Efficiency Enhancement of an Ammonia-Based Solar Thermochemical Energy Storage System Implemented with Hydrogen Permeation Membrane
Abstract
1. Introduction
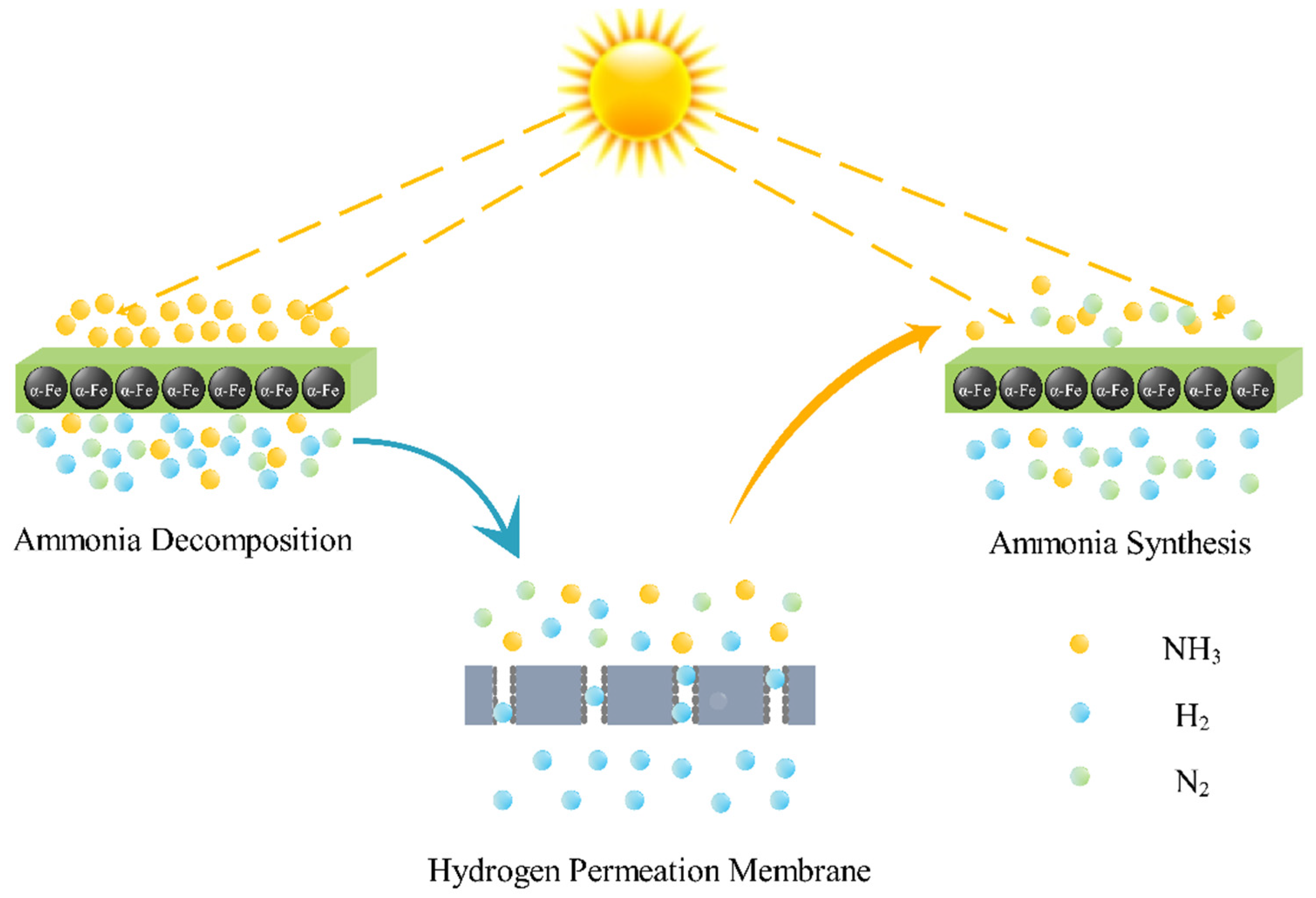
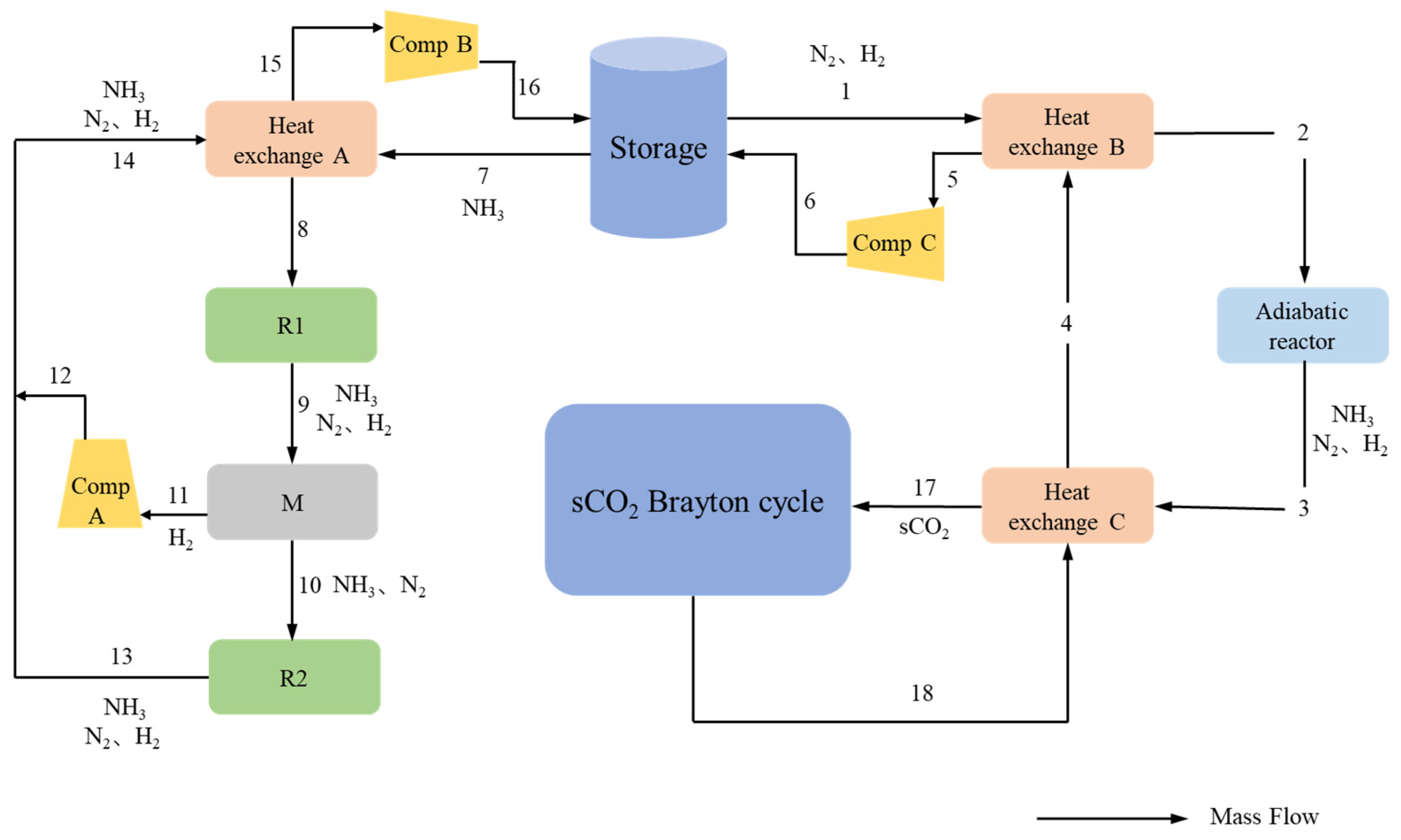
2. Modeling
- The endothermic reactor is assumed to be a blackbody cavity-receiver with the effective absorptivity and emissivity to be 1;
- The charging time is 8 h, considering a daytime of 8 h (clear sky) [38].
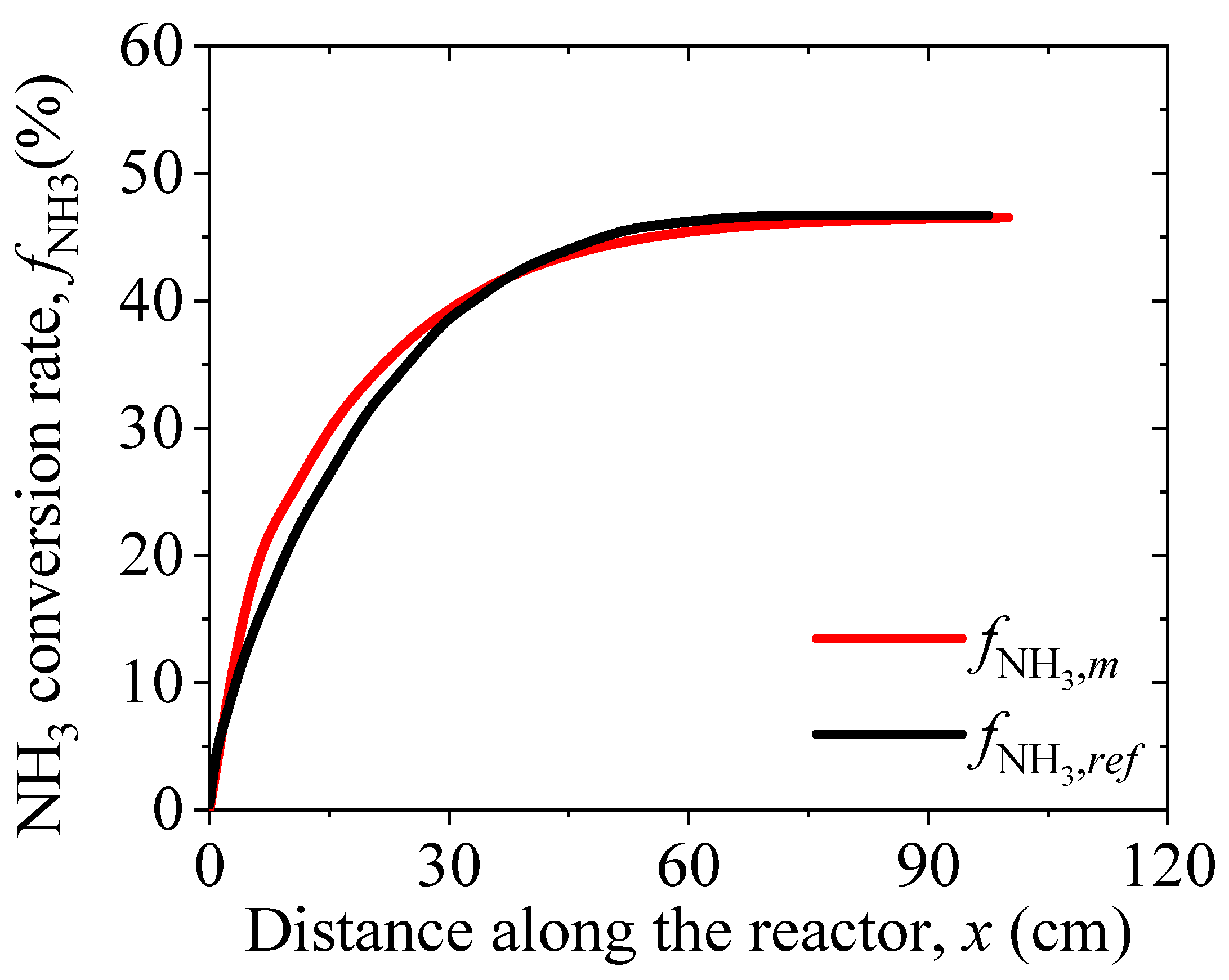
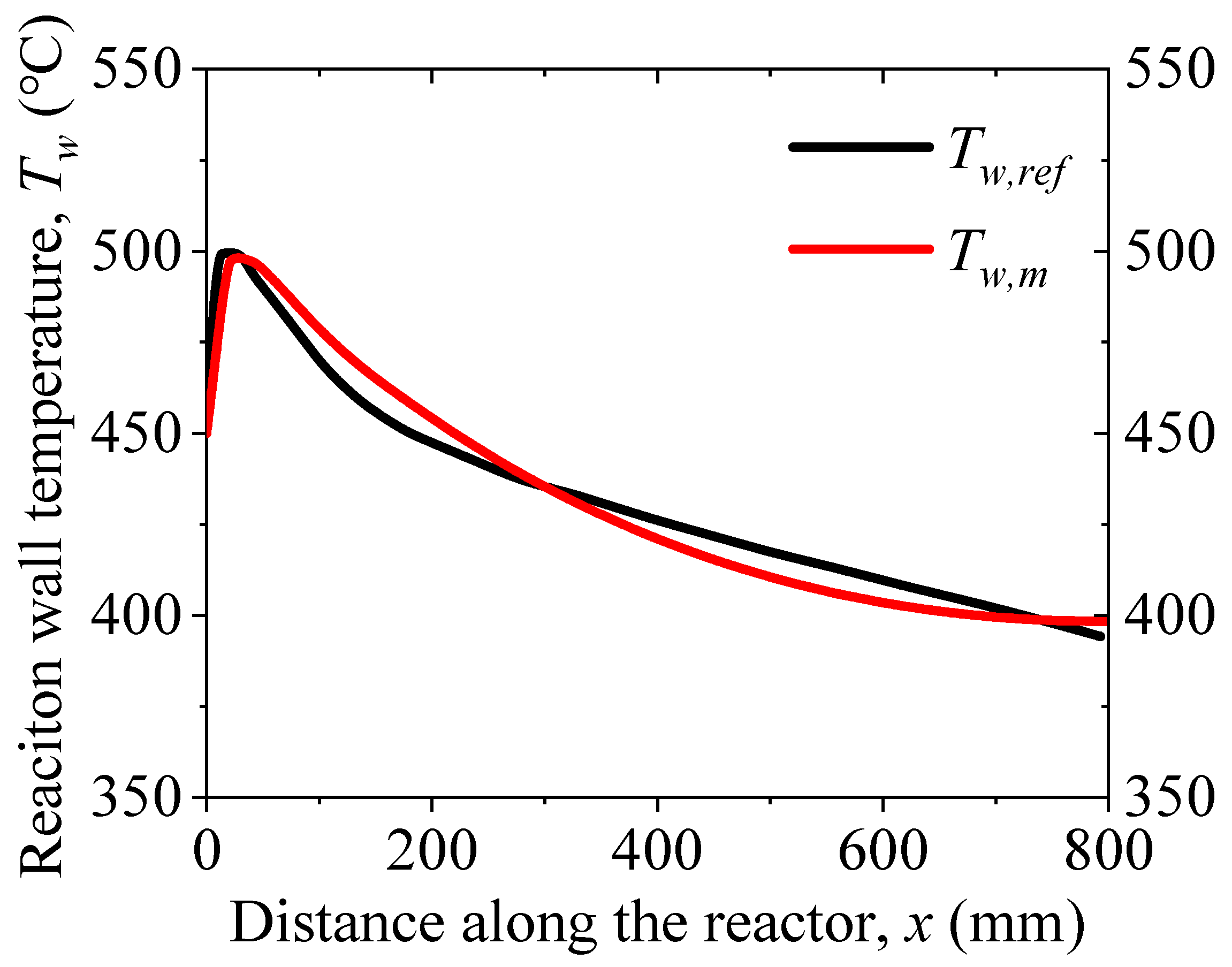
3. Model Validation
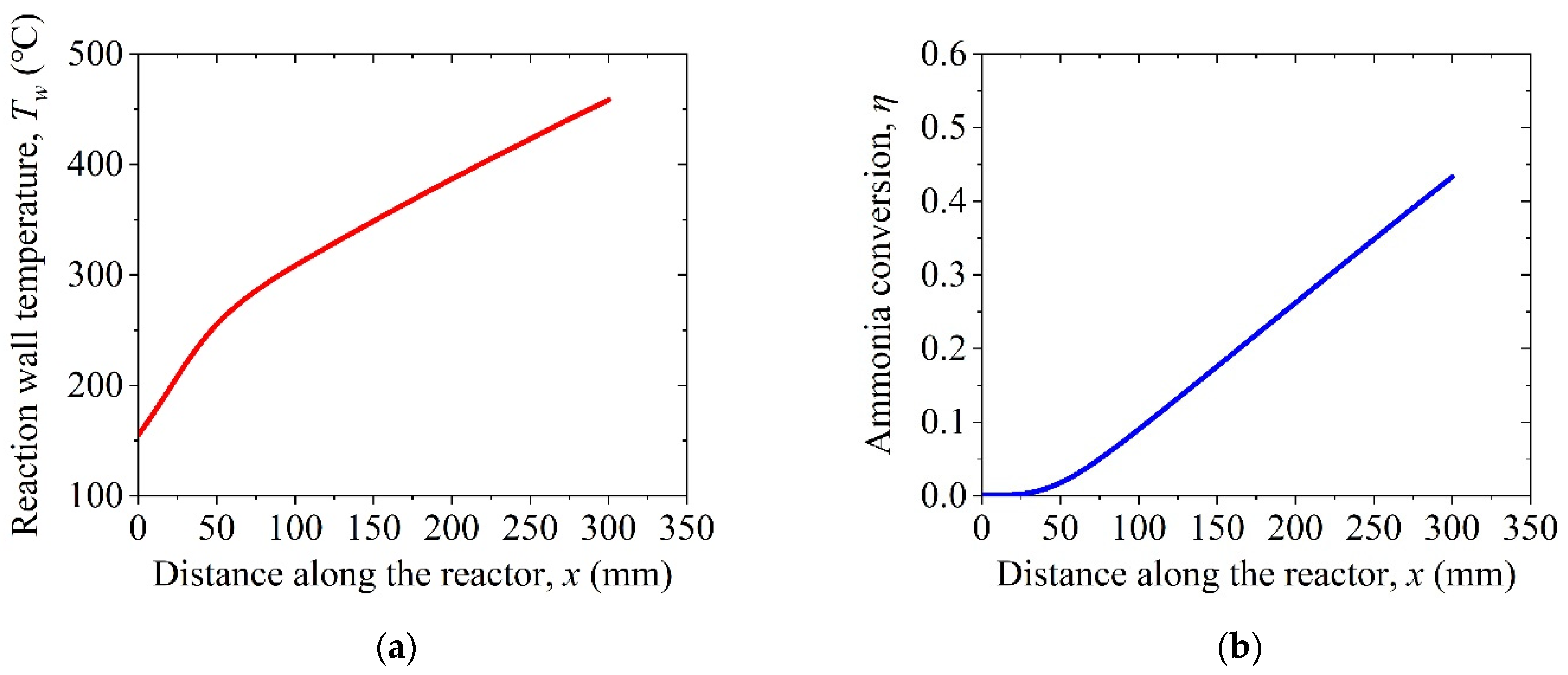
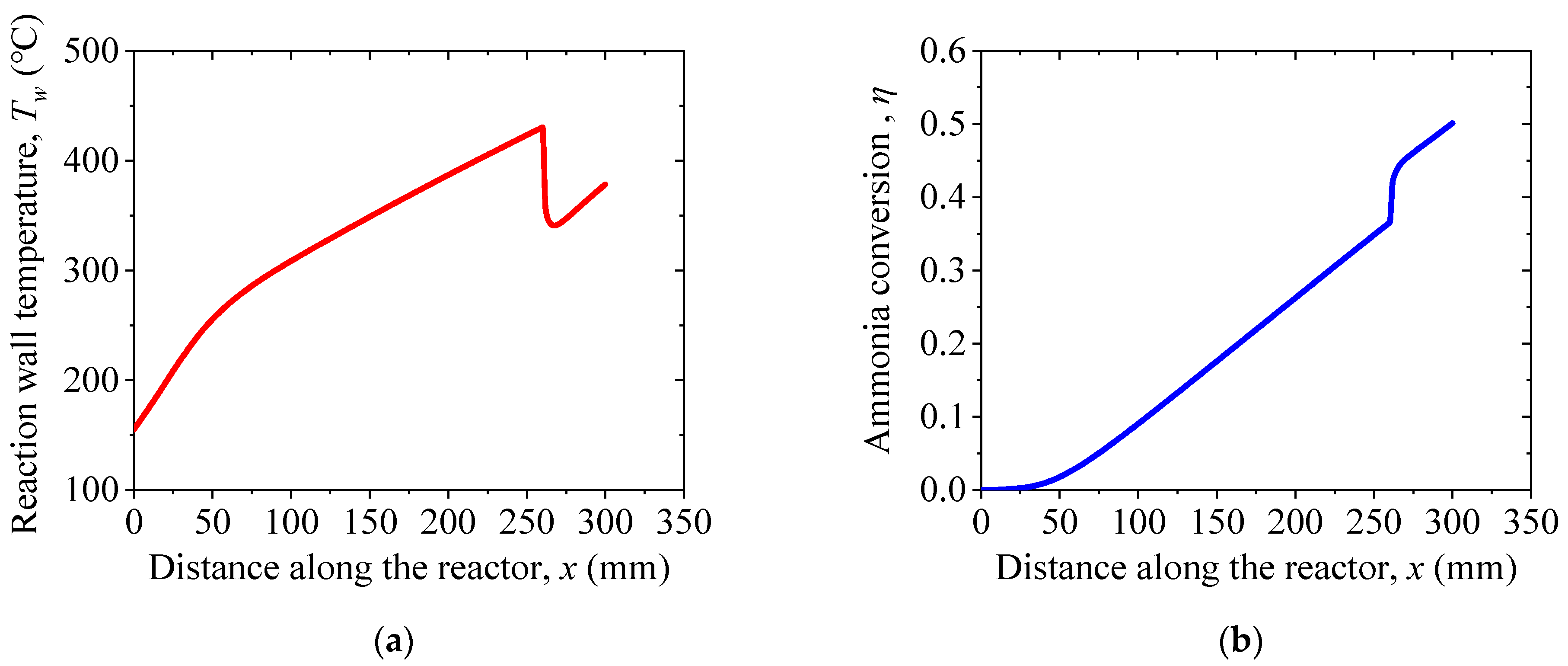
4. Results and Discussion
4.1. Thermodynamic Analysis of the Conventional System
4.2. Thermodynamic Analysis of the Proposed System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, X.; Root, T.W.; Maravelias, C.T. Storing solar energy with chemistry: The role of thermochemical storage in concentrating solar power. Green Chem. 2017, 19, 2427–2438. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Stehfest, E.; Gernaat, D.E.H.J.; Berg, M.V.D.; Bijl, D.L.; De Boer, H.S.; Daioglou, V.; Doelman, J.C.; Edelenbosch, O.Y.; Harmsen, M.; et al. Alternative pathways to the 1.5 °C target reduce the need for negative emission technologies. Nat. Clim. Chang. 2018, 8, 391–397. [Google Scholar] [CrossRef]
- Alamoudi, A.; Saaduddin, S.M.; Munir, A.B.; Muhammad-Sukki, F.; Abu-Bakar, S.H.; Yasin, S.H.M.; Karim, R.; Bani, N.A.; Mas’Ud, A.A.; Ardila-Rey, J.A.; et al. Using Static Concentrator Technology to Achieve Global Energy Goal. Sustainability 2019, 11, 3056. [Google Scholar] [CrossRef]
- Shao, J.; Chen, H.; Zhu, T. Solar Energy Block-Based Residential Construction for Rural Areas in the West of China. Sustainability 2016, 8, 362. [Google Scholar] [CrossRef]
- Bouaddi, S.; Fernández-García, A.; Sansom, C.; Sarasua, J.A.; Wolfertstetter, F.; Bouzekri, H.; Sutter, F.; Azpitarte, I. A Review of Conventional and Innovative- Sustainable Methods for Cleaning Reflectors in Concentrating Solar Power Plants. Sustainability 2018, 10, 3937. [Google Scholar] [CrossRef]
- Carrillo, A.J.; González-Aguilar, J.; Romero, M.; Coronado, J.M. Solar Energy on Demand: A Review on High Temperature Thermochemical Heat Storage Systems and Materials. Chem. Rev. 2019, 119, 4777–4816. [Google Scholar] [CrossRef]
- Zeng, Q.; Lai, Y.; Jiang, L.; Liu, F.; Hao, X.; Wang, L.; Green, M.A. Integrated Photorechargeable Energy Storage System: Next-Generation Power Source Driving the Future. Adv. Energy Mater. 2020, 10, 1903930. [Google Scholar] [CrossRef]
- Miguel, G.S.; Corona, B. Economic viability of concentrated solar power under different regulatory frameworks in Spain. Renew. Sustain. Energy Rev. 2018, 91, 205–218. [Google Scholar] [CrossRef]
- Fernández, A.G.; Gomez-Vidal, J.; Oró, E.; Kruizenga, A.; Solé, A.; Cabeza, L.F. Mainstreaming commercial CSP systems: A technology review. Renew. Energy 2019, 140, 152–176. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Prasad, J.S.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- Sarbu, I.; Sebarchievici, C. A Comprehensive Review of Thermal Energy Storage. Sustainability 2018, 10, 191. [Google Scholar] [CrossRef]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the art on the high-temperature thermochemical energy storage systems. Energy Convers. Manag. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Humphries, T.D.; Møller, K.T.; Rickard, W.D.A.; Sofianos, M.V.; Liu, S.; Buckley, C.E.; Paskevicius, M. Dolomite: A low cost thermochemical energy storage material. J. Mater. Chem. A 2019, 7, 1206–1215. [Google Scholar] [CrossRef]
- Harries, D.N.; Paskevicius, M.; Sheppard, D.A.; Price, T.E.C.; Buckley, C. Concentrating Solar Thermal Heat Storage Using Metal Hydrides. Proc. IEEE 2012, 100, 539–549. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.; Williamson, K.; Buckley, C. Metal hydride thermal heat storage prototype for concentrating solar thermal power. Energy 2015, 88, 469–477. [Google Scholar] [CrossRef]
- Chaise, A.; De Rango, P.; Marty, P.; Fruchart, D.; Miraglia, S.; Olives, R.; Garrier, S. Enhancement of hydrogen sorption in magnesium hydride using expanded natural graphite. Int. J. Hydrogen Energy 2009, 34, 8589–8596. [Google Scholar] [CrossRef]
- Rönnebro, E.C.E.; Whyatt, G.; Powell, M.R.; Westman, M.P.; Zheng, F.; Fang, Z.Z. Metal Hydrides for High-Temperature Power Generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef]
- Mejia, A.C.; Afflerbach, S.; Linder, M.; Schmidt, M. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor. Appl. Therm. Eng. 2020, 169, 114961. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Lanza, M.; Milone, C. Strategies for the enhancement of heat storage materials performances for MgO/H2O/Mg(OH)2 thermochemical storage system. Appl. Therm. Eng. 2017, 120, 626–634. [Google Scholar] [CrossRef]
- Li, Y.-T.; Li, M.-T.; Xu, Z.-B.; Meng, Z.-H.; Wu, Q.-P. Dehydration kinetics and thermodynamics of ZrO(NO3)2-doped Ca(OH)2 for chemical heat storage. Chem. Eng. J. 2020, 399, 125841. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO redox cycle for solar thermochemical energy storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef]
- Zhou, X.; Mahmood, M.; Chen, J.; Yang, T.; Xiao, G.; Ferrari, M.L. Validated model of thermochemical energy storage based on cobalt oxides. Appl. Therm. Eng. 2019, 159, 113965. [Google Scholar] [CrossRef]
- Albrecht, K.J.; Jackson, G.; Braun, R. Thermodynamically consistent modeling of redox-stable perovskite oxides for thermochemical energy conversion and storage. Appl. Energy 2016, 165, 285–296. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Hosseini, H. A review of material screening in pure and mixed-metal oxide thermochemical energy storage (TCES) systems for concentrated solar power (CSP) applications. Renew. Sustain. Energy Rev. 2018, 98, 9–26. [Google Scholar] [CrossRef]
- Yuan, Q.; Ding, J.; Lu, J.; Yu, T.; Wang, W. Heat Transfer and Energy Storage Performance of Methane Reforming with Carbon Dioxide in Semi-cavity Reactor. Procedia Eng. 2016, 157, 365–373. [Google Scholar] [CrossRef][Green Version]
- Fuqiang, W.; Jianyu, T.; Huijian, J.; Yu, L. Thermochemical performance analysis of solar driven CO2 methane reforming. Energy 2015, 91, 645–654. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Y.; Aryafar, H.; Wen, T.; Lavine, A.S. Performance of conical ammonia dissociation reactors for solar thermochemical energy storage. Appl. Energy 2019, 255, 113785. [Google Scholar] [CrossRef]
- Lovegrove, K.; Luzzi, A.; Soldiani, I.; Kreetz, H. Developing ammonia based thermochemical energy storage for dish power plants. Sol. Energy 2004, 76, 331–337. [Google Scholar] [CrossRef]
- Chen, C.; Aryafar, H.; Warrier, G.; Lovegrove, K.M.; LaVine, A.S. Ammonia synthesis for producing supercritical steam in the context of solar thermochemical energy storage. AIP Conf. Proc. 2016, 1734, 050010. [Google Scholar]
- Chen, C.; Lovegrove, K.M.; Sepulveda, A.; Lavine, A.S. Design and optimization of an ammonia synthesis system for ammonia-based solar thermochemical energy storage. Sol. Energy 2018, 159, 992–1002. [Google Scholar] [CrossRef]
- Luzzi, A.; Lovegrove, K.; Filippi, E.; Fricker, H.; Schmitz-Goeb, M.; Chandapillai, M.; Kaneff, S. Techno-Economic Analysis of a 10 MWe Solar Thermal Power Plant Using Ammonia-Based Thermochemical Energy Storage. Sol. Energy 1999, 66, 91–101. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, J.; Wang, W. High Temperature Energy Storage Performances of Methane Reforming with Carbon Dioxide in Tubular Packed Reactor. Energy Procedia 2014, 61, 407–410. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F.; Paturzo, L. Hydrogen production from methanol by oxidative steam reforming carried out in a membrane reactor. Catal. Today 2005, 104, 251–259. [Google Scholar] [CrossRef]
- Ardaneh, M.; Abolhasani, M.; Esmaeili, M. CFD modeling of two-stage H2 recovery process from ammonia purge stream by industrial hollow fiber membrane modules. Int. J. Hydrogen Energy 2019, 44, 4851–4867. [Google Scholar] [CrossRef]
- Chacartegui, R.; Alovisio, A.; Ortiz, C.; Valverde, J.M.; Verda, V.; Becerra, J. Thermochemical energy storage of concentrated solar power by integration of the calcium looping process and a CO2 power cycle. Appl. Energy 2016, 173, 589–605. [Google Scholar] [CrossRef]
- Chen, C.; Xia, Q.; Feng, S.; Liu, Q. A novel solar hydrogen production system integrating high temperature electrolysis with ammonia based thermochemical energy storage. Energy Convers. Manag. 2021, 237, 114143. [Google Scholar] [CrossRef]
- Wang, B.; Kong, H.; Wang, H.; Wang, Y.; Hu, X. Kinetic and thermodynamic analyses of mid/low-temperature ammonia decomposition in solar-driven hydrogen permeation membrane reactor. Int. J. Hydrogen Energy 2019, 44, 26874–26887. [Google Scholar] [CrossRef]
- Kreetz, H.; Lovegrove, K. Exergy analysis of an ammonia synthesis reactor in a solar thermochemical power system. Sol. Energy 2002, 73, 187–194. [Google Scholar] [CrossRef]
- Jones, J. Concentrating solar power, CSP lifts off 2007. Renew Energy World 2007, 10, 36–38. [Google Scholar]

| Activation Energy, Ea J/mol | Pre-Exponential Constant, ko,m kmol/m3∙s |
|---|---|
| 4.57 × 105 | 2.645 × 1011 |
| Parameters | Decomposition Reactor | Synthesis Reactor |
|---|---|---|
| Reactor length, mm | 110 | 800 |
| Reactor outer diameter, cm | 1 | - |
| Reactor inner diameter, cm | 0.95 | 0.15 |
| Mass flow rate, g/s | 0.0075 | 0.10 |
| Reactor wall temperature, °C | 150 | - |
| Reactor inlet temperature, °C | - | 450 |
| Ammonia Mass Flow Rate, mc (g/s) | Catalyst Bed Length, L (mm) | Catalyst Bed Diameter, D (mm) |
|---|---|---|
| 1.8 | 300 | 30 |
| Items | Exergy Loss, E (kW) |
|---|---|
| Charging loop | |
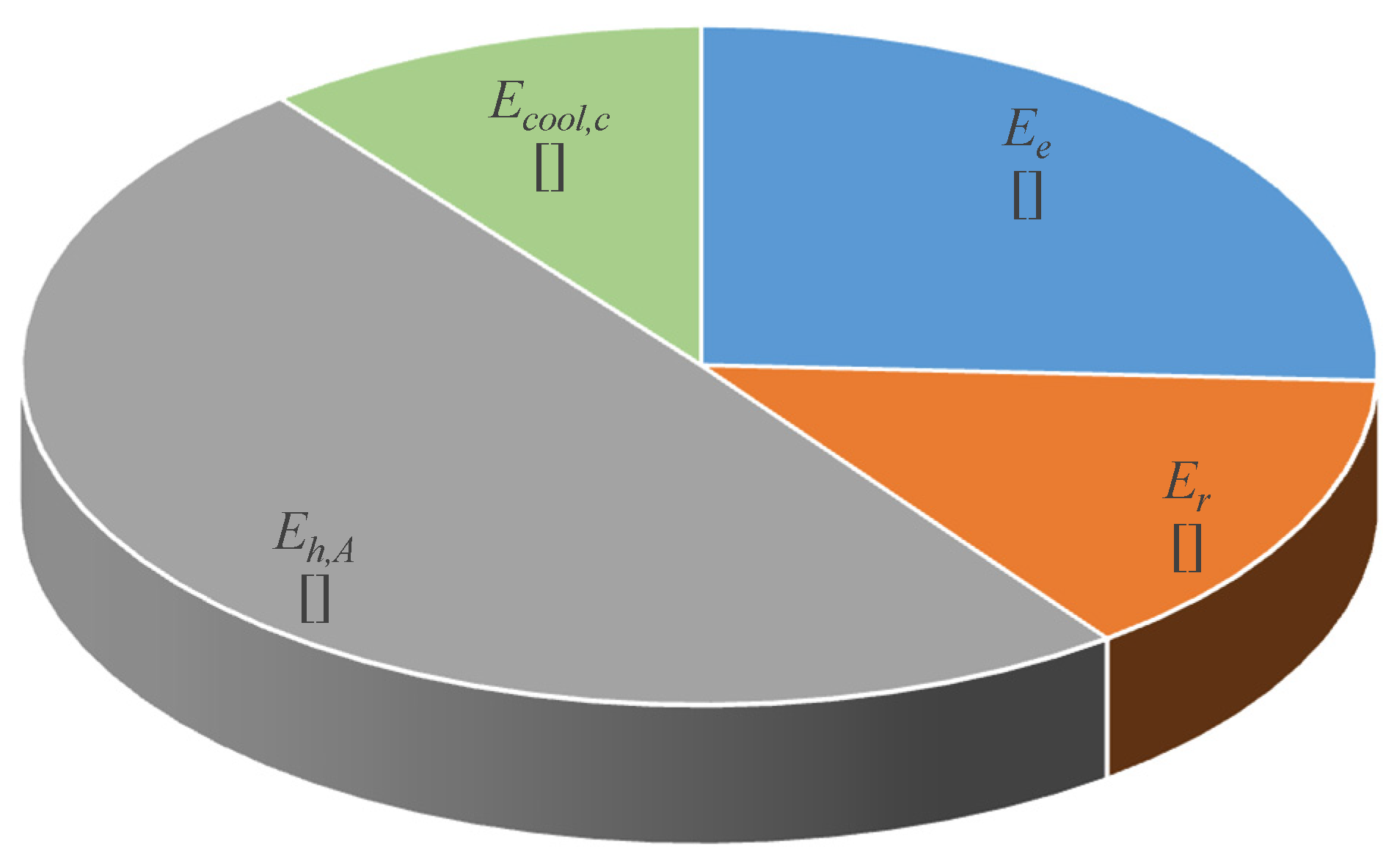
| Endothermic reaction exergy loss, Ee | 0.2587 |
| Re-radiation exergy loss, Er | 0.1419 |
| Heat exchanger A exergy loss, Eh,A | 0.4980 |
| Compressor B exergy loss, Ecomp,B | <1% |
| Charging loop cooling exergy loss, Ecool,c | 0.1068 |
| Total of the Charging loop | 1.0055 |
| Discharging loop | |
| Adiabatic reaction exergy loss, Ea | 0.1612 |
| Heat exchanger B exergy loss, Eh,B | 0.1403 |
| Heat exchanger C exergy loss, Eh,C | 0.0519 |
| Compressor C exergy loss, Ecomp,C | <1% |
| Discharging loop cooling exergy loss, Ecool,d | 0.0002 |
| Total of the discharging loop | 0.3537 |
| Overall system exergy loss | 1.3592 |
| Wnet | 0.8751 |
| ηste | 19.3% |
| Items | Exergy Loss, E (kW) |
|---|---|
| Charging loop | |
| Endothermic reaction exergy loss, Ee | 0.2801 |
| Re-radiation exergy loss, Er | 0.1172 |
| Heat exchanger A exergy loss, Eh,A | 0.3290 |
| Compressor A exergy loss, Ecomp,A | <1% |
| Pressure exergy loss, Epre | 0.0080 |
| Compressor B exergy loss, Ecomp,B | <1% |
| Charging loop cooling exergy loss, Ecool,c | 0.0376 |
| Total of the Charging loop | 0.7727 |
| Discharging loop | |
| Adiabatic reaction exergy loss, Ea | 0.2017 |
| Heat exchanger B exergy loss, Eh,B | 0.1755 |
| Heat exchanger C exergy loss, Eh,C | 0.0650 |
| Compressor C exergy loss, Ecomp,C | <1% |
| Discharging loop cooling exergy loss, Ecool,d | 0.0003 |
| Total of the discharging loop | 0.4426 |
| Overall system exergy loss | 1.1777 |
| Wnet | 1.0737 |
| ηste | 23.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Q.; Feng, S.; Kong, M.; Chen, C. Efficiency Enhancement of an Ammonia-Based Solar Thermochemical Energy Storage System Implemented with Hydrogen Permeation Membrane. Sustainability 2021, 13, 12783. https://doi.org/10.3390/su132212783
Xia Q, Feng S, Kong M, Chen C. Efficiency Enhancement of an Ammonia-Based Solar Thermochemical Energy Storage System Implemented with Hydrogen Permeation Membrane. Sustainability. 2021; 13(22):12783. https://doi.org/10.3390/su132212783
Chicago/Turabian StyleXia, Qi, Shuaiming Feng, Mingmin Kong, and Chen Chen. 2021. "Efficiency Enhancement of an Ammonia-Based Solar Thermochemical Energy Storage System Implemented with Hydrogen Permeation Membrane" Sustainability 13, no. 22: 12783. https://doi.org/10.3390/su132212783
APA StyleXia, Q., Feng, S., Kong, M., & Chen, C. (2021). Efficiency Enhancement of an Ammonia-Based Solar Thermochemical Energy Storage System Implemented with Hydrogen Permeation Membrane. Sustainability, 13(22), 12783. https://doi.org/10.3390/su132212783






