Influence of Anthropogenic Noise for Predicting Cinereous Vulture Nest Distribution
Abstract
:1. Introduction
2. Material and Methods
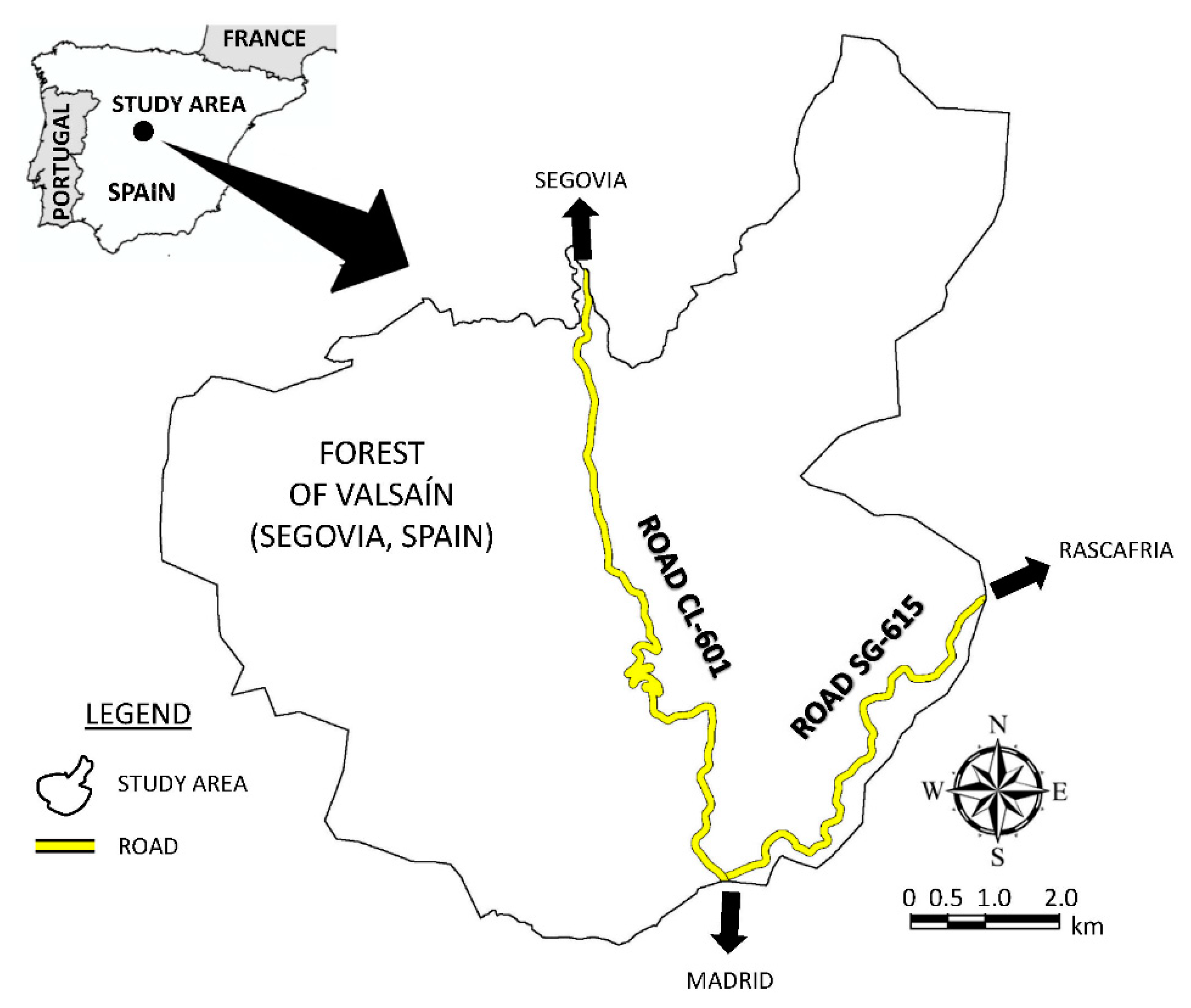
2.1. Study Area
2.2. Definition of Scenarios
2.3. Noise Modeling
2.4. Cinereous Vulture Potential Distribution Modeling
3. Results
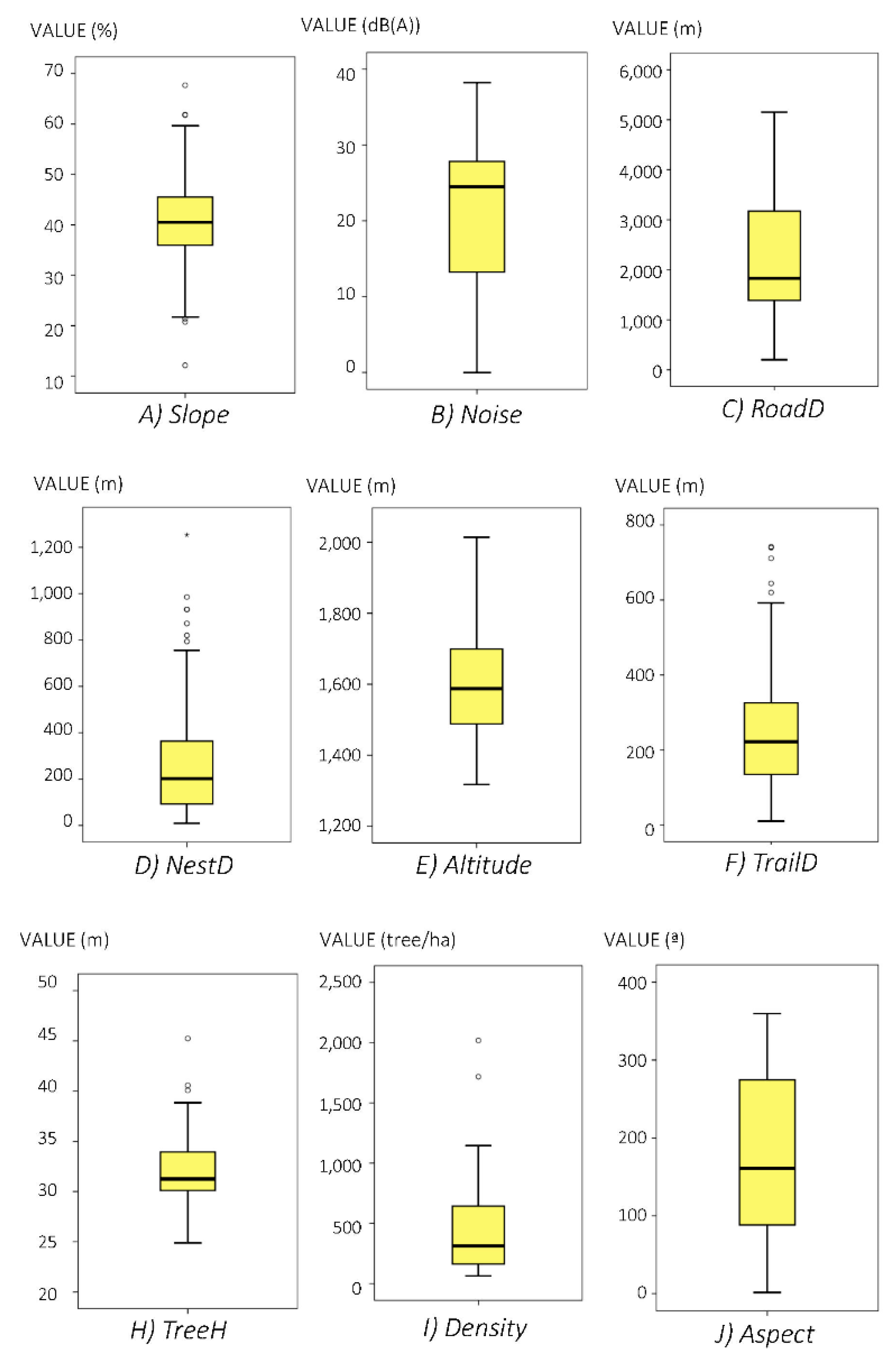
3.1. Descriptive Results
3.2. Applicability of the Model and Percent Contribution.
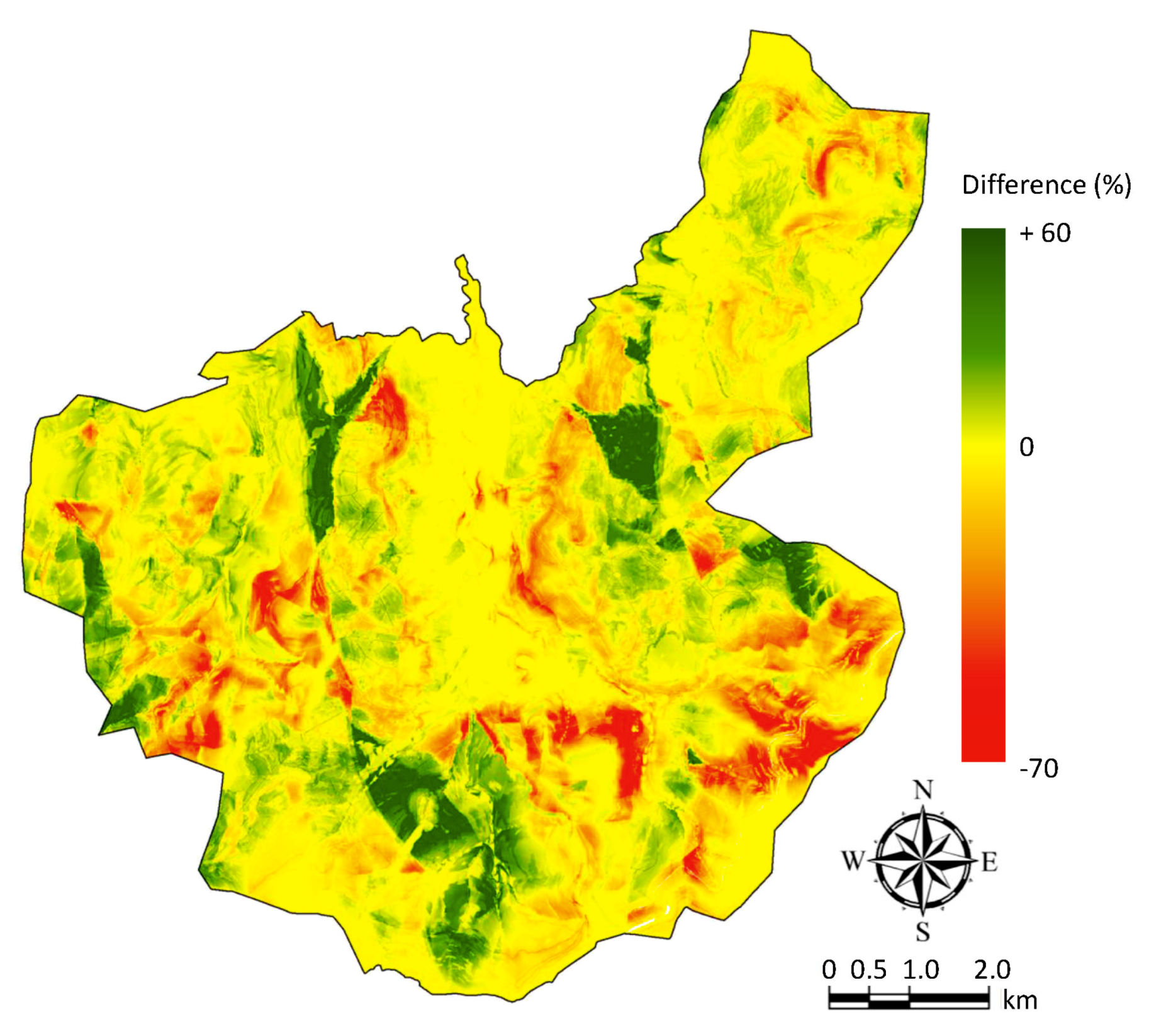
3.3. Potential Nesting Areas
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turner, A.; Fischer, M.; Tzanopoulos, J. Sound-mapping a coniferous forest—Perspectives for biodiversity monitoring and noise mitigation. PLoS ONE 2018, 13, e0189843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesmerises, F.; Dussault, C.; St-Laurent, M.H. Wolf habitat selection is shaped by human activities in a highly managed boreal forest. For. Ecol. Manag. 2012, 276, 125–131. [Google Scholar] [CrossRef]
- Ciuti, S.; Northrup, J.M.; Muhly, T.B.; Simi, S.; Musiani, M.; Pitt, J.A.; Boyce, M.S. Effects of humans on behaviour of wildlife exceed those of natural predators in a landscape of fear. PLoS ONE 2012, 7, e50611. [Google Scholar] [CrossRef] [PubMed]
- Basille, M.; Van Moorter, B.; Herfindal, I.; Martin, J.; Linnell, J.D.C.; Odden, J.; Andersen, R.; Gaillard, J.-M. Selecting habitat to survive: The impact of road density on survival in a large carnivore. PLoS ONE 2013, 8, e65493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Ree, R.; Jaeger, J.A.; van der Grift, E.; Clevenger, A. Effects of roads and traffic on wildlife populations and landscape function: Road ecology is moving toward larger scales. Ecol. Soc. 2011, 16, 48. [Google Scholar] [CrossRef]
- Ramp, D.; Wilson, V.K.; Croft, D.B. Assessing the impacts of roads in peri-urban reserves: Road-based fatalities and road usage by wildlife in the Royal National Park, New South Wales, Australia. Boil. Conserv. 2006, 129, 348–359. [Google Scholar] [CrossRef]
- Geneletti, D. Biodiversity impact assessment of roads: An approach based on ecosystem rarity. Environ. Impact Assess. Rev. 2003, 23, 343–365. [Google Scholar] [CrossRef]
- Iglesias-Merchán, C.; Diaz-Balteiro, L.; de la Puente, J. Road traffic noise impact assessment in a breeding colony of cinereous vultures (Aegypius monachus) in Spain. J. Acoust. Soc. Am. 2016, 139, 1124–1131. [Google Scholar] [CrossRef]
- Psaralexi, M.K.; Votsi, N.E.P.; Selva, N.; Mazaris, A.D.; Pantis, J.D. Importance of roadless areas for the European Conservation Network. Front. Ecol. Evol. 2017, 5, 311. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.; Jaeger, J.A.; Alonso, J.C. Assessing large-scale wildlife responses to human infrastructure development. Proc. Natl. Acad. Sci. USA 2016, 113, 8472–8477. [Google Scholar] [CrossRef] [Green Version]
- Forman, R.T.T.; Sperling, D.; Bissonette, J.A.; Clevenger, A.P.; Cutshall, C.D.; Dale, V.H.; Fahrig, L.; France, R.; Goldman, C.R.; Heanue, K.; et al. Road Ecology: Science and Solutions; Island Press: Washington, DC, USA, 2003. [Google Scholar]
- Forman, R.T.T.; Alexander, L.E. Roads and their major ecological effects. Annu. Rev. Ecol. Syst. 1998, 29, 207–231. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Dai, Q.; Gu, X.; Zhang, Z.; Zhou, J.; Qi, D.; Gu, X.; Yang, X.; Zhang, W.; Yang, B.; et al. Effects of roads on giant panda distribution: A mountain range scale evaluation. Sci. Rep. 2019, 9, 1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrete, M.; Grande, J.M.; Tella, J.L.; Sánchez-Zapata, J.A.; Donázar, J.A.; Díaz-Delgado, R.; Romo, A. Habitat, human pressure, and social behavior: Partialling out factors affecting large-scale territory extinction in an endangered vulture. Biol. Conserv. 2007, 136, 143–154. [Google Scholar] [CrossRef] [Green Version]
- McGregor, P.K.; Horn, A.G.; Leonard, M.L.; Thomsen, F. Anthropogenic Noise and Conservation. In Animal Communication and Noise; Springer: Berlin/Heidelberg, Germany, 2013; pp. 409–444. ISBN 978-3-642-41493-0. [Google Scholar]
- Iglesias Merchan, C.; Diaz-Balteiro, L. Noise pollution mapping approach and accuracy on landscape scales. Sci. Total Environ. 2013, 449, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Shannon, G.; McKenna, M.F.; Angeloni, L.M.; Crooks, K.R.; Fristrup, K.M.; Brown, E.; Warner, K.A.; Nelson, M.D.; White, C.; Briggs, J.; et al. A synthesis of two decades of research documenting the effects of noise on wildlife. Biol. Rev. 2016, 91, 982–1005. [Google Scholar] [CrossRef] [PubMed]
- Iglesias Merchan, C.; Horcajada-Sánchez, F.; Diaz-Balteiro, L.; Escribano-Ávila, G.; Lara-Romero, C.; Virgos, E.; Planillo, A.; Barja, I. A new large-scale index (AcED) for assessing traffic noise disturbance on wildlife: Stress response in a roe deer (Capreolus capreolus) population. Environ. Monit. Assess. 2018, 190, 185. [Google Scholar] [CrossRef]
- Gontier, M.; Balfors, B.; Mörtberg, U. Biodiversity in environmental assessment—Current practice and tools for prediction. Environ. Impact Assess. Rev. 2006, 26, 268–286. [Google Scholar] [CrossRef]
- Kumar, S.; Stohlgren, T.J. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 2009, 1, 094–098. [Google Scholar]
- Dettki, H.; Löfstrand, R.; Edenius, L. Modeling habitat suitability for moose in coastal northern Sweden: Empirical vs. process-oriented approaches. Ambio 2003, 32, 549–556. [Google Scholar] [CrossRef]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Monclús, L.; Lopez-Bejar, M.; De la Puente, J.; Covaci, A.; Jaspers, V.L. Can variability in corticosterone levels be related to POPs and OPEs in feathers from nestling cinereous vultures (Aegypius monachus)? Sci. Total Environ. 2019, 650, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Donázar, J.A.; Blanco, G.; Hiraldo, F.; Soto-Largo, E.; Oria, J. Effects of forestry and other land-use practices on the conservation of cinereous vultures. Ecol. Appl. 2002, 12, 1445–1456. [Google Scholar] [CrossRef]
- Lourenço, P.M.; Curado, N.; Loureiro, F.; Godino, A.; Santos, E. Selecting key areas for conservation at the regional level: The case of the globally ‘Near Threatened’ Cinereous Vulture Aegypius monachus in south-east Portugal. Bird Conserv. Int. 2013, 23, 168–183. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Opo, R.; Fernández-Olalla, M.; Margalida, A.; Arredondo, Á.; Guil, F. Effect of Methodological and Ecological Approaches on Heterogeneity of Nest-Site Selection of a Long-Lived Vulture. PLoS ONE 2012, 7, e33469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraldo Cano, F. El buitre negro (Aegypius monachus monachus L.) en la Península Ibérica. Población, biología general, uso de recursos e interacciones con otras aves. Ph.D. Thesis. 1977. Available online: https://idus.us.es/xmlui/handle/11441/72716 (accessed on 8 January 2020).
- Morán-López, R.; Sánchez Guzmán, J.M.; Costillo Borrego, E.; Villegas Sánchez, A. Nest-site selection of endangered cinereous vulture (Aegypius monachus) populations affected by anthropogenic disturbance: Present and future conservation implications. Anim. Conserv. 2006, 9, 29–37. [Google Scholar] [CrossRef]
- Poirazidis, K.; Goutner, V.; Skartsi, T.; Stamou, G. Modelling nesting habitat as a conservation tool for the Eurasian black vulture (Aegypius monachus) in Dadia Nature Reserve, northeastern Greece. Biol. Conserv. 2004, 118, 235–248. [Google Scholar] [CrossRef]
- Fernández-Bellon, D.; Cortés-Avizanda, A.; Arenas, R.; Donázar, J.A. Density-dependent productivity in a colonial vulture at two spatial scales. Ecology 2016, 97, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Opo, R.; Fernández-Olalla, M.; Margalida, A.; Arredondo, Á.; Guil, F. Influence of Environmental Factors on the Breeding Success of Cinereous Vultures Aegypius monachus. Acta Ornithol. 2013, 48, 187–193. [Google Scholar] [CrossRef]
- Fargallo, J.A.; Blanco, G.; Soto-Largo, E. Forest management effects on nesting habitat selected by Eurasian black vultures (Aegypius monachus) in central Spain. J. Raptor Res. 1998, 32, 202–207. [Google Scholar]
- Mihoub, J.B.; Jiguet, F.; Lécuyer, P.; Eliotout, B.; Sarrazin, F. Modelling nesting site suitability in a population of reintroduced Eurasian black vultures Aegypius monachus in the Grands Causses, France. Oryx 2013, 48, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Margalida, A.; Moreno-Opo, R.; Arroyo, B.E.; Arredondo, A. Reconciling the conservation of endangered species with economically important anthropogenic activities: Interactions between cork exploitation and the cinereous vulture in Spain. Anim. Conserv. 2011, 14, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Triantakonstantis, D.P.; Kollias, V.J.; Kalivas, D.P. Forest re-growth since 1945 in the Dadia forest nature reserve in northern Greece. New For. 2006, 32, 51–69. [Google Scholar] [CrossRef]
- Kirazli, C. The impact of some spatial factors on disturbance and reaction distances on nest occupation by the near threatened Cinereous Vulture (Aegypius monachus). North-Western J. Zool. 2016, 12, 304–313. [Google Scholar]
- D’Elia, J.; Haig, S.M.; Johnson, M.; Marcot, B.G.; Young, R. Activity-specific ecological niche models for planning reintroductions of California condors (Gymnogyps californianus). Biol. Conserv. 2015, 184, 90–99. [Google Scholar] [CrossRef]
- Mateo-Tomás, P.; Olea, P.P. Anticipating Knowledge to Inform Species Management: Predicting Spatially Explicit Habitat Suitability of a Colonial Vulture Spreading Its Range. PLoS ONE 2010, 5, e12374. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. S 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; En Chee, Y.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Mateo-Tomás, P.; Olea, P.P.; Sánchez-Barbudo, I.S.; Mateo, R. Alleviating human–wildlife conflicts: Identifying the causes and mapping the risk of illegal poisoning of wild fauna. J. Appl. Ecol. 2012, 49, 376–385. [Google Scholar] [CrossRef]
- Iglesias-Merchán, C.; Ortiz-urbina, E.; Ezquerro, M.; Diaz-Balteiro, L. Incorporating acoustic objectives into Forest Management Planning when sensitive bird species are relevant. PeerJ 2019, 7, e6922. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Balteiro, L.; Alonso, R.; Martínez-Jaúregui, M.; Pardos, M. Selecting the best forest management alternative by aggregating ecosystem services indicators over time: A case study in central Spain. Ecol. Indic. 2017, 72, 322–329. [Google Scholar] [CrossRef]
- Ezquerro, M.; Pardos, M.; Diaz-Balteiro, L. Integrating variable retention systems into strategic forest management to deal with conservation biodiversity objectives. For. Ecol. Manag. 2019, 433, 585–593. [Google Scholar] [CrossRef]
- López, I.; Pardo, M. Socioeconomic Indicators for the Evaluation and Monitoring of Climate Change in National Parks: An Analysis of the Sierra de Guadarrama National Park (Spain). Environments 2018, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Pater, L.L.; Grubb, T.G.; Delaney, D.K. Recommendations for improved assessment of noise impacts on wildlife. J. Wildl. Manag. 2009, 73, 788–795. [Google Scholar] [CrossRef]
- Guerrero-Casado, J.; Arenas, R.; Tortosa, F.S. Modelling the nesting-habitat of the Cinereous Vulture Aegypius monachus on a fine scale for conservation purposes. Bird Study 2013, 60, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Morán-López, R.; Sánchez, J.M.; Costillo, E.; Corbacho, C.; Villegas, A. Spatial variation in anthropic and natural factors regulating the breeding success of the cinereous vulture (Aegypius monachus) in the SW Iberian Peninsula. Biol. Conserv. 2006, 130, 169–182. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, G. Climatic Suitability of the Geographic Distribution of Stipa breviflora in Chinese Temperate Grassland under Climate Change. Sustainability 2018, 10, 3767. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef] [Green Version]
- Qin, A.; Liu, B.; Guo, Q.; Bussmann, R.W.; Ma, F.; Jian, Z.; Xu, G.; Pei, S. Maxent modeling for predicting impacts of climate change on the potential distribution of Thuja sutchuenensis Franch., an extremely endangered conifer from southwestern China. Glob. Ecol. Conserv. 2017, 10, 139–146. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapire, R.E. A Maximum Entropy Approach to Species Distribution Modeling. In Proceedings of the Twenty-First International Conference on Machine Learning, Banff, AL, Canada, 4–8 July 2004; pp. 655–662. [Google Scholar]
- Yiwen, Z.; Wei, L.B.; Yeo, D.C.J. Novel methods to select environmental variables in MaxEnt: A case study using invasive crayfish. Ecol. Model. 2016, 341, 5–13. [Google Scholar] [CrossRef]
- Pearson, R.G.; Raxworthy, C.J.; Nakamura, M.; Townsend Peterson, A. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J. Biogeogr. 2007, 34, 102–117. [Google Scholar] [CrossRef]
- Phillips, S. A Brief Tutorial on Maxent. Lessons Conserv. 2010, 3, 108–135. [Google Scholar]
- Poirazidis, K.; Goutner, V.; Tsachalidis, E.; Kati, V. Comparison of nest-site selection patterns of different sympatric raptor species as a tool for their conservation. Anim. Biodivers. Conserv. 2007, 30, 131–145. [Google Scholar]
- He, Q.; Zhou, G. Climate-associated distribution of summer maize in China from 1961 to 2010. Agric. Ecosyst. Environ. 2016, 232, 326–335. [Google Scholar] [CrossRef]
- Yamac, E.L.I.F. Roosting tree selection of Cinereous Vulture Aegypius monachus in breeding season in Turkey. Podoes 2007, 2, 30–36. [Google Scholar]
- Austin, M. Species distribution models and ecological theory: A critical assessment and some possible new approaches. Ecol. Model. 2007, 200, 1–19. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Acevedo, P.; Jiménez-Valverde, A.; Lobo, J.M.; Real, R. Delimiting the geographical background in species distribution modelling. J. Biogeogr. 2012, 39, 1383–1390. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Peterson, A.T.; Soberón, J.; Overton, J.M.; Aragón, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Boil. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Farcas, A.; Thompson, P.M.; Merchant, N.D. Underwater noise modelling for environmental impact assessment. Environ. Impact Assess. Rev. 2016, 57, 114–122. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Marivela, I.; Morales, M.B.; Iglesias-Merchán, C.; Delgado, M.P.; Tarjuelo, R.; Traba, J. Traffic noise pollution does not influence habitat selection in the endangered little bustard. Ardeola 2018, 65, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Keyel, A.C.; Reed, S.E.; McKenna, M.F.; Wittemyer, G. Modeling anthropogenic noise propagation using the Sound Mapping Tools ArcGIS toolbox. Environ. Model. Softw. 2017, 97, 56–60. [Google Scholar] [CrossRef]
- Monclús, L.; Lopez-Bejar, M.; De la Puente, J.; Covaci, A.; Jaspers, V.L. First evaluation of the use of down feathers for monitoring persistent organic pollutants and organophosphate ester flame retardants: A pilot study using nestlings of the endangered cinereous vulture (Aegypius monachus). Environ. Pollut. 2018, 238, 413–420. [Google Scholar] [CrossRef]
- Barrio, I.C.; Bueno, C.G.; Tortosa, F.S. Improving predictions of the location and use of warrens in sensitive rabbit populations. Anim. Conserv. 2009, 12, 426–433. [Google Scholar] [CrossRef]
- Iglesias-Merchan, C.; Ortiz-Urbina, E.; Ezquerro, M.; Diaz-Balteiro, L. Managing and designing soundscape experiences in nature: The influence of humans in natural sound sources (biophony). In INTER-NOISE and NOISE-CON Congress and Conference Proceedings; Institute of Noise Control Engineering: Madrid, Spain, 2019; pp. 1457–1464. [Google Scholar]
- Van Langevelde, F.; Jaarsma, C.F. Modeling the effect of traffic calming on local animal population persistence. Ecol. Soc. 2009, 14, 39. [Google Scholar] [CrossRef]
- Francis, C.D.; Ortega, C.P.; Cruz, A. Noise pollution changes avian communities and species interactions. Curr. Boil. 2009, 19, 1415–1419. [Google Scholar] [CrossRef] [Green Version]
- Arce-Gomez, A.; Donovan, J.D.; Bedggood, R.E. Social impact assessments: Developing a consolidated conceptual framework. Environ. Impact Assess. Rev. 2015, 50, 85–94. [Google Scholar] [CrossRef]
- Joseph, C.; Zeeg, T.; Angus, D.; Usborne, A.; Mutrie, E. Use of significance thresholds to integrate cumulative effects into project-level socio-economic impact assessment in Canada. Environ. Impact Assess. Rev. 2017, 67, 1–9. [Google Scholar] [CrossRef]


| Variables | Source | Abrev. | Sc #1 | Sc #2 | Description |
|---|---|---|---|---|---|
| Anthropogenic | |||||
| distance to roads | [24,25,26,29,31,32,35] | RoadD | ✓ | Distance from each nest tree to the nearest paved road (m) | |
| Noise | Noise | ✓ | Road traffic noise level (in dB(A)) in nesting sites due to road traffic | ||
| Distance to trails | [24,26,28,29,37,49,50,51] | TrailD | ✓ | ✓ | Distance from each nest tree to the nearest unpaved track (m) |
| Biologic | |||||
| Distance to nest | [24,26,28,29,37,50,51] | NestD | ✓ | ✓ | Distance from each nest tree to the nearest nest tree (m) |
| Geomorphologic | |||||
| Slope | [24,26,28,29,49,51] | Slope | ✓ | ✓ | Terrain slope (%) |
| Aspect | [26,29,31,33,35] | Aspect | ✓ | ✓ | Geographical orientation of the slope ‘N’,’S’,’W’,’E’ in degrees (°) |
| Altitude | [24,25,26,28,29,31,32,33,35,49,50] | Altitude | ✓ | ✓ | Altitude (m) above sea level in nesting locations |
| Forestry | |||||
| Trees density | [26,29,35] | TreeD | ✓ | ✓ | Average number of trees within each stand (trees/ha) |
| Tree height | [26,29,31,32] | TreeH | ✓ | ✓ | Average tree height (m) at stand level |
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. Noise | - | ||||||||
| 2. TreeD | −0.035 | - | |||||||
| 3. TreeH | 0.279 ** | 0.095 | - | ||||||
| 4. NestD | −0.124 * | 0.054 | 0.002 | - | |||||
| 5. Aspect | −0.019 | 0.097 | 0.005 | 0.034 | - | ||||
| 6. Slope | −0.189 ** | 0.133 * | −0.131 * | −0.015 | −0.017 | - | |||
| 7. Altitude | −0.377 ** | 0.168 ** | −0.325 ** | 0.026 | 0.014 | 0.252 ** | - | ||
| 8. RoadD | −0.685 ** | 0.045 | −0.339 ** | 0.052 | −0.091 | 0.182 ** | 0.379 ** | - | |
| 9. TrailD | 0.179 ** | −0.016 | 0.180 ** | −0.018 | −0.041 | −0.044 | −0.063 | −0.112 | - |
| Variable Order in Scenario #1 | Percent Contribution Scenario #1 | Percent Contribution Scenario #2 | Variable Order in Scenario #2 |
|---|---|---|---|
| Slope | 30.1% | 31.8% | Slope |
| Noise | 19.4% | 27.0% | RoadD |
| NestD | 14.7% | 12.5% | NestD |
| Altitude | 10.9% | 7.4% | TrailD |
| TrailD | 8.4% | 6.8% | Altitude |
| TreeH | 5.8% | 5.4% | TreeH |
| Density | 5.5% | 4.8% | Aspect |
| Aspect | 5.1% | 4.3% | Density |
| AUC | 0.71 | 0.71 | AUC |
| Probability of Presence (%) | Scenario #1 (Surface Area in %) | Scenario #2 (Surface Area in %) |
|---|---|---|
| 0–10 | 44.0 | 45.3 |
| 10–20 | 16.7 | 15.9 |
| 20–30 | 11.7 | 10.6 |
| 30–40 | 8.2 | 8.6 |
| 40–50 | 6.1 | 6.4 |
| 50–60 | 4.6 | 4.9 |
| 60–70 | 4.3 | 3.6 |
| 70–80 | 2.4 | 2.4 |
| 80–90 | 1.4 | 1.7 |
| 90–1000 | 0.6 | 0.7 |
| Total | 100.0 | 100.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Urbina, E.; Diaz-Balteiro, L.; Iglesias-Merchan, C. Influence of Anthropogenic Noise for Predicting Cinereous Vulture Nest Distribution. Sustainability 2020, 12, 503. https://doi.org/10.3390/su12020503
Ortiz-Urbina E, Diaz-Balteiro L, Iglesias-Merchan C. Influence of Anthropogenic Noise for Predicting Cinereous Vulture Nest Distribution. Sustainability. 2020; 12(2):503. https://doi.org/10.3390/su12020503
Chicago/Turabian StyleOrtiz-Urbina, Esther, Luis Diaz-Balteiro, and Carlos Iglesias-Merchan. 2020. "Influence of Anthropogenic Noise for Predicting Cinereous Vulture Nest Distribution" Sustainability 12, no. 2: 503. https://doi.org/10.3390/su12020503
APA StyleOrtiz-Urbina, E., Diaz-Balteiro, L., & Iglesias-Merchan, C. (2020). Influence of Anthropogenic Noise for Predicting Cinereous Vulture Nest Distribution. Sustainability, 12(2), 503. https://doi.org/10.3390/su12020503







