1. Introduction
The reuse of industrial by-products, which can be addressed to new materials or other industrial sectors and thus exploited as an energetic or non-energetic secondary raw material, represents a fundamental answer to the needs for developing sustainable economic growth, based on the decrease of natural resource exploitation (primary raw materials) and the minimization of waste output [
1]. This approach converts many waste materials into energetic or non-energetic resources, valorizing the so-called industrial by-products for: (i) enhancing the sustainability of raw material exploitation and management, (ii) limiting the greenhouse gas emissions, and (iii) reducing the waste disposal in landfills.
Although aluminum is one of the most common elements on earth, it is too reactive with other elements to occur naturally. The natural raw material for primary aluminum production is, therefore, the bauxite ores, with an average composition of ca. 40–60 wt.% Al
2O
3, 0.8–17 wt.% SiO
2, up to ca. 22 wt.% Fe
2O
3, and minor amounts of TiO
2, MnO, Cr
2O
3, V
2O
5, P
2O
5, and moisture ranging between 5 and 30 wt.%. The most representative minerals of a bauxite deposit are: gibbsite—Al(OH)
3, boehmite or diaspore—AlO(OH), hematite—Fe
2O
3, goethite—α-FeO(OH), magnetite—Fe
2+Fe
3+2O
4, siderite—FeCO
3, ilmenite—FeTiO
3, rutile, anatase or brookite—TiO
2, kaolinite—Al
2Si
2O
5(OH)
4, and quartz—SiO
2 [
2]. The Bayer process, invented and patented in 1887, is the primary process by which alumina is extracted from bauxite ore and separated from red mud [
3,
4]. The Hall–Héroult process (simultaneously discovered in 1886 by Charles Martin Hall and Paul Héroult) then allows for aluminum to be refined from alumina by means of electrolysis. The efforts in recovering (and minimizing) wastes from mining exploitation of bauxite ores to the end of the Bayer process is growing, to assure that secondary Al raw materials and by-products would meet all stakeholders’ requirements [
5]. Nevertheless, the primary aluminum industry is less sustainable than secondary aluminum production, which uses recycled scraps (e.g., beverage cans and domestic appliances) instead of natural raw geomaterial (i.e., the bauxite ores for the primary aluminum industry). The process of secondary aluminum production involves scrap gathering, their mixing, comminution, screening, and pyrolysis treatments to produce high-quality Al-rich material that can be subsequently melted, refined, and casted. In this way, secondary aluminum production has increased exponentially in the last few decades to ensure sustainability is applied to optimize reserves, decrease energy consumption, and narrow the gap between supply and demand [
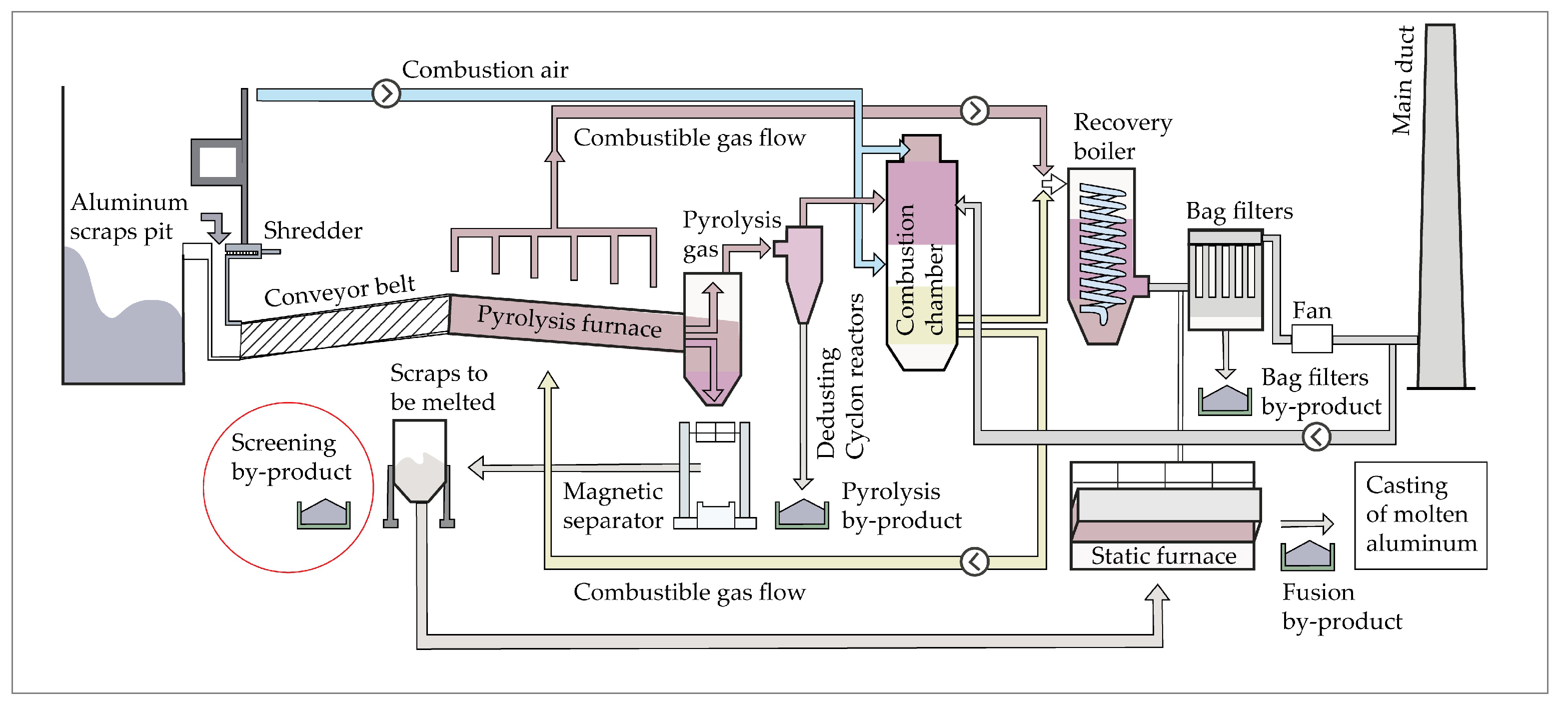
6]. Efficient plants have been developed over time, and the processes are constantly improved (
Figure 1).
The first step in aluminum recycling concerns the storage, shredding, and handling of the material through the separation of the non-metallic components from the metallic ones [
7,
8,
9] by applying a combination of various screening techniques, namely: (i) magnetic separation, (ii) Eddy current separation, and (iii) density separation [
8,
9,
10,
11,
12,
13,
14]. Aluminum scraps are loaded into hoppers by conveyor belt and processed by a pyrolysis combustor at a temperature between 500 °C and 580 °C. The pyrolysis process provides a significant (95%) reduction of water, oils, paints, and other organic substance contents from the material surface, avoiding possible explosions and oxidation during the successive melting [
15]. The powders and gases that are produced are exhausted by two parallel lines of treatment. Gas neutralization occurs at a temperature of 850 °C by employing a refractory afterburner (combustion chamber) and exhaust gas is conditioned at 580 °C through a heat exchanger. Gases are then conveyed to (i) the quencher, where the temperature is rapidly brought down (to 220 °C) by nebulized water, (ii) the fume purification system, for the abatement of residual dust through bag filters and of SOx, HCl, and HF gases by lime, and finally, (iii) the post-burner, to remove CO and Total Organic Carbon (TOC). The selected material is subsequently sent to the static melting furnace, employing the minimum possible use of energy and, therefore, the minimum oxidation. The melting process occurs using O
2 and low NOx emission burners, through refining and degassing systems and agitators with Ar/Cl
2 or N
2/Cl
2 mixtures [
16] of inert gases to minimize the energy required to reach aluminum’s melting temperature (Tm = 660.3 °C). Waste elements, specifically alkali metal impurities, hydrogen, non-metallic inclusions, and alloy elements (Mg), are reduced during the melting stage by refining processes [
17]. The compounds derived from the fusion process, such as HCl, Cl
2, and HF, are chemically neutralized, removed through a dedicated plant, and then conveyed to the bag filters for being treated in the cyclone reactor with lime. The lamination and solidification processes are improved to avoid volatile organic compound (VOC) emissions through cyclone fans with a wet scrubber for powders and biofilters. Around 70% of recycled aluminum is transformed into aluminum–silicon alloy castings used for the automotive industry. Aluminum alloys are materials where different kinds of other elements (up to 15% of the total weight) are added to the pure molten aluminum, increasing its properties and efficiency [
18].
In the framework of secondary aluminum production, the present study is of paramount importance because it is addressed to solving the problem of the management of by-products from the screening processes of scraps (
Figure 1). In fact, due to their physical-chemical features (see sub-chapter 1.1), these by-products cannot be disposed in landfills for non-hazardous waste if treatments to eliminate environmental impacts are not planned and carried out in advance. Experiments of chemical neutralization of these highly reactive by-products are the main focus of the present paper.
By-Products of the Secondary Aluminum Industry
The aluminum-rich scraps available worldwide can be approximately predicted, depending on the Life Cycle Assessment (LCA) of products containing aluminum. The recycled aluminum currently amounts to about 35% of the total primary aluminum production and requires 10 to 15 times less energy [
7,
19,
20]. The classification of aluminum scraps is dictated by the standards EN 12258 and EN 13980 [
21]: old scraps are mostly derived from used beverage cans (UBCs; containing about 94 wt.% Al, 0.8 wt.% oxides and 5.2 wt.% other inclusions) [
22,
23], representing one of the most important sources of secondary aluminum and the most economically recycled material (770–820€/ton) [
24]. The total energy saved for each ton of recycled aluminum is estimated to be around 14,000 kWh, and the avoided greenhouse gas emission of CO
2 is ca. 350 kg [
25].
By-products from the secondary aluminum industry mainly come from (i) the screening processes of the scraps in order to separate the material not suitable for the melting process, and (ii) the final (after melting) industrial by-product which is usually a salt-slag [
8]. This article is aimed at by-products coming from (i) the screening processes of the aluminum scraps represented by some metallic aluminum-rich solid material which is non-compliant to disposal in a non-hazardous waste landfill, under the EU criteria defined for their admissibility. In fact, the EU regulations classify these aluminum-rich by-products as special hazardous wastes capable of developing flammable gases and forming explosive mixtures with air (hazard class codes: HP10-HP11-HP12-HP13; European Waste Code, EWC: 100323*, where EWC marked with an asterisk "*" are hazardous waste under the Directive 91/689/EEC). These EWC 100323* by-products are hazardous because they often contain relatively high amounts of metallic aluminum (and other metal species) and are therefore a potential source for hydrogen release. This is the reason that contact with air or water might develop flammable gases. As already pointed out by the literature [
26], the chemical process is explained by the reaction (1):
The sodium hydroxide, used as a reaction catalyst, is also generated from sodium aluminate, as reported in the reaction (2):
The reactions responsible for the material oxidation (3) occur at 575 °C and can be expressed by combining aluminum hydroxide molecules that are produced during the previous reaction:
The aluminum oxide continuous hydration generates boehmite, which becomes bayerite, again interacting with water. However, the reactions suffer from chemical-physical limitations that prevent a precise quantification of the amount of hydrogen produced by the reaction of metal with the aqueous alkaline solution, compared to the water-splitting contribution in extreme thermodynamic and kinetic contexts.
Other reactions (below) tend to evolve at the operating temperatures of the metallurgical process. Aluminum sulfide can produce hydrogen sulfide (4); aluminum carbide may generate methane (5); whereas aluminum phosphide can produce phosphine (6):
In this work, experiments were planned to achieve the complete neutralization of the dangerous metallic aluminum-rich by-products, which are highly water-reactive and release significant amounts of gases, including H
2, CH
4, and possibly, minor amounts of CO from the organic components that are still present. As they are not suitable for non-hazardous waste landfill disposal, the neutralization process of these by-products from the secondary aluminum industry is mandatory through the appropriate treatments for safe storage and/or for utilization as a secondary raw material. The experiments to obtain chemical neutralization were performed through the mixing of the aluminum-rich by products with an aqueous alkaline solution in a stainless-steel compact mini-reactor equipped with gas inlet and outlet valves, a liquid sampling valve, and an internal thermocouple (
Figure 2). All the experimental runs were carried out at the Istituto di Geoscienze e Georisorse (IGG) of the National Research Council (CNR) and the Dipartimento di Scienze della Terra of the University of Florence.
2. Materials and Methods
The by-products that are investigated in the present work come from the screening processes of the aluminum scraps of an anonymous secondary aluminum industry specializing in recycling beverage cans and domestic appliances for the production of aluminum profiles, pipes, and laminates for different sectors, such as construction, automotive, electronics, and mechanics [
7,
8]. A mixture of approximately 50% domestic appliances (V.FG) and 50% beverage cans from urban waste collection materials (V.UBC) was chosen (
Table 1) as the starting material for the experiments because of its relatively high amount of metallic Al content.
To identify the main mineralogical phases of the investigated by-products before and after the chemical neutralization (i.e., oxidation), powder X-ray diffraction analyses were carried out by a Bruker D8 Advance diffractometer at CRI.ST (centro di servizi di CRIstallografia STrutturale, University of Florence). The grain size of the starting materials was determined through laser beam particle analyses (Hydro 2000MU analyzer, at the University of Milano Bicocca). Mass variation due to thermal treatment and changes in relation to thermodynamic conditions were obtained through thermogravimetric analyses with a Netzsch-Gerätebau GmbH-STA 409 CD simultaneous thermal analyzer (MISE—Direzione generale per le risorse minerarie ed energetiche, Rome, Italy).
Chemical analyses (major, minor, and trace elements) of the mixture of the two kinds of by-products (50% V.FG and 50% V.UBC;
Table 1) were performed by: (i) ICP-OES-MS with near-total multi-acid (hydrofluoric, nitric, and perchloric acids) digestion. After the digestion and dehydration, samples were brought into the solution using aqua regia, solubilizing only certain phases, and analyzed using a Varian ICP and Perkin Elmer Sciex ELAN ICP-MS at the Activation Laboratories Ltd.—Actlabs (Ancaster, ON, Canada), with ten sample duplicates and eight reference materials. Loss on Ignition (LOI) was also determined at Actlabs. (ii) IR using an ELTRA instrument to quantify total (graphitic and organic) carbon, CO
2, total sulfur (S), and SO
4 (Actlabs). To determine CO
2 content, 0.2 g samples were decomposed in a pure nitrogen environment furnace at 1000 °C. H
2O and other gases were removed before the detection of carbon dioxide in the IR cell. For total C, S, and SO
4, the inductive elements of the samples and an accelerator material were coupled with the high-frequency field of induction of a pure oxygen environment furnace. During combustion, carbon and sulfur elements were reduced, forming CO, CO
2, and SO
2. Carbon and sulfur were measured as gases flowed through the IR cells.
Other organic compounds were analyzed at the Laboratorio Arca SRL (Fano, Italy) according to UNI EN 14039:2005, EPA 3580A 1992, and EPA 8015C 2007 standard methods.
The Experimental Procedure
In order to quantify the volume of gases produced by metal oxidation within a specific time frame and to analyze its composition, experimental runs were performed in a mini-reactor by reacting solid metallic aluminum-rich by products (50% domestic appliances V.FG and 50% beverage cans from urban waste collection materials V.UBC;
Table 1) with an aqueous alkaline solution. Among the experimental parameters, a pH of 11 for the alkaline solution and a solid–liquid mass ratio of 1.27 were maintained as a constant during the four different experiments (Parr 174, 202, 204, 212), whereas grain size (<63 μm or 125–250 μm), liquid volume (4 or 8 mL), temperature (40 or 70 °C), and durations (24–25 or 94 h) of the experimental runs were varied in order to investigate the influence of these physical parameters on the gas production reactions. The solid samples (mass 10.17–10.18 or 5.10 g; density 2.78 g/mL) and the aqueous alkaline solution were continuously mixed inside the vessel using a magnetic stirrer set to 400–600 rounds per minute (
Table 2). During the runs, the temperature was kept constant until the material residues and gases were sampled and analyzed. In particular, the variation of pressure due to gas production was a direct and important parameter taken into consideration.
The mini-reactor assemblage (series 5500 HP T316, Parr Instrument Company;
Figure 2) consists of a vessel (autoclave) of ca. 25 mL of volume (ca. 5 cm
2 section area and ca. 4.5 cm height) equipped with a compact and variable-speed stirrer drive, capable of assuring the adequate mixing of the fluid–solid slurry at viscosities up to 10,000 PI (Pa·s). The maximum working pressure of this reactor reaches 200 bars while the actual maximum operating temperature (ranging from 225 up to 350 °C) depends on the type of gasket seal selected. The instrument is equipped with gas inlet and outlet valves, a liquid sampling valve, a pressure gauge, a safety rupture disc, and an internal thermocouple. The vessel support also acts as a heater for the reactor. It is an aluminum block that provides an excellent thermal uniformity (a stainless-steel heat shield is mounted for safety around the whole heating block). The gas that came from the reaction was analyzed for N
2, Ar, O
2, CO, and H
2 contents by using a Shimadzu 15A and a Thermo Focus gas chromatograph equipped with Thermal Conductivity Detectors (TCD), whereas CH
4 was analyzed by using a Shimadzu 14A gas chromatograph equipped with a Flame Ionization Detector (FID). Gas sampling was obtained through a needle which was inserted into the gas-out valve. When sampling the gas, the out valve was slowly opened and, after 2 s, the needle was injected into an Exetainer
® vial sealed with a porous membrane. The vial was initially filled with de-aired (by N
2 bubbling for at least 4 h) Milli-Q
® water and, finally, the gas species were analyzed through the gas chromatographic method.
Before starting the runs, (except for the Parr 174 experiment) the aqueous alkaline solutions were degassed through nitrogen “bubbling” for 24 h, whereas the free volume of air inside the vessel of the mini-reactor was displaced by flushing N2 for 3 min. This gas-displacement or “degassing” procedure was applied to reduce to a minimum any oxygen contamination from the air in order to constrain the gas production processes. However, effective reactions are theoretical, given the extreme experimental settings: relatively low T, high dynamic pressure, and significantly reduced redox conditions.
The ideal gas law was adopted as a theoretical model to quantify the moles of gases generated during the reaction. The free volume inside the experimental setup was estimated by subtracting the reactant’s volume from the vessel’s known total capacity. In this way, it was possible to relate each gas species’ partial pressure to the mass amount of gas produced. The reference model (7) developed to quantify gas moles produced inside the vessel, on the basis of the pressure that is generated, can be expressed by the following expressions:
where:
—moles of each gas produced (mol);
—total pressure inside the vessel (atm);
—total volume of the vessel corresponding to 0.025 (L);
—volume of the aqueous solution (L);
—mass of the starting material (g);
—density of the starting material (g/L);
—gas percentage quantified by GC analyses (%); R—gas constant (L atm/K mol) = 0.082057; T—temperature (K).
Concerning the way the gases fill the volume inside the vessel, as the gas generation process during the chemical neutralization reaction was progressively optimized, the saturation point was reached more rapidly. The pressure–time curve depends on the metal oxidation rate that reaches its maximum when at least one reagent was completely consumed.
4. Discussion
The current experimental tests were developed to investigate the efficiency of the oxidation of the solid material during reactions to recover hazardous aluminum-rich by-products and to therefore obtain laboratory data for the possible industrialization of a method that is capable of improving sustainability and workplace safety in secondary aluminum plants. The chemical neutralization involves the complete oxidation of metals, as it was already known that the interaction of metallic aluminum with an alkaline aqueous solution gives rise to the release of flammable and toxic gases [
27]. In the experimental tests performed with the mini-reactor, we exactly quantified gas production at a constant solid–aqueous alkaline (pH 11) solution mass ratio of 1.27. This also allowed for a comparison of the different procedural setups of the runs.
The generated mixture of gas during the experiments at the mini-reactor was primarily identified as hydrogen and minor components that are represented by methane and carbon monoxide, with the temperature, pH, and time of the experiments being clearly the main relevant driving factors of the neutralization reaction of the metallic aluminum-rich industrial by-products [
28]. Experimental results show that a quite efficient reaction leads to the highest gas production at 70 °C, with a coarse grain size starting material (125–250 µm; Parr 212 run), using high stirring speed, and a run duration of 94 h. Nevertheless, according to the XRD analysis, the complete chemical neutralization is reached in the experiment Parr 204 with a grain size of < 63 µm, thus emphasizing the strong influence of particle size on the reaction process.
In the present discussion, we will also address the relation between the Loss on Ignition (LOI) of the aluminum-rich by-products sample and that of the residual materials after the chemical neutralization in the mini-reactor. The LOI value is obtained as mass variation of the sample after heating at 1000 °C for 2 h. This involves the loss of volatile components (H
2O, hydrates, CO, and CO
2 groups). Therefore, the mass increase represented by negative LOI values is a strong indication of the presence of metallic content that can be evaluated by separating the contribution of different components, such as (i) hygroscopic water (H
2O
–, at 105 °C), (ii) the contribution of water to the crystalline structure of the mineral phases (H
2O
+, at 700–950 °C), (iii) the decomposition of organic matter (at 400 °C), and (iv) the decarbonization of the carbonates (at 350°C for magnesium carbonates and at 825 °C for calcium carbonates). Indeed, it can be inferred that the most significant increase of the mass by LOI is due to the oxidation process of the metallic aluminum. At a temperature higher than its melting point (660.3 °C), Al becomes more reactive with O
2 as a phase state change occurs. LOI values of (i) the by-products sample of the screening processes and (ii) the experimental residues of the four experimental runs have been compared in
Figure 9.
The starting material (50% V.FG + 50% V.UBC), having an aluminum content of 14 wt.% and an Al phase detected by XRD (
Figure 4), shows a negative Loss on Ignition of −31.05 wt.%. By contrast, the residual products from the experimental runs (Parr 174, 202, 204, 212) have LOIs of −6.4 wt.%, −10.4 wt.%, 7.0 wt.%, and 0.04 wt.%, respectively. It is worth noting that the experiments Parr 204 and 212, which apparently produced the highest amount of gas, do not show negative LOIs. The highest positive LOI value (7.0 wt.%) of the Parr 204 residue also matches with the absence of metallic aluminum, as proven by XRD phase composition (
Figure 8). Despite the loss of volatiles, mainly H
2O and CO
2, the starting by-products sample (V.FG + V.UBC) increases its mass due to oxidation (very high negative LOI;
Figure 9), whereas when the more chemical neutralization during the experiments occurs, the more the final by-products’ residues show positive LOI values. LOIs ≥ zero therefore seem to represent good indicators of by-products which have virtually become non-reactive due to the absence of metallic aluminum.
The present experimental research emphasizes the feasibility of hydrogen production (that could be potentially stored and/or reused as fuel) from the secondary aluminum by-products (waste) coming from the screening processes of scraps, mainly beverage cans and domestic appliances. The hydrogen recovery could really represent an important energetic (fuel) integration for the melting furnaces in the same secondary aluminum plants. Alternatively, the gaseous mixture production from the metallic aluminum by-products could be mixed with a silica component (e.g., from the glass industry) allowing for the production of foamed geopolymers, with pockets of hydrogen trapped in pores throughout the body of a material with high strength and hardness, which is suitable not only as an insulation material but also for industrial applications. It is worth noting that a fundamental physical characteristic of geopolymers is their resistance to mechanical, thermal, and atmospheric degradation, and they are also suitable for entrapping polluting and toxic substances [
29,
30,
31].
The creation and implementation of registries of Al-rich wastes and by-products [
5], coupled with platforms such as the Bureau of International Recycling [
32], will strongly help the possibility of acting as matchmaking between (i) producers/holders and (ii) potential industries seeking secondary Al raw materials to enhance the sustainability and performance of their products (e.g., as filler or for cement binders). If the present laboratory procedure of chemical neutralization of the metallic aluminum-rich by-products will be transferred to an industrialized process, the recovered residual material could certainly be recorded in such registries and therefore the most appropriate use and synergies between different industrial sectors and chains could be easily found.
5. Conclusions
The investigated industrial by-products that come from the pre-treatment screening processes of beverage cans and domestic appliances recycled in the industry of secondary aluminum production are generally classified by EU law as solid wastes containing dangerous substances and that can potentially develop flammable gases and explosive mixtures. This hazard mainly comes from the metallic aluminum that is still present in these by-products, which are therefore highly reactive in water-rich environments, which consequently facilitate the production of hydrogen-rich gas.
The experiments in the mini-reactor between by-products and aqueous alkaline solutions at pH 11 determined the composition of the hydrogen-rich gas (up to 96% of the total gases) and the oxidation rate, leading to the elimination of the highly reactive (and dangerous) metallic aluminum. Although only four experimental runs were carried out (at constant solid-aqueous alkaline solution mass ratio of 1.27), the results clearly emphasize the efficiency of the method in transforming the by-products into non-reactive material. Further laboratory investigations should be, however, addressed to the best fit of the various physical conditions (solid grain size, pH, temperature, stirring speed, and time) which were only partially controlled in the present paper.
In the framework of a circular economy and sustainability, we believe the metallic aluminum-rich by-products could potentially become, if chemically neutralized, a new secondary raw material resource addressed to the energetic (i.e., hydrogen as fuel) or non-energetic (e.g., “foamed geopolymers”) use. The secondary aluminum industry must increase workplace safety by reducing the risks of the storage of the metallic aluminum-rich hazardous by-products. In this way, it will be of paramount importance that the knowledge acquired from the chemical process of neutralization performed in the mini-reactor could be deepened further and, finally, transferred from the laboratory to the prototyping and industrialization phases.