Sustainable Management of Animal Genetic Resources to Improve Low-Input Livestock Production: Insights into Local Beninese Cattle Populations
Abstract
1. Introduction
2. Genetic Resources in the Context of Cattle Production in Benin
2.1. Diversity of Cattle Breeds in Benin
2.1.1. The Indigenous Cattle Breeds of Benin
2.1.2. Other Cattle Breeds Reared in Benin
| Category | Breed Name * | Presence in Benin # | Geographical Distribution in Africa | Frequently Desired Attributes by Farmers | Weakness | Observations | References |
|---|---|---|---|---|---|---|---|
| Savannah shorthorn | Somba | Northwest Benin (Boukombe) | Northeast Togo | Tolerance to diseases and feed and water shortages; good milk and meat qualities; good temperament and draught ability; ease of management; high sociocultural value | Small size, low milk and meat productivity | Reduction of population size due to diseases and lower effect of admixture | [31,33] |
| Dwarf shorthorn | Lagune | Southern and central Benin | Congo, Zambia and Gabon (known as Dahomey cattle) | Moderately affected by admixture with Zebu | [31,43] | ||
| Indigenous crossbreeds | Borgou | Across the country | Known as Ketekou or Keteka in West Africa | Tolerance to trypanosomiasis and feed and water shortages; endurance and draught ability | Lower milk and meat productivity than the Zebu | Highly affected by admixture with Zebu | [14,31,35,52] |
| Pabli | Northwest Benin (Kerou) | - | Share similar characteristics with the Borgou | - | Less investigated and reported as extinct | [31,69] | |
| Lyre-horned Zebu | White Fulani or White Bororo 1 | First most reported Zebu cattle in Benin | Central and western Africa | More resistant to diseases and tolerant to heat than other Zebu are; good performances in milk, meat and draught | Exigent in feed and water resources: practice integral and low grazing. | Large diversity and admixture within the population Its adaptive features are barely investigated | [10,36,51,70,71,72]. |
| M’Bororo or Red Fulani 2 | Third most reported Zebu cattle in Benin | Central and western Africa | Ability to walk long distances; intelligent animal, docile and attached to its owner (less susceptible to theft) | Less trypanotolerant, very exigent in feeding: practice selective grazing | Poorly investigated for population genetic characterization | [10,72,73,74] | |
| Shorthorn Zebu | Gudali 3 | Second most reported Zebu cattle in Benin | Nigeria, Ghana, Cameroon, Central African Republic, and Mali | Large size, growth, and milk performance; exploits large variety of feed resources in the dry season | Poor carcass yield: less than that of White Fulani Limited in walking | Large genetic diversity: many subpopulations; current distribution unknown in Benin | [52,54,55,56,72,75,76] |
| Azawak 4 | Very rare in extensive herds and crossbreed in state-owned farms | Mali, Niger, and Nigeria | Best milk performance within indigenous WA cattle, good meat performance, and excellent adaptation to drought | Requires high-quantity and qualified feed; slow in walking long distances | Currently promoted for crossbreeding with the indigenous breeds in extensive and state-owned farms The breed and resulting crossbreeds remain largely undescribed | [53,56,57,58,77] | |
| Undescribed (crossbreed) Zebu | Djeli or Nigerian Fulani 5 | More rare than other Zebu | Niger | Good reproductive and milk performance, weight gain, and docility | Less trypanotolerant, with medium size and body weight | Remains largely undescribed No study on genetic diversity and relationship with other breeds | [52,58,77] |
| Longhorn African taurine | N’Dama | Mainly kept on state-owned farms | Western and central Africa | Trypanotolerance and resistance to diseases | Less productive than the Borgou and Zebu are | High inbreeding in the current population on state-owned farms | [61,62,63,72,78] |
| Tropical crossbreed | Girolando | Kept on Kpinnou and Okpara state- owned farms | Ivory Coast, Burkina Faso, and Senegal | High milk performance | Very susceptible to disease pressures and exigent in feed and water resources | Low productivity in Benin due to inadequate environmental conditions and source of tick invasion in West Africa | [60,66,67,79,80] |
2.2. Management of Cattle Genetic Resources in Benin
2.2.1. Cattle Production Systems and Major Constraints
2.2.2. Institutional Management of AnGR in Benin
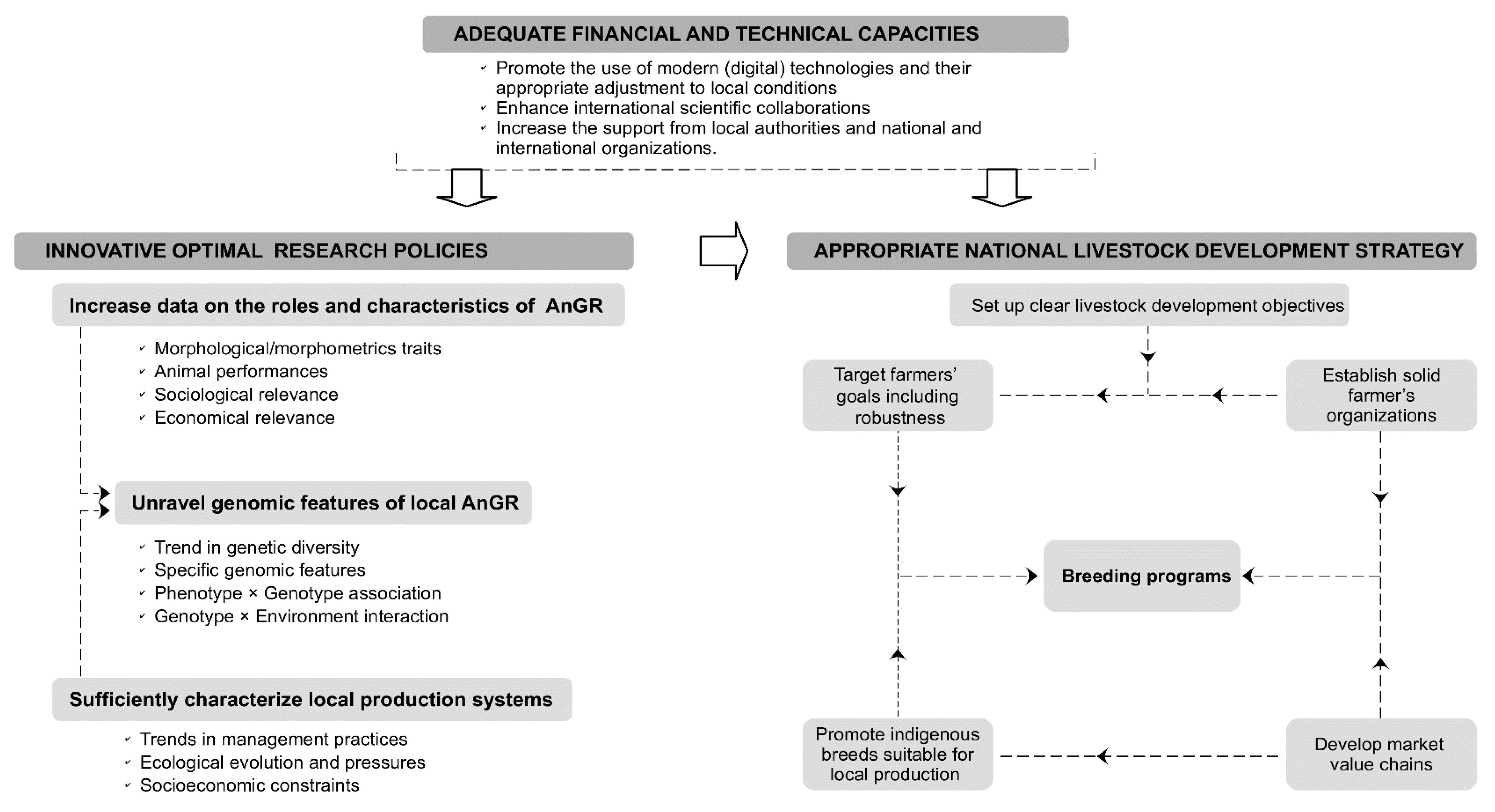
3. Pathways for the Management and Improvement of Cattle Genetic Resources in Benin
3.1. Livestock Development Objectives and Strategies
3.1.1. Breeding Goals of Smallholders
3.1.2. Optimization of Phenotyping and Genotyping Strategies
3.1.3. Structured Interventions with Appropriate Farmer and Market Organizations
3.2. Breeding Programs
3.2.1. Conserving and Building on Local Cattle Genetic Resources
3.2.2. Targeting Selection for Robust Cattle
3.2.3. Improving Farmer Management Practices
3.2.4. Promoting Institutional Supports
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thornton, P.K.; van de Steeg, J.; Notenbaert, A.; Herrero, M. The impacts of climate change on livestock and livestock systems in developing countries: A review of what we know and what we need to know. Agric. Syst. 2009, 101, 113–127. [Google Scholar] [CrossRef]
- Toulmin, C. Climate Change in Africa; Zed Books; Bloomsbury Publishing: London, UK, 2021; ISBN 9781350219229. [Google Scholar]
- Moritz, M. Livestock transfers, risk management, and human careers in a West African pastoral system. Hum. Ecol. 2013, 41, 205–219. [Google Scholar] [CrossRef]
- Bett, B.; Kiunga, P.; Gachohi, J.; Sindato, C.; Mbotha, D.; Robinson, T.; Lindahl, J.; Grace, D. Effects of climate change on the occurrence and distribution of livestock diseases. Prev. Vet. Med. 2017, 137, 119–129. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Wurzinger, M.; Mirkena, T.; Sölkner, J. Animal breeding strategies in Africa: Current issues and the way forward. J. Anim. Breed. Genet. 2014, 131, 327–328. [Google Scholar] [CrossRef]
- Vanlauwe, B.; Coyne, D.; Gockowski, J.; Hauser, S.; Huising, J.; Masso, C.; Nziguheba, G.; Schut, M.; van Asten, P. Sustainable intensification and the African smallholder farmer. Curr. Opin. Environ. Sustain. 2014, 8, 15–22. [Google Scholar] [CrossRef]
- Sunderland, T. Food security: Why is biodiversity important? Int. Forest. Rev. 2011, 13, 265–274. [Google Scholar] [CrossRef]
- Kim, K.; Kwon, T.; Dessie, T.; Yoo, D.; Mwai, O.A.; Jang, J.; Sung, S.; Lee, S.; Salim, B.; Jung, J.; et al. The mosaic genome of indigenous African cattle as a unique genetic resource for African pastoralism. Nat. Genet. 2020, 52, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Boutrais, J. Eleveurs, bétail et environnement. In Dynamique des Systèmes Agraires: À la Croisée des Parcours: Pasteurs, Éleveurs, Cultivateurs; Blanc-Pamard, C., Boutrais, J., Eds.; ORSTOM: Paris, France, 1994; pp. 304–319. ISBN 2709912287. [Google Scholar]
- Mirkena, T.; Duguma, G.; Haile, A.; Tibbo, M.; Okeyo, A.M.; Wurzinger, M.; Sölkner, J. Genetics of adaptation in domestic farm animals: A review. Livest. Sci. 2010, 132, 1–12. [Google Scholar] [CrossRef]
- Navès, M.; Alexandre, G.; Mahieu, M.; Gourdine, J.L.; Mandonnet, N. Les races animales locales: Bases du développement innovant et durable de l’élevage aux Antilles. In Proceedings of the CIAG 2011 Carrefours de L’innovation Agronomique, Guadeloupe, France, 3 November 2011; pp. 193–205. [Google Scholar]
- Jones, P.G.; Thornton, P.K. Croppers to livestock keepers: Livelihood transitions to 2050 in Africa due to climate change. Environ. Sci. Policy 2009, 12, 427–437. [Google Scholar] [CrossRef]
- Houessou, S.O.; Dossa, L.H.; Diogo, R.V.; Houinato, M.; Buerkert, A.; Schlecht, E. Change and continuity in traditional cattle farming systems of West African Coast countries: A case study from Benin. Agric. Syst. 2019, 168, 112–122. [Google Scholar] [CrossRef]
- Hoffmann, I.; Boerma, D.; Scherf, B. The Global Plan of Action for Animal Genetic Resources—The road to common understanding and agreement. Lives. Sci. 2011, 136, 7–14. [Google Scholar] [CrossRef]
- FAO (Ed.) The Second Report on the State of the World’s Animal Genetic Resources for Food and Agriculture; Commission on Genetic Resources for Food and Agriculture, Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; ISBN 978-92-5-108820-3. [Google Scholar]
- Wollny, C.B. The need to conserve farm animal genetic resources in Africa: Should policy makers be concerned? Ecol. Econ. 2003, 45, 341–351. [Google Scholar] [CrossRef]
- Marandure, T.; Bennett, J.; Dzama, K.; Makombe, G.; Gwiriri, L.; Mapiye, C. Advancing a holistic systems approach for sustainable cattle development programmes in South Africa: Insights from sustainability assessments. Agroecol. Sustain. Food Syst. 2020, 44, 827–858. [Google Scholar] [CrossRef]
- Ouédraogo, D.; Soudré, A.; Yougbaré, B.; Ouédraogo-Koné, S.; Zoma-Traoré, B.; Khayatzadeh, N.; Traoré, A.; Sanou, M.; Mészáros, G.; Burger, P.A.; et al. Genetic improvement of local cattle breeds in West Africa: A review of breeding programs. Sustainability 2021, 13, 2125. [Google Scholar] [CrossRef]
- Leroy, G.; Baumung, R.; Boettcher, P.; Scherf, B.; Hoffmann, I. Review: Sustainability of crossbreeding in developing countries; definitely not like crossing a meadow…. Animal 2016, 10, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Marshall, K.; Gibson, J.P.; Mwai, O.; Mwacharo, J.M.; Haile, A.; Getachew, T.; Mrode, R.; Kemp, S.J. Livestock genomics for developing countries—African examples in practice. Front. Genet. 2019, 10, 297. [Google Scholar] [CrossRef]
- Brown, A.; Ojango, J.; Gibson, J.; Coffey, M.; Okeyo, M.; Mrode, R. Short communication: Genomic selection in a crossbred cattle population using data from the Dairy Genetics East Africa Project. J. Dairy Sci. 2016, 99, 7308–7312. [Google Scholar] [CrossRef]
- Ibeagha-Awemu, E.M.; Peters, S.O.; Bemji, M.N.; Adeleke, M.A.; Do, D.N. Leveraging available resources and stakeholder involvement for improved productivity of African livestock in the era of genomic breeding. Front. Genet. 2019, 10, 357. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat/en/#home (accessed on 16 April 2021).
- Sounon, A.K.; Ickowicz, A.; Lesnoff, M.; Messad, S.; Valls-Fox, H.; Houinato, M.R. Impact de la sédentarisation des éleveurs sur la production bovine au nord du Bénin. Rev. D’élevage Médecine Vétérinaire Pays Trop. 2019, 72, 93. [Google Scholar] [CrossRef]
- Kinkpe, T.A.; Diogo, R.V.; Kpade, C.P.; Yabi, J.A.; Dossa, L.H. The role of cattle attributes in buyers’ choices in Benin. Afr. J. Agric. Resour. Econ. 2019, 14, 56–71. [Google Scholar] [CrossRef]
- Dean, A.S.; Fournié, G.; Kulo, A.E.; Boukaya, G.A.; Schelling, E.; Bonfoh, B. Potential risk of regional disease spread in West Africa through cross-border cattle trade. PLoS ONE 2013, 8, e75570. [Google Scholar] [CrossRef] [PubMed]
- Van Ufford, Q.; John, P.E. Trade and Traders: The Making of the Cattle Market in Benin. Ph.D. Thesis, Universiteit van Amsterdam, Amsterdam, The Netherlands, 1999. [Google Scholar]
- van den Akker, E. Major Crops and Their Regional Distribution in Benin; Atlas of Natural and Agronomic Resources of Niger and Benin; Germany University of Hohenheim: Hohenheim, Germany, 2000. [Google Scholar]
- Houessou, S.O.; Dossa, L.H.; Assogba, C.A.; Diogo, R.V.C.; Vanvanhossou, S.F.U.; Schlecht, E. The role of cross-border transhumance in influencing resident herders’ cattle husbandry practices and use of genetic resources. Animal 2020, 14, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Scheper, C.; Bohlouli, M.; Brügemann, K.; Weimann, C.; Vanvanhossou, S.F.U.; König, S.; Dossa, L.H. The role of agro-ecological factors and transboundary transhumance in shaping the genetic diversity in four indigenous cattle populations of Benin. J. Anim. Breed. Genet. 2020, 137, 622–640. [Google Scholar] [CrossRef] [PubMed]
- Rust, J.M.; Rust, T. Climate change and livestock production: A review with emphasis on Africa. S. Afr. J. Anim. Sci. 2013, 43, 255. [Google Scholar] [CrossRef]
- Dossa, L.H.; Vanvanhossou, F.U.S. The indigenous Somba cattle of the hilly Atacora region in North-West Benin: Threats and opportunities for its sustainable use. Trop. Anim. Health Prod. 2016, 48, 349–359. [Google Scholar] [CrossRef]
- Vanvanhossou, S.F.U.; Koura, I.B.; Dossa, L.H. The implications of herd entrustment practice for the sustainable use of cattle genetic resources in the (agro)-pastoral systems of West Africa: A case study from Benin. Pastoralism 2021, 11, 8. [Google Scholar] [CrossRef]
- Houessou, S.O.; Dossa, L.H.; Diogo, R.V.C.; Ahozonlin, M.C.; Dahouda, M.; Schlecht, E. Confronting pastoralists’ knowledge of cattle breeds raised in the extensive production systems of Benin with multivariate analyses of morphological traits. PLoS ONE 2019, 14, e0222756. [Google Scholar] [CrossRef]
- Rege, J.E.; Aboagye, G.S.; Tawah, C.L. Shorthorn cattle of West and Central Africa. I. Origin, distribution, classification and population statistics. World Anim. Rev. 1994, 78, 2–13. [Google Scholar]
- Mwai, O.; Hanotte, O.; Kwon, Y.J.; Cho, S. African indigenous cattle: Unique genetic resources in a rapidly changing world. Asian-Australas. J. Anim. Sci. 2015, 28, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.J.G.; Gnaho, L.K.; Meghen, C. Une enquête sur la race bovine Somba au Bénin. Rev. D’élevage Médecine Vétérinaire Pays Trop. 1995, 48, 77–83. [Google Scholar] [CrossRef]
- Felius, M.; Beerling, M.L.; Buchanan, D.; Theunissen, B.; Koolmees, P.; Lenstra, J. On the history of cattle genetic resources. Diversity 2014, 6, 705–750. [Google Scholar] [CrossRef]
- Porter, V.; Alderson, L.; Hall, S.J.G.; Sponenberg, D.P. Mason’s World Encyclopedia of Livestock Breeds and Breeding; CABI: Wallingford, UK, 2016. [Google Scholar]
- Vanvanhossou, S.F.U.; Diogo, R.V.C.; Dossa, L.H. Estimation of live bodyweight from linear body measurements and body condition score in the West African Savannah Shorthorn cattle in North-West Benin. Cogent Food Agric. 2018, 4, 1549767. [Google Scholar] [CrossRef]
- Ahozonlin, M.C.; Dossa, L.H. Diversity and resilience to socio-ecological changes of smallholder Lagune cattle farming systems of Benin. Sustainability 2020, 12, 7616. [Google Scholar] [CrossRef]
- Ahozonlin, M.C.; Dossa, L.H.; Dahouda, M.; Gbangboche, A.B. Morphological divergence in the West African shorthorn Lagune cattle populations from Benin. Trop. Anim. Health Prod. 2020, 52, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Ahozonlin, M.C.; Koura, I.B.; Dossa, L.H. Determinants of crossbreeding practices by cattle farmers in South Benin, West Africa: Implications for the sustainable use of the indigenous Lagune cattle population. Sustain. Agric. Res. 2019, 8, 101. [Google Scholar] [CrossRef]
- Flori, L.; Thevenon, S.; Dayo, G.K.; Senou, M.; Sylla, S.; Berthier, D.; Moazami-Goudarzi, K.; Gautier, M. Adaptive admixture in the West African bovine hybrid zone: Insight from the Borgou population. Mol. Ecol. 2014, 23, 3241–3257. [Google Scholar] [CrossRef] [PubMed]
- Aïsso, R.C.; Aïssi, M.V.; Youssao, A.I.; Soumanou, M.M. Caractéristiques physico-chimiques du fromage Peulh produit dans les conditions optimales de coagulation à partir du lait de deux races de vaches du Bénin. Nat. Technol. 2016, 14, 37–43. [Google Scholar]
- Djenontin, A.J.; Amidou, M.; Baco, N.M. Diagnostic gestion du troupeau: Gestion des ressources pastorales dans les départements de l’Alibori et du Borgou au nord Bénin. Bull. Rech. Agron. Bénin 2004, 43, 30–45. [Google Scholar]
- Pecaud, M.G. L’élevage des Animaux Domestiques au Dahomey; Imp. du Gvt. Général de l’A.O.F: Gorée, Senegal, 1912; p. 157. [Google Scholar]
- Atchy, A.A. Contribution à L’étude de la Transhumance en Republique Populaire du Bénin. Ph.D. Thesis, Université de Dakar, Dakar, Senegal, 1976. [Google Scholar]
- Murray, C.; Huerta-Sanchez, E.; Casey, F.; Bradley, D.G. Cattle demographic history modelled from autosomal sequence variation. Philos. Trans. R. Soc. B 2010, 365, 2531–2539. [Google Scholar] [CrossRef]
- Tawah, C.L.; Rege, J. White Fulani cattle of West and Central Africa. Anim. Genet. Resour. Inf. 1996, 17, 127–145. [Google Scholar] [CrossRef]
- Tamou, C.; de Boer, I.J.; Ripoll-Bosch, R.; Oosting, S.J. Understanding roles and functions of cattle breeds for pastoralists in Benin. Livest. Sci. 2018, 210, 129–136. [Google Scholar] [CrossRef]
- Kassa, S.K.; Dayo, G.K.C.; Yapi-Gnaoré, V.; Sylla, S.; Konkobo, M.; Youssao, A.K.I. Genetic diversity of Benin cattle populations using microsatellite markers. Int. J. Anim. Sci. Technol. 2019, 3, 7. [Google Scholar] [CrossRef]
- Tawah, C.L.; Rege, J.E.O. Gudali cattle of West and Central Africa. Anim. Genet. Resour. Inf. 1996, 17, 147–164. [Google Scholar] [CrossRef]
- Umar, D.S.B.; Nwagu, B.I.; Umar, U.A.; Rufina, O.O.; Saleh, I.; Onotu, S.I.; Ugwu, L. On-station performance evaluation of indigenous breeds of cattle for dairy production systems in Nigeria. Asian J. Res. Agric. For. 2020, 30–38. [Google Scholar] [CrossRef]
- Rege, J.; Tawah, C.L. The state of African cattle genetic resources II. Geographical distribution, characteristics and uses of present-day breeds and strains. Anim. Genet. Resour. Inf. 1999, 26, 1–25. [Google Scholar] [CrossRef]
- Barassounon Amadou, I. Valorisation du Zébu Azawak par Croisement avec les Taurins Borgou et Évaluation des Facteurs de Risques liés à son Adaptation au Bénin. Master’s Thesis, Université d’Abomey-Calavi, Abomey-Calavi, Benin, 2018. [Google Scholar]
- Belli, P.; Turini, J.; Harouna, A.; Garba, I.A.; Pistocchini, E.; Zecchini, M. Critères de sélection des bovins laitiers par les éleveurs autour de Niamey au Niger. Rev. D’élevage Médecine Vétérinaire Pays Trop. 2008, 61, 51. [Google Scholar] [CrossRef]
- Alassane, Y.; Ahounou, S.; Toleba, S.; Adjakpa, A.; Dotche, I.; Houaga, I.; Moula, N.; Antoine-Moussiaux, N.; Hornick, J.; Youssao, A. Zootechnical performance of Girolando cattle at Kpinnou Breeding Farm, South-West of Benin Republic. J. Adv. Vet. Anim. Res. 2018, 5, 123. [Google Scholar] [CrossRef]
- Alkoiret, I.T.; Mama Yari, H.; Gbangboche, A.B.; Lokossou, R. Reproductive performance and milk production of Girolando cows in the Ranch of Kpinnou, South-West of Benin Republic. J. Anim. Vet. Adv. 2011, 10, 2588–2592. [Google Scholar] [CrossRef]
- Youssao, A.; Ahissou, A.; Toure, Z. Introduction de la race bovine N’Dama à la Ferme Elevage de l’Okpara au Bénin. Quelques performances zootechniques. Anim. Genet. Resour. Inf. 2000, 27, 17–25. [Google Scholar] [CrossRef][Green Version]
- Gbangboché, A.B.; Alkoiret, T.I. Reproduction et production de lait des bovins de race Borgou et N’Dama au Bénin. J. Appl. Biosci. 2011, 46, 3185–3194. [Google Scholar]
- Alkoiret, I.T.; Awohouedji, D.Y.; Yacoubou, A.M. Paramètres démographiques des cheptels de bovins Borgou et N’Dama à la Ferme d’Elevage de l’Okpara au nord-est du Bénin. Int. J. Biol. Chem. Sci. 2011, 4. [Google Scholar] [CrossRef][Green Version]
- da Costa, A.N.L.; Feitosa, J.V.; Montezuma, P.A.; de Souza, P.T.; de Araújo, A.A. Rectal temperatures, respiratory rates, production, and reproduction performances of crossbred Girolando cows under heat stress in northeastern Brazil. Int. J. Biometeorol. 2015, 59, 1647–1653. [Google Scholar] [CrossRef]
- Kassa, S.K.; Salifou, C.F.A.; Dayo, G.K.; Ahounou, S.; Dotché, O.I.; Issifou, T.M.; Houaga, I. Assessment of milk production and resilience of Girolando cattle, reared in semi-improved breeding system in Benin. J. Vet. Adv. 2016, 6, 1269. [Google Scholar] [CrossRef]
- Adakal, H.; Biguezoton, A.; Zoungrana, S.; Courtin, F.; de Clercq, E.M.; Madder, M. Alarming spread of the Asian cattle tick Rhipicephalus microplus in West Africa-another three countries are affected: Burkina Faso, Mali and Togo. Exp. Appl. Acarol. 2013, 61, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Madder, M.; Thys, E.; Achi, L.; Touré, A.; de Deken, R. Rhipicephalus (Boophilus) microplus: A most successful invasive tick species in West-Africa. Exp. Appl. Acarol. 2011, 53, 139–145. [Google Scholar] [CrossRef]
- Madder, M.; Adehan, S.; de Deken, R.; Adehan, R.; Lokossou, R. New foci of Rhipicephalus microplus in West Africa. Exp. Appl. Acarol. 2012, 56, 385–390. [Google Scholar] [CrossRef]
- Belemsaga, D.M.; Lombo, Y.; Thevenon, S.; Sylla, S. Inventory analysis of West African cattle breeds. In Applications of Gene-Based Technologies for Improving Animal Production and Health in Developing Countries; Springer: New York, NY, USA, 2005; pp. 167–173. [Google Scholar]
- Norezzine, A.; Duksi, F.; Tsvetkova, A.D.; Ulybina, E.A.; Gins, M.S.; Yacer, R.N.; Klenovitsky, A.A.; Nikishov, A.A.; Amirshoev, F.; Digha, J.; et al. Genetic characterization of White Fulani cattle in Nigeria: A comparative study. J. Adv. Vet. Anim. Res. 2019, 6, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ibeagha-Awemu, E.M.; Jann, O.C.; Weimann, C.; Erhardt, G. Genetic diversity, introgression and relationships among West/Central African cattle breeds. Genet. Sel. Evol. 2004, 36, 673–690. [Google Scholar] [CrossRef] [PubMed]
- Dessie, T.; Mwai, O.A. The Story of Cattle in Africa: Why Diversity Matters; ILRI, Rural Development Administration, Republic of Korea and AU-IBAR: Nairobi, Kenya, 2019; ISBN 92-9146-583-3. [Google Scholar]
- Ayantunde, A.A.; Kango, M.; Hiernaux, P.; Udo, H.M.J.; Tabo, R. Herders’ perceptions on ruminant livestock breeds and breeding management in southwestern Niger. Hum. Ecol. 2007, 35, 139–149. [Google Scholar] [CrossRef][Green Version]
- Ibeagha-Awemu, E.M.; Erhardt, G. An evaluation of genetic diversity indices of the Red Bororo and White Fulani cattle breeds with different molecular markers and their implications for current and future improvement options. Trop. Anim. Health Prod. 2006, 38, 431–441. [Google Scholar] [CrossRef]
- Domingo, A.M. Contribution à L’étude de la Population Bovine des Etats du Golfe du Bénin. Ph.D. Thesis, Ecole Inter-Etats des Sciences et Medecine Veterinaires de Dakar, Dakar, Senegal, 1976. [Google Scholar]
- Boutrais, J. The Fulani and cattle breeds: Crossbreeding and heritage strategies. Africa 2007, 77, 18–36. [Google Scholar] [CrossRef]
- Payne, W.J.A.; Hodges, J. Tropical Cattle: Origins, Breeds and Breeding Policies; Blackwell Science: London, UK, 1997; ISBN 0632040483. [Google Scholar]
- Starkey, P.H. N’Dama cattle—A productive trypanotolerant breed. World. Anim. Rev. 1984, 50, 2–15. [Google Scholar]
- Toukourou, Y.; Senou, M. Performances zootechniques de la vache girolando à la ferme de kpinnou au Bénin. Ann. Sci. Agron. 2011, 14. [Google Scholar] [CrossRef]
- Bouyer, F.; Seck, M.T.; Dicko, A.H.; Sall, B.; Lo, M.; Vreysen, M.J.B.; Chia, E.; Bouyer, J.; Wane, A. Ex-ante benefit-cost analysis of the elimination of a Glossina palpalis gambiensis population in the Niayes of Senegal. PLoS Negl. Trop. Dis. 2014, 8, e3112. [Google Scholar] [CrossRef] [PubMed]
- Blench, R. Traditional Livestock Breeds: Geographical Distribution and Dynamics in Relation to the Ecology of West Africa; Overseas Development Institute: London, UK, 1999. [Google Scholar]
- Rege, J. The state of African cattle genetic resources I. Classification framework and identification of threatened and extinct breeds. Anim. Genet. Resour. Inf. 1999, 25, 1–25. [Google Scholar] [CrossRef]
- Djohy, G.L.; Sounon Bouko, B. Mobilité pastorale et ses dynamiques spatio-temporelles dans la commune de Tchaourou au Bénin. Ann. l’Université Moundou 2020, 7, 6–28. Available online: http://aflash-revue-mdou.org/wp-content/uploads/2020/12/1_article_Djohy-et-Sounon-Bouko_aflash_731.pdf (accessed on 31 August 2021).
- Alkoiret, I.; Awohouedji, D.; Akossou, A.; Bosma, R. Typologie des systèmes d’élevage bovin de la commune de Gogounou au nord-est du Bénin. Ann. Sci. Agron. 2010, 12. [Google Scholar] [CrossRef]
- Azalou, M.; Assani, A.; Alkoiret, I. Typology of cattle herds in transhumance in the municipality of Djidja in Southern Benin. HAYA-SJLS 2017, 2, 65–72. [Google Scholar]
- Djenontin, J.A.; Madjidou, O.; Houinato, M.R.; Mensah, G.A.; Sinsin, B.A. Extensive herding pastoral calendar in North-East Benin: A tool for cattle herd management. Sci. Chang. Planétaires/Sécheresse 2012, 23, 261–270. [Google Scholar] [CrossRef]
- Tamou, C.; Ripoll-Bosch, R.; de Boer, I.J.M.; Oosting, S.J. Pastoralists in a changing environment: The competition for grazing land in and around the W Biosphere Reserve, Benin Republic. Ambio 2018, 47, 340–354. [Google Scholar] [CrossRef] [PubMed]
- De Haan, L.J. Gestion de Terroir at the Frontier: Village level management of peasants and pastoralists in Benin. In The Arid Frontier: Interactive Management of Environment and Development; Bruins, H.J., Lithwick, H., Eds.; Kluwer Academic: Boston, MA, USA, 1998; pp. 209–227. [Google Scholar]
- Topanou, O.L.; Okou, C.; Boko, M. Durabilité agro-écologique des exploitations agricoles dans la commune de Gogounou au Bénin. Afr. Sci. Rev. Int. Sci. Technol. 2015, 11, 129–137. [Google Scholar] [CrossRef]
- Zakari, S.; Tente, B.A.H.; Yabi, I.; Imorou, I.T.; Tabou, T.; Afouda, F.; n’Bessa, B. Vulnérabilité des troupeaux transhumants aux mutations climatiques: Analyse des perceptions et adaptations locales dans le bassin de la Sota à Malanville. Afr. Sci. Rev. Int. Sci. Technol. 2015, 11, 211–228. [Google Scholar] [CrossRef]
- Ahokpossi, Y. Analysis of the rainfall variability and change in the Republic of Benin (West Africa). Hydrol. Sci. J. 2018, 63, 2097–2123. [Google Scholar] [CrossRef]
- Kperou Gado, B.O.; Toko Imorou, I.; Arouna, O.; Sidi Imorou, H.; Oumorou, M. Déterminants des itinéraires de transhumance à la périphérie de la réserve de biosphère transfrontalière du W au Bénin. J. Appl. Biosci. 2020, 152, 15650–15666. [Google Scholar] [CrossRef]
- Kperou Gado, B.O.; Toko Imorou, I.; Arouna, O.; Oumorou, M. Caractérisation des parcours de transhumance à la périphérie de la réserve de biosphère transfrontalière du W au Bénin. Int. J. Biol. Chem. Sci. 2020, 14, 333–352. [Google Scholar] [CrossRef]
- Kate, S.; Amagnide, A.; Hounmenou, C.G.; Hounkpatin, E.L.B.; Sinsin, B. Changements climatiques et gestion des ressources pastorales en zone agropastorale au Nord-Bénin: Cas de la commune de Banikoara. Afr. Sci. Rev. Int. Sci. Technol. 2015, 11, 201–215. [Google Scholar]
- Abdoulaye, I.M.; Ayena, M.; Yabi, A.J.; Dedehouanou, H.; Biaou, G.; Houinato, M. Gouvernance locale des infrastructures pastorales et agropastorales dans le Borgou au Nord-Est du Bénin: Quels modes pour une gestion durable des infrastructures? Int. J. Innov. Appl. Stud. 2018, 24, 1312–1323. [Google Scholar]
- Gorna, K.; Houndjè, E.; Romey, A.; Relmy, A.; Blaise-Boisseau, S.; Kpodékon, M.; Saegerman, C.; Moutou, F.; Zientara, S.; Bakkali Kassimi, L. First isolation and molecular characterization of foot-and-mouth disease virus in Benin. Vet. Microbiol. 2014, 171, 175–181. [Google Scholar] [CrossRef]
- Dehoux, J.P.; Hounsou-Ve, G. Epizootie de fièvre aphteuse au Nord-Bénin durant la saison sèche 1990–1991. Rev. Elev. Med. Vet. Pays Trop. 1991, 44, 261–262. [Google Scholar] [CrossRef]
- Houndjè, E.; Kpodekon, M.; Moutou, F.; Blaise-Boisseau, S.; Bakkali-Kassimi, L.; Berkvens, D.; Zientara, S.; Saegerman, C. Principales caractéristiques épidémiologiques et impact économique de la fièvre aphteuse en Afrique: Synthèse bibliographique. Ann. Med. Vet. 2013, 157, 120–134. [Google Scholar]
- Adesokan, H.K.; Streicher, E.M.; van Helden, P.D.; Warren, R.M.; Cadmus, S.I.B. Genetic diversity of Mycobacterium tuberculosis complex strains isolated from livestock workers and cattle in Nigeria. PLoS ONE 2019, 14, e0211637. [Google Scholar] [CrossRef]
- Doko, A.; Guedegbe, B.; Baelmans, R.; Demey, F.; N’Diaye, A.; Pandey, V.S.; Verhulst, A. Trypanosomiasis in different breeds of cattle from Benin. Vet. Parasitol. 1991, 40, 1–7. [Google Scholar] [CrossRef]
- Zannou, O.M.; Ouedraogo, A.S.; Biguezoton, A.S.; Lempereur, L.; Patrick Yao, K.; Abatih, E.; Zoungrana, S.; Lenaert, M.; Toe, P.; Farougou, S.; et al. First digital characterization of the transhumance corridors through Benin used by cattle herds from Burkina Faso and associated risk scoring regarding the invasion of Rhipicephalus (Boophilus) microplus. Transbound. Emerg. Dis. 2021, 68, 2079–2093. [Google Scholar] [CrossRef]
- Assogba, M.N.; Youssao, A.K.I. Prévalence de la fasciolose bovine à Fasciola gigantica (Cobbold, 1885) dans les principaux abattoirs du Bénin. Rev. Méd. Vét. 2001, 152, 699–704. [Google Scholar]
- Zerbo, L.H.; Dahourou, L.D.; Sidi, M.; Ouoba, L.B.; Ouandaogo, S.H.; Bazimo, G.; N’paton Sie, B.; Traore, K.Z.A.; Tapsoba, M.; Ouedraogo, A.; et al. Seroprevalence and determinants of contagious bovine pleuropneumonia in cattle in Burkina Faso. Trop. Anim. Health Prod. 2020, 53, 39. [Google Scholar] [CrossRef] [PubMed]
- Ankeli, P.I.; Raji, M.A.; Kazeem, H.M.; Tambuwal, F.M.; Francis, M.I.; Ikpa, L.T.; Fagbamila, I.O.; Luka, P.D.; Nwankpa, N.D. Seroprevalence of contagious bovine pleuropneumonia in Plateau state, North-central Nigeria. Bull. Anim. Health Prod. Afr. 2017, 65, 359–368. [Google Scholar] [CrossRef]
- Alhaji, N.B.; Babalobi, O.O.; Saidu, S. Using sero-positivity to assess geospatial burden of contagious bovine pleuropneumonia on pastoral cattle herds of north-central Nigeria. Pastoralism 2016, 6, 982. [Google Scholar] [CrossRef]
- Dognon, S.R.; Antoine-Moussiaux, N.; Douny, C.; Gustin, P.; Moula, N.; Scippo, M.L.; Youssao, A.K.I. The use of antibiotics in cattle in North-East Benin: Pharmaceutical inventory and risk practices of cattle breeders. Trop. Anim. Health Prod. 2018, 50, 1683–1699. [Google Scholar] [CrossRef] [PubMed]
- Chabi China, T.F.; Olounlade, P.A.; Salifou, S. Ethnobotanical study of endogenous methods used for the treatment of diseases of Somba cattle breed in northen Benin. J. Drug Deliv. Ther. 2014, 4, 91–99. [Google Scholar] [CrossRef]
- Vitouley, H.S.; Sidibe, I.; Bengaly, Z.; Marcotty, T.; van den Abbeele, J.; Delespaux, V. Is trypanocidal drug resistance a threat for livestock health and production in endemic areas? Food for thoughts from Sahelian goats infected by Trypanosoma vivax in Bobo Dioulasso (Burkina Faso). Vet. Parasitol. 2012, 190, 349–354. [Google Scholar] [CrossRef]
- Vitouley, H.S.; Bengaly, Z.; Adakal, H.; Sidibé, I.; van Abbeele, J.; Delespaux, V. Epidemiological monitoring network of chemoresistance to trypanocidal and acaricides drugs in West Africa (RESCAO). Tropicultura 2013, 31, 205–212. [Google Scholar]
- Moazami Goudarzi, K.; Belemsaga, D.M.; Ceriotti, G.; Laloë, D.; Fagbohoum, F.; Kouagou, N.T.; Sidibé, I.; Codjia, V.; Crimella, M.C.; Grosclaude, F.; et al. Caractérisation de la race bovine Somba à l’aide de marqueurs moléculaires. Rev. Elev. Med. Vet. Pays Trop. 2001, 54, 129. [Google Scholar] [CrossRef][Green Version]
- Freeman, A.R.; Meghen, C.M.; MacHugh, D.E.; Loftus, R.T.; Achukwi, M.D.; Bado, A.; Sauveroche, B.; Bradley, D.G. Admixture and diversity in West African cattle populations. Mol. Ecol. 2004, 13, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Dzama, K.; Rees, D.J.G.; Muchadeyi, F.C. Tropically adapted cattle of Africa: Perspectives on potential role of copy number variations. Anim. Genet. 2016, 47, 154–164. [Google Scholar] [CrossRef]
- Vanvanhossou, S.F.U.; Scheper, C.; Dossa, L.H.; Yin, T.; Brügemann, K.; König, S. A multi-breed GWAS for morphometric traits in four Beninese indigenous cattle breeds reveals loci associated with conformation, carcass and adaptive traits. BMC Genom. 2020, 21, 783. [Google Scholar] [CrossRef]
- FAO-Benin. Rapport National d’aide à la Préparation du Deuxième Rapport sur l’État des Ressources Zoogénétiques pour L’alimentation et L’Agriculture Dans le Monde, Incluant des Données Spécifiques au Secteur Servant Pour l’État de la Biodiversité Pour L’Alimentation et L’Agriculture dans le Monde. 2013. Available online: http://www.fao.org/3/i4787e/i4787f02.pdf (accessed on 21 May 2021).
- Hestin, T. Les stratégies de Développement de L’élevage Bovin au Bénin au Travers de la Mise en Place de Deux Projets Consécutifs (le P.D.E puis le P.A.F.I.L.A.V). Ph.D. Thesis, Claude Bernard University Lyon 1, Lyon, France, 2012. [Google Scholar]
- McDermott, J.J.; Staal, S.J.; Freeman, H.A.; Herrero, M.; van de Steeg, J.A. Sustaining intensification of smallholder livestock systems in the tropics. Livest. Sci. 2010, 130, 95–109. [Google Scholar] [CrossRef]
- Chabi Toko, R.; Adegbidi, A.; Lebailly, P. Valorisation des produits laitiers dans les ménages Peul du Nord-Est du Bénin. Int. J. Biol. Chem. Sci. 2016, 9, 2716. [Google Scholar] [CrossRef]
- Merks, J.W.M.; Mathur, P.K.; Knol, E.F. New phenotypes for new breeding goals in pigs. Animal 2012, 6, 535–543. [Google Scholar] [CrossRef]
- Molotsi, A.; Dube, B.; Oosting, S.; Marandure, T.; Mapiye, C.; Cloete, S.; Dzama, K. Genetic traits of relevance to sustainability of smallholder sheep farming systems in South Africa. Sustainability 2017, 9, 1225. [Google Scholar] [CrossRef]
- Bassett, T.J.; Turner, M.D. Sudden Shift or migratory drift? FulBe herd movements to the Sudano-Guinean region of West Africa. Hum. Ecol. 2007, 35, 33–49. [Google Scholar] [CrossRef]
- FAO. Surveying and Monitoring of Animal Genetic Resources; FAO: Rome, Italy, 2011; ISBN 978-92-5-106973-8. [Google Scholar]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual framework for drought phenotyping during molecular breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef]
- Mrode, R.; Ekine Dzivenu, C.; Marshall, K.; Chagunda, M.G.G.; Muasa, B.S.; Ojango, J.; Okeyo, A.M. Phenomics and its potential impact on livestock development in low-income countries: Innovative applications of emerging related digital technology. Anim. Front. 2020, 10, 6–11. [Google Scholar] [CrossRef]
- König, S.; May, K. Invited review: Phenotyping strategies and quantitative-genetic background of resistance, tolerance and resilience associated traits in dairy cattle. Animal 2019, 13, 897–908. [Google Scholar] [CrossRef]
- Lindblom, J.; Lundström, C.; Ljung, M.; Jonsson, A. Promoting sustainable intensification in precision agriculture: Review of decision support systems development and strategies. Precis. Agric. 2017, 18, 309–331. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Bellah, F.; Chiari, T. Repeatable genotype × location interaction and its exploitation by conventional and GIS-based cultivar recommendation for durum wheat in Algeria. Eur. J. Agron. 2006, 24, 70–81. [Google Scholar] [CrossRef]
- Justice, C.; Townshend, J.; Vermote, E.; Masuoka, E.; Wolfe, R.; Saleous, N.; Roy, D.; Morisette, J. An overview of MODIS Land data processing and product status. Remote Sens. Environ. 2002, 83, 3–15. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Brazier, R.E.; Anderson, K. Ultra-fine grain landscape-scale quantification of dryland vegetation structure with drone-acquired structure-from-motion photogrammetry. Remote Sens. Environ. 2016, 183, 129–143. [Google Scholar] [CrossRef]
- Tomlinson, M.; Solomon, W.; Singh, Y.; Doherty, T.; Chopra, M.; Ijumba, P.; Tsai, A.C.; Jackson, D. The use of mobile phones as a data collection tool: A report from a household survey in South Africa. BMC Med. Inform. Decis. Mak. 2009, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Houle, D.; Govindaraju, D.R.; Omholt, S. Phenomics: The next challenge. Nat. Rev. Genet. 2010, 11, 855–866. [Google Scholar] [CrossRef]
- Resende, R.T.; Piepho, H.P.; Rosa, G.J.M.; Silva-Junior, O.B.; Silva, F.F.; de Resende, M.D.V.; Grattapaglia, D. Enviromics in breeding: Applications and perspectives on envirotypic-assisted selection. Theor. Appl. Genet. 2021, 134, 95–112. [Google Scholar] [CrossRef]
- FAO. Molecular Genetic Characterization of Animal Genetic Resources; FAO: Rome, Italy, 2011; ISBN 9789251070321. [Google Scholar]
- Ducrocq, V.; Laloe, D.; Swaminathan, M.; Rognon, X.; Tixier-Boichard, M.; Zerjal, T. Genomics for ruminants in developing countries: From principles to practice. Front. Genet. 2018, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Kruska, R.L.; Reid, R.S.; Thornton, P.K.; Henninger, N.; Kristjanson, P.M. Mapping livestock-oriented agricultural production systems for the developing world. Agric. Syst. 2003, 77, 39–63. [Google Scholar] [CrossRef]
- FAO. Breeding Strategies for Sustainable Management of Animal Genetic Resources; Food and Agriculture Organization of the United Nations: Rome, Italy, 2010; ISBN 978-92-5-106391-0. [Google Scholar]
- Haile, A.; Mirkena, T.; Duguma, G.; Wurzinger, M.; Rischkowsky, B.; Tibbo, M.; Okeyo, M.; Sölkner, J. Community based sheep breeding programs: Tapping into indigenous knowledge. Livest. Res. Rural Dev. 2013, 25, 219. [Google Scholar]
- Mueller, J.P.; Rischkowsky, B.; Haile, A.; Philipsson, J.; Mwai, O.; Besbes, B.; Valle Zárate, A.; Tibbo, M.; Mirkena, T.; Duguma, G.; et al. Community-based livestock breeding programmes: Essentials and examples. J. Anim. Breed. Genet. 2015, 132, 155–168. [Google Scholar] [CrossRef]
- Wurzinger, M.; Sölkner, J.; Iñiguez, L. Important aspects and limitations in considering community-based breeding programs for low-input smallholder livestock systems. Small Rumin. Res. 2011, 98, 170–175. [Google Scholar] [CrossRef]
- Pierre, C. Identités peules en mosaïque agropastorale au Bénin. Anthropodev. Développement 2015, 42–43, 133–159. [Google Scholar] [CrossRef]
- Floquet, A.; Nansi, J. Plus de Place en Ville Pour les Bœufs: La Filière Bovine Face à L’expansion Urbaine à Abomey et Bohicon (Bénin); Document de Travail Ecocité; Cebedes: Cotonou, Benin, 2005. [Google Scholar]
- Soule, A.H. Analyse du Système de Production de lait dans les Élevages Bovins et Fonctionnement des Mini-Laiteries Installées au Nord-Bénin. Ph.D. Thesis, Université d’Abomey-Calavi, Abomey-Calavi, Benin, 2015. [Google Scholar]
- Duteurtre, G. Les produits laitiers africains à l’épreuve de la libéralisation: Des traditions laitières en danger, un patrimoine à valoriser. In Proceedings of the Rencontres Internationales sur le Lait, Vecteur de Développement, Dakar, Sénégal, 12–13 June 2019; INRA-ISRA: Dakar, Senegal, 2019. [Google Scholar]
- Shilomboleni, H. A sustainability assessment framework for the African green revolution and food sovereignty models in southern Africa. Cogent Food Agric. 2017, 3, 1328150. [Google Scholar] [CrossRef]
- Gautier, M.; Flori, L.; Riebler, A.; Jaffrézic, F.; Laloé, D.; Gut, I.; Moazami-Goudarzi, K.; Foulley, J.L. A whole genome Bayesian scan for adaptive genetic divergence in West African cattle. BMC Genom. 2009, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Ellegren, H.; Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 2016, 17, 422–433. [Google Scholar] [CrossRef]
- Burrow, H.M. Utilization of diverse breed resources for tropical beef production. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Minas Gerais, Brazil, 13–18 August 2006; p. 32-01. [Google Scholar]
- Knap, P.W.; Doeschl-Wilson, A. Why breed disease-resilient livestock, and how? Genet. Sel. Evol. 2020, 52, 60. [Google Scholar] [CrossRef]
- Marshall, K. Optimizing the use of breed types in developing country livestock production systems: A neglected research area. J. Anim. Breed. Genet. 2014, 131, 329–340. [Google Scholar] [CrossRef]
- Hayes, B.J.; Lewin, H.A.; Goddard, M.E. The future of livestock breeding: Genomic selection for efficiency, reduced emissions intensity, and adaptation. Trends Genet. 2013, 29, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Friggens, N.C.; Blanc, F.; Berry, D.P.; Puillet, L. Review: Deciphering animal robustness. A synthesis to facilitate its use in livestock breeding and management. Animal 2017, 11, 2237–2251. [Google Scholar] [CrossRef]
- Rauw, W.M.; Gomez-Raya, L. Genotype by environment interaction and breeding for robustness in livestock. Front. Genet. 2015, 6, 310. [Google Scholar] [CrossRef]
- Calus, M.; Berry, D.P.; Banos, G.; de Haas, Y.; Veerkamp, R.F. Genomic selection: The option for new robustness traits? Adv. Anim. Biosci. 2013, 4, 618–625. [Google Scholar] [CrossRef]
- Knap, P.W. Breeding robust pigs. Aust. J. Exp. Agric. 2005, 45, 763. [Google Scholar] [CrossRef]
- Calus, M.; Groen, A.F.; de Jong, G. Genotype × environment interaction for protein yield in Dutch dairy cattle as quantified by different models. J. Dairy Sci. 2002, 85, 3115–3123. [Google Scholar] [CrossRef]
- Neser, F.W.; van Wyk, J.B.; Ducrocq, V. A preliminary investigation into genotype x environment interaction in South African Holstein cattle for reproduction and production traits. S. Afr. J. Anim. Sci. 2015, 44, 75–79. [Google Scholar] [CrossRef]
- Mrode, R.; Ojango, J.M.K.; Okeyo, A.M.; Mwacharo, J.M. Genomic selection and use of molecular tools in breeding programs for indigenous and crossbred cattle in developing countries: Current status and future prospects. Front. Genet. 2018, 9, 694. [Google Scholar] [CrossRef]
- Chaudhry, A.S. Forage based animal production systems and sustainability, an invited keynote. Rev. Bras. Zootec. 2008, 37, 78–84. [Google Scholar] [CrossRef]
- Lenné, J.M.; Thomas, D. Integrating crop—Livestock research and development in sub-Saharan Africa. Outlook Agric. 2006, 35, 167–175. [Google Scholar] [CrossRef]
- Holt-Giménez, E.; Altieri, M.A. Agroecology, food sovereignty and the New Green Revolution. J. Sustain. Agric. 2012, 120904081412003. [Google Scholar] [CrossRef]
- Lovell, S.T.; DeSantis, S.; Nathan, C.A.; Olson, M.B.; Ernesto Méndez, V.; Kominami, H.C.; Erickson, D.L.; Morris, K.S.; Morris, W.B. Integrating agroecology and landscape multifunctionality in Vermont: An evolving framework to evaluate the design of agroecosystems. Agric. Syst. 2010, 103, 327–341. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Zobeck, T.M.; Allen, V. Soil microbial, chemical and physical properties in continuous cotton and integrated crop-livestock systems. Soil Sci. Soc. Am. J. 2004, 68, 1875–1884. [Google Scholar] [CrossRef]
- Letty, B.; Alcock, R. Crop–livestock interactions: Implications for policy-makers and for farmers. Afr. J. Range Forage Sci. 2013, 30, 45–50. [Google Scholar] [CrossRef]
- Moritz, M. Pastoral intensification in West Africa: Implications for sustainability. J. R. Anthropol. Inst. 2012, 18, 418–438. [Google Scholar] [CrossRef]
- Moritz, M.; Hamilton, I.M.; Yoak, A.J.; Scholte, P.; Cronley, J.; Maddock, P.; Pi, H. Simple movement rules result in ideal free distribution of mobile pastoralists. Ecol. Modell. 2015, 305, 54–63. [Google Scholar] [CrossRef]
- Le Meur, P.Y.; Hochet, P. Property relations by other means: Conflict over dryland resources in Benin and Mali. Eur. J. Dev. Res. 2010, 22, 643–659. [Google Scholar] [CrossRef]
- Shyma, K.P.; Gupta, J.P.; Singh, V. Breeding strategies for tick resistance in tropical cattle: A sustainable approach for tick control. J. Parasit. Dis. 2015, 39, 1–6. [Google Scholar] [CrossRef]
- Diogo, R.V.C.; Dossa, L.H.; Vanvanhossou, S.F.U.; Abdoulaye, B.D.; Dosseh, K.H.; Houinato, M.; Schlecht, E.; Buerkert, A. Farmers’ and herders’ perceptions on rangeland management in two agroecological zones of Benin. Land 2021, 10, 425. [Google Scholar] [CrossRef]


| Agro-Pastoralists | Mobile Pastoralists | |
|---|---|---|
| Production | Meat quality Draught ability | Live weight Milk yield Milking easiness Milk quality (cheese making) |
| Reproduction | Calving ease Calf survival | Fertility (short calving interval) First calving at young age |
| Robustness | Ability to produce with limited feed and water (quantity and quality) Resistance to diseases (Trypanosomiasis) Longevity | Moderate exigence in feed and water Ability to walk long distances Tolerant to heat stress |
| Temperament | Docility Ease of keeping | Aggressiveness (against theft) |
| External appearance | Coat color | Large conformation |
| References | [14,33,34,38,42,44] | [14,34,35,44,52,76,117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanvanhossou, S.F.U.; Dossa, L.H.; König, S. Sustainable Management of Animal Genetic Resources to Improve Low-Input Livestock Production: Insights into Local Beninese Cattle Populations. Sustainability 2021, 13, 9874. https://doi.org/10.3390/su13179874
Vanvanhossou SFU, Dossa LH, König S. Sustainable Management of Animal Genetic Resources to Improve Low-Input Livestock Production: Insights into Local Beninese Cattle Populations. Sustainability. 2021; 13(17):9874. https://doi.org/10.3390/su13179874
Chicago/Turabian StyleVanvanhossou, Sèyi Fridaïus Ulrich, Luc Hippolyte Dossa, and Sven König. 2021. "Sustainable Management of Animal Genetic Resources to Improve Low-Input Livestock Production: Insights into Local Beninese Cattle Populations" Sustainability 13, no. 17: 9874. https://doi.org/10.3390/su13179874
APA StyleVanvanhossou, S. F. U., Dossa, L. H., & König, S. (2021). Sustainable Management of Animal Genetic Resources to Improve Low-Input Livestock Production: Insights into Local Beninese Cattle Populations. Sustainability, 13(17), 9874. https://doi.org/10.3390/su13179874






