An Effective Control for Lead-Acid Performance Enhancement in a Hybrid Battery-Supercapacitor System Used in Transport Vehicles †
Abstract
:1. Introduction
2. Related Works
- Currently, LABs have seen minimal adoption for use in modern vehicle applications as compared to lithium-ion. The biggest challenge of well-established LABs is its lifespan being about five years due to extra strain by high current demand on the battery during starting, and it leads to reducing the lifespan. Therefore, to enhance battery lifespan from 5 years to about eight years, hybridisation is needed. The research article presents a new application for LABs to meet these vehicles requirements by hybridising it with supercapacitor. The supercapacitor supplies a significant starting current (i.e., 400 A for up to 3 s); thus, it enhances LAB’s lifespan;
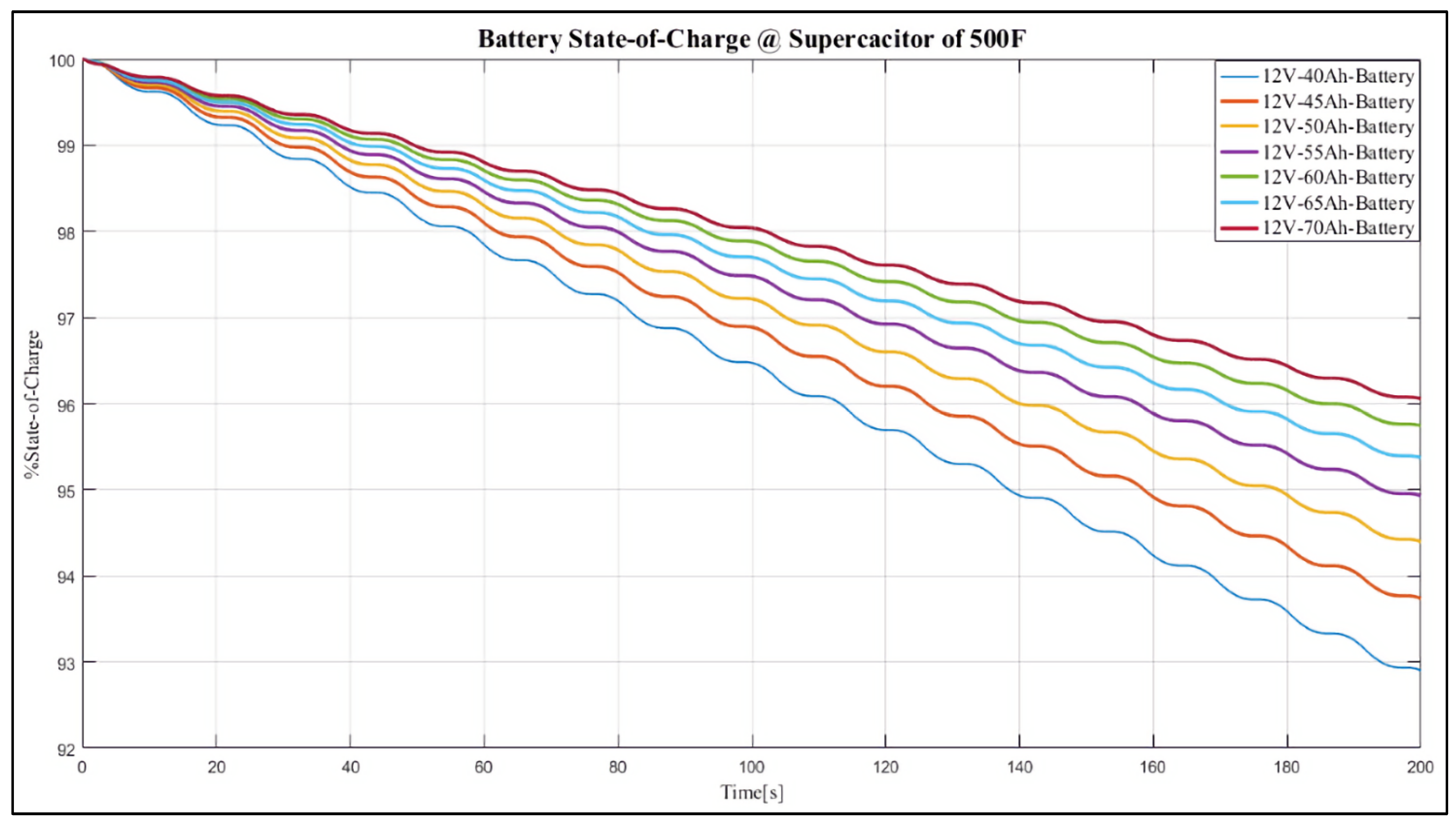
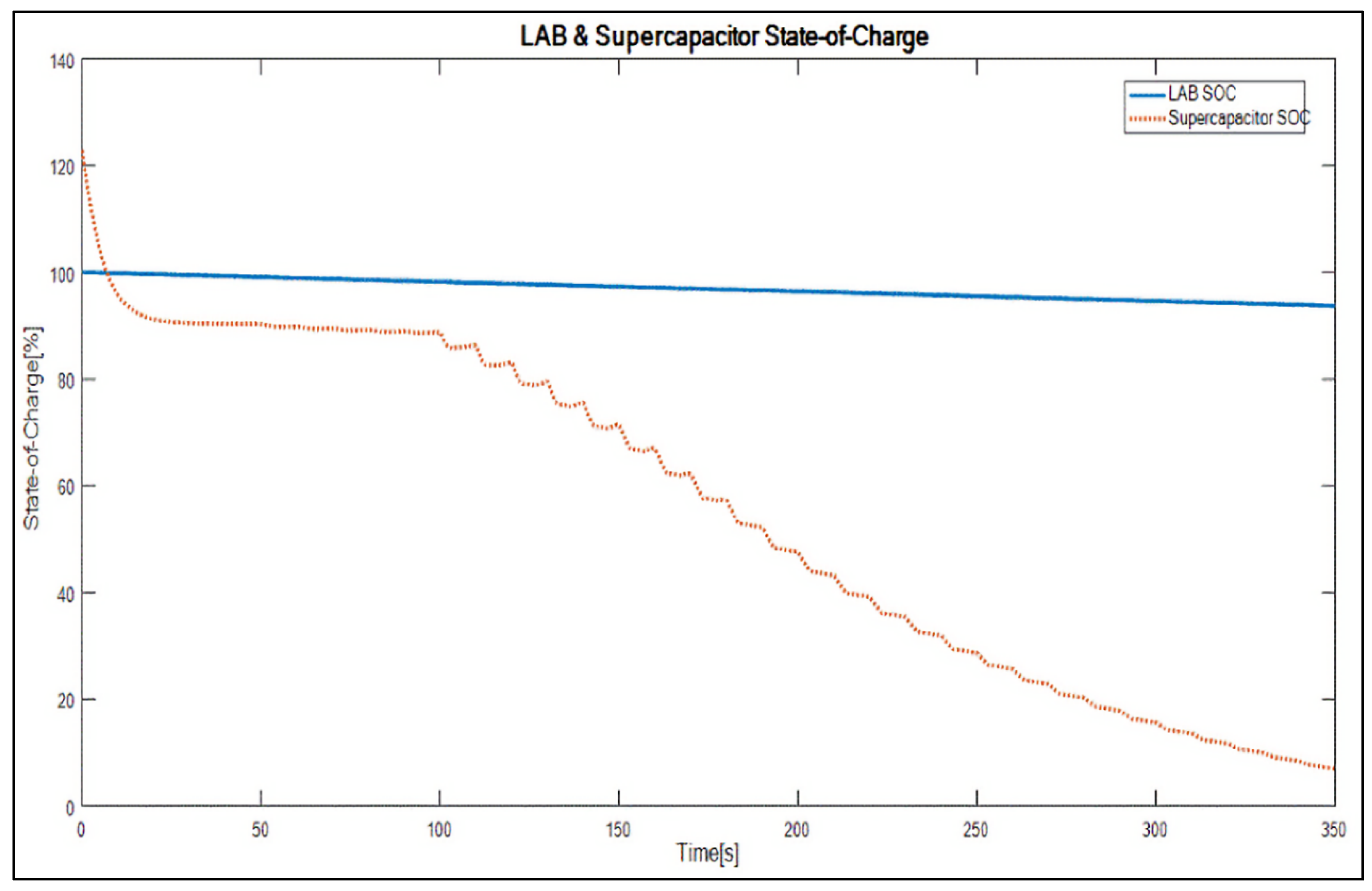
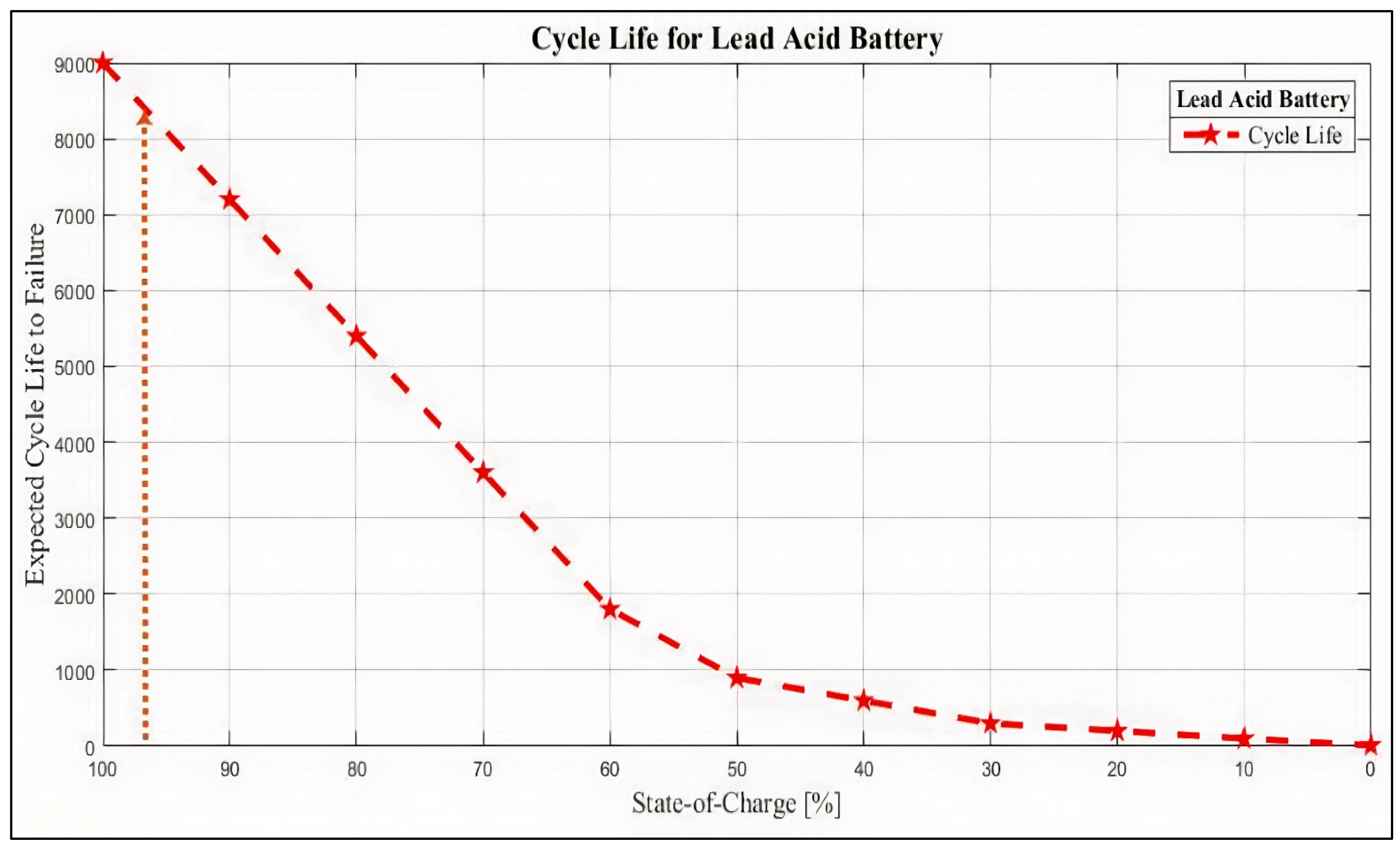
- It maintains the battery SoC at the highest level (i.e., 90%–95% SoC) to mitigate sulphation and allow at least 4% DCA;
- It enhances battery-cycling lifespan by 8200 cycles;
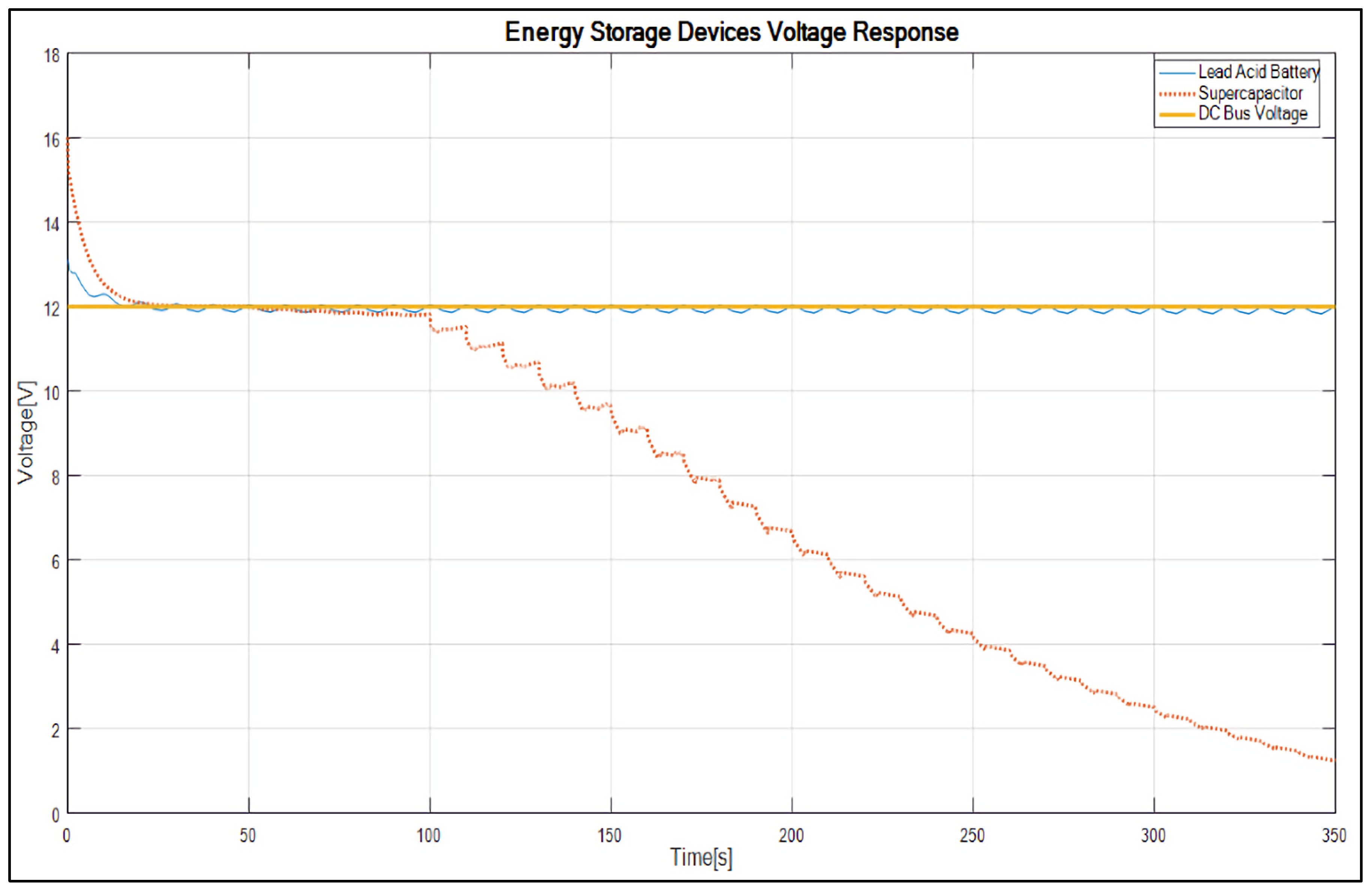
- It reduces the converter output voltage attenuation and maintains the DC bus voltage link at 12 V.
3. Materials and Methods
3.1. LAB Modelling
3.2. Modelling of a Supercapacitor
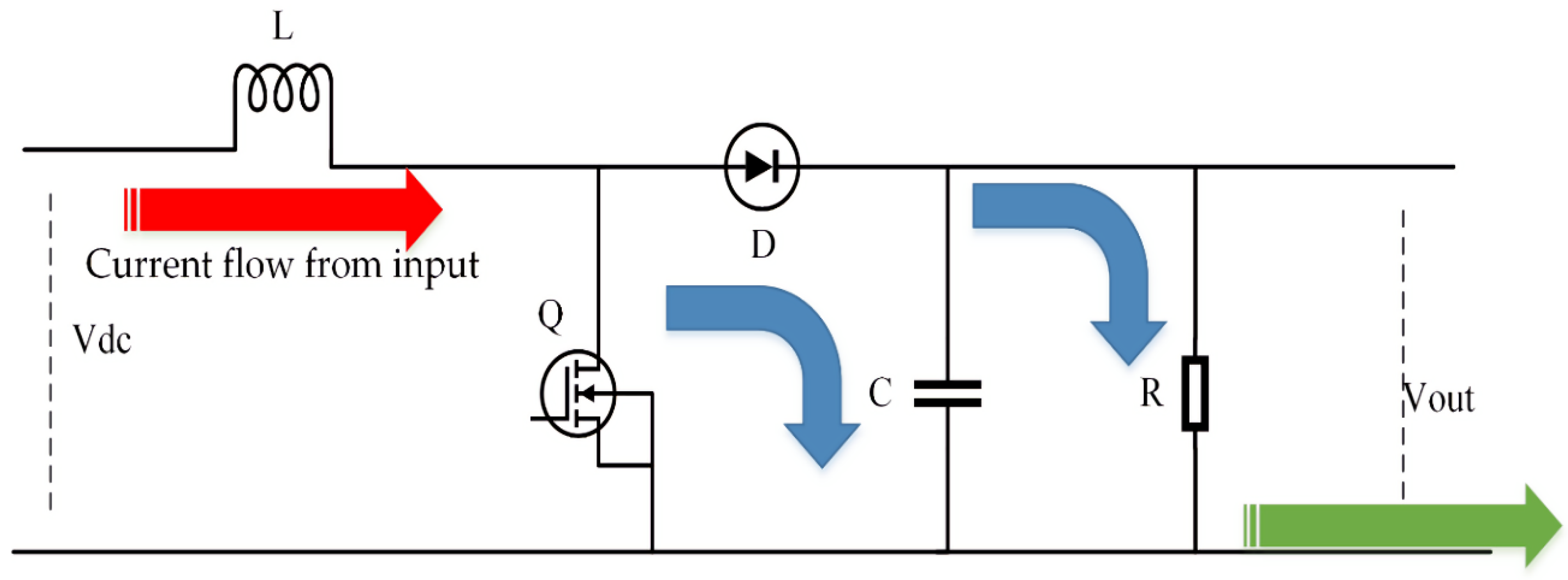
3.3. LAB Boost Converter
3.4. Step-Up/Step-Down Converter for a Supercapacitor
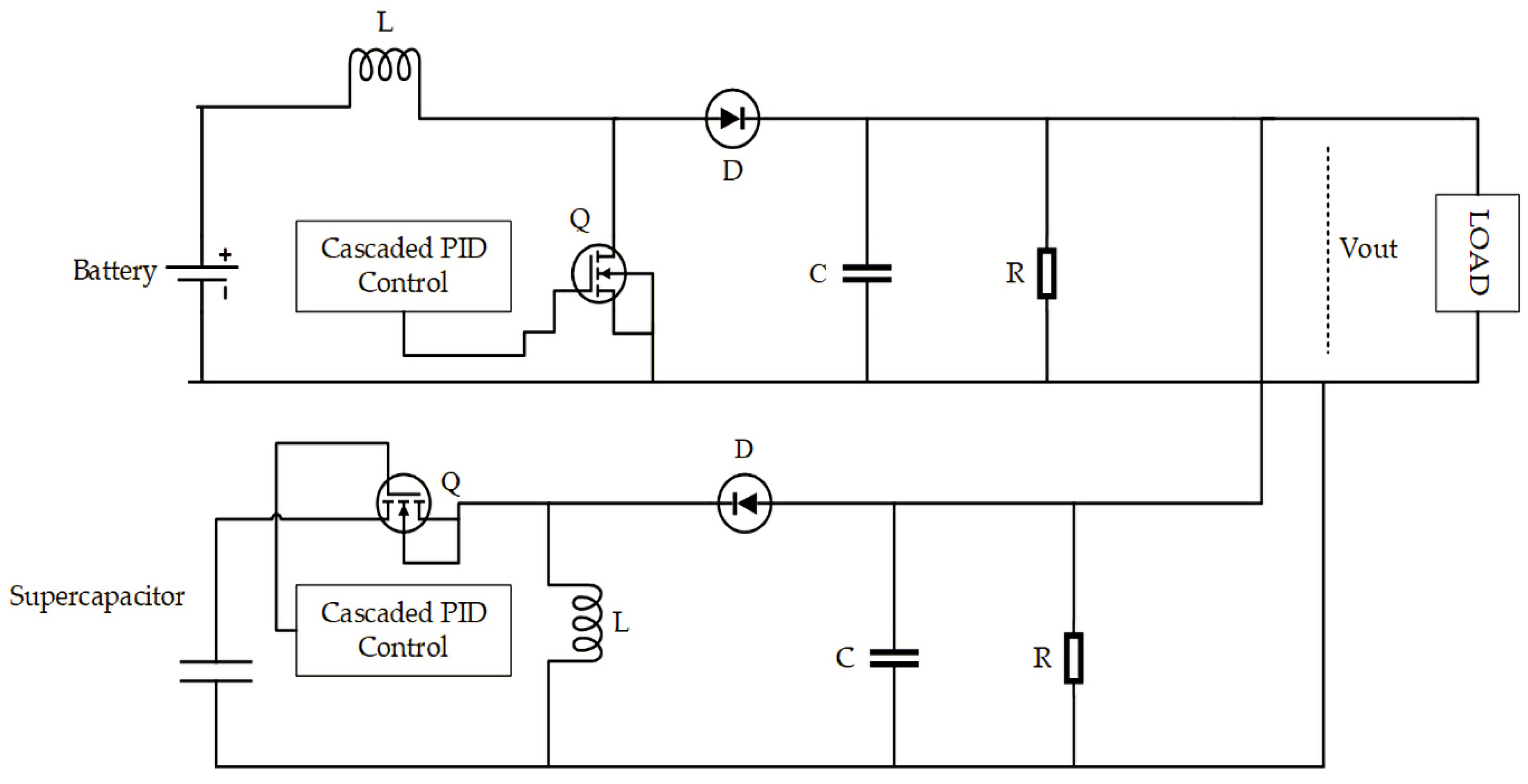
3.5. Control Enhancement
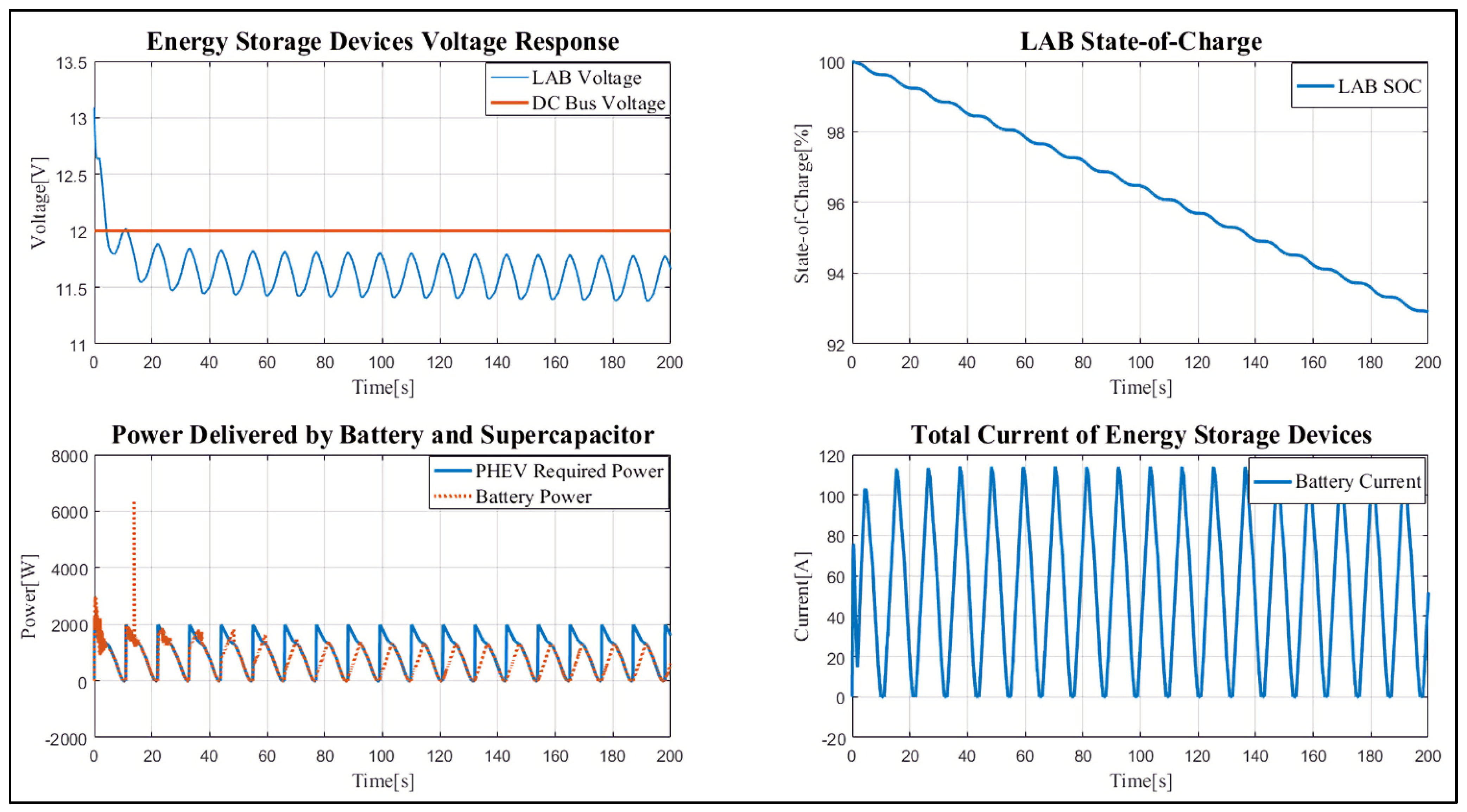
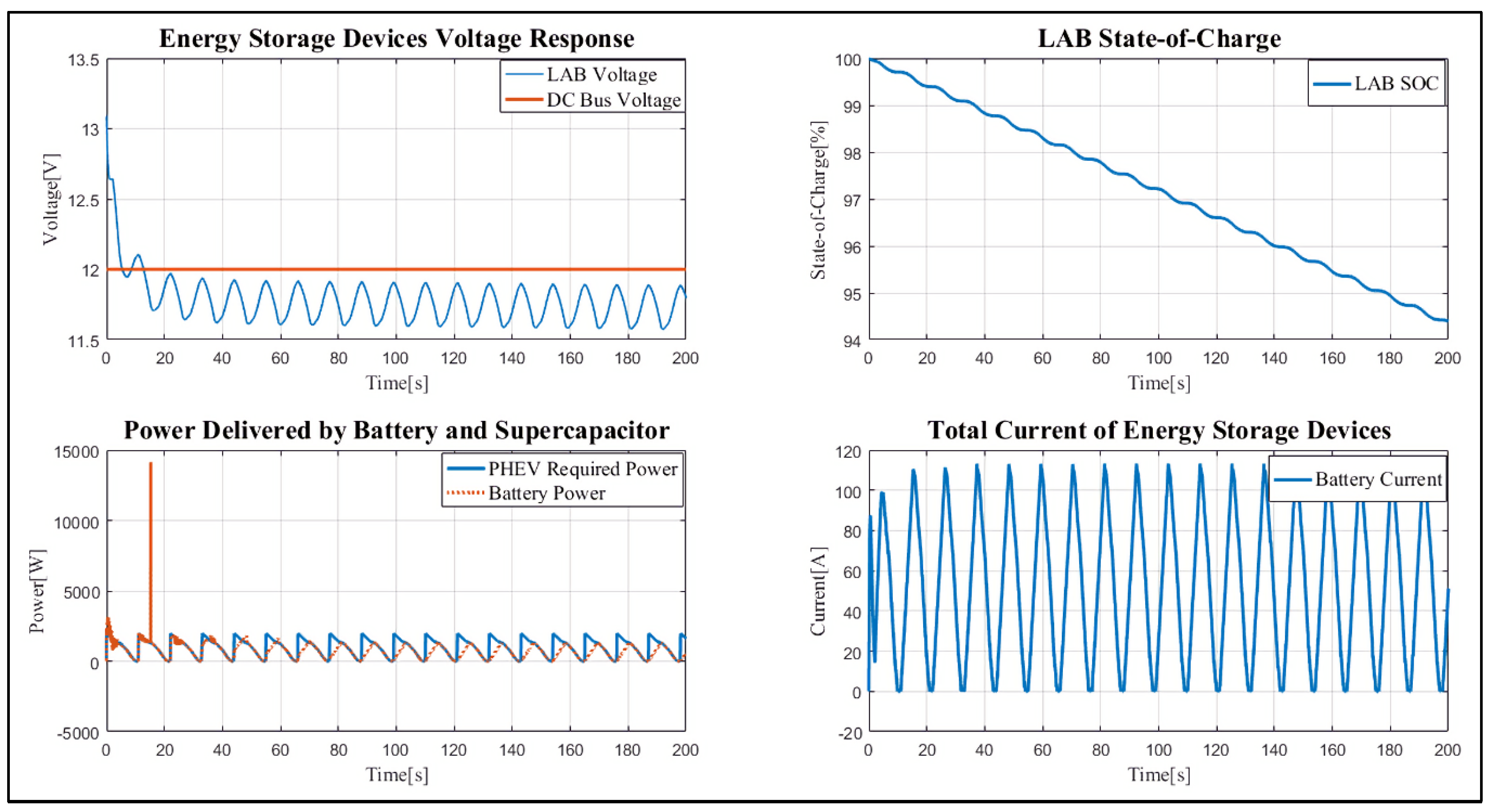
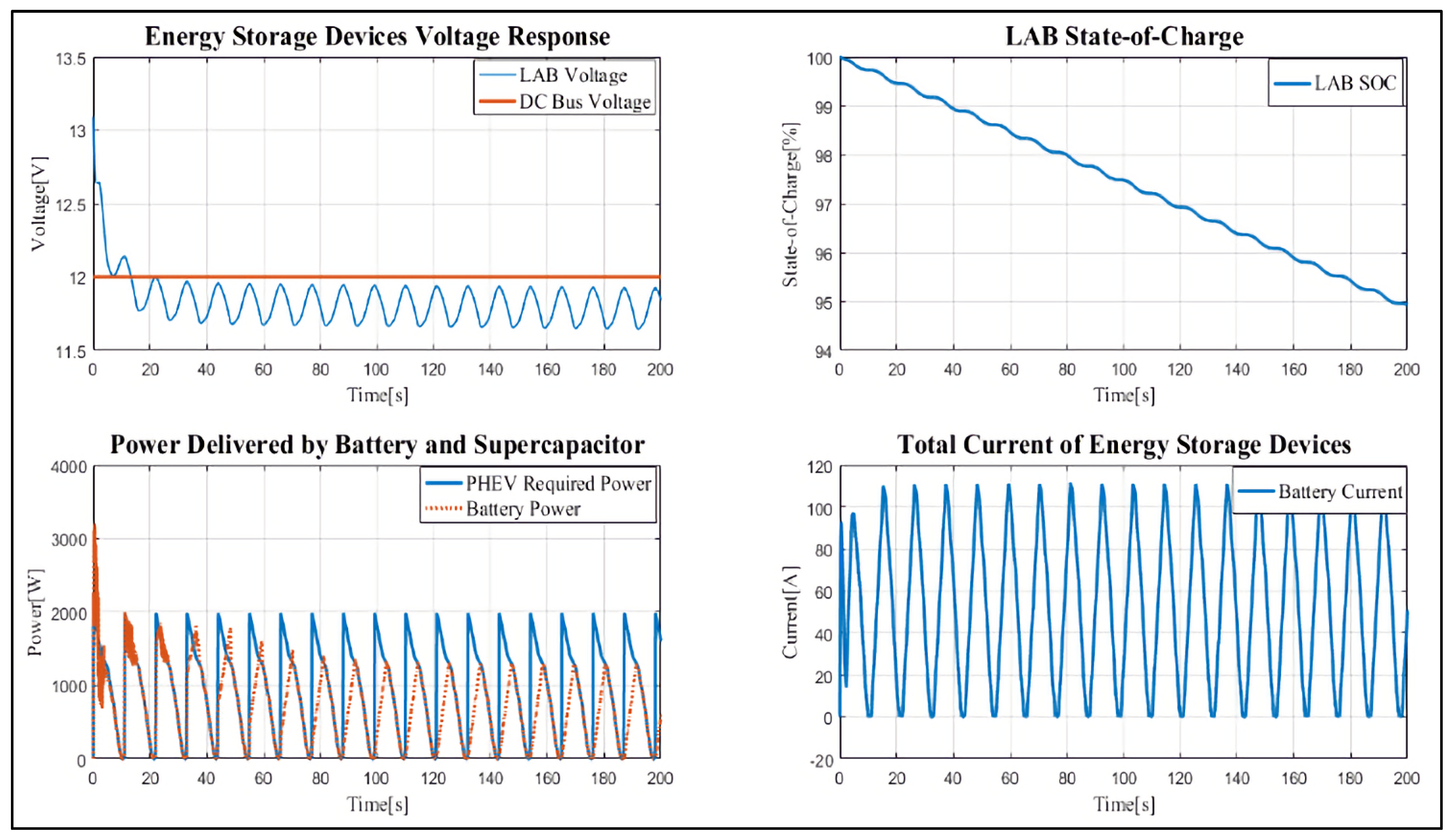
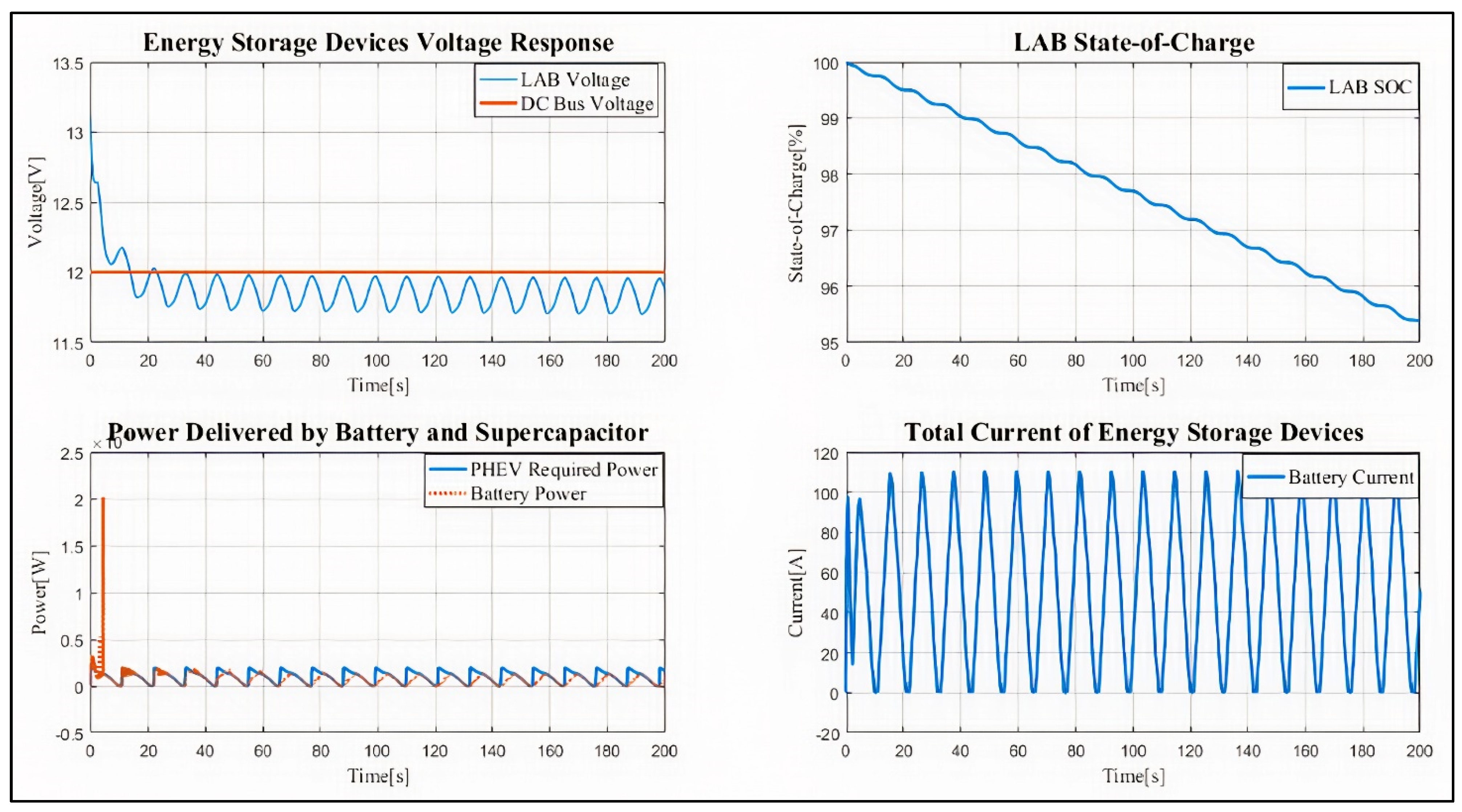
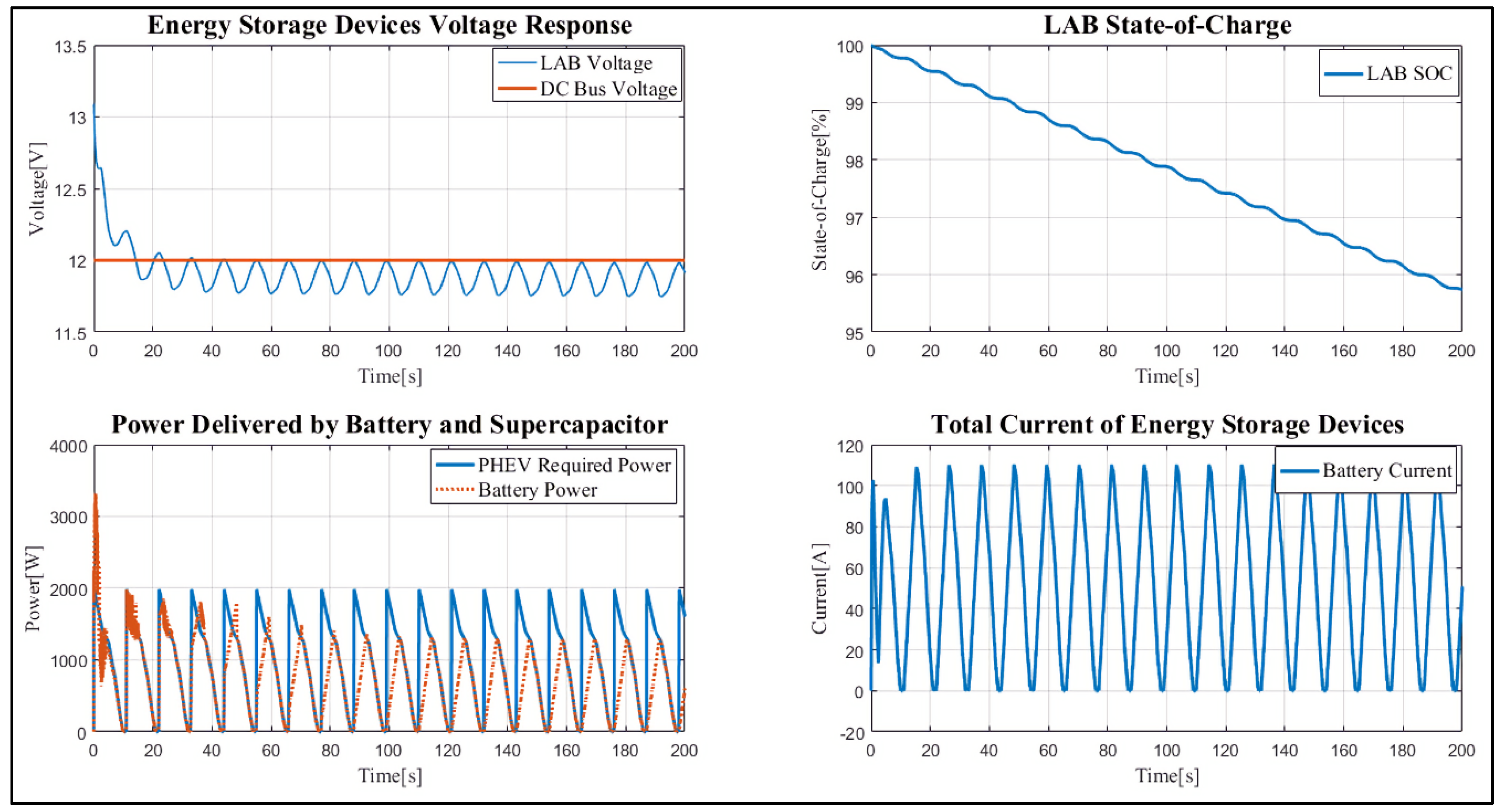
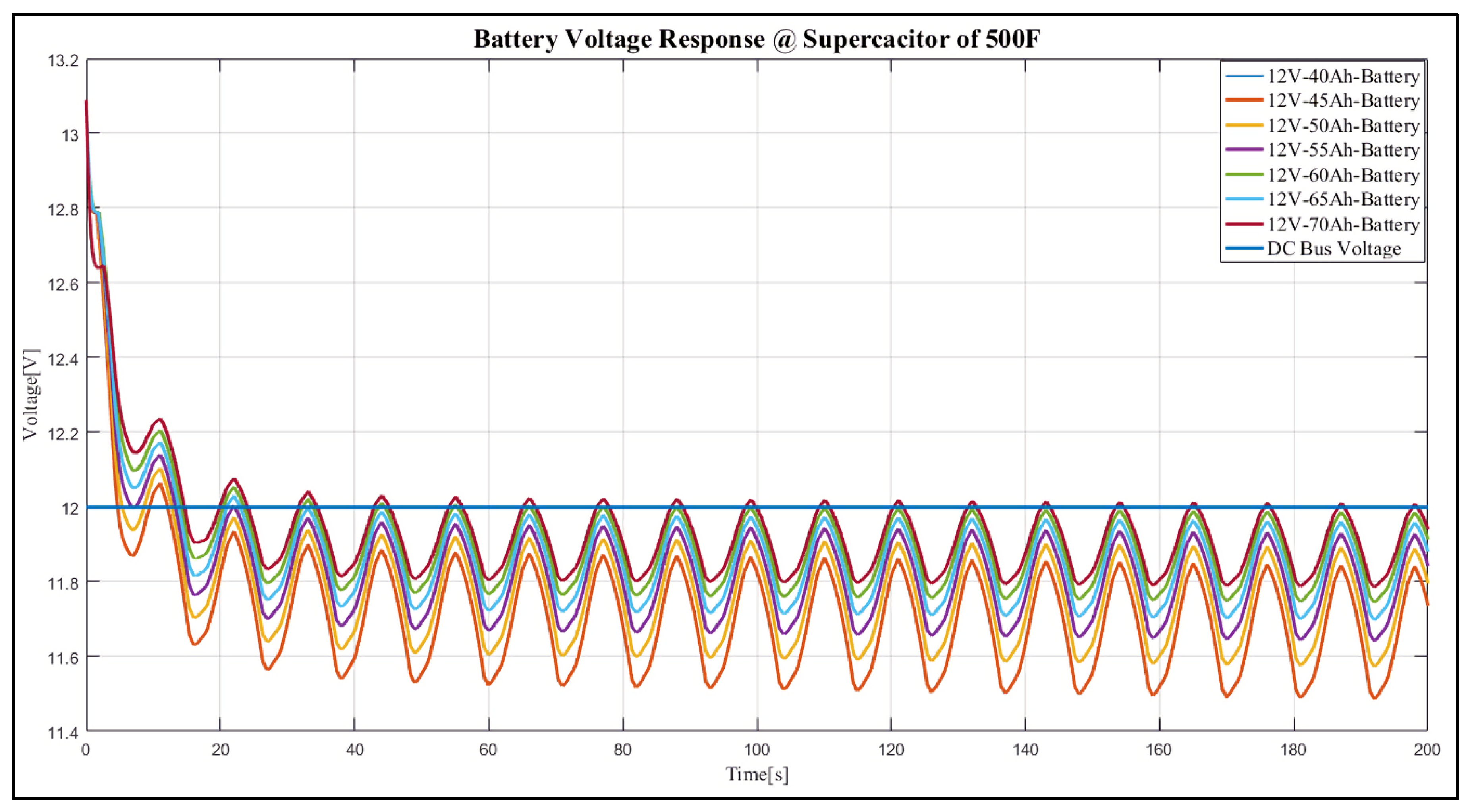
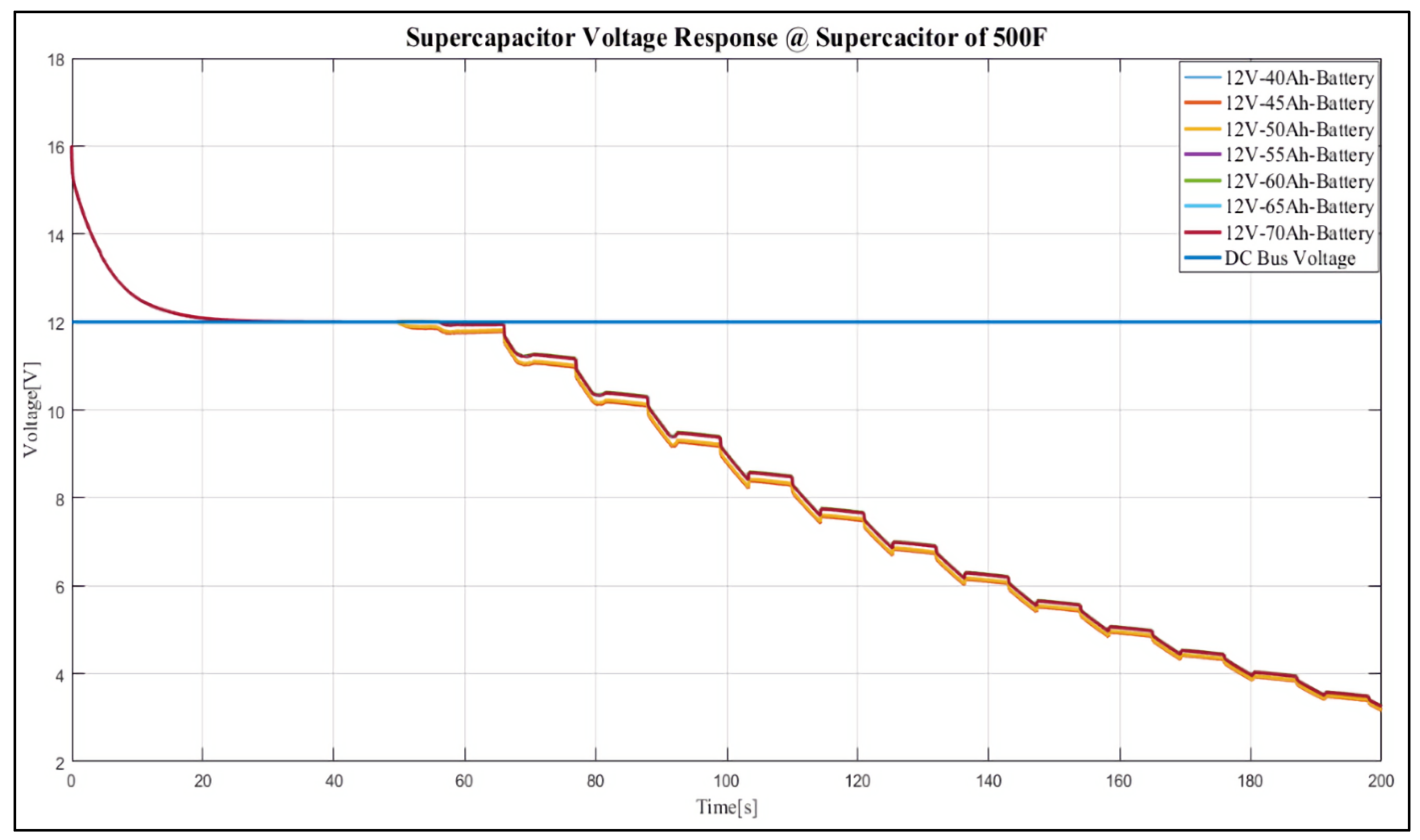
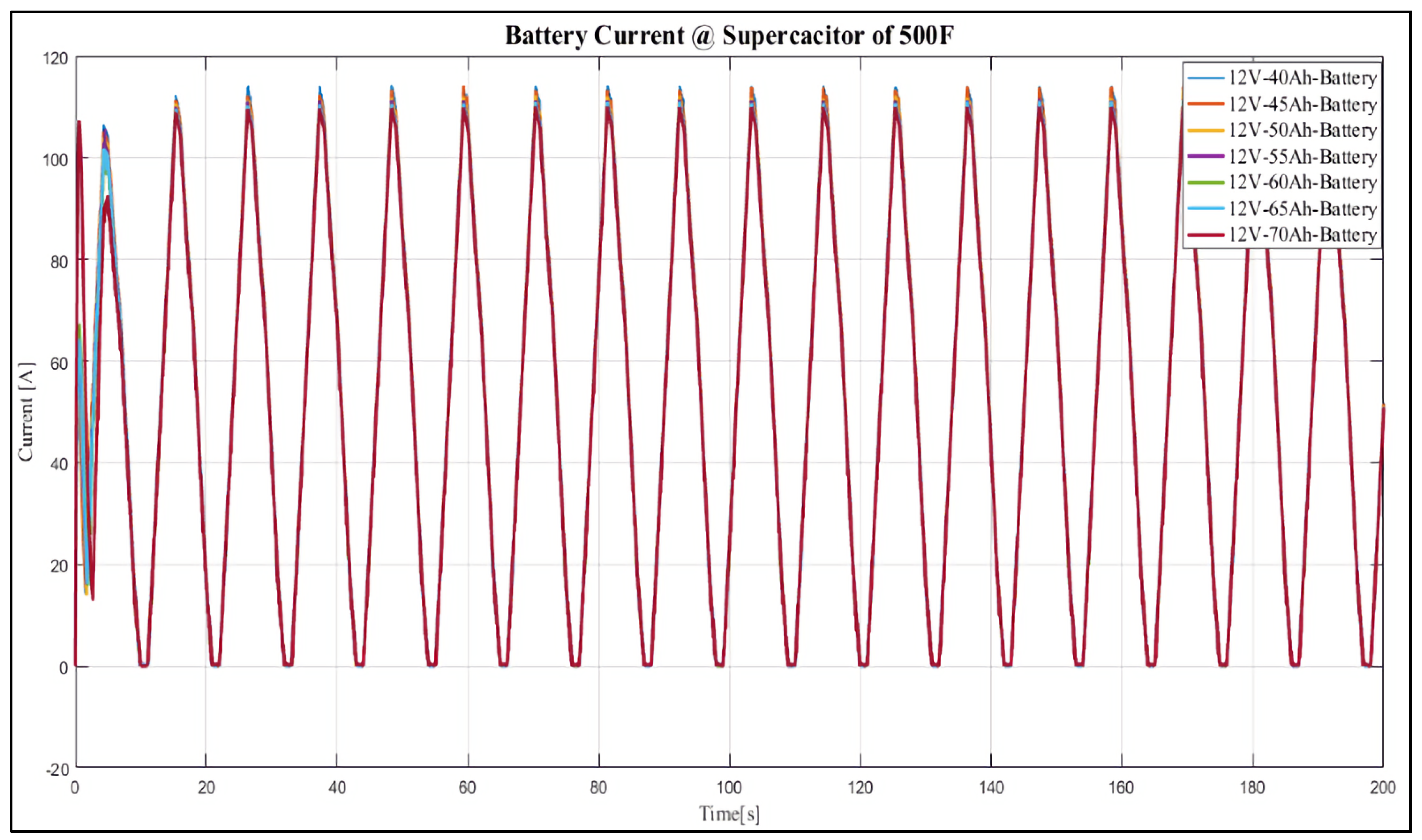
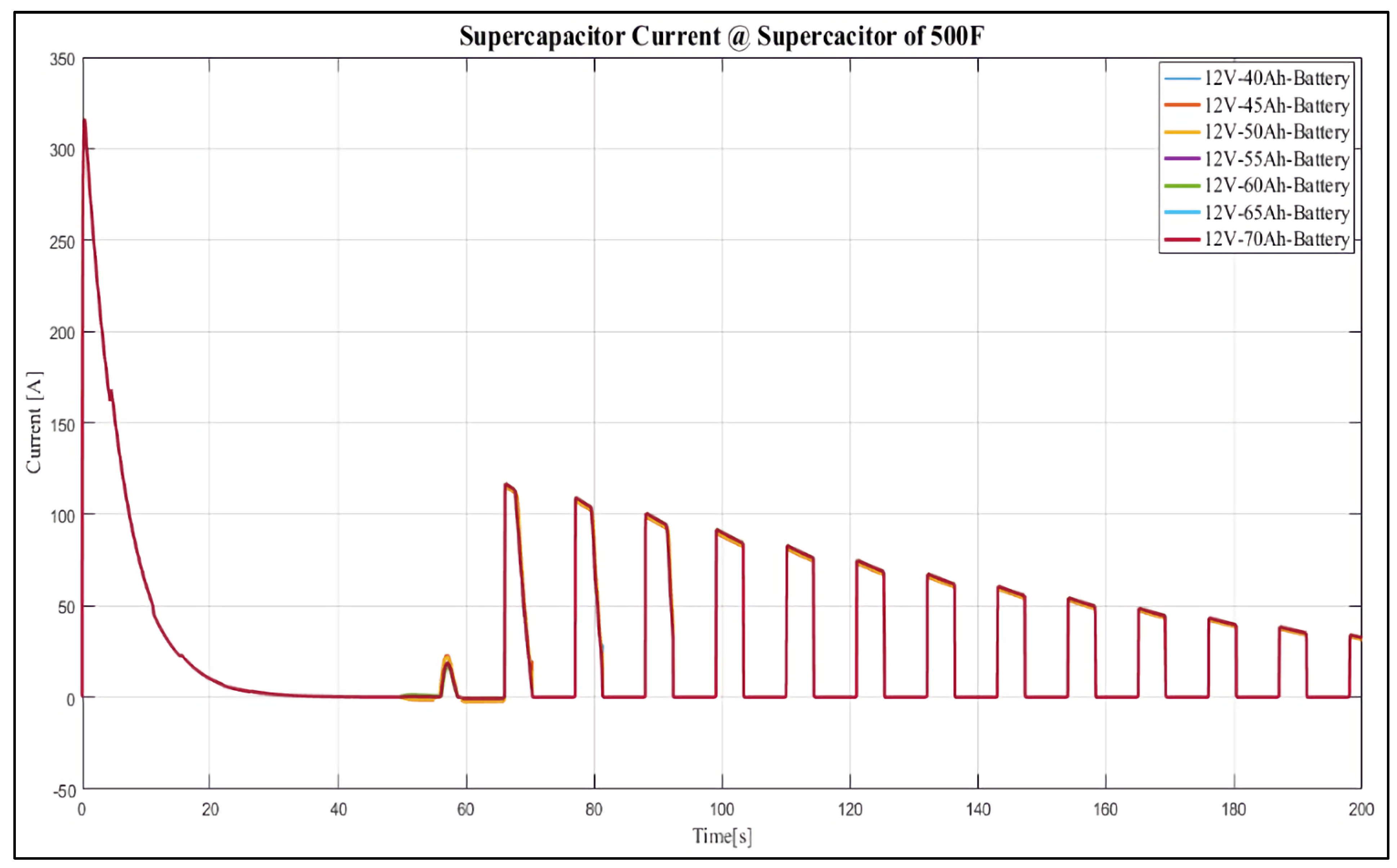
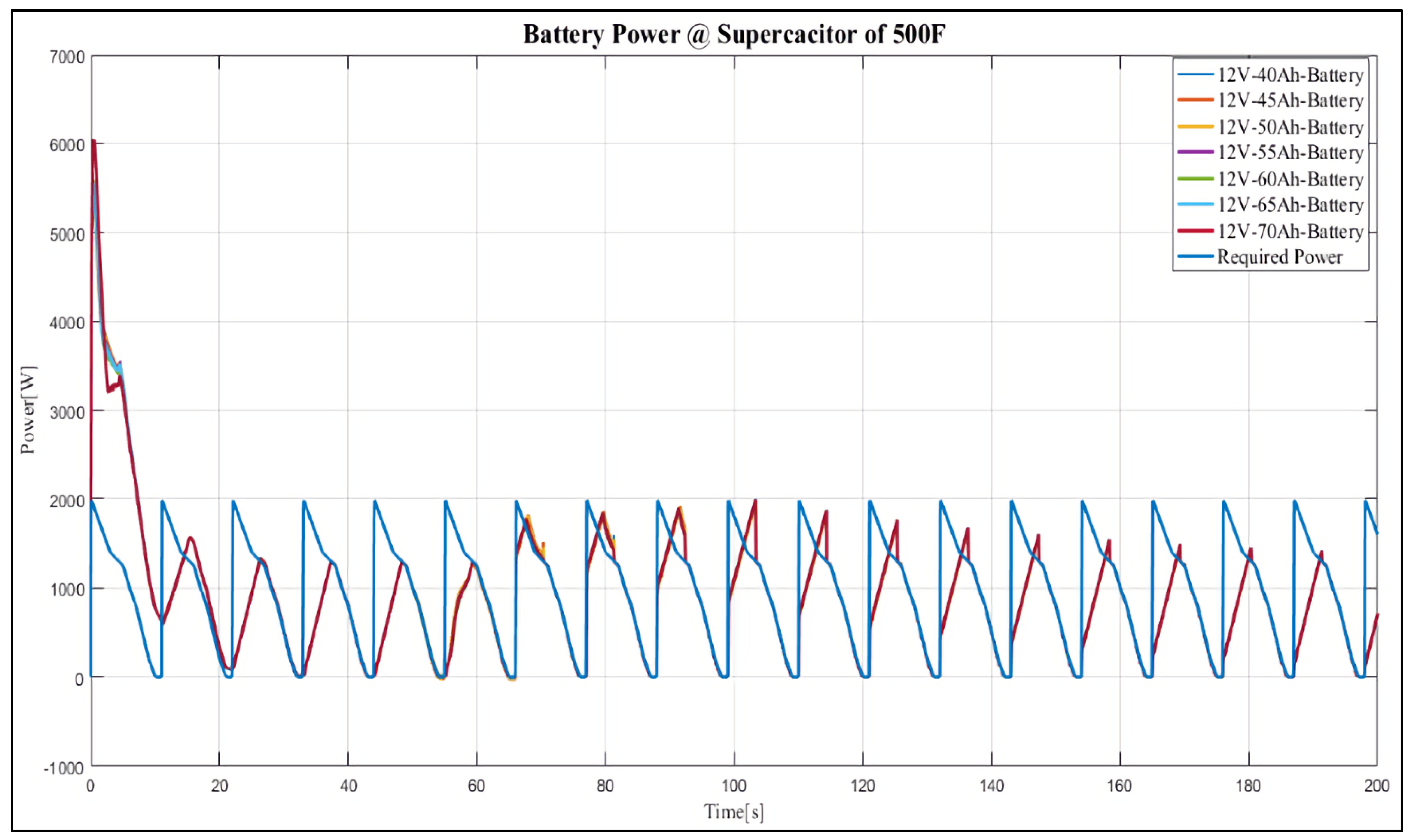
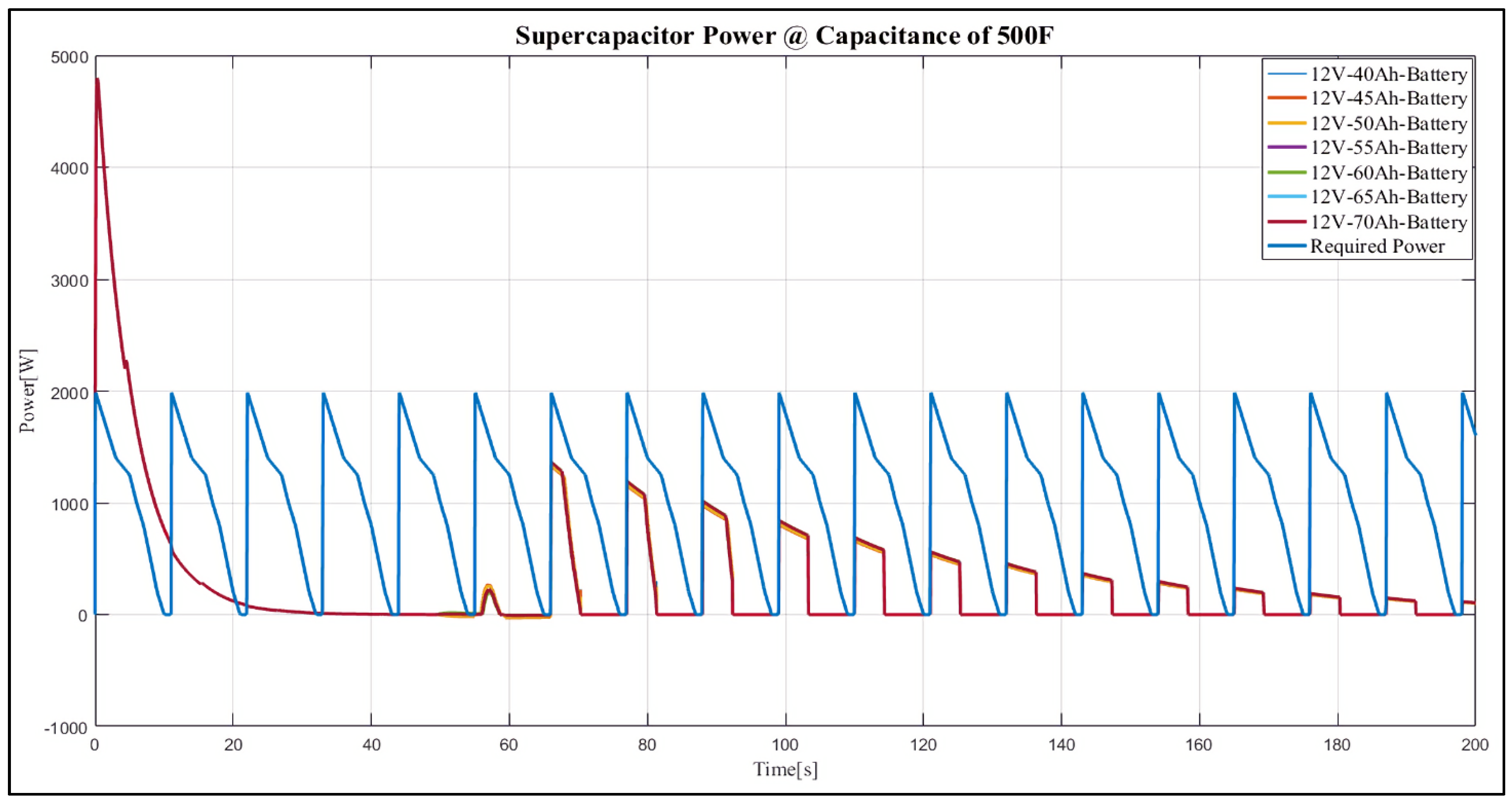
4. Results and Discussion
Case Study
- 12 V, 40 Ah;
- 12 V, 45 Ah;
- 12 V, 50 Ah;
- 12 V, 55 Ah;
- 12 V, 60 Ah;
- 12 V, 65 Ah;
- 12 V, 70 Ah.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| A | Amperes |
| AC | Alternating Current |
| Ah | Ampere-hours |
| BEV | Battery Electric Vehicle |
| BMS | Battery Management System |
| CA | Charge Acceptance |
| DC | Direct-Current |
| DC/DC | Direct-Current/Direct-Current |
| DCA | Dynamic Charge Acceptance |
| ESS | Energy Storage System |
| F | Farad |
| FOPID | Fractional Order Proportional Integrate-Derivative |
| FPGA | Field-Programmable Generic Array |
| HEV | The Hybrid Electric Vehicle |
| ICE | The Internal Combustion Engine |
| ICEE | The Internal Combustion Engine Energy |
| LA | Lead-Acid |
| LAB/s | Lead-Acid Battery/ies |
| MATLAB/Simulink | Mathematical Laboratory/Simulation-link |
| PHEV | A Plug-in Hybrid Electric Vehicle |
| PID | Proportional-Integrate-Derivative |
| PSoC | The Partial State-of-Charge |
| PWM | Pulse width modulation |
| SoC | State-of-Charge |
| TV/s | Transport Vehicle/s |
| USA | The United States of America |
| D | Control Duty Cycle |
| Battery Voltage | |
| Battery open-circuit voltage | |
| Electronic constant | |
| PID control compensation factor | |
| Maximum battery charge current | |
| Minimum battery charge current | |
| Maximum battery voltage response | |
| Minimum battery voltage response | |
| Current flowing into the battery | |
| Actual battery charge | |
| Battery stored charge | |
| Battery charging current | |
| State-of-Charge | |
| The total ampere flows through a supercapacitor | |
| Amperes going through branch in the equivalent circuit of supercapacitor | |
| Supercapacitor voltage/Converter input voltage | |
| Converter output voltage | |
| Converter switching-on time | |
| The temperature in °C | |
| Voltage across branch of the supercapacitor | |
| The proportional term | |
| The integrate the term | |
| The derivative term | |
| The compensation tuning factor | |
| Battery internal resistance |
References
- Lencwe, M.J.; Chowdhury, S.D.; Olwal, T.O. Towards performance enhancement of lead-acid battery for modern transport vehicles. In Proceedings of the 2020 IEEE PES/IAS PowerAfrica, Nairobi, Kenya, 25–28 August 2020; pp. 1–5. [Google Scholar]
- Kurzweil, P.; Garche, J. 2-Overview of batteries for future automobiles. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–96. ISBN 978-0-444-63700-0. [Google Scholar]
- Ariza Chacón, H.E.; Banguero, E.; Correcher, A.; Pérez-Navarro, Á.; Morant, F. Modelling, parameter identification, and experimental validation of a lead acid battery bank using evolutionary algorithms. Energies 2018, 11, 2361. [Google Scholar] [CrossRef]
- Cheng, C.-S.; Lau, R.W.-H.; Rathi, N.K.; Chung, H.S.-H. Extraction of intrinsic parameters of lead–acid batteries using energy recycling technique. IEEE Trans. Power Electron. 2019, 34, 4765–4779. [Google Scholar] [CrossRef]
- Garche, J.; Karden, E.; Moseley, P.T.; Rand, D.A.J. Lead-Acid Batteries for Future Automobiles; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-444-63703-1. [Google Scholar]
- Badeda, J.; Huck, M.; Sauer, D.U.; Kabzinski, J.; Wirth, J. 16-Basics of lead-acid battery modelling and simulation. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 463–507. ISBN 978-0-444-63700-0. [Google Scholar]
- Singh, A.; Karandikar, P.B.; Kulkarni, N.R. Mitigation of sulfation in lead acid battery towards life time extension using ultra capacitor in hybrid electric vehicle. J. Energy Storage 2021, 34, 102219. [Google Scholar] [CrossRef]
- Ibrahim, N.M.P.A.; Rahman, R.A.M.M.; Yassin, A.M.E.A.; Aziz, M.H. Parameters observation of restoration capacity of industrial lead acid battery using high current pulses. Int. J. Power Electron. Drive Syst. 2020, 11, 1596–1602. [Google Scholar] [CrossRef]
- Kwiecien, M.; Badeda, J.; Huck, M.; Komut, K.; Duman, D.; Sauer, D.U. Determination of SoH of lead-acid batteries by electrochemical impedance spectroscopy. Appl. Sci. 2018, 8, 873. [Google Scholar] [CrossRef]
- Yang, S.; Li, R.; Cai, X.; Xue, K.; Yang, B.; Hu, X.; Dai, C. Enhanced cycle performance and lifetime estimation of lead-acid batteries. New J. Chem. 2018, 42, 8900–8904. [Google Scholar] [CrossRef]
- Mekonnen, Y.; Aburbu, H.; Sarwat, A. Life cycle prediction of sealed lead acid batteries based on a weibull model. J. Energy Storage 2018, 18, 467–475. [Google Scholar] [CrossRef]
- Kumar, B.; Khare, N.; Chaturvedi, P.K. Advanced Battery Management System Using MATLAB/Simulink. In Proceedings of the 2015 IEEE International Telecommunications Energy Conference (IN℡EC), Osaka, Japan, 18–22 October 2015; pp. 1–6. [Google Scholar]
- Mahmoudzadeh Andwari, A.; Pesiridis, A.; Rajoo, S.; Martinez-Botas, R.; Esfahanian, V. A Review of battery electric vehicle technology and readiness levels. Renew. Sustain. Energy Rev. 2017, 78, 414–430. [Google Scholar] [CrossRef]
- Manzetti, S.; Mariasiu, F. Electric vehicle battery technologies: From present state to future systems. Renew. Sustain. Energy Rev. 2015, 51, 1004–1012. [Google Scholar] [CrossRef]
- Ogura, K.; Kolhe, M.L. 4-Battery technologies for electric vehicles. In Electric Vehicles: Prospects and Challenges; Muneer, T., Kolhe, M.L., Doyle, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 139–167. ISBN 978-0-12-803021-9. [Google Scholar]
- Rabalais, A. Drive down to electric avenue: Taking electric vehicle incentives higher. LSU J. Energy Law Resour. 2017, 5, 427. [Google Scholar]
- Albers, J.; Meissner, E. 6-Automotive absorptive glass-mat lead-acid batteries: State of the art. In Lead-Acid Batteries for Future Automobiles; Garche, J., Karden, E., Moseley, P.T., Rand, D.A.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 185–211. ISBN 978-0-444-63700-0. [Google Scholar]
- Farjah, A.; Ghanbari, T.; Seifi, A.R. Contribution management of lead-acid battery, li-ion battery, and supercapacitor to handle different functions in EVs. Int. Trans. Electr. Energy Syst. 2020, 30, e12155. [Google Scholar] [CrossRef]
- Jankowska, E.; Kopciuch, K.; Błażejczak, M.; Majchrzycki, W.; Piórkowski, P.; Chmielewski, A.; Bogdziński, K. Hybrid Energy Storage Based on Ultracapacitor and Lead Acid Battery: Case Study. In Proceedings of the Automation 2018, Warsaw, Poland, 21–23 March 2018; Springer: Cham, Switzerland, 2018; pp. 339–349. [Google Scholar]
- Smith, M.J.; Gladwin, D.T.; Stone, D.A. An analysis of the influence of high-frequency ripple currents on dynamic charge acceptance in lead-acid batteries. J. Energy Storage 2019, 22, 27–35. [Google Scholar] [CrossRef]
- Lencwe, M.J.; Chowdhury, S.P.D.; Olwal, T.O. Performance Studies of Lead Acid Batteries for Transport Vehicles. In Proceedings of the 2017 IEEE PES PowerAfrica, Accra, Ghana, 27–30 June 2017; pp. 528–532. [Google Scholar]
- Lencwe, M.J.; Chowdhury, S.P.; Olwal, T.O. A multi-stage approach to a hybrid lead acid battery and supercapacitor system for transport vehicles. Energies 2018, 11, 2888. [Google Scholar] [CrossRef]
- Kumar, B.; Khare, N.; Chaturvedi, P.K. FPGA-based design of advanced BMS implementing SoC/SoH estimators. Microelectron. Reliab. 2018, 84, 66–74. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Sreedhar, S.; Siegel, J.B.; Choi, S. Topology Comparison for 48V Battery-Supercapacitor Hybrid Energy Storage System. IFAC-Pap. 2017, 50, 4733–4738. [Google Scholar] [CrossRef]
- Song, Z.; Hou, J.; Hofmann, H.; Li, J.; Ouyang, M. Sliding-mode and lyapunov function-based control for battery/supercapacitor hybrid energy storage system used in electric vehicles. Energy 2017, 122, 601–612. [Google Scholar] [CrossRef]
- Bai, Z.; Yan, Z.; Wu, X.; Xu, J.; Cao, B.H. Control for battery/supercapacitor hybrid energy storage system used in electric vehicles. Int. J. Automot. Technol. 2019, 20, 1287–1296. [Google Scholar] [CrossRef]
- Ferg, E.E.; Mgangato, S. The performance of Pb-acid cells linked to supercapacitors under partial state of capacity cycling onditions. J. Energy Storage 2017, 14, 49–55. [Google Scholar] [CrossRef]
- Benabdelaziz, K.; Maaroufi, M. Battery dynamic energy model for use in electric vehicle simulation. Int. J. Hydrog. Energy 2017, 42, 19496–19503. [Google Scholar] [CrossRef]
- Fiorenti, S.; Guanetti, J.; Onori, S.; Guezennec, Y.; Madella, N.; Saletti, A.; Bovo, S. Modeling and experimental validation of PbA battery-supercapacitor energy storage system. In Proceedings of the 7th IFAC Symposium on Advances in Automotive Control, Tokyo, Japan, 4–7 September 2013; Volume 46, pp. 307–312. [Google Scholar] [CrossRef]
- Dos Santos, E.; da Silva, E.R. Control Strategies for Power Converters. In Advanced Power Electronics Converters: PWM Converters Processing AC Voltages; IEEE: Piscataway, NJ, USA, 2014; pp. 264–294. ISBN 978-1-118-96822-2. [Google Scholar]
- Daowd, M.; Omar, N.; Van Den Bossche, P.; Van Mierlo, J. Passive and active battery balancing comparison based on MATLAB simulation. In Proceedings of the 2011 IEEE Vehicle Power and Propulsion Conference, Chicago, IL, USA, 6–9 September 2011; pp. 1–7. [Google Scholar]
- Seim, L.H. Modeling, Control and Experimental Testing of a Supercapacitor/Battery Hybrid System: Passive and Semi-Active Topologies. Master’s Thesis, Nowergian University of Life Sciences, As, Norway, 2012; pp. 1–133. [Google Scholar]
- Bopche, L.M.; Deosant, A.A.; Ahmad, M. Combination of Parallel Connected Supercapacitor Amp; Amp; Battery for Enhancing Battery Life. In Proceedings of the 2016 International Conference on Automatic Control and Dynamic Optimization Techniques (ICACDOT), Pune, India, 9–10 September 2016; pp. 77–82. [Google Scholar]
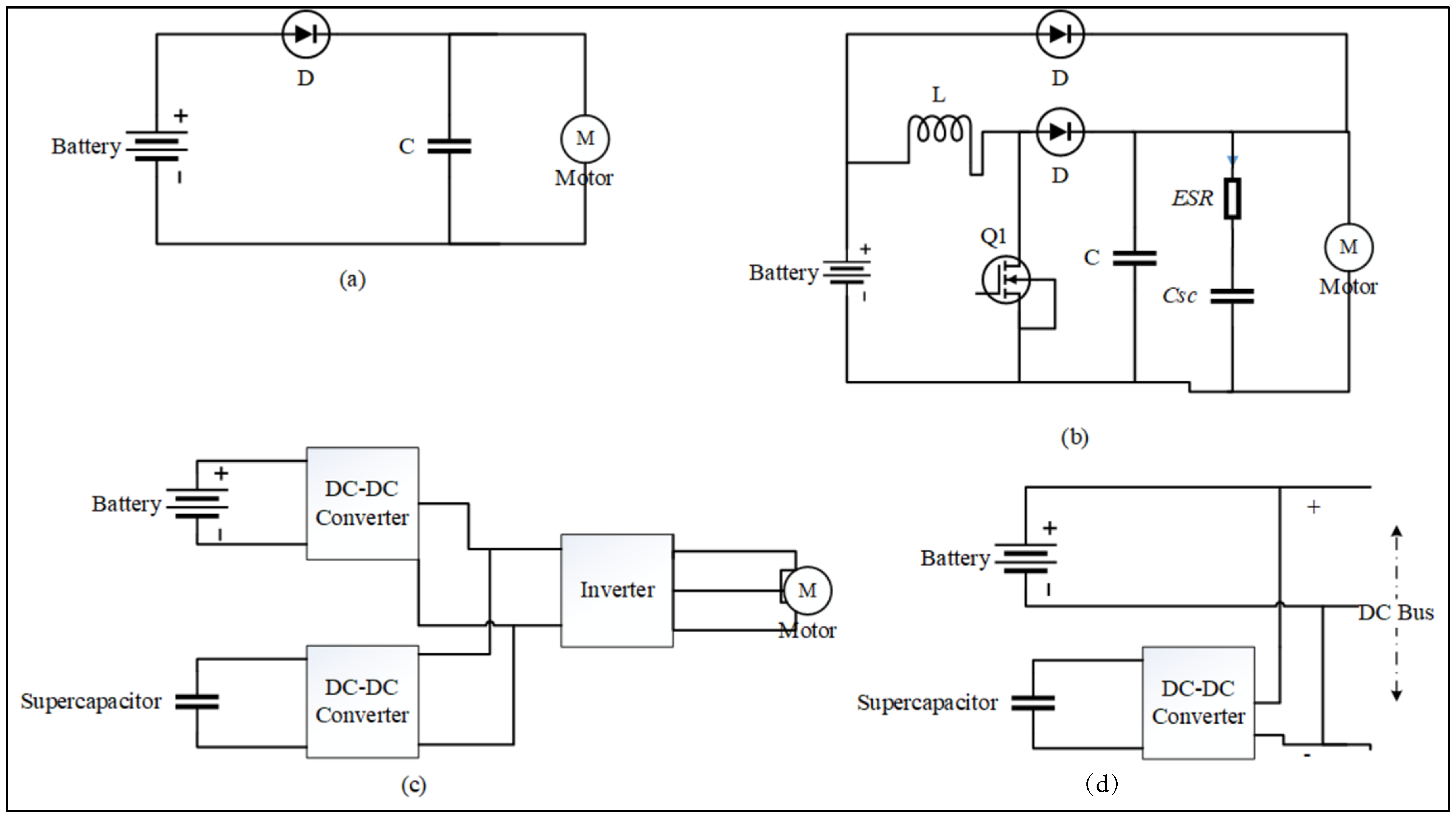
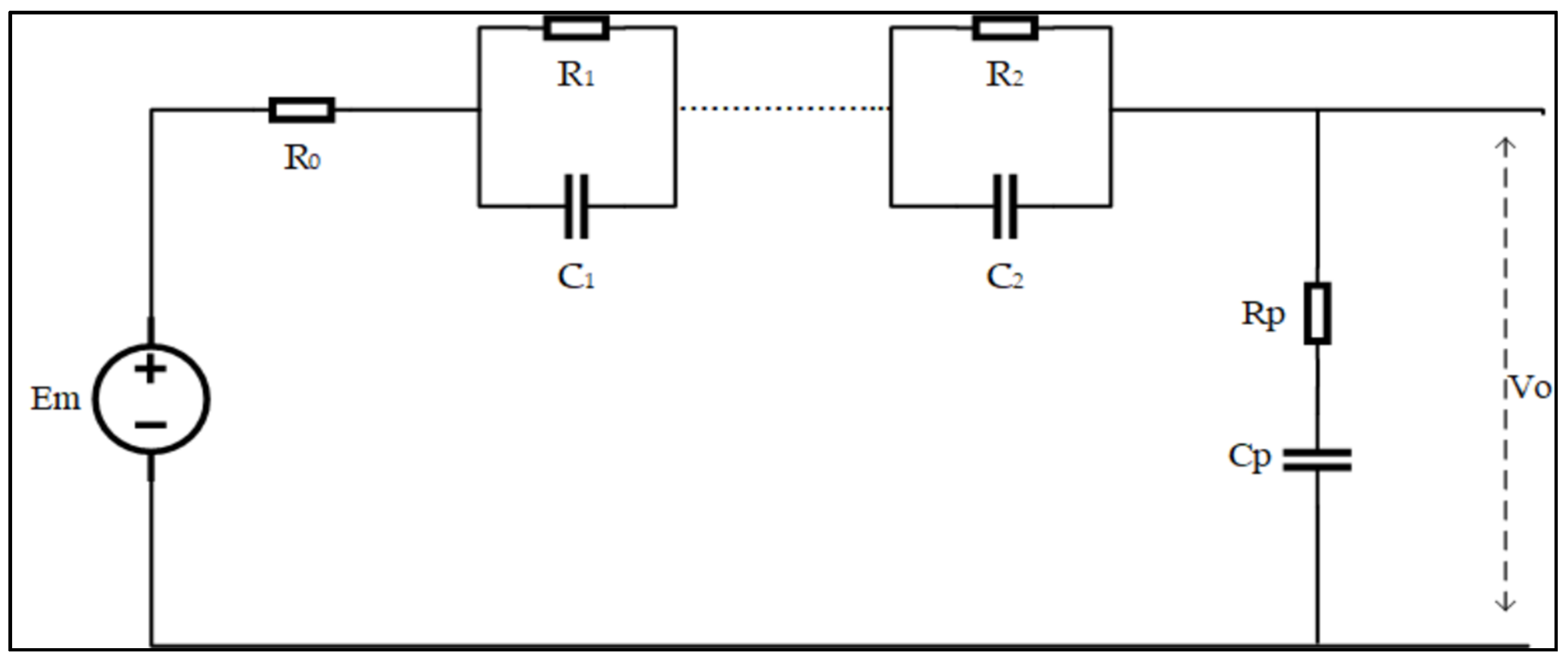
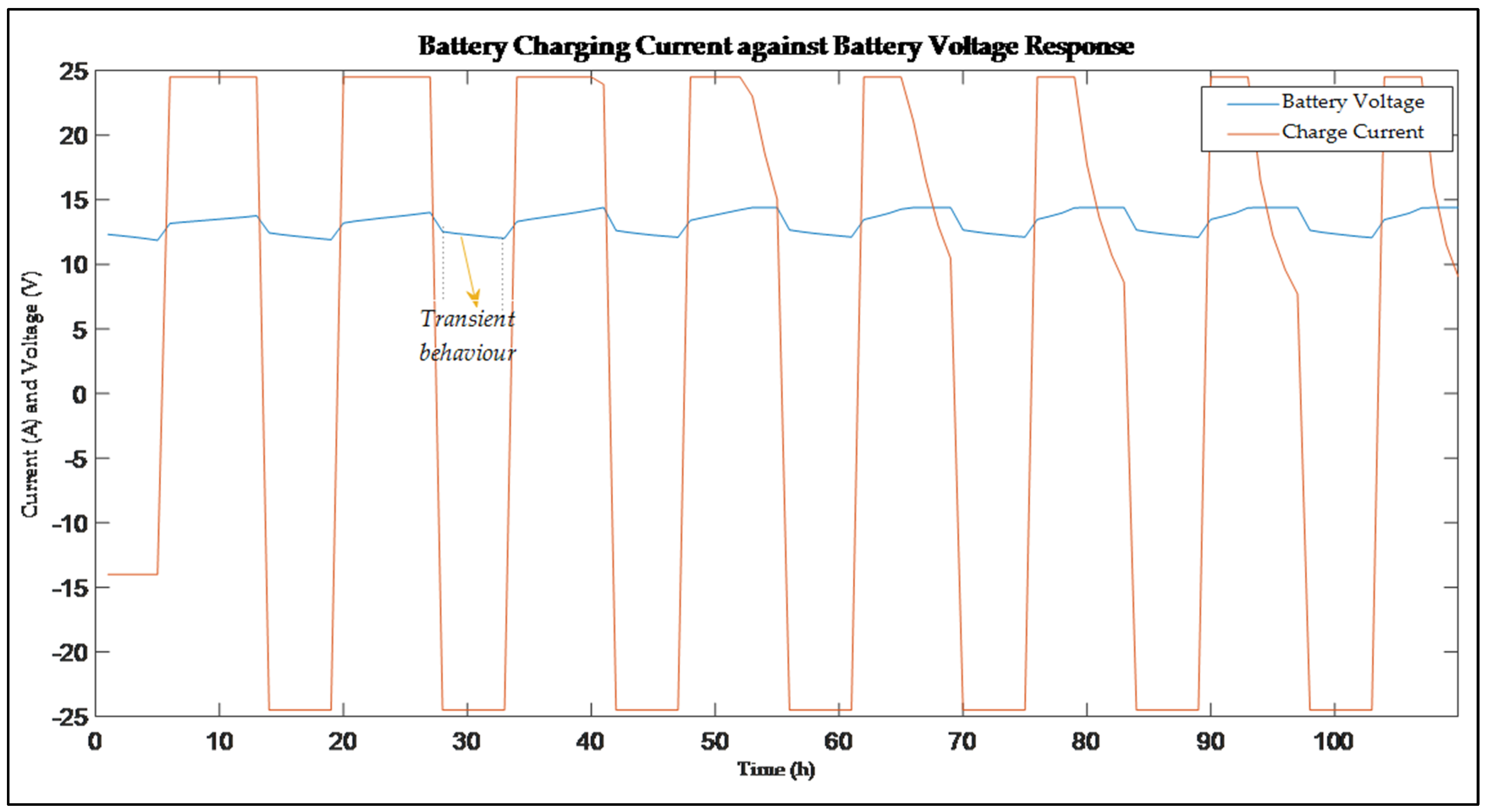
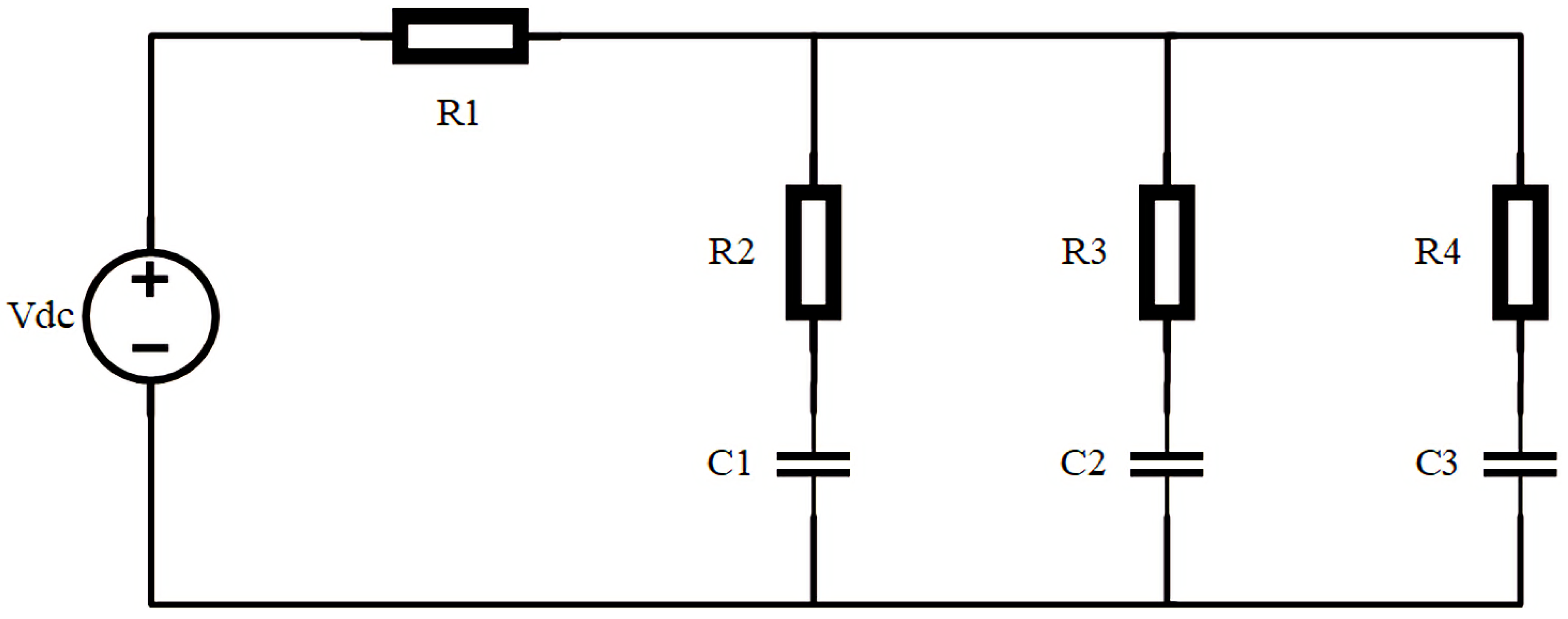




| LABs | Pros | Cons |
|---|---|---|
| Flooded/Vented | It does not require additional water. There is no acid leakage. There are no insignificant acid smokes. Simple for carriage. It does not require special airing. Horizontal placement is possible. Staking on top of each other is feasible. It has a small phenomenon of acid stratification. At 25 °C, it needs fewer overcharges. Virtuous capability for high-rate discharge. | Cautious charging is required. Careful temperature supervision is needed. It has enormous top-of-charge voltage variations. At high temperatures, it needs amplified over-charge. Poorer lifecycle at deep discharge. Explicit enormity is not measured. Unreachable dry-charge state. Shelf life is two years. |
| AGM and Gel | Provide compact cyclical damage. Oxygen re-mixture conquers hydrogen re-mixture. There is no acid leakage. No water loss. It is vigorous. | It has lessened operational volume. It releases gases. It cannot withstand rapid charging. |
| Supercap. Capacitance (F) | LAB Current (A) | LAB Power (W) | LAB_SOC (%) | LAB Voltage (V) | SC Current (A) | SC_SOC (%) | SC Voltage (V) | SC Power (W) |
|---|---|---|---|---|---|---|---|---|
| 50 | 57.0209 | 685.9421 | 98.0562 | 11.9252 | 1.7560 | 5.2361 | 1.0664 | 8.5347 |
| 100 | 54.9272 | 681.2823 | 98.0912 | 11.9194 | 4.6783 | 14.8988 | 2.2566 | 29.0278 |
| 150 | 56.9789 | 728.3355 | 97.9593 | 11.9130 | 7.8508 | 19.3916 | 2.7954 | 51.7433 |
| 200 | 57.3473 | 753.9346 | 98.0022 | 11.9120 | 10.6334 | 28.7286 | 4.0163 | 73.0338 |
| 250 | 58.0500 | 783.6551 | 97.9600 | 11.9105 | 13.4528 | 34.6446 | 4.8227 | 94.4330 |
| 300 | 57.7716 | 802.5973 | 97.9767 | 11.9108 | 15.9796 | 42.4262 | 5.8717 | 116.6760 |
| 350 | 56.7324 | 811.3848 | 97.9928 | 11.9126 | 17.7695 | 49.4759 | 6.8000 | 137.7858 |
| 400 | 58.6096 | 857.3102 | 97.9597 | 11.9091 | 20.2304 | 53.8175 | 7.3648 | 161.4549 |
| 450 | 55.9299 | 828.6651 | 97.9431 | 11.9110 | 20.0024 | 59.3998 | 8.0830 | 164.8698 |
| 500 | 56.1081 | 842.8436 | 97.9310 | 11.9105 | 20.7826 | 62.6387 | 8.4954 | 176.9535 |
| 550 | 55.4630 | 850.4630 | 97.9252 | 11.9116 | 21.5369 | 66.7698 | 9.0169 | 191.8746 |
| 600 | 56.318 | 877.0163 | 98.1201 | 11.9129 | 21.3818 | 74.4037 | 9.9738 | 205.8309 |
| 650 | 56.5564 | 893.3687 | 97.9282 | 11.9095 | 23.0541 | 73.6330 | 9.8771 | 222.1349 |
| 700 | 56.6487 | 906.0791 | 98.0526 | 11.9115 | 22.9291 | 78.4828 | 10.4829 | 233.6138 |
| 750 | 55.6472 | 894.6524 | 97.9161 | 11.9161 | 23.0638 | 77.6213 | 10.3763 | 234.2114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lencwe, M.J.; Chowdhury, S.P.D.; Olwal, T.O. An Effective Control for Lead-Acid Performance Enhancement in a Hybrid Battery-Supercapacitor System Used in Transport Vehicles. Sustainability 2021, 13, 13971. https://doi.org/10.3390/su132413971
Lencwe MJ, Chowdhury SPD, Olwal TO. An Effective Control for Lead-Acid Performance Enhancement in a Hybrid Battery-Supercapacitor System Used in Transport Vehicles. Sustainability. 2021; 13(24):13971. https://doi.org/10.3390/su132413971
Chicago/Turabian StyleLencwe, Mpho J., S. P. Daniel Chowdhury, and Thomas O. Olwal. 2021. "An Effective Control for Lead-Acid Performance Enhancement in a Hybrid Battery-Supercapacitor System Used in Transport Vehicles" Sustainability 13, no. 24: 13971. https://doi.org/10.3390/su132413971
APA StyleLencwe, M. J., Chowdhury, S. P. D., & Olwal, T. O. (2021). An Effective Control for Lead-Acid Performance Enhancement in a Hybrid Battery-Supercapacitor System Used in Transport Vehicles. Sustainability, 13(24), 13971. https://doi.org/10.3390/su132413971







