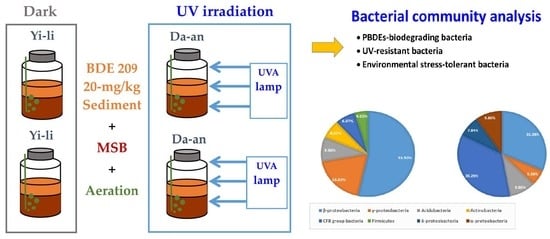
Variation of Microbial Communities in Aquatic Sediments under Long-Term Exposure to Decabromodiphenyl Ether and UVA Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. River Sediments
2.3. BDE-209-Contaminated Microcosms and Exposure to UVA Irradiation
2.4. PBDEs Analysis
2.5. Bacteria Community Analysis
3. Results
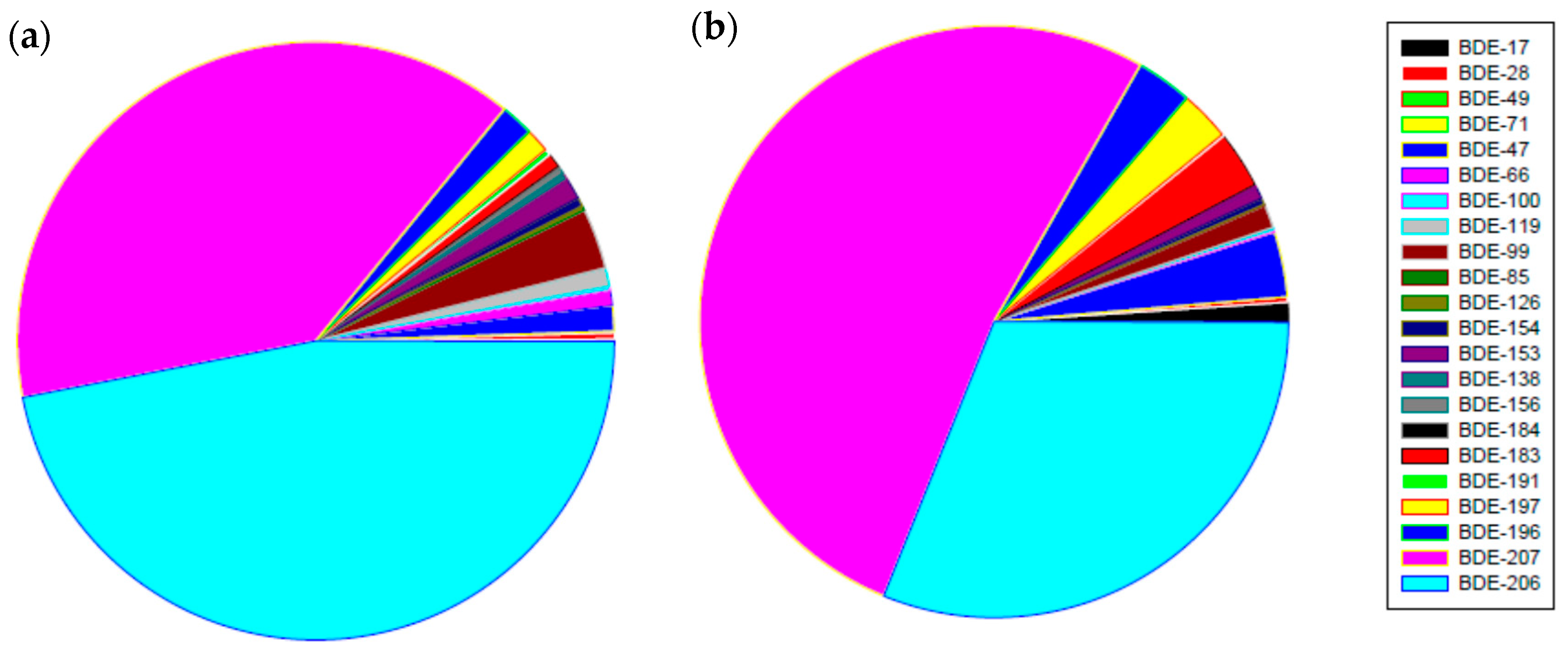
3.1. PBDEs Concentrations in River Sediments
3.2. Bacterial Communities of Microcosms Containing BDE-209-Contaminated Yi-Li Sediment
3.3. Bacterial Communities of Microcosms Containing BDE-209-Contaminated Da-An Sediment
4. Discussion
4.1. Bacterial Species Involved in PBDE Biodegradation in the Microcosms
4.2. The Effects UVA Irradiation and the Presence of of BDE-209 on the Bacterial Communities
4.3. The Influence of Sediment Heterogeneity on the Bacterial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, P.; Cotrufo, M.F.; Rumpel, C.; Paustian, K.; Kuikman, P.J.; Elliott, J.A.; McDowell, R.; Griffiths, R.I.; Asakawa, S.; Bustamante, M.; et al. Biogeochemical cycles and biodiversity as key drivers of ecosystem services provided by soils. Soil 2015, 1, 665–685. [Google Scholar] [CrossRef]
- Lee, E.; Kim, T.H.; Choi, J.S.; Nabanata, P.; Kim, N.Y.; Ahn, M.Y.; Jung, K.K.; Kang, I.H.; Kim, T.S.; Kwack, S.J.; et al. Evaluation of liver and thyroid toxicity in Sprague-Dawley rats after exposure to polybrominated diphenyl ether BDE-209. J. Toxicol Sci. 2010, 35, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Drage, D.; Mueller, J.F.; Birch, G.; Eaglesham, G.; Hearn, L.K.; Harrad, S. Historical trends of PBDEs and HBCDs in sediment cores from Sydney estuary, Australia. Sci. Total Environ. 2015, 512–513, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, X.; Liang, C.; Leung, J.Y.S.; Li, H.; Chen, S.; Mai, B.; Miao, S.; Chen, Y.; Wu, Z.; et al. Historical trends and ecological risks of polybrominated diphenyl ethers (PBDEs) and alternative halogenated flame retardants (AHFRs) in a mangrove in South China. Sci. Total Environ. 2017, 599, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Chokwe, T.B.; Magubane, M.N.; Abafe, O.A.; Okonkwo, J.O.; Sibiya, I.V. Levels, distributions, and ecological risk assessments of polybrominated diphenyl ethers and alternative flame retardants in river sediments from Vaal River, South Africa. Environ. Sci. Pollut. Res. 2019, 26, 7156–7163. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Duan, H.; Song, Q.; Liu, Y.; Li, Y.; Li, J.; Shen, W.; Luo, J.; Wang, J. Characterization of brominated flame retardants from e-waste components in China. Waste Manag. 2017, 68, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Liao, W.C.; Chen, W.C.; Wang, Y.S. Interaction of polybrominated diphenyl ethers (PBDEs) with anaerobic mixed bacterial cultures isolated from river sediment. J. Hazard. Mater. 2009, 165, 518–524. [Google Scholar] [CrossRef]
- Wu, P.; Wang, Y.-S.; Sun, C.-C.; Sun, F.-L.; Wang, Y.-T. Microbial community shift with decabromodiphenyl ether (BDE 209) in sediments of the Pearl River estuary, China. Biologia 2013, 68, 788–796. [Google Scholar] [CrossRef]
- Ma, W.; Yan, Y.; Ma, M.; Zhang, Y.; Cheng, X. Migration and biodegradation of BDE-99 in different river-based natural groundwater recharge modes with treated municipal wastewater. Process Saf. Environ. 2016, 104, 531–540. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Kumar, R.A.; Tyagi, M.B.; Sinha, R.P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 2010, 592980. [Google Scholar] [CrossRef]
- Hunting, E.R.; White, C.M.; van Gemert, M.; Mes, D.; Stam, E.; van der Geest, H.G.; Kraak, M.H.; Admiraal, W. UV radiation and organic matter composition shape bacterial functional diversity in sediments. Front. Microbiol. 2013, 4, 317. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, C.E.W.; Paul, A. Photolysis. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Amsterdam, The Netherland, 2008; Volume 1, pp. 2724–2732, ISSN 978-0-08-045405-4. [Google Scholar]
- Pan, Y.; Tsang, D.C.W.; Wang, Y.; Li, Y.; Yang, X. The photodegradation of polybrominated diphenyl ethers (PBDEs) in various environmental matrices: Kinetics and mechanisms. Chem. Eng. Sci. 2016, 297, 74–96. [Google Scholar] [CrossRef]
- Söderström, G.; Sellström, U.; de Wit, C.A.; Tysklind, M. Photolytic debromination of decabromodiphenyl ether (BDE 209). Environ. Sci. Technol. 2004, 38, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.-W.; Buettner, G.R.; Venkataraman, S.; Treime, S.E.; Robertson, L.W.; Ludewig, G. UVA/B-Induced formation of free radicals from decabromodiphenyl ether. Environ. Sci. Technol. 2009, 43, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.-Y.; Filley, T.R.; Jafvert, C.T.; Nies, L.; Hua, I.; Bezares-Cruz, J. Photodegradation of decabromodiphenyl ether adsorbed onto clay minerals, metal oxides, and sediment. Environ. Sci. Technol. 2006, 40, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.-L.; Chang, Y.-T.; Liao, Y.-F.; Lin, C.-H. Biodegradation of decabromodiphenyl ether (BDE-209) by bacterial mixed cultures in the soil/water system. Int. Biodeterior. Biodegrad. 2013, 85, 671–682. [Google Scholar] [CrossRef]
- Yu, C.-C.; Chang, T.-C.; Liao, C.-S.; Chang, Y.-T. A comparison of the microbial community and functional genes present in free-living and soil particle-attached bacteria from an aerobic bioslurry reactor treating high-molecular-weight PAHs. Sustainability 2019, 11, 1088. [Google Scholar] [CrossRef]
- Chou, H.-L.; Hwa, M.-Y.; Lee, Y.-C.; Chang, Y.-J.; Chang, Y.-T. Microbial degradation of decabromodiphenyl ether (DBDE) in soil slurry microcosms. Environ. Sci. Pollut. Res. 2016, 23, 5255–5267. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, H.; Wen, C.; Guo, Y.; Guo, J.; Xu, M.; Li, X. A comparative study of bacterial community structures in the sediments from brominated flame retardants contaminated river and noncontaminated reservoir. Afr. J. Microbiol. Res. 2012, 6, 3248–3260. [Google Scholar]
- Liu, J.; He, X.; Lin, X.; Chen, W.; Zhou, Q.; Shu, W.; Huang, L. Ecological effects of combined pollution associated with e-waste recycling on the composition and diversity of soil microbial communities. Environ. Sci. Technol. 2015, 49, 6438–6447. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Shu, H.; Lin, X.; Zhou, Q.; Bramryd, T.; Shu, W.; Huang, L. Microbial community structure and function in sediments from e-waste contaminated rivers at Guiyu area of China. Environ. Pollut. 2018, 235, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, W.; Xiao, L.; Yang, L. Effect of decabromodiphenyl ether (BDE 209) and dibromodiphenyl ether (BDE 15) on soil microbial activity and bacterial community composition. J. Hazard. Mater. 2011, 186, 883–890. [Google Scholar] [CrossRef]
- Chang, Y.T.; Lo, T.; Chou, H.-L.; Laio, Y.-F.; Lin, C.C.; Chen, H.-T. Anaerobic biodegradation of BDE-209-contaminated sediment by organic composts. Int. Biodeterior. Biodegrad. 2016, 113, 228–237. [Google Scholar] [CrossRef]
- Shi, G.; Yin, H.; Ye, J.; Peng, H.; Li, J.; Luo, C. Aerobic biotransformation of decabromodiphenyl ether (PBDE-209) by Pseudomonas aeruginosa. Chemosphere 2013, 93, 1487–1493. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, A.-J.; Qiu, L.-N.; Li, J.-R.; Li, F.-K. Biodegradation of decabromodiphenyl ether (BDE-209) by crude enzyme extract from Pseudomonas aeruginosa. Int. J. Environ. Res. Public Health 2015, 12, 11829–11847. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, X.; Xia, N.; Wu, S.; Gao, F.; Zhou, W. Identification and biodegradation efficiency of a newly isolated 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) aerobic degrading bacterial strain. Int. Biodeterior. Biodegrad. 2013, 76, 24–31. [Google Scholar] [CrossRef]
- Xin, J.; Liu, X.; Liu, W.; Zheng, X.L. Aerobic transformation of BDE-47 by a Pseudomonas putida sp. strain TZ-1 Isolated from PBDEs-contaminated sediment. Bull. Environ. Contam. Toxicol. 2014, 93, 483–488. [Google Scholar] [CrossRef]
- Lv, Y.; Li, L.; Chen, Y.; Tang, Z.; Hu, Y. Effects of glucose and biphenyl on aerobic cometabolism of polybrominated diphenyl ethers by Pseudomonas putida: Kinetics and degradation mechanism. Int. Biodeterior. Biodegrad. 2016, 108, 76–84. [Google Scholar] [CrossRef]
- Xu, M.; Chen, X.; Qiu, M.; Zeng, X.; Xu, J.; Deng, D.; Sun, G.; Li, X.; Guo, J. Bar-coded pyrosequencing reveals the responses of PBDE-degrading microbial communities to electron donor amendments. PLoS ONE 2012, 7, e30439. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Qiu, M.; Sun, G.; Guo, J.; Xu, M. Synergistic degradation of deca-BDE by an enrichment culture and zero-Valent iron. Environ. Sci. Pollut. Res. 2014, 21, 7856–7862. [Google Scholar] [CrossRef]
- Schmidt, S.; Wittich, R.M.; Erdmann, D.; Wilkes, H.; Francke, W.; Fortnagel, P. Biodegradation of diphenyl ether and its monohalogenated derivatives by Sphingomonas sp. strain SS3. Appl. Environ. Microbiol. 1992, 58, 2744–2750. [Google Scholar] [PubMed]
- Schmidt, S.; Fortnagel, P.; Wittich, R.M. Biodegradation and transformation of 4,4’-dihalodiphenyl and 2,4-dihalodiphenyl ethers by Sphingomonas sp. strain SS33. Appl. Environ. Microbiol. 1993, 59, 3931–3933. [Google Scholar] [PubMed]
- Kim, Y.M.; Nam, I.H.; Murugesan, K.; Schnidt, S.; Crowley, D.E.; Chang, Y.S. Biodegradation of diphenyl ether and transformation of selected brominated congeners by Sphingomonas sp. PH-07. Appl. Microbiol. Biotechnl. 2007, 77, 187–194. [Google Scholar] [CrossRef]
- Kim, Y.M.; Murugesan, K.; Chang, Y.Y.; Kim, E.J.; Chang, Y.S. Degradation of polybrominated diphenyl ethers by a sequential treatment with nanoscale zero valent iron and aerobic biodegradation. J. Chem. Technol. Biotechnol. 2012, 87, 216–224. [Google Scholar] [CrossRef]
- Tian, R.M.; Lee, O.O.; Wang, Y.; Cai, L.; Bougouffa, S.; Chiu, J.M.; Wu, R.S.; Qian, P.Y. Effect of polybrominated diphenyl ether (PBDE) treatment on the composition and function of the bacterial community in the sponge Haliclona cymaeformis. Front. Microbiol. 2015, 5, 799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shih, Y.-H.; Chou, H.-L.; Peng, Y.-H. Microbial degradation of 4-monobrominated diphenyl ether with anaerobic sludge. J. Hazard. Mater. 2012, 213, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Kielak, A.M.; Barreto, C.C.; Kowalchuk, G.A.; van Veen, J.A.; Kuramae, E.E. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016, 7, 744. [Google Scholar] [CrossRef]
- Goosen, N.; Moolenaar, G.F. Repair of UV damage in bacteria. DNA Repair 2008, 7, 353–379. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Q.; Wan, R.; Xie, S. Changes in bacterial community of anthracene bioremediation in municipal solid waste composting soil. J. Zhejiang Univ. Sci. B 2011, 12, 760–768. [Google Scholar] [CrossRef]
- Agogué, H.; Joux, F.; Obernosterer, I.; Lebaron, P. Resistance of marine bacterioneuston to solar radiation. Appl. Environ. Microbiol. 2005, 71, 5282–5289. [Google Scholar] [CrossRef]
- Liu, H.; Yan, J.; Wang, Q.; Karlson, U.G.; Zou, G.; Yuan, Z. Biodegradation of methyl tert-butyl ether by enriched bacterial culture. Curr. Microbiol. 2009, 59, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Romanovskaia, V.A.; Rokitko, P.V.; Malashenko, IuR. Unique properties of highly radioresistant bacteria. Mikrobiol. Z. 2000, 62, 40–63. [Google Scholar] [PubMed]
- Jolivet, E.; L’Haridon, S.; Corre, E.; Forterre, P.; Prieur, D. Thermococcus gammatolerans sp. nov., a hyperthermophilic archaeon from a deep-sea hydrothermal vent that resists ionizing radiation. Int. J. Syst. Evol. Microbiol. 2003, 53, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liu, Q.; Liu, L.; Chen, J. Cloning and characterization of dichloromethane dehalogenase from Methylobacterium rhodesianum for dichloromethane degradation. Bioremediation J. 2017, 21, 71–80. [Google Scholar] [CrossRef]
- Liang, S.H.; Liu, J.K.; Lee, K.H.; Kuo, Y.C.; Kao, C.M. Use of specific gene analysis to assess the effectiveness of surfactant-enhanced trichloroethylene cometabolism. J. Hazard. Mater. 2011, 198, 323–330. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kang, M.-S.; Kim, G.; Lee, C.; Lim, S.; Lee, J.; Roh, S.H.; Kang, H.; Ha, J.M.; Bae, S.; et al. Flavisolibacter tropicus sp. nov., isolated from tropical soil. Int. J. Syst. Evol. Microbiol. 2016, 66, 3413–3419. [Google Scholar] [CrossRef]
- Kim, M.; Sohn, E.-H.; Jung, H.-Y.; Srinivasan, S. Complete genome sequence of Flavisolibacter tropicus, a radiation resistant bacterium. Korean J. Microbiol. 2018, 54, 87–89. [Google Scholar]
- Thelusmond, J.-R.; Strathmarin, T.J.; Cupples, A.M. The identification of carbamazepine biodegrading phylotypes and phylotypes sensitive to carbamazepine exposure in two soil microbial communities. Sci. Total Environ. 2016, 571, 1241–1252. [Google Scholar] [CrossRef]
- Lin, Z.; Zhen, Z.; Ren, L.; Yang, J.; Luo, C.; Zhong, L.; Hu, H.; Liang, Y.; Li, Y.; Zhang, D. Effects of two ecological earthworm species on atrazine degradation performance and bacterial community structure in red soil. Chemosphere 2018, 196, 467–475. [Google Scholar] [CrossRef]
- Hemkemeyer, M.; Dohrmann, A.B.; Christensen, B.T.; Tebbe, C.C. Bacterial preferences for specific soil particle size fractions revealed by community analyses. Front. Microbiol. 2018, 9, 149. [Google Scholar] [CrossRef]
- Sessitsch, A.; Weilharter, A.; Gerzabek, M.H.; Kirchmann, H.; Kandeler, E. Microbial population structures in soil particle size fractions of a long-term fertilizer field experiment. Appl. Environ. Microbiol. 2001, 67, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- Franzluebbers, A.J. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 2002, 66, 95–106. [Google Scholar] [CrossRef]
- Yan, F.; McBratney, A.B.; Copeland, L. Functional substrate biodiversity of cultivated and uncultivated a horizons of vertisols in NW New South Wales. Geoderma 2000, 96, 321–343. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Lloyd, J.; Herms, D.A.; Hoitink, H.A.J.; Michel, F.C., Jr. Effects of mulching and fertilization on soil nutrients, microbial activity and rhizosphere bacterial community structure determined by analysis of TRFLPs of PCR-amplified 16S rRNA genes. Appl. Soil Ecol. 2002, 21, 31–48. [Google Scholar] [CrossRef]
- Liu, T.X.; Ji, X.E. Effect of crop straw burning on soil organic matter and soil microbes. Soils 2003, 4, 347–348, ISSN 0253-9829. [Google Scholar]
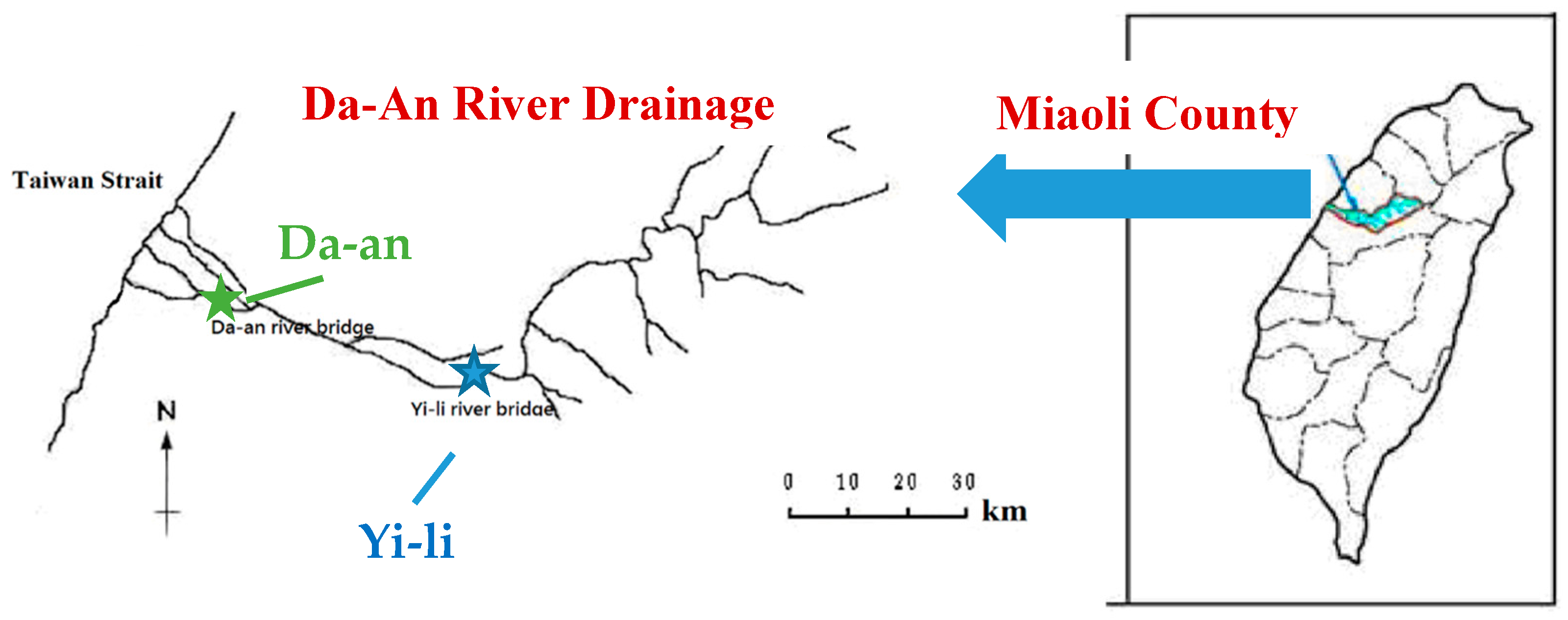


| River Sediment | Water Conc. (%) | Organic Matter (%) | Texture | Particle Fraction 2 | ||
|---|---|---|---|---|---|---|
| Sand (%) | Silt (%) | Clay (%) | ||||
| Yi-li | 30.0 | 0.474 | Sand | 89.2 | 3.9 | 6.9 |
| Da-an | 30.1 | 0.749 | Silt loam | 28.4 | 50.8 | 20.8 |
| Microcosm in the Dark Environment | Microcosm in the UVA Irradiation | ||||
|---|---|---|---|---|---|
| No. | Bacterial Strains | Proportion 1 (%) | No. | Bacterial Strains | Proportion 2 (%) |
| 1 | Methyloversatilis sp. | 23.25 | 1 | Methyloversatilis sp. | 23.53 3 |
| 2 | Comamonadaceae bacterium | 11.62 | 2 | Sediminibacterium ginsengisoli | 11.76 |
| 3 | Acidovorax sp. | 9.30 | 3 | Acidobacteriaceae bacterium | 9.80 |
| 4 | Xanthomonadaceae bacterium | 9.30 | 4 | Terrimonas ferruginea | 9.80 |
| 5 | Acidobacteria bacterium | 9.30 | 5 | Ferruginibacter alkalilentus | 7.84 |
| 6 | Pseudomonas sp. | 6.97 | 6 | Anaeromyxobacter sp. | 7.84 |
| 7 | Propionibacterium sp. | 6.97 | 7 | Alpha proteobacterium | 5.88 |
| 8 | Ferruginibacter sp. | 6.97 | 8 | Lishizhenia sp. | 5.88 |
| 9 | Clostridium akagii | 4.65 | 9 | Caedibacter sp. | 5.88 |
| 10 | Alcaligenes sp. | 4.65 | 10 | Rhodocyclaceae bacterium | 3.92 |
| 11 | Hydrogenophaga sp. | 4.65 | 11 | Rhizobiales bacterium | 3.92 |
| 12 | Pseudoxanthomonas sp. | 2.32 | 12 | Comamonas sp. | 3.92 |
| Microcosm in the Dark Environment | Microcosm in the UVA Irradiation | ||||
|---|---|---|---|---|---|
| No. | Bacterial Strains | Proportion 1 (%) | No. | Bacterial Strains | Proportion 2 (%) |
| 1 | Acidobacteria bacterium | 15.00 | 1 | Flavisolibacter sp. | 35.29 6 |
| 2 | Gemmatimonas sp. | 15.00 3 | 2 | Hyphomicrobium sp. | 13.73 7 |
| 3 | Comamonas sp. | 10.00 | 3 | Alpha proteobacterium | 13.73 |
| 4 | Azoarcus sp. | 10.00 4 | 4 | Sphingobacteria bacterium | 9.82 |
| 5 | Methylibium sp. | 8.33 5 | 5 | Acidobacteria bacterium | 5.88 |
| 6 | Pseudoxanthomonas sp. | 5.00 | 6 | Xanthomonadaceae bacterium | 5.88 |
| 7 | Rhodocyclaceae bacterium | 5.00 | 7 | Sphingomonas sp. | 5.88 |
| 8 | Lautropia sp. | 3.33 | 8 | Parvibaculum sp. | 3.92 |
| 9 | Firmicutes bacterium | 3.33 | 9 | Terrimonas sp. | 3.92 |
| 10 | Hydrogenophaga sp. | 3.33 | |||
| 11 | Pseudomonas sp. | 3.33 | |||
| 12 | Alpha proteobacterium | 3.33 | |||
| 13 | Parvibaculum lavamentivorans | 3.33 | |||
| 14 | Flavobacteriia bacterium | 3.33 | |||
| 15 | Hyphomicrobium zavarzinii | 3.33 | |||
| 16 | Xanthomonas sp. | 1.66 | |||
| 17 | Nitrosomonas sp. | 1.66 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Chou, H.-L.; Li, H.; Boyd, S. Variation of Microbial Communities in Aquatic Sediments under Long-Term Exposure to Decabromodiphenyl Ether and UVA Irradiation. Sustainability 2019, 11, 3773. https://doi.org/10.3390/su11143773
Chang Y-T, Chou H-L, Li H, Boyd S. Variation of Microbial Communities in Aquatic Sediments under Long-Term Exposure to Decabromodiphenyl Ether and UVA Irradiation. Sustainability. 2019; 11(14):3773. https://doi.org/10.3390/su11143773
Chicago/Turabian StyleChang, Yi-Tang, Hsi-Ling Chou, Hui Li, and Stephen Boyd. 2019. "Variation of Microbial Communities in Aquatic Sediments under Long-Term Exposure to Decabromodiphenyl Ether and UVA Irradiation" Sustainability 11, no. 14: 3773. https://doi.org/10.3390/su11143773
APA StyleChang, Y.-T., Chou, H.-L., Li, H., & Boyd, S. (2019). Variation of Microbial Communities in Aquatic Sediments under Long-Term Exposure to Decabromodiphenyl Ether and UVA Irradiation. Sustainability, 11(14), 3773. https://doi.org/10.3390/su11143773