Terrestrial Vertebrate Biodiversity Loss under Future Global Land Use Change Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Future Scenarios
2.2. Land Use Data
2.3. Projecting Species Extinctions
2.4. Projecting Evolutionary History Loss
2.5. Drivers of Evolutionary History Loss in Each Ecoregion
3. Results
3.1. Future Natural Habitat Loss
3.2. Projected Species Richness and Evolutionary History Loss
3.3. Land Use Drivers of Biodiversity Loss
3.4. Rates of Biodiversity Loss
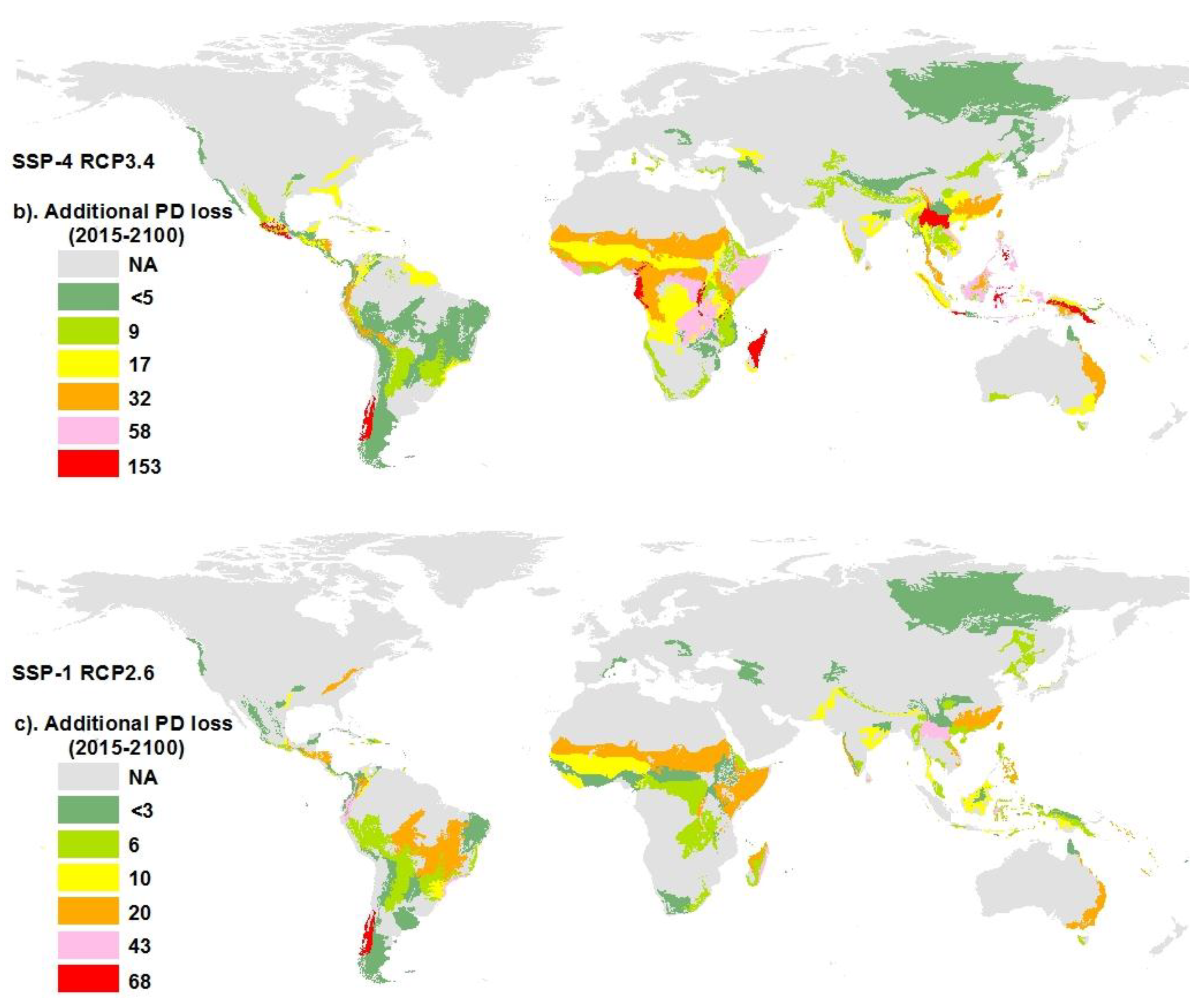
3.5. Hotspots of Biodiversity Loss
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bennett, E.M.; Cramer, W.; Begossi, A.; Cundill, G.; Díaz, S.; Egoh, B.N.; Geijzendorffer, I.R.; Krug, C.B.; Lavorel, S.; Lazos, E.; et al. Linking biodiversity, ecosystem services, and human well-being: Three challenges for designing research for sustainability. Curr. Opin. Environ. Sustain. 2015, 14, 76–85. [Google Scholar] [CrossRef]
- United Nations General Assembly. Transforming Our World: The 2030 Agenda for Sustainable Development. United Nations: New York, NY, USA, 2015. Available online: http://www.un.org/ga/search/view_doc.asp?symbol=A/RES/70/1&Lang=E (accessed on 1 June 2018).
- Convention on Biological Diversity. Conference of the Parties Decision X/2: Strategic Plan for Biodiversity 2011–2020. 2011. Available online: www.cbd.int/decision/cop?id=12268 (accessed on 5 December 2017).
- Kok, M.T.; Kok, K.; Peterson, G.D.; Hill, R.; Agard, J.; Carpenter, S.R. Biodiversity and ecosystem services require IPBES to take novel approach to scenarios. Sustain. Sci. 2017, 12, 177–181. [Google Scholar] [CrossRef]
- Ferrier, S.; Ninan, K.N.; Leadley, P.; Alkemade, R.; Acosta, L.A.; Akçakaya, H.R.; Brotons, L.; Cheung, W.W.L.; Christensen, V.; Harhash, K.A.; et al. The Methodological Assessment Report on Scenarios and Models of Biodiversity and Ecosystem Services; Secretariat of the Intergovernmental Platform for Biodiversity and Ecosystem Services: Bonn, Germany, 2016. [Google Scholar]
- Maxwell, S.L.; Fuller, R.A.; Brooks, T.M.; Watson, J.E. Biodiversity: The ravages of guns, nets and bulldozers. Nature 2016, 536, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Titeux, N.; Henle, K.; Mihoub, J.B.; Regos, A.; Geijzendorffer, I.R.; Cramer, W.; Verburg, P.H.; Brotons, L. Biodiversity scenarios neglect future land-use changes. Glob. Chang. Biol. 2016, 22, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.; Sala, O.; Pereira, H. The future of vascular plant diversity under four global scenarios. Ecol. Soc. 2006, 11, 2. Available online: https://www.ecologyandsociety.org/vol11/iss2/art25/ (accessed on 5 August 2018).
- Jantz, S.M.; Barker, B.; Brooks, T.M.; Chini, L.P.; Huang, Q.; Moore, R.M.; Noel, J.; Hurtt, G.C. Future habitat loss and extinctions driven by land-use change in biodiversity hotspots under four scenarios of climate-change mitigation. Conserv. Biol. 2015, 29, 1122–1131. [Google Scholar] [CrossRef] [PubMed]
- Hurtt, G.C.; Chini, L.P.; Frolking, S.; Betts, R.A.; Feddema, J.; Fischer, G.; Fisk, J.P.; Hibbard, K.; Houghton, R.A.; Janetos, A.; et al. Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim. Chang. 2011, 109, 117. Available online: https://doi.org/10.1007/s10584-011-0153-2 (accessed on 5 August 2018).
- Harfoot, M.; Tittensor, D.P.; Newbold, T.; McInerny, G.; Smith, M.J.; Scharlemann, J.P. Integrated assessment models for ecologists: The present and the future. Glob. Ecol. Biogeogr. 2014, 23, 124–143. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Edmonds, J.; Kainuma, M.; Riahi, K.; Thomson, A.; Hibbard, K.; Hurtt, G.C.; Kram, T.; Krey, V.; Lamarque, J.F.; et al. The representative concentration pathways: An overview. Clim. Chang. 2011, 109, 5. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Chanin, M.L.; Angell, J.; Barnett, J.; Gaffen, D.; Gelman, M.; Keckhut, P.; Koshelkov, Y.; Labitzke, K.; Lin, J.J.; et al. Stratospheric temperature trends: Observations and model simulations. Rev. Geophys. 2001, 39, 71–122. [Google Scholar] [CrossRef] [Green Version]
- IUCN. IUCN Red List of Threatened Species Gland; International Union for Conservation of Nature: Gland, Switzerland, 2017; Available online: www.iucnredlist.org (accessed on 1 June 2018).
- Chaudhary, A.; Burivalova, Z.; Koh, L.P.; Hellweg, S. Impact of forest management on species richness: Global meta-analysis and economic trade-offs. Sci. Rep. 2016, 6, 23954. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.P.; Kriegler, E.; O’Neill, B.C.; Ebi, K.L.; Riahi, K.; Carter, T.R.; Edmonds, J.; Hallegatte, S.; Kram, T.; Mathur, R.; et al. A new scenario framework for climate change research: Scenario matrix architecture. Clim. Chang. 2014, 122, 373–386. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Ebi, K.L.; Kemp-Benedict, E.; Riahi, K.; Rothman, D.S.; van Ruijven, B.J.; van Vuuren, D.P.; Birkmann, J.; Kok, K.; et al. The roads ahead: Narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Chang. 2015, 42, 169–180. [Google Scholar] [CrossRef]
- Riahi, K.; Van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Popp, A.; Calvin, K.; Fujimori, S.; Havlik, P.; Humpenöder, F.; Stehfest, E.; Bodirsky, B.L.; Dietrich, J.P.; Doelmann, J.C.; Gusti, M.; et al. Land-use futures in the shared socio-economic pathways. Glob. Environ. Chang. 2017, 42, 331–345. [Google Scholar] [CrossRef]
- Kriegler, E.; Edmonds, J.; Hallegatte, S.; Ebi, K.L.; Kram, T.; Riahi, K.; Winkler, H.; Van Vuuren, D.P. A new scenario framework for climate change research: The concept of shared climate policy assumptions. Clim. Chang. 2014, 122, 401–414. [Google Scholar] [CrossRef]
- Hurtt, G.; Chini, L.; Sahajpal, R.; Frolking, S.; Calvin, K.; Fujimori, S.; Klein Goldewijk, K.; Hasegawa, T.; Havlik, P.; Heinemann, A.; et al. Harmonization of global land-use change and management for the period 850–2100. Geosci. Model Dev. (in preparation). Available online: http://luh.umd.edu/data.shtml (accessed on 2 November 2017).
- Chaudhary, A.; Brooks, T.M. National Consumption and Global Trade Impacts on Biodiversity. World Dev. 2017, in press. [Google Scholar] [CrossRef]
- Pereira, H.M.; Ziv, G.; Miranda, M. Countryside Species–Area Relationship as a Valid Alternative to the Matrix-Calibrated Species–Area Model. Conserv. Biol. 2014, 28, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Verones, F.; de Baan, L.; Hellweg, S. Quantifying land use impacts on biodiversity: Combining species–area models and vulnerability indicators. Environ. Sci. Technol. 2015, 49, 9987–9995. [Google Scholar] [CrossRef] [PubMed]
- IUCN. IUCN Habitat Classification Scheme Version, 3.1; International Union for Conservation of Nature: Gland, Switzerland, 2015; Available online: http://www.iucnredlist.org/technical-documents/classification-schemes/habitats-classification-scheme-ver3 (accessed on 2 June 2018).
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial Ecoregions of the World: A New Map of Life on Earth: A new global map of terrestrial ecoregions provides an innovative tool for conserving biodiversity. BioScience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Chaudhary, A.; Kastner, T. Land use biodiversity impacts embodied in international food trade. Glob. Environ. Chang. 2016, 38, 195–204. [Google Scholar] [CrossRef]
- Chaudhary, A.; Carrasco, L.R.; Kastner, T. Linking national wood consumption with global biodiversity and ecosystem service losses. Sci. Total Environ. 2017, 586, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Crozier, R.H. Preserving the information content of species: Genetic diversity, phylogeny, and conservation worth. Ann. Rev. Ecol. Syst. 1997, 28, 243–268. [Google Scholar] [CrossRef]
- Mace, G.M.; Gittleman, J.L.; Purvis, A. Preserving the tree of life. Science 2003, 300, 1707–1709. [Google Scholar] [CrossRef] [PubMed]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Redding, D.W.; Hartmann, K.; Mooers, A.O. Global distribution and conservation of evolutionary distinctness in birds. Curr. Biol. 2014, 24, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Collen, B.; Turvey, S.T.; Waterman, C.; Meredith, H.M.; Kuhn, T.S.; Baillie, J.E.; Isaac, N.J. Investing in evolutionary history: Implementing a phylogenetic approach for mammal conservation. Philos. Trans. Royal Soc. B Biol. Sci. 2011, 366, 2611–2622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadotte, M.W. Experimental evidence that evolutionarily diverse assemblages result in higher productivity. Proc. Natl. Acad. Sci. USA 2013, 110, 8996–9000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faith, D.P. The PD Phylogenetic Diversity Framework: Linking Evolutionary History to Feature Diversity for Biodiversity Conservation. In Biodiversity Conservation and Phylogenetic Systematics; Pellens, R., Grandcolas, P., Eds.; Springer: Basel, Switzerland, 2016; pp. 39–56. [Google Scholar]
- Martyn, I.; Kuhn, T.S.; Mooers, A.O.; Moulton, V.; Spillner, A. Computing evolutionary distinctiveness indices in large scale analysis. Algorithms Mol. Biol. 2012, 7, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaac, N.J.; Redding, D.W.; Meredith, H.M.; Safi, K. Phylogenetically-informed priorities for amphibian conservation. PLoS ONE 2012, 7, e43912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, A.; Pourfaraj, V.; Mooers, A.O. Projecting global land use-driven evolutionary history loss. Divers. Distrib. 2018, 24, 158–167. [Google Scholar] [CrossRef]
- Steel, M.; Pourfaraj, V.; Chaudhary, A.; Mooers, A. Evolutionary Isolation and Phylogenetic Diversity Loss under Random Extinction Events. J. Theor. Biol. 2017, 438, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Isaac, N.J.; Turvey, S.T.; Collen, B.; Waterman, C.; Baillie, J.E. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS ONE 2007, 2, e296. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, D.P.; Stehfest, E.; den Elzen, M.G.; Kram, T.; van Vliet, J.; Deetman, S.; Isaac, M.; Goldewijk, K.K.; Hof, A.; Beltran, A.M.; et al. RCP2. 6: Exploring the possibility to keep global mean temperature increase below 2 C. Clim. Chang. 2011, 109, 95–116. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Stehfest, E.; Gernaat, D.E.; Doelman, J.C.; Van den Berg, M.; Harmsen, M.; de Boer, H.S.; Bouwman, L.F.; Daioglou, V.; Edelenbosch, O.Y.; et al. Energy, land-use and greenhouse gas emissions trajectories under a green growth paradigm. Glob. Environ. Chang. 2017, 42, 237–250. [Google Scholar] [CrossRef]
- Calvin, K.; Bond-Lamberty, B.; Clarke, L.; Edmonds, J.; Eom, J.; Hartin, C.; Kim, S.; Kyle, P.; Link, R.; Moss, R.; et al. The SSP4: A world of deepening inequality. Glob. Environ. Chang. 2017, 42, 284–296. [Google Scholar] [CrossRef]
- Kriegler, E.; Bauer, N.; Popp, A.; Humpenöder, F.; Leimbach, M.; Strefler, J.; Baumstark, L.; Bodirsky, B.L.; Hilaire, J.; Klein, D.; Mouratiadou, I. Fossil-fueled development (SSP5): An energy and resource intensive scenario for the 21st century. Glob. Environ. Chang. 2017, 42, 297–315. [Google Scholar] [CrossRef]
- Fujimori, S.; Hasegawa, T.; Masui, T.; Takahashi, K.; Herran, D.S.; Dai, H.; Hijioka, Y.; Kainuma, M. SSP3: AIM implementation of shared socioeconomic pathways. Glob. Environ. Chang. 2017, 42, 268–283. [Google Scholar] [CrossRef]
- Fricko, O.; Havlik, P.; Rogelj, J.; Klimont, Z.; Gusti, M.; Johnson, N.; Kolp, P.; Strubegger, M.; Valin, H.; Amann, M.; et al. The marker quantification of the Shared Socioeconomic Pathway 2: A middle-of-the-road scenario for the 21st century. Glob. Environ. Chang. 2017, 42, 251–267. [Google Scholar] [CrossRef] [Green Version]
- Drakare, S.; Lennon, J.J.; Hillebrand, H. The imprint of the geographical, evolutionary and ecological context on species—Area relationships. Ecol. Lett. 2006, 9, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate Chang. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L.E. Global warming and extinctions of endemic species from biodiversity hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Segan, D.B.; Murray, K.A.; Watson, J.E. A global assessment of current and future biodiversity vulnerability to habitat loss–climate change interactions. Glob. Ecol. Conserv. 2016, 5, 12–21. [Google Scholar] [CrossRef]
- Mantyka-Pringle, C.S.; Martin, T.G.; Rhodes, J.R. Interactions between climate and habitat loss effects on biodiversity: A systematic review and meta-analysis. Glob. Chang. Biol. 2012, 18, 1239–1252. [Google Scholar] [CrossRef]
- Visconti, P.; Bakkenes, M.; Baisero, D.; Brooks, T.; Butchart, S.H.; Joppa, L.; Alkemade, R.; Di Marco, M.; Santini, L.; Hoffmann, M.; et al. Projecting global biodiversity indicators under future development scenarios. Conserv. Lett. 2016, 9, 5–13. [Google Scholar] [CrossRef]
- McLellan, R.; Iyengar, L.; Jeffries, B.; Oerlemans, N. (Eds.) Living Planet Report 2014: Species and Spaces, People and Places; World Wide Fund for Nature: Gland, Switzerland, 2014. [Google Scholar]
- Pearson, R.G.; Dawson, T.P. Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akcakaya, H.R.; et al. Assessing species vulnerability to climate Change. Nat. Clim. Chang. 2015, 5, 215. [Google Scholar] [CrossRef] [Green Version]
- Hanski, I.; Zurita, G.A.; Bellocq, M.I.; Rybicki, J. Species–fragmented area relationship. Proc. Natl. Acad. Sci. USA 2013, 110, 12715–12720. [Google Scholar] [CrossRef] [PubMed]
- Millennium Ecosystem Assessment. Ecosystems and Human Wellbeing: A Framework for Assessment; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G.; et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Jetz, W.; Wilcove, D.S.; Dobson, A.P. Projected impacts of climate and land-use change on the global diversity of birds. PLoS Biol. 2007, 5, e157. [Google Scholar] [CrossRef] [PubMed]
- Newbold, T.; Hudson, L.N.; Hill, S.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global effects of land use on local terrestrial biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz, S.; Fargione, J.; Chapin, F.S.; Tilman, D. Biodiversity loss threatens human well-being. PLoS Biol. 2006, 4, e277. [Google Scholar] [CrossRef] [PubMed]
- Venter, O.; Fuller, R.A.; Segan, D.B.; Carwardine, J.; Brooks, T.; Butchart, S.H.; Di Marco, M.; Iwamura, T.; Joseph, L.; O’Grady, D.; et al. Targeting global protected area expansion for imperiled biodiversity. PLoS Biol. 2014, 12, e1001891. [Google Scholar] [CrossRef] [PubMed]
- Possingham, H.P.; Bode, M.; Klein, C.J. Optimal conservation outcomes require both restoration and protection. PLoS Biol. 2015, 13, e1002052. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Gustafson, D.; Mathys, A. Multi-indicator sustainability assessment of global food systems. Nat. Commun. 2018, 9, 848. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A.; Pfister, S.; Hellweg, S. Spatially explicit analysis of biodiversity loss due to global agriculture, pasture and forest land use from a producer and consumer perspective. Environ. Sci. Technol. 2016, 50, 3928–3936. [Google Scholar] [CrossRef] [PubMed]
- Pollock, L.J.; Thuiller, W.; Jetz, W. Large conservation gains possible for global biodiversity facets. Nature 2017, 546, 141. [Google Scholar] [CrossRef] [PubMed]
- Waldron, A.; Miller, D.C.; Redding, D.; Mooers, A.; Kuhn, T.S.; Nibbelink, N.; Roberts, J.T.; Tobias, J.A.; Gittleman, J.L. Reductions in global biodiversity loss predicted from conservation spending. Nature 2017, 551, 24295. [Google Scholar] [CrossRef] [PubMed]


| Year | Scenario | Projected Endemic Extinctions | Projected PD Loss (MY) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| M | B | A | T | M | B | A | T | ||
| 850 | Past | 6 | 5 | 15 | 25 | 0 | 3 | 20 | 24 |
| 1900 | Past | 74 | 86 | 212 | 372 | 268 | 271 | 1137 | 1675 |
| 2015 | Present | 199 | 222 | 602 | 1023 | 948 | 828 | 3493 | 5270 |
| 2050 | RCP2.6 SSP-1 | 219 | 236 | 665 | 1120 | 1039 | 872 | 3875 | 5787 |
| RCP4.5 SSP-2 | 239 | 255 | 720 | 1214 | 1155 | 952 | 4212 | 6319 | |
| RCP7.0 SSP-3 | 249 | 260 | 746 | 1255 | 1202 | 976 | 4394 | 6572 | |
| RCP3.4 SSP-4 | 267 | 270 | 764 | 1301 | 1338 | 1022 | 4517 | 6876 | |
| RCP6.0 SSP-4 | 252 | 253 | 735 | 1241 | 1272 | 943 | 4391 | 6606 | |
| RCP8.5 SSP-5 | 241 | 255 | 747 | 1244 | 1157 | 920 | 4421 | 6499 | |
| 2100 | RCP2.6 SSP-1 | 241 | 256 | 734 | 1232 | 1226 | 941 | 4293 | 6459 |
| RCP4.5 SSP-2 | 297 | 317 | 816 | 1430 | 1455 | 1230 | 4756 | 7441 | |
| RCP7.0 SSP-3 | 302 | 301 | 883 | 1485 | 1523 | 1150 | 5203 | 7876 | |
| RCP3.4 SSP-4 | 398 | 408 | 1035 | 1841 | 1997 | 1575 | 6100 | 9672 | |
| RCP6.0 SSP-4 | 320 | 319 | 883 | 1522 | 1581 | 1203 | 5273 | 8057 | |
| RCP8.5 SSP-5 | 278 | 281 | 825 | 1385 | 1365 | 1043 | 4878 | 7286 | |
| Land Use Type | Past | Present | % of Total Projected Extinctions in 2100AD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 850 AD | 1900 AD | 2015 AD | RCP2.6 SSP-1 | RCP2.6 SSP-2 | RCP7.0 SSP-3 | RCP3.4 SSP-4 | RCP6.0 SSP-4 | RCP8.5 SSP-5 | |
| Sec. Veg. (forests) | 0 | 28 | 28 | 35 | 35 | 28 | 21 | 30 | 35 |
| Sec. Veg. (non-forests) | 0 | 19 | 9 | 13 | 11 | 8 | 8 | 8 | 11 |
| Pasture | 7 | 13 | 21 | 11 | 13 | 17 | 15 | 20 | 16 |
| Rangeland | 44 | 16 | 11 | 8 | 7 | 10 | 8 | 10 | 8 |
| Urban | 0 | 0 | 2 | 3 | 3 | 3 | 3 | 4 | 3 |
| C3 annual crops | 20 | 9 | 11 | 9 | 12 | 13 | 11 | 11 | 10 |
| C3 permanent crops | 13 | 7 | 9 | 11 | 10 | 10 | 13 | 8 | 8 |
| C4 annual crops | 9 | 4 | 5 | 3 | 5 | 6 | 4 | 4 | 6 |
| C4 permanent crops | 2 | 1 | 1 | 5 | 1 | 1 | 13 | 3 | 1 |
| C3 Nitrogen fixing crops | 4 | 2 | 2 | 2 | 3 | 3 | 2 | 2 | 3 |
| Metric | Period | Scenario | EAP | EU&CA | LAC | MENA | N. America | S. Asia | SSA |
|---|---|---|---|---|---|---|---|---|---|
| SR | 2015–2050 | SSP-1 RCP2.6 | 30 | 0 | 28 | 0 | 3 | 14 | 13 |
| SSP-2 RCP4.5 | 60 | 2 | 68 | 1 | 3 | 22 | 34 | ||
| SSP-3 RCP7.0 | 58 | 0 | 59 | 0 | 3 | 27 | 77 | ||
| SSP-4 RCP3.4 | 96 | 0 | 49 | 0 | 4 | 32 | 88 | ||
| SSP-4 RCP6.0 | 55 | 0 | 40 | 0 | 4 | 27 | 82 | ||
| SSP-5 RCP8.5 | 50 | 0 | 66 | 0 | 3 | 29 | 62 | ||
| 2050–2100 | SSP-1 RCP2.6 | 45 | 1 | 41 | 0 | 2 | 8 | 17 | |
| SSP-2 RCP4.5 | 176 | 6 | 110 | 2 | 4 | 26 | 79 | ||
| SSP-3 RCP7.0 | 65 | 2 | 63 | 0 | 1 | 3 | 95 | ||
| SSP-4 RCP3.4 | 281 | 7 | 100 | 1 | 1 | 7 | 137 | ||
| SSP-4 RCP6.0 | 101 | 4 | 42 | 2 | 0 | 6 | 127 | ||
| SSP-5 RCP8.5 | 65 | 3 | 31 | 0 | 1 | 4 | 43 | ||
| PD | 2015–2050 | SSP-1 RCP2.6 | 139 | 5 | 167 | 0 | 16 | 117 | 73 |
| SSP-2 RCP4.5 | 314 | 13 | 368 | 2 | 14 | 153 | 187 | ||
| SSP-3 RCP7.0 | 257 | 16 | 338 | 1 | 16 | 184 | 482 | ||
| SSP-4 RCP3.4 | 535 | 10 | 281 | 1 | 23 | 206 | 544 | ||
| SSP-4 RCP6.0 | 330 | 5 | 268 | 1 | 24 | 186 | 518 | ||
| SSP-5 RCP8.5 | 286 | 5 | 363 | 1 | 15 | 203 | 354 | ||
| 2050–2100 | SSP-1 RCP2.6 | 269 | 0 | 232 | 1 | 10 | 27 | 130 | |
| SSP-2 RCP4.5 | 884 | 31 | 597 | 2 | 23 | 175 | 446 | ||
| SSP-3 RCP7.0 | 403 | 8 | 335 | 1 | 2 | 6 | 557 | ||
| SSP-4 RCP3.4 | 1358 | 44 | 513 | 1 | 12 | 50 | 784 | ||
| SSP-4 RCP6.0 | 494 | 28 | 179 | 1 | 2 | 26 | 695 | ||
| SSP-5 RCP8.5 | 306 | 23 | 169 | 0 | 1 | 8 | 275 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaudhary, A.; Mooers, A.O. Terrestrial Vertebrate Biodiversity Loss under Future Global Land Use Change Scenarios. Sustainability 2018, 10, 2764. https://doi.org/10.3390/su10082764
Chaudhary A, Mooers AO. Terrestrial Vertebrate Biodiversity Loss under Future Global Land Use Change Scenarios. Sustainability. 2018; 10(8):2764. https://doi.org/10.3390/su10082764
Chicago/Turabian StyleChaudhary, Abhishek, and Arne O. Mooers. 2018. "Terrestrial Vertebrate Biodiversity Loss under Future Global Land Use Change Scenarios" Sustainability 10, no. 8: 2764. https://doi.org/10.3390/su10082764
APA StyleChaudhary, A., & Mooers, A. O. (2018). Terrestrial Vertebrate Biodiversity Loss under Future Global Land Use Change Scenarios. Sustainability, 10(8), 2764. https://doi.org/10.3390/su10082764





