Environmental and Economic Water Management in Shale Gas Extraction
Abstract
:1. Introduction
2. Life Cycle Assessment Methodology
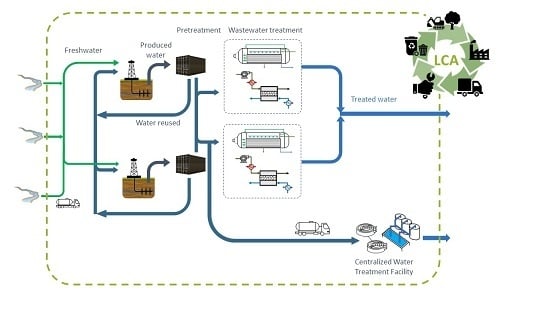
2.1. Wastewater Treatment
2.1.1. Initial Wastewater Pre-treatment
2.1.2. Thermal-Based Technologies for the Wastewater Treatment
2.1.3. Membrane Distillation Technology for the Wastewater Treatment
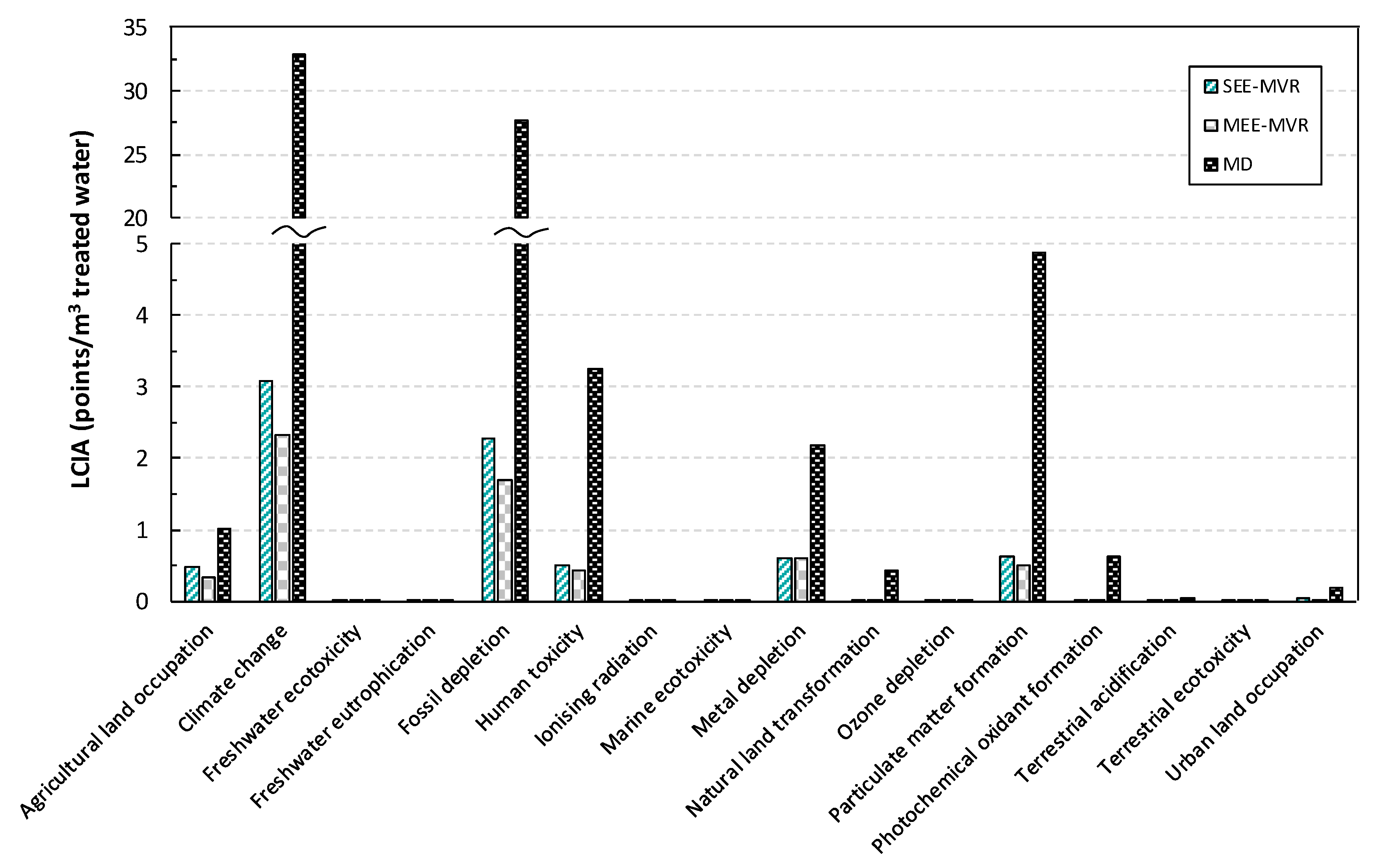
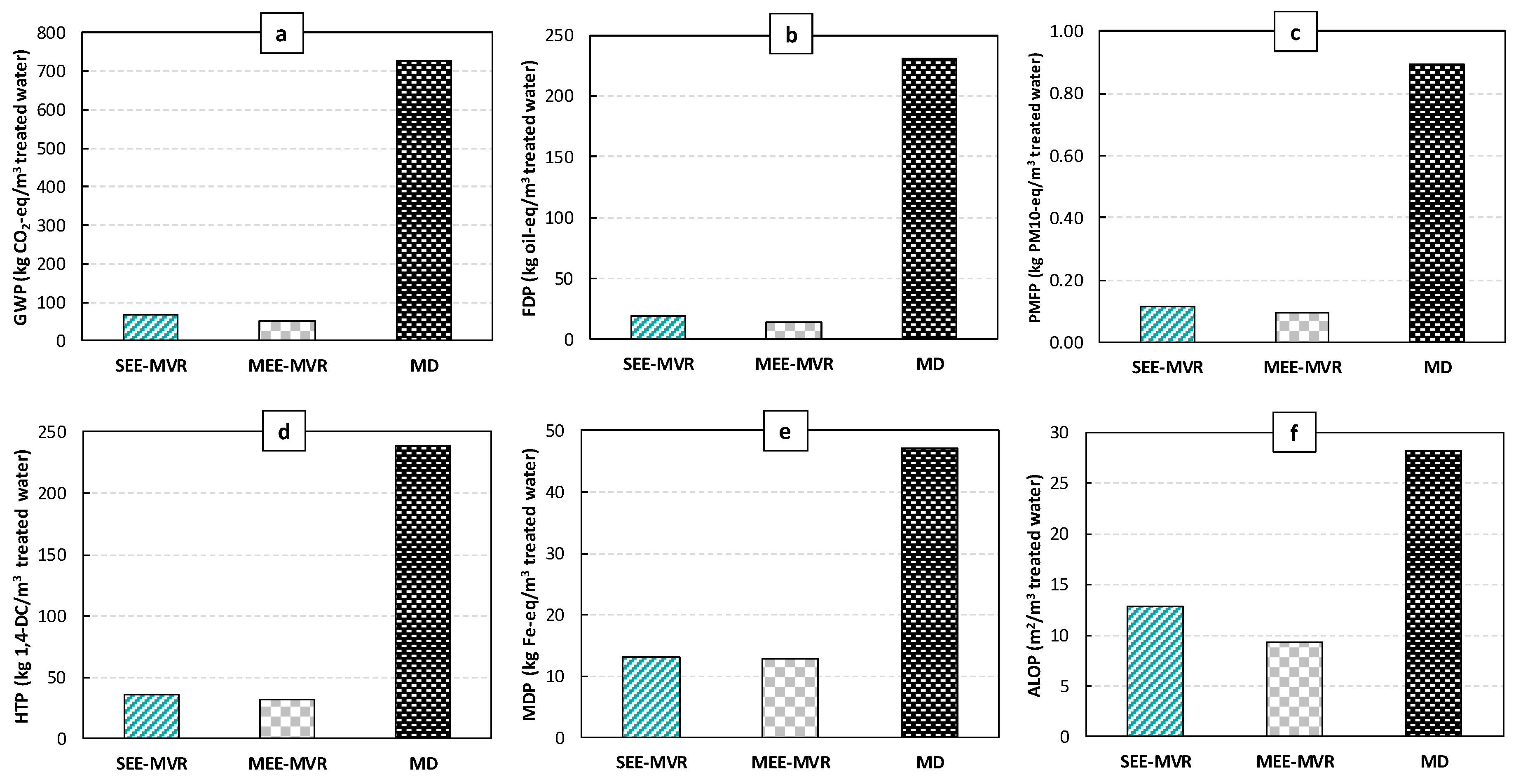
2.2. Wastewater Treatment Results
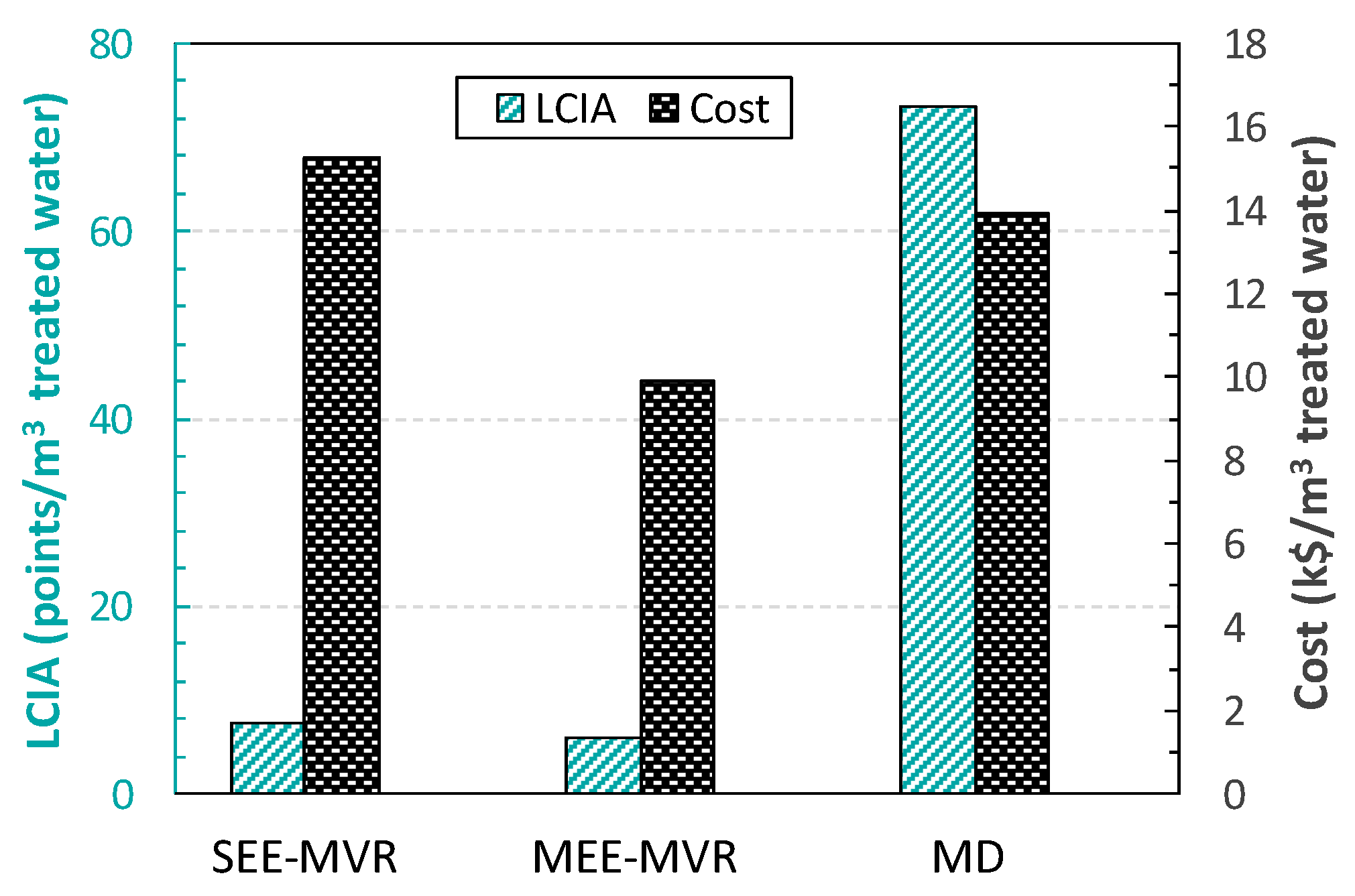
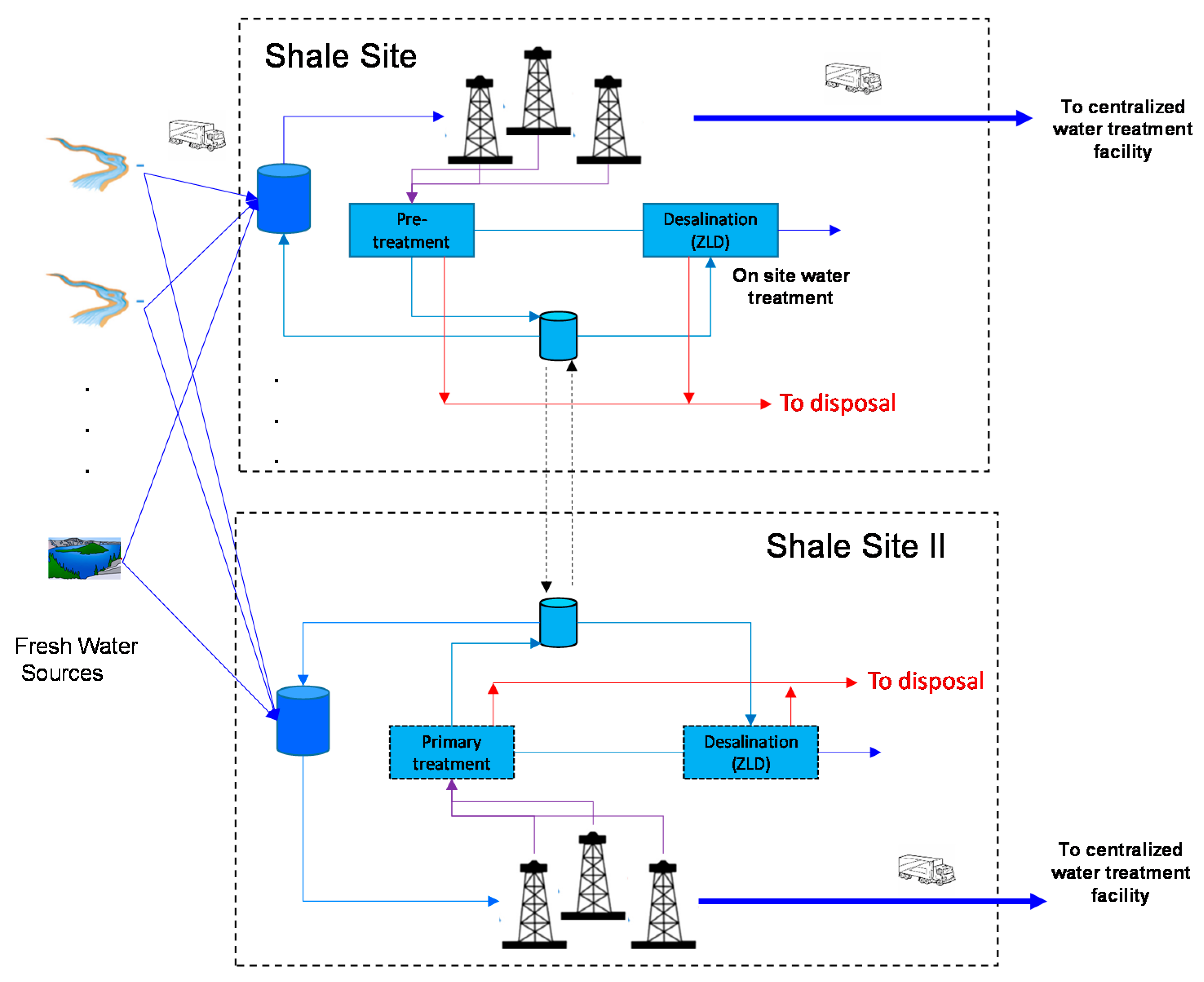
3. Economic vs. LCA of the Complete Water Management in Shale Gas Exploitation
- A fixed time period is discretized into weeks as time intervals.
- Water transportation is only executed by trucks (the model can be easily extended to deal with transportation by pipes as well).
- The volume of water used to fracture a well must be available when needed—this includes the possibility of storage in tanks, or a ‘just in time water availability’—including water required in drilling, construction, and completion.
- The amount of water needed to carry out all the operations, as well as the variation in flowback water with time after the wells are turned in operation is known a priory.
- The well is turned in operation immediately after the drilled activities are finished.
- The amount of gas that releases and its variation with time after the wells are turned in operation are known a priori.
- Forecasts of gas prices for the complete time period are known a priori.
Case Study
4. Conclusions
Supplementary Materials
- Comparison between thermal and membrane-based technologies for all the subcategories of impact using ReCiPe Midpoint (H).
- Waste Water Management: Comprehensive Mathematical Model Formulation.
- Case Study: Data and Results for the best environmental solution; best economic solution (maximum gross profit) and the best solution for minimum freshwater consumption.
Author Contributions
Funding
Conflicts of Interest
References
- International Energy Agency. Global Energy & CO2 Status Report—The Latest Trends in Energy and Emissions 2018.; IEA Publications: Paris, France, 2019. [Google Scholar]
- BP. BP Statistical Review of World Energy Statistical Review of World; Pureprint Group Limited: London, UK, 2019. [Google Scholar]
- Stevens, P. The ‘Shale Gas Revolution’: Developments and Changes; Chatham House: London, UK, 2012; pp. 2–3. [Google Scholar]
- U.S. Energy Information Administration. Annual Energy Outlook 2019 with Projections to 2050; U.S. Energy Information Administration: Washington, DC, USA, 2019; Volume January. [Google Scholar]
- Lira-Barragán, L.F.; Ponce-Ortega, J.M.; Guillén-Gosálbez, G.; El-Halwagi, M.M. Optimal Water Management under Uncertainty for Shale Gas Production. Ind. Eng. Chem. Res. 2016, 55, 1322–1335. [Google Scholar] [CrossRef]
- Nicot, J.-P.; Scanlon, B.R. Water use for shale gas production in Texas, U.S. U.S. Environ. Sci. Technol. 2012, 46, 3580–3586. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Valle, J.E.; Riera-Palou, X. Modeling the relative GHG emissions of conventional and shale gas production. Environ. Sci. Technol. 2011, 45, 10757–10764. [Google Scholar] [CrossRef]
- Mauter, M.S.; Alvarez, P.J.J.; Burton, G.A.; Cafaro, D.C.; Chen, W.; Gregory, K.B.; Jiang, G.; Li, Q.; Pittock, J.; Reible, D.; et al. Regional Variation in Water Related Impacts of Shale Gas Development and Implications for Emerging International Plays. Environ. Sci. Technol. 2014, 48, 8298–8306. [Google Scholar] [CrossRef]
- Weber, C.L.; Clavin, C. Life cycle carbon footprint of shale gas: Review of evidence and implications. Environ. Sci. Technol. 2012, 46, 5688–5695. [Google Scholar] [CrossRef]
- Kuhn, M.; Umbach, F. Strategic Perspectives of Unconventional Gas: A Game Changer with Implications for the EU’s Energy Security; EUCERS: London, UK, 2011; Volume 1, ISBN 9780956903303. [Google Scholar]
- UNCTAD (United Nations Conference on Trade and Development). Commodities at a Glance: Special Issue on Shale Gas; UNCTAD: New York, NY, USA; Geneva, Switzerland, 2018. [Google Scholar]
- Bickle, M.; Goodman, D.; Mair, R.; Roberts, J.; Selley, R.; Shipton, Z.; Thomas, H.; Younger, P. Shale Gas Extraction in the UK: A Review of Hydraulic Fracturing; The Royal Academy of Engineering: London, UK, 2012; pp. 1–76. [Google Scholar]
- Vinson&Elkins. Shale Development in Denmark. Available online: http://fracking.velaw.com/shaledevelopment- (accessed on 6 June 2016).
- Martor, B. France: Evolutions in the Legal Framework for Shale Oil and Gas. Available online: http://www.shale-gas-information-platform.org/categories/legislation/expert-articles/martor-article.html (accessed on 8 July 2016).
- EPA. Technical Development Document for the Effluent Limitations Guidelines and Standards for the oil and Gas Extraction Point Source Category; Environmental Protection Agency: Washingtong, DC, USA, 2016. [Google Scholar]
- Hammond, G.P.; O’Grady, Á. Indicative energy technology assessment of UK shale gas extraction. Appl. Energy 2017, 185, 1907–1918. [Google Scholar] [CrossRef] [Green Version]
- Prpich, G.; Coulon, F.; Anthony, E.J. Review of the scientific evidence to support environmental risk assessment of shale gas development in the UK. Sci. Total Environ. 2016, 563, 731–740. [Google Scholar] [CrossRef]
- Lutz, B.D.; Lewis, A.N.; Doyle, M.W. Generation, transport, and disposal of wastewater associated with Marcellus Shale gas development. Water Resour. Res. 2013, 49, 647–656. [Google Scholar] [CrossRef]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Yang, L.; Grossmann, I.E.; Manno, J. Optimization models for shale gas water management. AIChE J. 2014, 60, 3490–3501. [Google Scholar] [CrossRef]
- Balaba, R.S.; Smart, R.B. Total arsenic and selenium analysis in Marcellus shale, high-salinity water, and hydrofracture flowback wastewater. Chemosphere 2012, 89, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Carter, K.E. Water usage for natural gas production through hydraulic fracturing in the United States from 2008 to 2014. J. Environ. Manage. 2016, 170, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Estrada, J.M.; Bhamidimarri, R. A review of the issues and treatment options for wastewater from shale gas extraction by hydraulic fracturing. Fuel 2016, 182, 292–303. [Google Scholar] [CrossRef]
- Ellsworth, W.L. Injection-Induced Earthquakes. Science 2013, 341, 1225942. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and Reuse of High-Salinity Shale Gas Produced Water: Drivers, Technologies, and Future Directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef]
- Michel, M.M.; Reczek, L. Pre-Treatment of Flowback Water To Desalination. Membr. Membr. Process. Environ. Prot. Monogr. Environ. Eng. Committee. Polish Acad. Sci. 2014, 119, 309–321. [Google Scholar]
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Salcedo-Díaz, R.; Ruiz-Femenia, R.; Carrero-Parreño, A.; Onishi, V.C.; Reyes-Labarta, J.A.; Caballero, J.A. Combining Forward and Reverse Osmosis for Shale Gas Wastewater Treatment to Minimize Cost and Freshwater Consumption. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 40, pp. 2725–2730. [Google Scholar]
- Shaffer, D.L.; Werber, J.R.; Jaramillo, H.; Lin, S.; Elimelech, M. Forward osmosis: Where are we now? Desalination 2015, 356, 271–284. [Google Scholar] [CrossRef]
- Bartholomew, T.V.; Mauter, M.S. Multiobjective Optimization Model for Minimizing Cost and Environmental Impact in Shale Gas Water and Wastewater Management. ACS Sustain. Chem. Eng. 2016, 4, 3728–3735. [Google Scholar] [CrossRef]
- Jiang, M.; Hendrickson, C.T.; VanBriesen, J.M. Life Cycle Water Consumption and Wastewater Generation Impacts of a Marcellus Shale Gas Well. Environ. Sci. Technol. 2014, 48, 1911–1920. [Google Scholar] [CrossRef] [Green Version]
- Pekney, N.J.; Veloski, G.; Reeder, M.; Tamilia, J.; Rupp, E.; Wetzel, A. Measurement of atmospheric pollutants associated with oil and natural gas exploration and production activity in Pennsylvania’s Allegheny National Forest. J. Air Waste Manage. Assoc. 2014, 64, 1062–1072. [Google Scholar] [CrossRef] [PubMed]
- Coday, B.D.; Miller-Robbie, L.; Beaudry, E.G.; Munakata-Marr, J.; Cath, T.Y. Life cycle and economic assessments of engineered osmosis and osmotic dilution for desalination of Haynesville shale pit water. Desalination 2015, 369, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.; Stamford, L.; Azapagic, A. Shale Gas: A Review of the Economic, Environmental, and Social Sustainability. Energy Technol. 2016, 4, 772–792. [Google Scholar] [CrossRef]
- Stamford, L.; Azapagic, A. Life cycle environmental impacts of UK shale gas. Appl. Energy 2014, 134, 506–518. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Clift, R.; Lettieri, P.; Chapman, C. Shale gas: a life-cycle perspective for UK production. Int. J. Life Cycle Assess. 2017, 22, 919–937. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; You, F. Shale Gas Supply Chain Design and Operations toward Better Economic and Life Cycle Environmental Performance: MINLP Model and Global Optimization Algorithm. ACS Sustain. Chem. Eng. 2015, 3, 1282–1291. [Google Scholar] [CrossRef]
- Cafaro, D.C.; Grossmann, I.E. Strategic planning, design, and development of the shale gas supply chain network. AIChE J. 2014, 60, 2122–2142. [Google Scholar] [CrossRef]
- Carrero-Parreño, A.; Reyes-Labarta, J.A.; Salcedo-Díaz, R.; Ruiz-Femenia, R.; Onishi, V.C.; Caballero, J.A.; Grossmann, I.E. Holistic Planning Model for Sustainable Water Management in the Shale Gas Industry. Ind. Eng. Chem. Res. 2018, 57, 13131–13143. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Sun, A.Y.; Duncan, I.J. Shale gas wastewater management under uncertainty. J. Environ. Manage. 2016, 165, 188–198. [Google Scholar] [CrossRef]
- Burnham, A.; Han, J.; Clark, C.E.; Wang, M.; Dunn, J.B.; Palou-Rivera, I. Life-cycle greenhouse gas emissions of shale gas, natural gas, coal, and petroleum. Environ. Sci. Technol. 2012, 46, 619–627. [Google Scholar] [CrossRef]
- Laurenzi, I.J.; Jersey, G.R. Life cycle greenhouse gas emissions and freshwater consumption of marcellus shale gas. Environ. Sci. Technol. 2013, 47, 4896–4903. [Google Scholar] [CrossRef] [PubMed]
- Howarth, R.W.; Santoro, R.; Ingraffea, A. Methane and the greenhouse-gas footprint of natural gas from shale formations. Clim. Change 2011, 106, 679–690. [Google Scholar] [CrossRef] [Green Version]
- Goedkoop, M.; Heijungs, R.; Huijbregts, M.; De Schryver, A.; Struijs, J.; Zelm, R. Van ReCiPe 2008: A Life Cycle Impact Assessment Method which Comprises Harmonised Category Indicators at the Midpoint and the Endpoint Level; National Institute for Public Health and the Environment (RIVM): Biithoven, The Netherlands, 2009. [Google Scholar]
- Wernet, G.; Bauer, C.; Steubing, B.; Reinhard, J.; Moreno-Ruiz, E.; Weidema, B. The ecoinvent database version 3 (part I): Overview and methodology. Int. J. Life Cycle Assess. 2016, 21, 1218–1230. [Google Scholar] [CrossRef]
- ISO (International Organization for Standarization). ISO 14040:2006 Environmental Management—Life Cycle Assessment—Principles and Framework; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO (International Organization for Standarization). ISO 14044:2006 Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Rebitzer, G.; Ekvall, T.; Frischknecht, R.; Hunkeler, D.; Norris, G.; Rydberg, T.; Schmidt, W.-P.; Suh, S.; Weidema, B.P.; Pennington, D.W. Life cycle assessment: Part 1: Framework, goal and scope definition, inventory analysis, and applications. Environ. Int. 2004, 30, 701–720. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Parreño, A.; Onishi, V.C.; Salcedo-Díaz, R.; Ruiz-Femenia, R.; Fraga, E.S.; Caballero, J.A.; Reyes-Labarta, J.A. Optimal Pretreatment System of Flowback Water from Shale Gas Production. Ind. Eng. Chem. Res. 2017, 56, 4386–4398. [Google Scholar] [CrossRef] [Green Version]
- Onishi, V.C.; Carrero-Parreño, A.; Reyes-Labarta, J.A.; Fraga, E.S.; Caballero, J.A. Desalination of shale gas produced water: A rigorous design approach for zero-liquid discharge evaporation systems. J. Clean. Prod. 2017, 140, 1399–1414. [Google Scholar] [CrossRef] [Green Version]
- Carrero-Parreño, A.; Onishi, V.C.; Ruiz-Femenia, R.; Salcedo-Díaz, R.; Caballero, J.A.; Reyes-Labarta, J.A. Optimization of multistage membrane distillation system for treating shale gas produced water. Desalination 2019, 460, 15–27. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, R.K.; Hauschild, M.Z.; Boulay, A.M.; Fantke, P.; Laurent, A.; Núñez, M.; Vieira, M. Life Cycle Impact Assessment; Springer: Frankfurt, Germany; Cincinnati, OH, USA, 2017; ISBN 9783319564753. [Google Scholar]
- Carrero-Parreño, A.; Quirante, N.; Ruiz-Femenia, R.; Reyes-Labarta, J.A.; Salcedo-Díaz, R.; Grossmann, I.E.; Caballero, J.A. Economic and environmental strategic water management in the shale gas industry: Application of cooperative game theory. AIChE J. 2019, 65, 1–15. [Google Scholar] [CrossRef]
- Mavrotas, G.; Florios, K. An improved version of the augmented s-constraint method (AUGMECON2) for finding the exact pareto set in multi-objective integer programming problems. Appl. Math. Comput. 2013, 219, 9652–9669. [Google Scholar] [CrossRef]
- Mavrotas, G. Effective implementation of the ε-constraint method in Multi-Objective Mathematical Programming problems. Appl. Math. Comput. 2009, 213, 455–465. [Google Scholar] [CrossRef]





| Parameter | Quantity | Units | Ecoinvent Input |
|---|---|---|---|
| Pretreatment plant * | |||
| Inlet flow | 1.001 | m3/m3 treated water | |
| Outlet flow | 1.000 | m3/m3 treated water | |
| Electrocoagulation | |||
| -Material-HDPE (Hihg density polyethylene) | 1.616 × 10−4 | kg/m3 treated water | [GLO] market for polyethylene, high density |
| -Electricity | 4.336 | kWh/m3 treated water | [GB] market for electricity, high voltage |
| Sedimentation | |||
| -Electricity | 8.599 × 10−3 | kWh/m3 treated water | [GB] market for electricity, high voltage |
| -Steel | 8.799 × 10−4 | kg/m3 treated water | [GLO] market for steel, chromium steel 18/8 |
| -HDPE | 5.799 × 10−6 | kg/m3 treated water | [GLO] market for polyethylene, high density |
| -Concrete | 9.999 × 10−6 | m3/m3 treated water | [RoW] concrete production, for civil engineering, with cement CEM II/B |
| Softening | |||
| -Material-Fiberglass | 4.483 × 10−4 | kg/m3 treated water | [GLO] market for glass fiber reinforced plastic, polyamide, injection molded |
| -Lime | 0.199 | kg/m3 treated water | [GLO] market for lime |
| -Soda | 0.570 | kg/m3 treated water | [GLO] market for soda ash, light, crystalline, heptahydrate |
| Filter press | |||
| -Sludge inlet | 1.218 | kg/m3 treated water | [RoW] drying, sewage sludge |
| -Sludge outlet | 0.616 | kg/m3 treated water | [GLO] market for sewage sludge, dried |
| -Material-Polypropylene | 4.320 × 10−5 | kg/m3 treated water | [GLO] market for polypropylene, granulate |
| -Electricity | 0.566 | kWh/m3 treated water | [GB] market for electricity, high voltage |
| Parameter | Quantity | Units |
|---|---|---|
| Pretreatment plant | ||
| Emissions | ||
| -Sand (silica, quartz) | 66.258 | kg/m3 treated water |
| -Hydrochloric acid | 1.469 | kg/m3 treated water |
| -Petroleum distillate | 0.367 | kg/m3 treated water |
| -Isopropanol | 0.367 | kg/m3 treated water |
| -Potassium chloride | 0.245 | kg/m3 treated water |
| -Hydroxyethyl cellulose | 0.245 | kg/m3 treated water |
| -Ethylene glycol | 0.184 | kg/m3 treated water |
| -Sodium potassium hydroxide | 4.898 × 10−2 | kg/m3 treated water |
| -Ammonium persulfate | 4.898 × 10−2 | kg/m3 treated water |
| -Borate salts | 4.898 × 10−2 | kg/m3 treated water |
| -Citric acid | 1.837 × 10−2 | kg/m3 treated water |
| -Glutaraldehyde | 4.898 × 10−-3 | kg/m3 treated water |
| -Formamide | 4.898 × 10−3 | kg/m3 treated water |
| -Diesel | 9.304 | kg/m3 treated water |
| -Polyacrylamide | 0.556 | kg/m3 treated water |
| -Sodium chloride | 5.831 × 10−5 | kg/m3 treated water |
| Parameter | Quantity | Units | Ecoinvent input |
|---|---|---|---|
| Single-Effect Evaporation with Mechanical Vapor Recompression (SEE-MVR) * | |||
| Feedwater | 1.304 | kg/s | |
| Nickel amount | 4.585 × 10−3 | kg/m3 treated water | [GLO] market for nickel, 99.5% |
| Chromium steel amount | 5.047 × 10−3 | kg/m3 treated water | [GLO] market for steel, chromium steel 18/8 |
| Electricity | 51.496 | kWh/m3 treated water | [GB] market for electricity, high voltage |
| Brine | 93.064 | kg/m3 treated water | [RER] sodium chloride production, brine solution |
| Treated water | 1.000 | m3/m3 treated water | |
| Multiple-Effect Evaporation with Mechanical Vapor Recompression (MEE-MVR) * | |||
| Feedwater | 1.304 | kg/s | |
| Nickel amount | 3.212 × 10−3 | kg/m3 treated water | [GLO] market for nickel, 99.5% |
| Chromium steel amount | 1.385 × 10−3 | kg/m3 treated water | [GLO] market for steel, chromium steel 18/8 |
| Electricity | 29.188 | kWh/m3 treated water | [GB] market for electricity, high voltage |
| Brine | 93.064 | kg/m3 treated water | [RER] sodium chloride production, brine solution |
| Treated water | 1.000 | m3/m3 treated water | |
| Parameter | Quantity | Units | Ecoinvent Input |
|---|---|---|---|
| Membrane technology * | |||
| Feedwater | 2.985 | m3/m3 treated water | |
| Electricity | 8.310 | kWh/m3 treated water | [GB] market for electricity, high voltage |
| Cooling water | 1.204 × 105 | kg/m3 treated water | [Europe without Switzerland] market for tap water |
| Steam | 1.847 × 103 | kg/m3 treated water | [GLO] market for steam, in the chemical industry |
| Membrane composition | |||
| -PTFE | 1.025 × 10−3 | kg/m3 treated water | [GLO] market for polypropylene, granulate |
| -PP | 1.377 × 10−2 | kg/m3 treated water | [GLO] market for tetrafluoroethylene |
| Brine | 607.433 | kg/m3 treated water | [RER] sodium chloride production, brine solution |
| Treated water | 1.000 | m3/m3 treated water | |
| Ecosystem Quality | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Units | Freshwater Ecotoxicity | Natural Land Transformation | Marine Ecotoxicity | Climate Change | Terrestrial Acidification | Terrestrial Ecotoxicity | Agricultural Land Occupation | Freshwater Eutrophication | Urban Land Occupation | |
| Transport | points/T·km | 4.197 × 10−7 | 1.416 × 10−4 | 2.170 × 10−7 | 1.597 × 10−3 | 6.217 × 10−6 | 1.951 × 10−5 | 4.405 × 10−5 | 6.753 × 10−7 | 4.131 × 10−4 |
| Water extraction | points/kg | 4.876 × 10−9 | 7.197 × 10−8 | 9.823 × 10−10 | 3.247 × 10−6 | 1.049 × 10−8 | 2.851 × 10−9 | 7.656 × 10−7 | 1.785 × 10−8 | 8.810 × 10−8 |
| Disposal | points/kg | 6.248 × 10−6 | 0.000 | 2.532 × 10−7 | 0.000 | 0.000 | 1.374 × 10−5 | 0.000 | 0.000 | 0.000 |
| Pretreatment | points/kg | 7.045 × 10−7 | 4.649 × 10−6 | 1.490 × 10−7 | 3.104 × 10−4 | 9.500 × 10−7 | 3.357 × 10−7 | 8.978 × 10−5 | 7.351 × 10−7 | 8.831 × 10−6 |
| Treatment | points/kg | 5.748 × 10−7 | 9.257 × 10−6 | 1.249 × 10−7 | 4.390 × 10−4 | 1.646 × 10−6 | 5.473 × 10−7 | 1.892 × 10−4 | 1.441 × 10−6 | 1.462 × 10−5 |
| Human Health | |||||||
|---|---|---|---|---|---|---|---|
| Units | Photochemical Oxidant Formation | Ozone Depletion | Particulate Matter Formation | Ionizing Radiation | Climate Change | Human Toxicity | |
| Transport | points/T·km | 2.705 × 10−5 | 9.087 × 10−7 | 1.385 × 10−3 | 2.400 × 10−6 | 2.527 × 10−3 | 4.319 × 10−4 |
| Water extraction | points/kg | 2.687 × 10−8 | 7.558 × 10−10 | 1.507 × 10−6 | 3.010 × 10−8 | 5.137 × 10−6 | 1.698 × 10−6 |
| Disposal | points/kg | 0.000 | 0.000 | 0.000 | 1.580 × 10−8 | 0.000 | 5.524 × 10−4 |
| Pretreatment | points/kg | 6.333 × 10−6 | 3.377 × 10−8 | 2.002 × 10−4 | 3.445 × 10−7 | 4.911 × 10−4 | 7.862 × 10−5 |
| Treatment | points/kg | 5.911 × 10−6 | 6.604 × 10−8 | 2.283 × 10−4 | 9.430 × 10−7 | 6.945 × 10−4 | 2.670 × 10−4 |
| Resource Depletion | |||
|---|---|---|---|
| Units | Metal Depletion | Fossil Depletion | |
| Transport | points/T·km | 1.195 × 10−4 | 4.300 × 10−3 |
| Water extraction | points/kg | 1.902 × 10−7 | 6.173 × 10−6 |
| Disposal | points/kg | 0.000 | 0.000 |
| Pretreatment | points/kg | 3.707 × 10−4 | 5.397 × 10−4 |
| Treatment | points/kg | 1.664 × 10−4 | 8.621 × 10−4 |
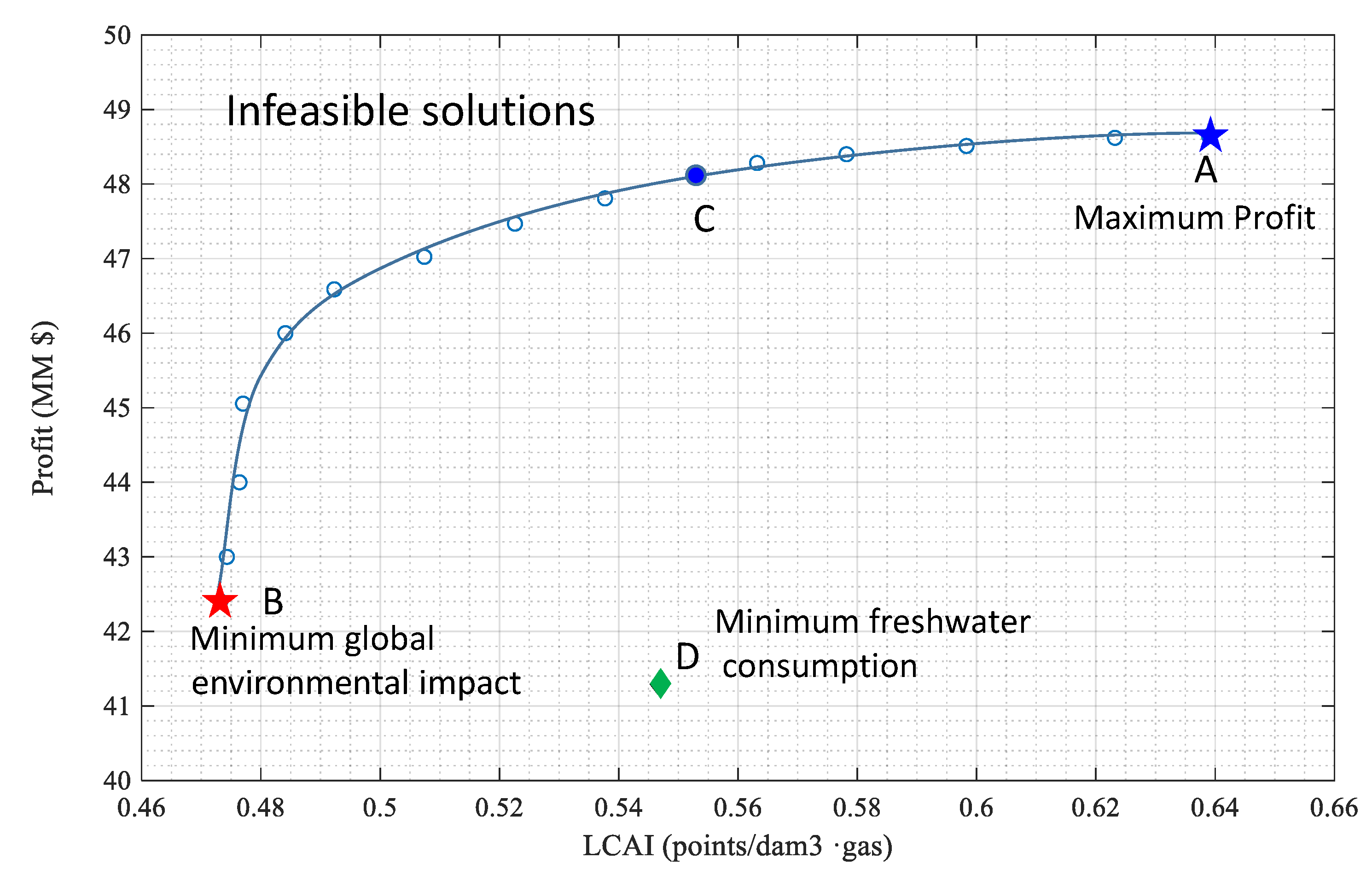
| Profit (k$) | Ecosystem Quality (points/dam3) | Human Health (points/dam3) | Resources Depletion (points/dam3) | Aggregated End-Point (points/dam3) | FreshWater Consumption (m3) |
|---|---|---|---|---|---|
| 48,643.0 | 0.1288 | 0.2522 | 0.2580 | 0.6390 | 178,893.8 |
| 48,620.0 | 0.1257 | 0.2459 | 0.2517 | 0.6232 | 178,893.8 |
| 48,510.7 | 0.1206 | 0.2360 | 0.2417 | 0.5983 | 178,893.8 |
| 48,400.1 | 0.1166 | 0.2282 | 0.2334 | 0.5782 | 167,470.0 |
| 48,282.6 | 0.1135 | 0.2222 | 0.2274 | 0.5632 | 167,470.0 |
| 48,129.5 | 0.1115 | 0.2182 | 0.2233 | 0.5529 | 164,989.5 |
| 47,807.7 | 0.1084 | 0.2122 | 0.2170 | 0.5377 | 157,453.7 |
| 47,468.1 | 0.1054 | 0.2063 | 0.2110 | 0.5226 | 155,745.1 |
| 47,024.1 | 0.1023 | 0.2003 | 0.2048 | 0.5074 | 149,376.2 |
| 46,588.4 | 0.0993 | 0.1944 | 0.1987 | 0.4923 | 146,314.2 |
| 46,000.0 | 0.0972 | 0.1913 | 0.1956 | 0.4841 | 144,920.0 |
| 45,055.0 | 0.0962 | 0.1883 | 0.1925 | 0.4770 | 141,947.8 |
| 44,000.0 | 0.0961 | 0.1881 | 0.1923 | 0.4764 | 141,882.5 |
| 43,000.0 | 0.0956 | 0.1873 | 0.1914 | 0.4743 | 141,105.5 |
| 42,404.0 | 0.0954 | 0.1870 | 0.1908 | 0.4732 | 139,713.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, J.A.; Labarta, J.A.; Quirante, N.; Carrero-Parreño, A.; Grossmann, I.E. Environmental and Economic Water Management in Shale Gas Extraction. Sustainability 2020, 12, 1686. https://doi.org/10.3390/su12041686
Caballero JA, Labarta JA, Quirante N, Carrero-Parreño A, Grossmann IE. Environmental and Economic Water Management in Shale Gas Extraction. Sustainability. 2020; 12(4):1686. https://doi.org/10.3390/su12041686
Chicago/Turabian StyleCaballero, José A., Juan A. Labarta, Natalia Quirante, Alba Carrero-Parreño, and Ignacio E. Grossmann. 2020. "Environmental and Economic Water Management in Shale Gas Extraction" Sustainability 12, no. 4: 1686. https://doi.org/10.3390/su12041686
APA StyleCaballero, J. A., Labarta, J. A., Quirante, N., Carrero-Parreño, A., & Grossmann, I. E. (2020). Environmental and Economic Water Management in Shale Gas Extraction. Sustainability, 12(4), 1686. https://doi.org/10.3390/su12041686