consumption Caused a Significant Decrease in Serum High-Density Lipoprotein (HDL)-Cholesterol and Apolipoprotein AI with the Atherogenic Changes of HDL in Middle-Aged Korean Women. Int. J. Mol. Sci. 2022, 23, 8623. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Q.; Bouwman, F.G.; van Baak, M.A.; Roumans, N.J.; Vink, R.G.; Mariman, E.C. Plasma Levels of Triglycerides and IL-6 Are Associated with Weight Regain and Fat Mass Expansion. J. Clin. Endocrinol. Metab. 2022, 107, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Nonogaki, K.; Fuller, G.M.; Fuentes, N.L.; Moser, A.H.; Staprans, I.; Grunfeld, C.; Feingold, K.R. Interleukin-6 stimulates hepatic triglyceride secretion in rats. Endocrinology 1995, 136, 2143–2149. [Google Scholar] [CrossRef]
- Zuliani, G.; Volpato, S.; Blè, A.; Bandinelli, S.; Corsi, A.M.; Lauretani, F.; Paolisso, G.; Fellin, R.; Ferrucci, L. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: The InChianti study. Atherosclerosis 2007, 192, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaraschi, M.; Basilico, N.; Sisto, F.; Taramelli, D.; Eligini, S.; Colli, S.; Sirtori, C.R.; Franceschini, G.; Calabresi, L. High-density lipoproteins attenuate interleukin-6 production in endothelial cells exposed to pro-inflammatory stimuli. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2005, 1736, 136–143. [Google Scholar] [CrossRef]
- Terkeltaub, R.; Bushinsky, D.A.; Becker, M.A. Recent developments in our understanding of the renal basis of hyperuricemia and the development of novel antihyperuricemic therapeutics. Arthritis Res. Ther. 2006, 8, S4. [Google Scholar] [CrossRef] [Green Version]
- Castell, J.V.; Gómez-Lechón, M.J.; David, M.; Andus, T.; Geiger, T.; Trullenque, R.; Fabra, R.; Heinrich, P.C. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989, 242, 237–239. [Google Scholar] [CrossRef] [Green Version]
- Chaiyasut, C.; Pengkumsri, N.; Sirilun, S.; Peerajan, S.; Khongtan, S.; Sivamaruthi, B.S. Assessment of changes in the content of anthocyanins, phenolic acids, and antioxidant property of Saccharomyces cerevisiae mediated fermented black rice bran. AMB Express 2017, 7, 114. [Google Scholar] [CrossRef]
- Shin, H.-Y.; Kim, S.-M.; Lee, J.H.; Lim, S.-T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Zhao, H.; Lan, Y.; Liu, H.; Zhu, Y.; Liu, W.; Zhang, J.; Jia, L. Antioxidant and hepatoprotective activities of polysaccharides from spent mushroom substrates (Laetiporus sulphureus) in acute alcohol-induced mice. Oxidative Med. Cell. Longev. 2017, 2017, 5863523. [Google Scholar] [CrossRef] [Green Version]
- Menéndez, R.; Amor, A.; González, R.; Jiménez, S.; Mas, R. Inhibition of rat microsomal lipid peroxidation by the oral administration of D002. Braz. J. Med. Biol. Res. 2000, 33, 85–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menéndez, R.; Más, R.; Amor, A.M.; Pérez, Y.; González, R.M.; Fernández, J.; Molina, V.; Jiménez, S. Antioxidant effects of D002 on the in vitro susceptibility of whole plasma in healthy volunteers. Arch. Med. Res. 2001, 32, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, I.; Illnait, J.; Terry, H.; Mas, R.; Fernández, L.; Fernández, J.C.; Gámez, R.; Mesa, M.; Mendoza, S.; Ruiz, D. Effects of Abexol®(beeswax alcohols) on gastrointestinal symptoms in middle-aged and older subjects. Rev. CENIC Cienc. Biol. 2009, 40, 147–154. [Google Scholar]
- Illnait, J.; Rodriguez, I.; Molina, V.; Mendoza, S.; Mas, R.; Fernandez, L.; Oyarzabal, A.; Perez, Y.; Mesa, M.; Fernández, J. Effects of D-002 (beeswax alcohols) on gastrointestinal symptoms and oxidative markers in middle-aged and older subjects. Lat. Am. J. Pharm. 2013, 32, 166–174. [Google Scholar]
- Puente, R.; Illnait, J.; Mas, R.; Carbajal, D.; Mendoza, S.; Fernández, J.C.; Mesa, M.; Gamez, R.; Reyes, P. Evaluation of the effect of D-002, a mixture of beeswax alcohols, on osteoarthritis symptoms. Korean J. Intern. Med. 2014, 29, 191. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, I.; Illnait, J.; Molina, V.; Oyarzabal, A.; Fernandez, L.; Mesa, M.; Más, R.; Mendoza, S.; Gámez, R.; Jiménez, S. Comparison of the antioxidant effects of D-002 (beeswax alcohols) and grape seed extract (GSE) on plasma oxidative variables in healthy subjects. Lat. Am. J. Pharm. 2010, 29, 255–262. [Google Scholar]
- Kim, S.P.; Lee, S.J.; Nam, S.H.; Friedman, M. The composition of a bioprocessed shiitake (Lentinus edodes) mushroom mycelia and rice bran formulation and its antimicrobial effects against Salmonella enterica subsp. enterica serovar Typhimurium strain SL1344 in macrophage cells and in mice. BMC Complement. Altern. Med. 2018, 18, 322. [Google Scholar] [CrossRef] [PubMed]
- Budilarto, E.S.; Kamal-Eldin, A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1095–1137. [Google Scholar] [CrossRef]
- Berger, A.; Rein, D.; Schäfer, A.; Monnard, I.; Gremaud, G.; Lambelet, P.; Bertoli, C. Similar cholesterol–lowering properties of rice bran oil, with varied γ–oryzanol, in mildly hypercholesterolemic men. Eur. J. Nutr. 2005, 44, 163–173. [Google Scholar] [CrossRef]
- Hargrove, J.L.; Greenspan, P.; Hartle, D.K. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med. 2004, 229, 215–226. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Braun, M.S.; Wetterauer, P.; Wetterauer, B.; Wink, M. Antioxidant, cytotoxic, and antimicrobial activities of Glycyrrhiza glabra L., Paeonia lactiflora Pall., and Eriobotrya japonica (Thunb.) Lindl. extracts. Medicines 2019, 6, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tania, U.H.; Hassan, M.R.; Eshita, N.J.; Akhter, R.; Shahriar, M. Evaluation of In vitro Antioxidant and In vivo Pharmacological Activity of Leaf Extracts of Hoya parasitica (Wall.). J. Appl. Pharm. Sci. 2016, 6, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Egharevba, E.; Chukwuemeke-Nwani, P.; Eboh, U.; Okoye, E.; Bolanle, I.O.; Oseghale, I.O.; Imieje, V.O.; Erharuyi, O.; Falodun, A. Evaluation of the Antioxidant and Hypoglycaemic Potentials of the Leaf Extracts of Stachytarphyta jamaicensis (Verbenaceae). Trop. J. Nat. Prod. Res. 2019, 3, 170–174. [Google Scholar] [CrossRef]
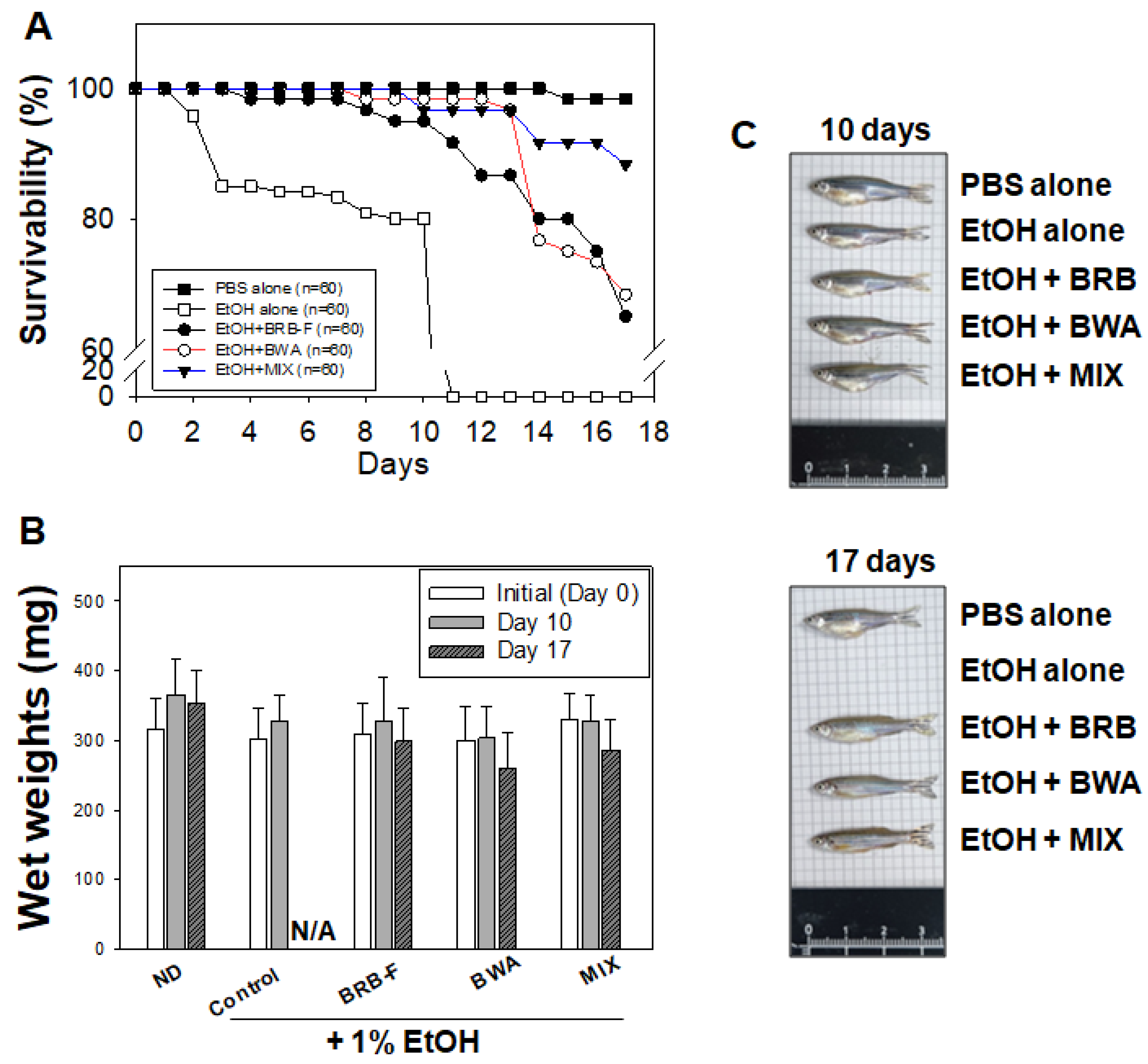


| Group | Diet Type | Wet Weight (mg) | ||
|---|---|---|---|---|
| Day 0 (n = 60) | Day 10 (n = 20) | Day 17 (n = 20) | ||
| PBS alone | Normal diet (ND) | 316 ± 43 | 365 ± 49 | 353 ± 47 |
| EtOH alone | ND | 303 ± 43 | 328 ± 38 | N/A |
| EtOH + BRB-F | ND with BRB-F (10% wt/wt) | 310 ± 45 | 328 ± 64 | 299 ± 48 |
| EtOH + BWA | ND with BWA (10%, wt/wt) | 300 ± 48 | 304 ± 45 | 261 ± 51 |
| EtOH + MIX | ND with Mixture of BRB-F and BWA (6:1, wt/wt) | 329 ± 39 | 327 ± 38 | 287 ± 44 |
| Group | n | TC (mg/dL) | TG (mg/dL) | AST (IU/L) | ALT (IU/L) | GGT (IU/L) |
|---|---|---|---|---|---|---|
| PBS control | 20 | 155 ± 17 ab | 183 ± 5 ab | 246 ± 15 ab | 109 ± 26 | 144 ± 12 |
| EtOH + BRB-F | 20 | 182 ± 23 b | 304 ± 25 b | 316 ± 14 b | 144 ± 9 | 166 ± 3 |
| EtOH + BWA | 20 | 182 ± 23 b | 233 ± 49 b | 251 ± 5 ab | 117 ± 18 | 162 ± 9 |
| EtOH + MIX (BRB-F: BWA, 6:1) | 20 | 130 ± 19 a | 146 ± 8 a | 202 ± 11 a | 100 ± 8 | 138 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Zee, S.; Cho, K.-H. Beeswax Alcohol and Fermented Black Rice Bran Synergistically Ameliorated Hepatic Injury and Dyslipidemia to Exert Antioxidant and Anti-Inflammatory Activity in Ethanol-Supplemented Zebrafish. Biomolecules 2023, 13, 136. https://doi.org/10.3390/biom13010136
Han Y, Zee S, Cho K-H. Beeswax Alcohol and Fermented Black Rice Bran Synergistically Ameliorated Hepatic Injury and Dyslipidemia to Exert Antioxidant and Anti-Inflammatory Activity in Ethanol-Supplemented Zebrafish. Biomolecules. 2023; 13(1):136. https://doi.org/10.3390/biom13010136
Chicago/Turabian StyleHan, Youngji, Seonggeun Zee, and Kyung-Hyun Cho. 2023. "Beeswax Alcohol and Fermented Black Rice Bran Synergistically Ameliorated Hepatic Injury and Dyslipidemia to Exert Antioxidant and Anti-Inflammatory Activity in Ethanol-Supplemented Zebrafish" Biomolecules 13, no. 1: 136. https://doi.org/10.3390/biom13010136







