Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops
Abstract
:1. Introduction
2. Global Market for PGPR-Based Biostimulants
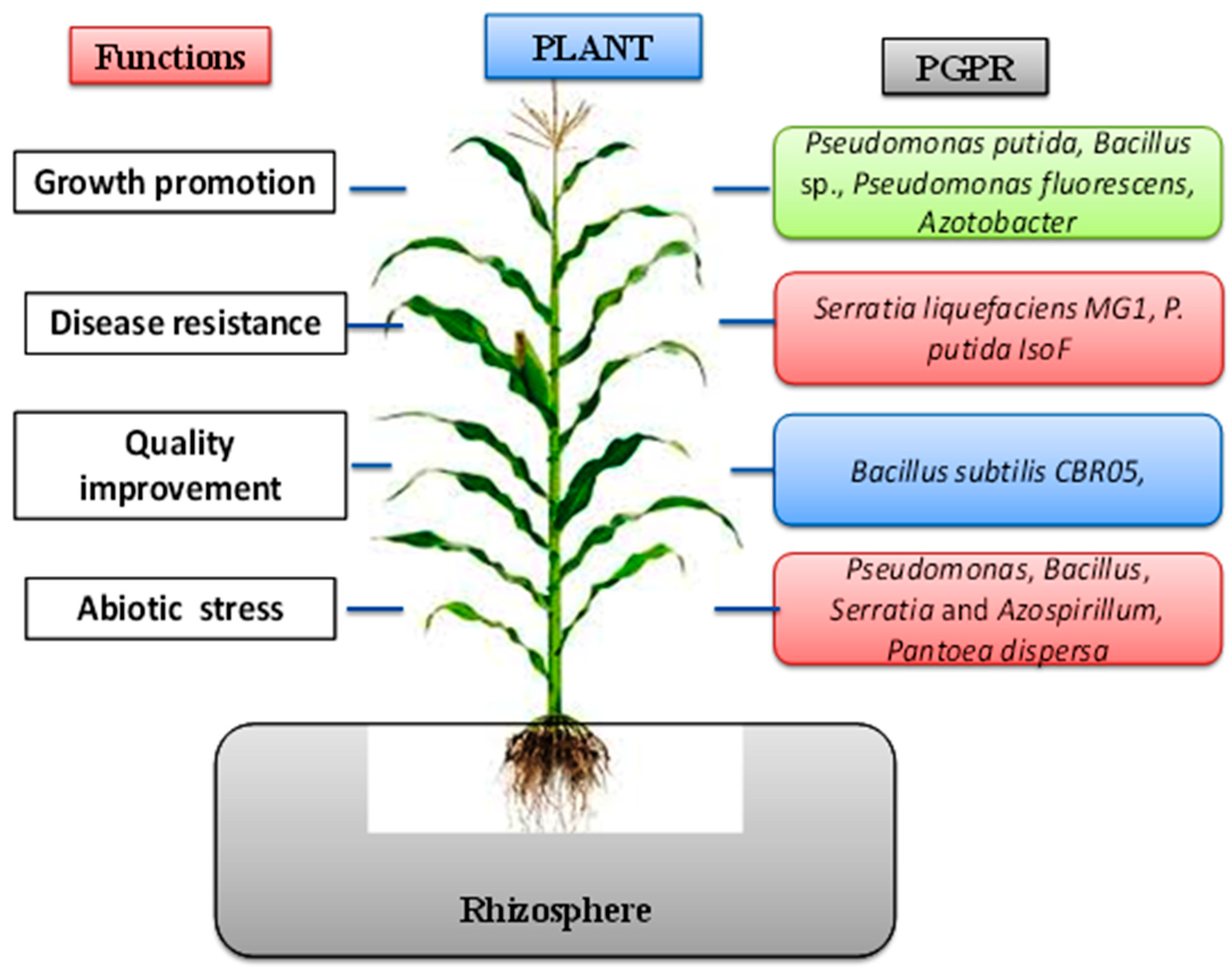
3. Bacterial Plant Biostimulants, Beneficial Effects, and Mode of Action
3.1. Plant Growth Promotion and Nutrient Acquisition
3.1.1. Phytohormone Stimulation
3.1.2. Nitrogen
3.1.3. Phosphorus
3.1.4. Potassium
3.1.5. Micronutrients
3.2. Quality Improvement of Crop and Yield by Bacterial Plant Biostimulants
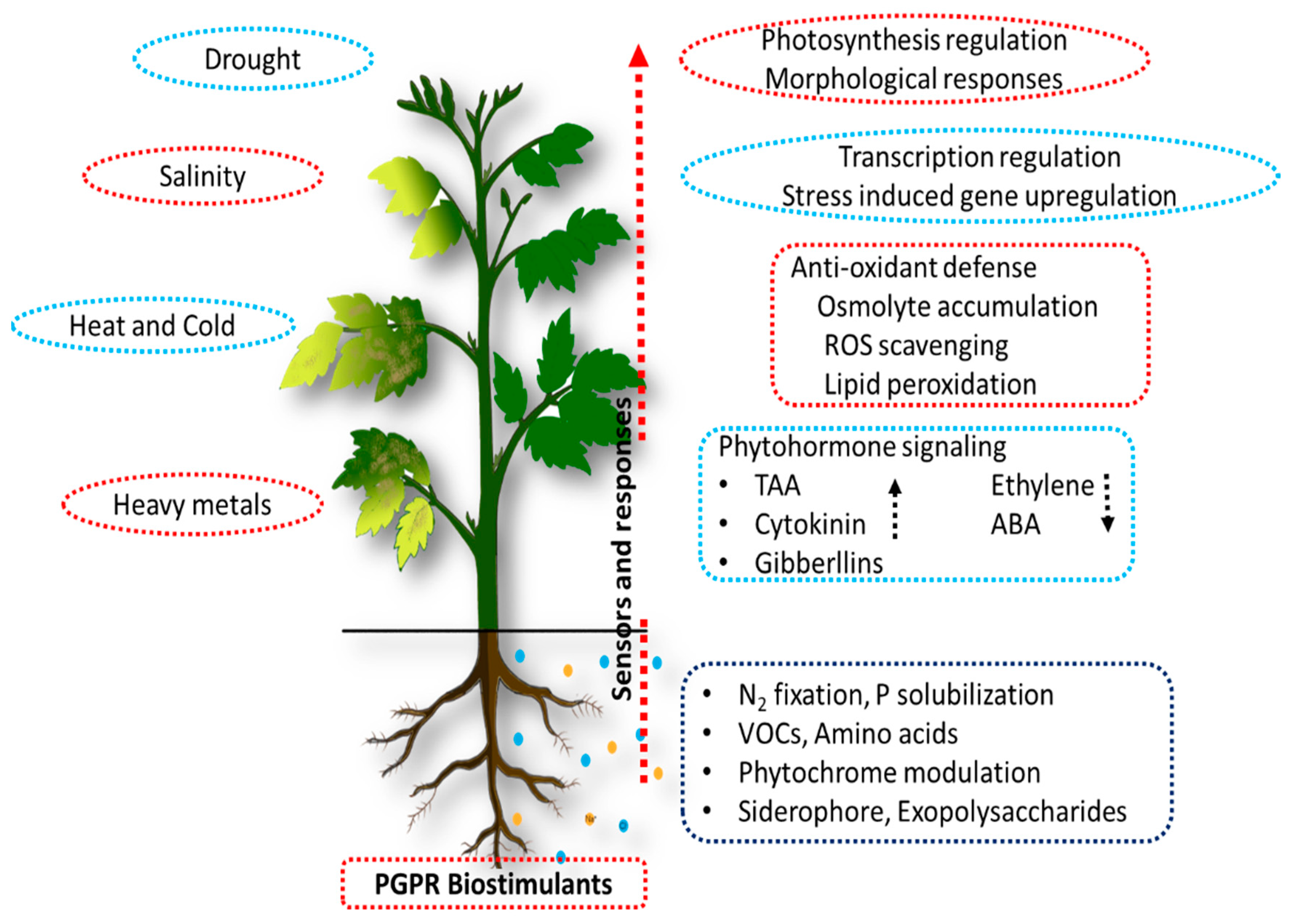
3.3. Abiotic Stress Tolerance Induced by Bacterial Plant Biostimulants
3.3.1. Drought Stress
3.3.2. Salinity Stress
3.3.3. Heat Stress
3.3.4. Cold Stress
3.3.5. Heavy Metal Stress
3.4. Disease Suppression/Defense against Plant Pathogens through Antagonism
3.4.1. Antibiosis
3.4.2. VOC Antagonism
3.4.3. Lysis by Extracellular Enzymes
3.4.4. Bacteriocins
3.4.5. Siderophores
3.5. Induction of Systemic Resistance (ISR)
3.5.1. Systemic Acquired Resistance (SAR)
3.5.2. Induced Systemic Resistance (ISR)
3.5.3. Induced Systemic Tolerance (IST)
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebert, A.W.; Engels, J.M. Plant Biodiversity and Genetic Resources Matter! Plants 2020, 9, 1706. [Google Scholar] [CrossRef]
- Shahzad, A.; Ullah, S.; Dar, A.A.; Sardar, M.F.; Mehmood, T.; Tufail, M.A.; Shakoor, A.; Haris, M. Nexus on climate change: Agriculture and possible solution to cope future climate change stresses. Environ. Sci. Pollut. Res. 2021, 7, 1–22. [Google Scholar]
- Sangiorgio, D.; Cellini, A.; Donati, I.; Pastore, C.; Onofrietti, C.; Spinelli, F. Facing climate change: Application of microbial biostimulants to mitigate stress in horticultural crops. Agronomy 2020, 10, 794. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total. Environ. 2020, 751, 141763. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [Green Version]
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of Climate Change on Agriculture and Its Mitigation Strategies: A Review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water scarcity and future challenges for food production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Kałużewicz, A.; Bosiacki, M.; Spiżewski, T. Influence of biostimulants on the content of macro-and micronutrients in broccoli plants exposed to drought stress. J. Elem. 2018, 23, 287–296. [Google Scholar]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Daneshmand, H.; Alaghmand, S.; Camporese, M.; Talei, A.; Yeh, P.J.F.; Daly, E. Long-term impacts of partial afforestation on water and salt dynamics of an intermittent catchment under climate change. Water 2020, 12, 1067. [Google Scholar] [CrossRef] [Green Version]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hort. 2015, 31, 1–17. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–2. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Toward sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- E.U. Regulation of the European Parliament and the Council Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No. 1107/2009 and Repealing Regulation (EC) No 2003/2003. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L:2019:170:TOC (accessed on 11 January 2021).
- Szczałba, M.; Kopta, T.; Gąstoł, M.; Sękara, A. Comprehensive insight into arbuscular mycorrhizal fu ngi, Trichoderma spp. and plant multilevel interactions with emphasis on biostimulation of horticultural crops. J. Appl. Microbiol. 2019, 127, 630–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozniak, E.; Blaszczak, A.; Wiatrak, P.; Canady, M. Biostimulant Mode of Action: Impact of Biostimulant on Whole-Plant Level. In The Chemical Biology of Plant Biostimulants; Geelan, D., Xu, L., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2020; pp. 205–227. [Google Scholar]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [Green Version]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hort. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Le Van Duhamel, M.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, I.A.; Al-Suod, H.; Ligor, M.; Monedeiro, F.; Buszewski, B. Effects of growth conditions and cultivability on the content of cyclitols in Medicago sativa. Int. J. Environ. Sci. Technol. 2020, 18, 33–48. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hort. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Lugtenberg, B. Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture; Lugtenberg, B., Ed.; Springer: Cham, Switzerland, 2015; pp. 1–15. [Google Scholar]
- Rouphael, Y.; Colla, G. Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Dunhamtrimmer.com. 2018. Available online: http://dunhamtrimmer.com/products-services (accessed on 11 January 2021).
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1801. [Google Scholar] [CrossRef]
- Le Mire, G.; Nguyen, M.; Fassotte, B.; du Jardin, P.; Verheggen, F.; Delaplace, P.; Jijakli, H. Implementing biostimulants and biocontrol strategies in the agroecological management of cultivated ecosystems. Biotechnol. Agron. Société Environ. 2016, 20, 1–15. [Google Scholar]
- Aamir, M.; Rai, K.K.; Zehra, A.; Dubey, M.K.; Kumar, S.; Shukla, V.; Upadhyay, R.S. Microbial bioformulation-based plant biostimulants: A plausible approach toward next generation of sustainable agriculture. In Microbial Endophytes; Woodhead Publishing: Cambridge, UK, 2020; pp. 195–225. [Google Scholar]
- Mishra, J.; Arora, N.K. Bioformulations for plant growth promotion and combating phytopathogens: A sustainable approach. In Bioformulations: For Sustainable Agriculture; Springer: New Delhi, India, 2016; pp. 3–33. [Google Scholar]
- Dellagi, A.; Quillere, I.; Hirel, B. Beneficial soil-borne bacteria, and fungi: A promising way to improve plant nitrogen acquisition. J. Exp. Bot. 2020, 7, 4469–4479. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, R.Z.; Patel, P.R.; Shaikh, S.S. Plant Growth Promotion and Root Colonization by EPS Producing Enterobacter sp. RZS5 under Heavy Metal Contaminated Soil. Indian J. Exp. Biol. 2015, 53, 116–123. [Google Scholar] [PubMed]
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Shaikh, S.S.; Sayyed, R.Z. Dynamism of PGPR in bioremediation and plant growth promotion in heavy metal contaminated soil. Indian J. Exp. Biol. 2016, 54, 286–290. [Google Scholar]
- Woźniak, M.; Gałązka, A.; Tyśkiewicz, R.; Jaroszuk-Ściseł, J. Endophytic Bacteria Potentially Promote Plant Growth by Synthesizing Different Metabolites and their Phenotypic/Physiological Profiles in the Biolog GEN III MicroPlateTM Test. Int. J. Mol. Sci. 2019, 20, 5283. [Google Scholar] [CrossRef] [Green Version]
- Kabiraj, A.; Majhi, K.; Halder, U.; Let, M.; Bandopadhyay, R. Role of Plant Growth-Promoting Rhizobacteria (PGPR) for crop stress management. In Sustainable Agriculture in the Era of Climate Change; Springer: Cham, Switzerland, 2020; pp. 367–389. [Google Scholar]
- Papik, J.; Folkmanova, M.; Polivkova, M.; Suman, J.; Uhlik, O. The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol. Adv. 2020, 44, 107614. [Google Scholar] [CrossRef]
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirb. Agric. 2015, 102, 465–478. [Google Scholar] [CrossRef] [Green Version]
- Wemheuer, F.; Kaiser, K.; Karlovsky, P.; Daniel, R.; Vidal, S.; Wemheuer, B. Bacterial endophyte communities of three agricultural important grass species differ in their response towards management regimes. Sci. Rep. 2017, 7, 1–13. [Google Scholar]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.; Schenk, P.M. Inner plant values: Diversity, colonization, and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Pii, Y.; Penn, A.; Gattullo, C.E.; Allegretta, I.; Terzano, R.; Crecchio, C.; Mimmo, T.; Cesco, S. The interactions between plant, microorganism, and soil affect Fe acquisition in cucumber plants. Center Soil Ecol. 2015, 87, 45–52. [Google Scholar]
- Sayyed, R.Z.; Arora, N.K.; Reddy, M.S. Rhizobacteria in Abiotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer Nature: Singapore, 2019; Volume 1, pp. 1–362. [Google Scholar]
- Nguyen, M.L.; Spaepen, S.; du Jardin, P.; Delaplace, P. Biostimulant effects of rhizobacteria on wheat growth and nutrient uptake depend on nitrogen application and plant development. Arch Agron. Soil Sci. 2019, 65, 58–73. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of Bacterial Inoculum and Fertigation Management on Nursery and Field Production of Lettuce Plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Pellegrini, M.; Spera, D.; Ercole, C.; del Gallo, M. Allium cepa L. seed inoculation with a consortium of plant growth-promoting bacteria: Effects on plant growth and development and soil fertility status and microbial community. Proceedings 2020, 6, 20. [Google Scholar]
- He, Y.; Pantigoso, H.A.; Wu, Z.; Vivanco, J.M. Co-inoculation of Bacillus sp. and Pseudomonas putida at different development stages acts as a biostimulant to promote growth, yield, and nutrient uptake of tomato. J. Appl. Microbiol. 2019, 127, 196–207. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chun, S.C.; Oh, J.W.; Paramasivan, M.; Saini, R.K.; Sahayarayan, J.J. Bacillus subtilis CBR05 for Tomato (Solanum lycopersicum) Fruits in South Korea as a Novel Plant Probiotic Bacterium (PPB): Implications from Total Phenolics, Flavonoids, and Carotenoids Content for Fruit Quality. Agronomy 2019, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Akanda, A.M.; Prova, A.; Islam, M.T.; Hossain, M.M. Isolation and identification of plant growth-promoting rhizobacteria from cucumber rhizosphere and their effect on plant growth promotion and disease suppression. Front. Microbiol. 2016, 6, 1360. [Google Scholar] [CrossRef] [Green Version]
- Mangmang, J.S.; Deaker, R.; Rogers, G. Optimal plant growth-promoting concentration of Azospirillum brasilense inoculated to cucumber, lettuce, and tomato seeds varies between bacterial strains. Isr. J. Plant Sci. 2015, 62, 145–152. [Google Scholar] [CrossRef]
- Jha, Y.; Subramanian, R.B. PGPR regulate the caspase-like activity, programmed cell death, and antioxidant enzyme activity in paddy under salinity. Physiol. Mol. Biol. Plants 2014, 20, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum Brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Hussain, S.; Matloob, A.; Khan, F.A.; Khaliq, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.; Ullah, N.; et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Reg. 2015, 75, 391–404. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Llorente, B.E.; Alasia, M.A.; Larraburu, E.E. Biofertilization with Azospirillum brasilense improves in vitro culture of Handroanthus ochraceus, a forestry, ornamental and medicinal plant. New Biotechnol. 2016, 33, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Faruque, O.M.; Miwa, H.; Yasuda, M.; Fujii, Y.; Kaneko, T.; Sato, S.; Okazaki, S. Identification of Bradyrhizobium elkanii genes involved in incompatibility with soybean plants carrying the Rj4 allele. Appl. Environ. Microbiol. 2015, 81, 6710–6717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-León, R.; Rojas-Solís, D.; Contreras-Pérez, M.; del Carmen Orozco-Mosqueda, M.; Macías-Rodríguez, L.I.; Reyes-de la Cruz, H.; Valencia-Cantero, E.; Santoyo, G. Characterization of the antifungal and plant growth-promoting effects of diffusible and volatile organic compounds produced by Pseudomonas fluorescens strains. Biol. Control 2015, 81, 83–92. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant growth-promoting rhizobacteria (PGPR): Current and prospects for the development of sustainable agriculture. J. Microbiol. Biochem. Technol. 2015, 7, 096–102. [Google Scholar]
- Jha, C.K.; Saraf, M. Plant growth-promoting rhizobacteria (PGPR): A review. J. Agric. Res. Dev. 2015, 5, 108–119. [Google Scholar]
- Heydarian, Z.; Yu, M.; Gruber, M.; Glick, B.R.; Zhou, R.; Hegedus, D.D. Inoculation of soil with plant growth-promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression of the corresponding acdS gene in transgenic plants increases salinity tolerance in Camelina sativa. Front. Microbiol. 2016, 7, 1966. [Google Scholar] [CrossRef] [PubMed]
- Bucao, D.S.; Yapit, R.H.; Gabriel, M.L.S. Biochemical Characterization of Microbials and Their Effects on the Growth and Yield of Multiplier Onion (Allium ascalonicum L.) in Northwestern Philippines. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Springer: Singapore, 2019; pp. 75–91. [Google Scholar]
- Kumar, R.; Saurabh, K.; Kumawat, N.; Sundaram, P.K.; Mishra, J.S.; Singh, D.K.; Hans, H.; Krishna, B.; Bhatt, B.P. Sustaining productivity through integrated use of microbes in agriculture. In Role of Microbial Communities for Sustainability; Springer: Singapore, 2021; pp. 109–145. [Google Scholar]
- Singh, M.; Dotaniya, M.L.; Mishra, A.; Dotaniya, C.K.; Regar, K.L.; Lata, M. Role of biofertilizers in conservation agriculture. In Conservation Agriculture; Bisht, J., Meena, V., Mishra, P., Pattanayak, A., Eds.; Springer: Singapore, 2016; pp. 113–134. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Raizada, M.N. A meta-analysis of the effectiveness of diverse rhizobia inoculants on soybean traits under field conditions. Soil. Bioland. Biochem. 2017, 105, 177–196. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth-promoting bacteria in agriculture: Two sides of a coin. Appl. Soil. Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Inoculant preparation, and formulations for Azospirillum spp. In Handbook for Azospirillum; Springer: Cham, Switzerland, 2015; pp. 469–485. [Google Scholar]
- Beattie, G.A. Microbiomes: Curating communities from plants. Nature 2015, 528, 340–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, M.; Kiran, S.; Gulati, A.; Singh, B.; Tewari, R. Isolation and identification of phosphate solubilizing bacteria able to enhance the growth and aloin-A biosynthesis of Aloe barbadensis Miller. Microbiol. Res. 2012, 167, 358–363. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils; Springer Plus: Berlin/Heidelberg, Germany, 2013; Volume 2, p. 587. [Google Scholar]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Suleman, M.; Yasmin, S.; Rasul, M.; Yahya, M.; Atta, B.M.; Mirza, M.S. Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS ONE 2018, 13, 0204408. [Google Scholar] [CrossRef] [Green Version]
- Bechtaoui, N.; Raklami, A.; Benidire, L.; Tahiri, A.I.; Göttfert, M.; Oufdou, K. Effects of PGPR co-inoculation on growth, phosphorus nutrition and phosphatase/phytase activities of faba bean under different phosphorus availability conditions. Pol. J. Environ. Stud. 2020, 29, 1557–1565. [Google Scholar] [CrossRef]
- Nath, D.; Maurya, B.R.; Meena, V.S. Documentation of five potassium-and phosphorus-solubilizing bacteria for their K and P-solubilization ability from various minerals. Biocatal. Agric. Biotechnol. 2017, 10, 174–181. [Google Scholar] [CrossRef]
- Meena, V.S.; Meena, S.K.; Verma, J.P.; Kumar, A.; Aeron, A.; Mishra, P.K.; Bisht, J.K.; Pattanayak, A.; Naveed, M.; Dotaniya, M.L. Plant beneficial rhizospheric microorganism (PBRM) strategies to improve nutrients use efficiency: A review. Ecol. Eng. 2017, 107, 8–32. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Parmar, P.; Phour, M.; Sehrawat, A. Potassium-solubilizing microorganisms (KSMs) and its effect on plant growth improvement. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 171–185. [Google Scholar]
- Bahadur, I.; Maurya, B.R.; Meena, V.S.; Saha, M.; Kumar, A.; Aeron, A. Mineral release dynamics of tricalcium phosphate and waste muscovite by mineral-solubilizing rhizobacteria isolated from Indo-Gangetic plain of India. Geomicrobiol. J. 2017, 34, 454–466. [Google Scholar] [CrossRef]
- Liu, J.; Aronsson, H.; Ulen, B.; Bergström, L. Potential phosphorus leaching from sandy topsoils with different fertilizer histories before and after application of pig slurry. Soil. Use Manag. 2012, 28, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Mehnaz, S. An overview of globally available bioformulations. In Bioformulations: Sustainable Agriculture; Arora, N.K., Mehnaz, S., Balestrini, R., Eds.; Springer: Singapore, 2016; pp. 267–281. [Google Scholar]
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Biores. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.; Pereira, W.; de Albuquerque Silva, P.; Baldani, J.I.; Boddey, R.M.; Alves, B.J.R.; Urquiaga, S.; Reis, V.M. Yield of sugarcane varieties and their sugar quality grown in different soil types and inoculated with a diazotrophic bacteria consortium. Plant Prod. Sci. 2018, 20, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Shakeel, M.; Rais, A.; Hassan, M.N.; Hafeez, F.Y. Root associated Bacillus sp. improves growth, yield, and zinc translocation for basmati rice (Oryza sativa) varieties. Front. Microbiol. 2015, 6, 1286. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Devi, S.H.; Basumatary, A.; Dutta, S.; Singh, L.K.; Kalita, P.; Bora, S.S.; Devi, S.R.; Saikia, A.; Sharma, P.; et al. Biostimulants: Potential and Prospects in Agriculture. Int. Res. J. Pure Appl. Chem. 2020, 21, 20–35. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hort. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Grzyb, Z.S.; Paszt, L.S.; Piotrowski, W.; Malusa, E. The influence of mycorrhizal fungi on the growth of apple and sour cherry maidens fertilized with different bioproducts in the organic nursery. J. Life Sci. 2015, 9, 221–228. [Google Scholar]
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: The importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caradonia, F.; Ronga, D.; Flore, A.; Barbieri, R.; Moulin, L.; Terzi, V.; Francia, E. Biostimulants and cherry rootstock increased tomato fruit yield and quality in sustainable farming systems. Ital. J. Agron. 2020, 15, 121–131. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Kitir, N.; Unek, C.; Nikerel, E.; Ozdemir, B.S.; Güneş, A.; Mokhtari, N.E.P. Beneficial role of plant growth-promoting bacteria in vegetable production under abiotic stress. In Microbial Strategies for Vegetable Production; Zaidi, A., Khan, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 151–166. [Google Scholar] [CrossRef]
- Ji, J.; Yuan, D.; Jin, C.; Wang, G.; Li, X.; Guan, C. Enhancement of growth and salt tolerance of rice seedlings (Oryza sativa L.) by regulating ethylene production with a novel halotolerant PGPR strain Glutamicibacter sp. YD01 containing ACC deaminase activity. Acta Physiol. Plant. 2020, 42, 1–17. [Google Scholar] [CrossRef]
- Ilyas, N.; Mazhar, R.; Yasmin, H.; Khan, W.; Iqbal, S.; Enshasy, H.E.; Dailin, D.J. Rhizobacteria isolated from saline soil induce systemic tolerance in wheat (Triticum aestivum L.) against salinity stress. Agronomy 2020, 10, 989. [Google Scholar] [CrossRef]
- Filgueiras, L.; Silva, R.; Almeida, I.; Vidal, M.; Baldani, J.I.; Meneses, C.H.S.G. Gluconacetobacter diazotrophicus mitigates drought stress in Oryza sativa L. Plant Soil. 2019, 451, 57–73. [Google Scholar] [CrossRef]
- Silva, R.; Filgueiras, L.; Santos, B.; Coelho, M.; Silva, M.; Estrada-Bonilla, G.; Meneses, C. Gluconacetobacter diazotrophicus changes the molecular mechanisms of root development in Oryza sativa L. growing under water stress. Int. J. Mol. Sci. 2020, 21, 333. [Google Scholar] [CrossRef] [Green Version]
- Garcia, J.E.; Maroniche, G.; Creus, C.; Suarez-Rodriguez, R.; Ramirez-Trujillo, J.A.; Groppa, M.D. In vitro PGPR properties and osmotic tolerance of different Azospirillum native strains and their effects on growth of maize under drought stress. Microbiol. Res. 2017, 202, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Galván, A.; Romero-Perdomo, F.A.; Estrada-Bonilla, G.; Meneses, C.H.S.G.; Bonilla, R.R. Dry-caribbean Bacillus spp. strains ameliorate drought stress in maize by a strain-specific antioxidant response modulation. Microorganisms 2020, 8, 823. [Google Scholar]
- Fatima, T.; Arora, N.K. Pseudomonas entomophila PE3 and its exopolysaccharides as biostimulants for enhancing growth, yield, and tolerance responses of sunflower under saline conditions. Microbiol. Res. 2021, 244, 126671. [Google Scholar] [CrossRef]
- Tiryaki, D.; Aydın, İ.; Atıcı, Ö. Psychrotolerant bacteria isolated from the leaf apoplast of cold-adapted wild plants improve the cold resistance of bean (Phaseolus vulgaris L.) under low temperature. Cryobiology 2019, 86, 111–119. [Google Scholar] [CrossRef]
- Qin, Y.; Fu, Y.; Kang, W.; Li, H.; Gao, H.; Vitalievitch, K.S.; Liu, H. Isolation and identification of a cold-adapted bacterium and its characterization for biocontrol and plant growth-promoting activity. Ecol. Eng. 2017, 105, 362–369. [Google Scholar] [CrossRef]
- Zerrouk, I.Z.; Benchabane, M.; Khelifi, L.; Yokawa, K.; Ludwig-Muller, J.; Baluska, F. Pseudomonas strain isolated from date-palm rhizospheres improves root growth and promotes root formation in maize exposed to salt and aluminum stress. J. Plant Physiol. 2016, 191, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Khan, M.S. Heavy metal-induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen-fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Ngumbi, E.; Kloepper, J. Bacterial-mediated drought tolerance: Current and future prospects. Appl. Soil Ecol. 2016, 10, 109–125. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; Ali, S.Z. Enhancement of drought stress tolerance in crops by plant growth-promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Jochum, M.D.; McWilliams, K.L.; Borrego, E.J.; Kolomiets, M.V.; Niu, G.; Pierson, E.A.; Jo, Y.K. Bioprospecting plant growth-promoting rhizobacteria that mitigate drought stress in grasses. Front. Microbiol. 2019, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Samrah, A.A.; Ikram, R.; Rizwan, M.; Akhtar, N.; Yasmin, H.; Sayyed, R.Z.; Ali, S.; Ilyas, N. 24-Epibrassinolide regulated antioxidants and osmolyte defense and endogenous hormones in two wheat varieties under drought stress. Physiol. Plantarum. 2020, 1–11. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for the cultivation of saline soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Smith, D.L. Plant growth-promoting rhizobacteria in amelioration of salinity stress: A systems biology perspective. Front. Plant Sci. 2017, 8, 1768. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Timmermans, J.; Chen, Q.; van Bodegom, P.M. A Review of Remote Sensing Challenges for Food Security with Respect to Salinity and Drought Threats. Remote Sens. 2021, 13, 6. [Google Scholar] [CrossRef]
- Fazeli-Nasab, B.; Sayyed, R.Z. Plant growth-promoting rhizobacteria and salinity stress: A journey into the soil. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 21–34. [Google Scholar]
- Pan, J.; Peng, F.; Xue, X.; You, Q.; Zhang, W.; Wang, T.; Huang, C. The growth promotion of two salt-tolerant plant groups with PGPR inoculation: A meta-analysis. Sustainability. 2019, 11, 378. [Google Scholar] [CrossRef] [Green Version]
- Malhotra, S.K. Horticultural crops and climate change—A review. Indian J. Agric. Sci. 2017, 87, 12–22. [Google Scholar]
- Pereira, F. Rhizobacteria as Bioprotectants against stress conditions. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 157–177. [Google Scholar]
- Canarini, A.; Dijkstra, F.A. Dry-rewetting cycles regulate wheat carbon rhizodeposition, stabilization, and nitrogen cycling. Soil Biol. Biochem. 2015, 81, 195–203. [Google Scholar] [CrossRef]
- Singh, J.; Singh, P.; Ray, S.; Rajput, R.S.; Singh, H.B. Plant growth-promoting rhizobacteria: Benign and useful substitute for mitigation of biotic and abiotic stresses. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 81–101. [Google Scholar]
- Huang, S.; Zuo, T.; Ni, W. Important roles of glycine betaine in stabilizing the structure and function of the photosystem II complex under abiotic stresses. Planta 2020, 251, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth-promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress-responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.M.; Asaf, S.; Khan, A.L.; Khan, A.; Mun, B.G.; Khan, M.A.; Gul, H.; Lee, I.J. Complete genome sequence of Pseudomonas psychrotolerans CS51, a plant growth-promoting bacterium, under heavy metal stress conditions. Microorganisms 2020, 8, 382. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef]
- Hassan, T.U.; Bano, A.; Naz, I. Alleviation of heavy metals toxicity by the application of plant growth-promoting rhizobacteria and effects on wheat grown in saline-sodic field. Int. J. Phytoremed. 2017, 19, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Niu, D.D.; Zheng, Y.; Zheng, L.; Jiang, C.H.; Zhou, D.M.; Guo, J.H. Application of PSX biocontrol preparation confers root-knot nematode management and increased fruit quality in tomato under field conditions. Biocont. Sci. Technol. 2016, 26, 174–180. [Google Scholar] [CrossRef]
- Munhoz, L.D.; Fonteque, J.P.; Santos, I.M.O.; Navarro, M.O.P.; Simionato, A.S.; Goya, E.T.; Rezende, M.I.; Balbi-Peña, M.L.; de Oliveira, A.G.; Andrade, G. Control of bacterial stem rot on tomato by extracellular bioactive compounds produced by Pseudomonas aeruginosa LV strain. Cogent Food Agric. 2017, 31, 1282592. [Google Scholar] [CrossRef]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic Strain Bacillus subtilis 26DCryChS Producing Cry1Ia Toxin from Bacillus thuringiensis Promotes Multifaceted Potato Defense against Phytophthora infestans (Mont.) de Bary and Pest Leptinotarsa decemlineata Say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef]
- Daranas, N.; Badosa, E.; Francés, J.; Montesinos, E.; Bonaterra, A. Enhancing water stress tolerance improves fitness in biological control strains of Lactobacillus plantarum in-plant environments. PLoS ONE 2018, 13, 0190931. [Google Scholar] [CrossRef] [Green Version]
- Meena, M.; Zehra, A. Tomato: A model plant to study plant-pathogen interactions. Food Sci. Nutr. Technol. 2019, 4, 000171. [Google Scholar]
- Liu, K.; Garrett, C.; Fadamiro, H.; Kloepper, J.W. Induction of systemic resistance in Chinese cabbage against black rot by plant growth-promoting rhizobacteria. Biol. Control. 2016, 99, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Zehra, A.; Meena, M.; Dubey, M.K.; Aamir, M.; Upadhyay, R.S. Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Bot. Stud. 2017, 58, 44. [Google Scholar] [CrossRef] [PubMed]
- Goutam, J.; Singh, R.; Vijayaraman, R.S.; Meena, M. Endophytic fungi: Carrier of potential antioxidants. In Fungi and Their Role in Sustainable Development: Current Perspectives; Gehlot, P., Singh, J., Eds.; Springer: Singapore, 2018; pp. 539–551. [Google Scholar]
- Dinesh, R.; Anandaraj, M.; Kumar, A.; Bini, Y.K.; Subila, K.P.; Aravind, R. Isolation, characterization, and evaluation of multi-trait plant growth-promoting rhizobacteria for their growth-promoting and disease suppressing effects on ginger. Microbiol. Res. 2015, 173, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, R.Z. Rhizobacteria in Biotic Stress Management. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer Nature: Singapore, 2019; Volume 2, pp. 1–419. [Google Scholar]
- Ulloa-Ogaz, A.L.; Muñoz-Castellanos, L.N.; Nevárez-Moorillón, G.V. Biocontrol of phytopathogens: Antibiotic production as a mechanism of control. In The Battle Against Microbial Pathogens: Basic Science, Technological Advances, and Educational Programs; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2015; pp. 305–309. [Google Scholar]
- Fernando, W.; Nakkeeran, S.; Zhang, Y.; Savchuk, S. Biological control of Sclerotinia Sclerotiorum (lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop. Prot. 2018, 26, 100–107. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, V.; Tripathi, R.B. Isolation of phosphate solubilizing microorganism (PSMs) from the soil. J. Microbiol. Bitechnol. Res. 2017, 1, 90–95. [Google Scholar]
- Ramadan, E.M.; AbdelHafez, A.A.; Hassan, E.A.; Saber, F.M. Plant growth-promoting rhizobacteria and their potential for biocontrol of phytopathogens. Afr. J. Microbiol. Res. 2016, 10, 486–504. [Google Scholar]
- Wang, X.; Mavrodi, D.V.; Ke, L.; Mavrodi, O.V.; Yang, M.; Thomashow, L.S.; Zhang, N.; Weller, D.M.; Zhang, J. Biocontrol and plant growth-promoting activity of rhizobacteria from Chinese fields with contaminated soils. Microb. Biotechnol. 2015, 8, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Yousaf, S.; Rajer, F.U. Plant growth-promoting activity of volatile organic compounds produced by biocontrol strains. Sci. Lett. 2016, 4, 40–43. [Google Scholar]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, S.S.; Sharma, R. Amelioration of biotic stress by application of rhizobacteria for agriculture sustainability. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Singapore, 2019; pp. 111–168. [Google Scholar]
- Suryadi, Y.; Susilowati, D.N.; Fauziah, F. Management of plant diseases by PGPR-mediated induced resistance with special reference to tea and rice crops. In Plant Growth Promoting Rhizobacteria for Sustainable Stress Management; Springer: Berlin/Heidelberg, Germany, 2019; pp. 65–110. [Google Scholar]
- Kang, B.R.; Anderson, A.J.; Kim, Y.C. Hydrogen cyanide produced by Pseudomonas chlororaphis o6 exhibits nematicidal activity against Meloidogyne Hapla. Plant Pathol. J. 2018, 34, 35–43. [Google Scholar] [CrossRef]
- Santoro, M.V.; Bogino, P.C.; Nocelli, N.; Cappellari, L.R.; Giordano, W.F.; Banchio, E. Analysis of plant growth-promoting effects of Fluorescent Pseudomonas strains isolated from Mentha piperita rhizosphere and effects of their volatile organic compounds on essential oil composition. Front. Microbiol. 2016, 7, 1085. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E. Plant defense by VOC-induced microbial priming. Trends Plant Sci. 2019, 24, 187–189. [Google Scholar] [CrossRef]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth-promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1–19. [Google Scholar] [CrossRef]
- Rakshiya, Y.S.; Verma, M.K.; Sindhu, S.S. Efficacy of antagonistic soil bacteria in the management of subterranean termites (Isoptera). Res. Environ. Life Sci. 2016, 9, 949–955. [Google Scholar]
- Rooney, W.M.; Grinter, R.W.; Correia, A.; Parkhill, J.; Walker, D.C.; Milner, J.J. Engineering bacteriocin-mediated resistance against the plant pathogen Pseudomonas syringae. Plant Biotechnol. J. 2020, 18, 1296–1306. [Google Scholar] [CrossRef] [Green Version]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of global trends in classification, methods of preparation and application of bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Príncipe, A.; Fernandez, M.; Torasso, M.; Godino, A.; Fischer, S. Effectiveness of tailocins produced by prin in controlling the bacterial-spot disease in tomatoes caused by Xanthomonas vesicatoria. Microbiol. Res. 2018, 213, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Rooney, W.M.; Chai, R.; Milner, J.J.; Walker, D. Bacteriocins targeting Gram-negative phytopathogenic bacteria: Plantibiotics of the future. Front. Microbiol. 2020, 11, 2283. [Google Scholar] [CrossRef]
- Riaz, U.; Murtaza, G.; Anum, W.; Samreen, T.; Sarfraz, M.; Nazir, M.Z. Plant Growth-Promoting Rhizobacteria (PGPR) as biofertilizers and biopesticides. In Microbiota and Biofertilizers; Springer: Cham, Switzerland, 2021; pp. 181–196. [Google Scholar]
- Reed, S.C.; Yang, X.; Thornton, P.E. Incorporating phosphorus cycling into global modeling efforts: A worthwhile, tractable endeavor. New Phytol. 2015, 208, 324–329. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Ilyas, N.; Tabassum, B.; Hashem, A.; Abd Allah, E.F.; Jadhav, H.P. Plausible role of plant growth-promoting Rhizobacteria in future climatic scenario. In Enviromet Biotechnology: For Sustainable Future; Sobti, R., Arora, N., Kothari, R., Eds.; Springer: Singapore, 2019; pp. 175–197. [Google Scholar]
- Abdul Malik, N.A.; Kumar, I.S.; Nadarajah, K. Elicitor and receptor molecules: Orchestrators of plant defense and immunity. Int. J. Mol. Sci. 2020, 21, 963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maithani, D.; Singh, H.; Sharma, A. Stress alleviation in plants using SAR and ISR: Current views on stress signaling network. In Microbes and Signaling Biomolecules Against Plant Stress; Springer: Singapore, 2021; pp. 7–36. [Google Scholar]
- Meena, M.; Swapnil, P.; Divyanshu, K.; Kumar, S.; Tripathi, Y.N.; Zehra, A.; Marwal, A.; Upadhyay, R.S. PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: Current perspectives. J. Basic. Microbiol. 2020, 60, 828–861. [Google Scholar] [PubMed]
- David, L.; Kang, J.; Dufresne, D.; Zhu, D.; Chen, S. Multi-Omics Revealed Molecular Mechanisms Underlying Guard Cell Systemic Acquired Resistance. Int. J. Mol. Sci. 2021, 22, 191. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef]
- Shaikh, S.S.; Sayyed, R.Z.; Reddy, M.S. Plant growth-promoting rhizobacteria: An eco-friendly approach for sustainable agroecosystem. In Plant, Soil, and Microbes; Springer: Cham, Switzerland, 2016; pp. 181–201. [Google Scholar]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, L.D. Plant growth-promoting rhizobacteria: Context, mechanisms of action, and roadmap to the commercialization of biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced systemic resistance (ISR) in plants: Mechanism of action. Ind. J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [Green Version]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid-dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, D.; Wang, X.; Wang, Y.; Song, X.; Wang, J.; Guo, J.; Zhao, H. Bacillus cereus AR156 activates PAMP-triggered immunity and induces a systemic acquired resistance through a NPR1-and SA-dependent signaling pathway. Biochem. Biophys. Res. 2016, 469, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of plants with microbial world: Exploring the regulatory networks for PGPR mediated defense signaling. Microb. Res. 2020, 23, 126486. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.; Song, Y.; Kloepper, J.W.; Fadamiro, H. Rhizobacteria activates (+)-δ-cadinene synthase genes and induces systemic resistance in cotton against beet armyworm (Spodoptera exigua). Plant Cell Environ. 2016, 39, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Khoshru, B.; Mitra, D.; Khoshmanzar, E.; Myo, E.M.; Uniyal, N.; Mahakur, B.; Mohapatra, P.K.D.; Panneerselvam, P.; Boutaj, H.; Alizadeh, M.; et al. Current scenario and future prospects of plant growth-promoting rhizobacteria: An economic valuable resource for the agriculture revival under stressful conditions. J. Plant Nutr. 2020, 43, 3062–3092. [Google Scholar] [CrossRef]
- Rashid, M.H.O.; Chung, Y.R. Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 2017, 8, 1816. [Google Scholar] [CrossRef] [Green Version]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.L.; Venkatasalam, E.P.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth-promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Serteyn, L.; Quaghebeur, C.; Ongena, M.; Cabrera, N.; Barrera, A.; Molina-Montenegro, A.M.; Francis, F.; Ramírez, C.C. Induced Systemic Resistance by a Plant Growth-Promoting Rhizobacterium Impacts Development and Feeding Behavior of Aphids. Insects 2020, 11, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.N.; Erb, M.; Hartley, S.E. Roots under attack: Contrasting plant responses to below- and aboveground insect herbivory. New Phytologist. 2016, 210, 413–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schikora, A.; Schenk, S.T.; Hartmann, A. Beneficial effects of bacteria plant communication based on quorum sensing molecules of the N-acyl homoserine lactone group. Plant Mol. Biol. 2016, 90, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2020, 18, 152–163. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.G.; Lee, K.E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monnier, N.; Furlan, A.; Botcazon, C.; Dahi, A.; Mongelard, G.; Cordelier, S. Rhamnolipids from Pseudomonas aeruginosa are elicitors triggering Brassica napus protection against Botrytis cinerea without physiological disorders. Front. Plant Sci. 2018, 9, 1170. [Google Scholar] [CrossRef] [Green Version]
- Mejri, S.; Siah, A.; Coutte, F.; Magnin-Robert, M.; Randoux, B.; Tisserant, B.; Krier, F.; Jacques, P.; Reignault, P.; Halama, P. Biocontrol of the wheat pathogen Zymoseptoria tritici using cyclic lipopeptides from Bacillus subtilis. Environ. Sci. Pollut. Res. 2018, 25, 29822–29833. [Google Scholar] [CrossRef] [PubMed]
- Pršić, J.; Ongena, M. Elicitors of plant immunity triggered by beneficial bacteria. Front. Plant. Sci. 2020, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Rodríguez, M.; Llamas, I.; Béjar, V.; Sampedro, I. Silencing of Phytopathogen Communication by the Halotolerant PGPR Staphylococcus Equorum Strain EN21. Microorganisms 2020, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Reyes, C.; Schenk, S.T.; Neumann, C.; Kogel, K.H.; Schikora, A. N-acyl-homoserine lactones-producing bacteria protect plants against plant and human pathogens. Microb. Biotechnol. 2014, 7, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Omoboye, O.O.; Oni, F.E.; Batool, H.; Yimer, H.Z.; De Mot, R.; Höfte, M. Pseudomonas cyclic lipopeptides suppress the rice blast fungus Magnaporthe oryzae by induced resistance and direct antagonism. Front. Plant Sci. 2019, 10, 901. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Ongena, M.; Höfte, M. The cyclic lipopeptide orfamide induces systemic resistance in rice to Cochliobolus miyabeanus but not to Magnaporthe oryzae. Plant Cell Rep. 2017, 36, 1731–1746. [Google Scholar] [CrossRef] [PubMed]
- Alquéres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for plant growth and mitigation of abiotic stresses: A metabolomics perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from plant and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Vaishnav, A.; Singh, J.; Singh, P.; Rajput, R.S.; Singh, H.B.; Sarma, B.K. Sphingo bacterium sp. BHU-AV3 Induces Salt Tolerance in tomato by enhancing antioxidant activities and energy metabolism. Front. Microbiol. 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.S.; Arshad, A.; Rajput, L.; Fatima, K.; Ullah, S.; Ahmad, M.; Imran, A. Growth-Stimulatory Effect of Quorum Sensing Signal Molecule N-Acyl-Homoserine Lactone-Producing Multi-Trait Aeromonas spp. on Wheat Genotypes Under Salt Stress. Front. Microbiol. 2020, 11, 553621. [Google Scholar] [CrossRef]
- Saif, S.; Khan, M.S. Assessment of toxic impact of metals on proline, antioxidant enzymes, and biological characteristics of Pseudomonas aeruginosa inoculated Cicer arietinum grown in chromium and nickel-stressed sandy clay loam soils. Environ. Monit. Assess. 2018, 190, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Bacillus velezensis 5113 Induced Metabolic and Molecular Reprogramming during Abiotic Stress Tolerance in Wheat. Sci. Rep. 2019, 9, 16282. [Google Scholar] [CrossRef] [Green Version]


| Commercial Products (Manufacturer) | PGPR Strains | Target Crops for Use | Target of Function |
|---|---|---|---|
| FZB24®fl Rhizovital 42® (ABiTEP GmbH, Germany) | Bacillus amyloliquefaciens and B. amyloliquefaciens sp. plantarum | Ornamentals, vegetable field crops | Phosphate availability and protection against pathogens |
| Inomix® Biostimulant, Inomix® phosphore, and Inomix® Biofertilisant (IAB (Iabiotec), Spain) | B. subtilis (IAB/BS/F1) and B. polymyxa (IAB/BP/01); Saccharomyces cerevisiae; B. megaterium and P. fluorescens; and Rhizobium leguminosarum, Azotobacter vinelandii, B. megaterium, and Saccharomyces cerevisiae | Cereals | Plant growth promotion increases root and shoot weight, strong root system |
| BactoFil B10® (AGRO.bio Hungary Kft., Hungary) | Azotobacter vinelandii, Azospirillum lipoferum, P. fluorescens, B. circulans, B. megaterium, and B. subtilis | Dicotyledons (potato, sunflower, rapeseed) | Soil amelioration; produce plant growth-promoting hormones auxin, gibberellins, and kinetin; N2 fixation; a biocontrol agent |
| Bio-Gold (BioPower, Sri Lanka) | Pseudomonas fluorescens and Azotobacter chroococcum | All agricultural and horticultural crops | Growth promotion via nitrogen fixation, drought tolerance, control of root rot and wilt diseases, phosphorus solubilization |
| Cedomon® (Lantmannen BioAgri AB, Sweden) | P. chlororaphis | Barley and oats | Highly effective against various types of seed-borne diseases |
| Rhizosum N Liquid PSA (Mapleton Agri Biotec Pty Limited, Australia) | Azotoformans (N2-fixing bacteria) and Pseudomonas sp | Wheat | Phosphate availability, N2 fixation, plant growth promotion |
| BactoFil A10® (AGRO.bio Hungary Kft., Hungary) | Azotobacter vinelandii, Azospirillum brasilense, P. fluorescens, B. polymyxa, and B. megaterium | Monocotyledons (cereals) | Increased soil nutrient content that results in plant growth promotion |
| Micosat F® Uno; Micosat F® Cereali (CCS Aosta Srl, Italy) | Agrobacterium radiobacter AR 39, Streptomyces sp. SB 14, and B. subtilis BA 41 | Fruits, vegetables, and flowers | Increased nutrient and water absorption, increases stress tolerance and enhances ISR |
| Paenibacillus durus PD 76, B. subtilis BR 62, and Streptomyces spp. ST 60 | Cereals, soybeans, beet, tomatoes, and sunflowers | ||
| Bioscrop BT16 (Motivos Campestres, Portugal) | Bacillus thuringiensis var. kurstaki | Deciduous fruit trees, horticultural brassicas, cotton, citrus, cauliflower, olives, pepper, banana, and tomato | Protection against pests (beetles) |
| Amase® (Lantmannen Bioagri, Sweden) | Rhizobium, Azotobacter, Pseudomonas, Bacillus, and Chaetomium | Cucumber, lettuce, tomato, pepper, eggplant, cabbage, and broccoli | Growth promotion, quick production of the large and strong root system, and increases stress tolerance |
| PGA® (Organica technologies, USA) | Bacillus sp. | Fruits and vegetables | Improved biomass accumulation, stress tolerance |
| Nitroguard® | Azorhizobium caulinodens NAB38, Azospirillum brasilense NAB317, Azoarcus indigens NAB04, and Bacillus sp. | Cereals, rapeseed, and sugar | Growth promotion via nitrogen fixation |
| TwinN® (Mapleton Agri Biotec Pty Ltd. Australia) | Azospirillum brasilense NAB317, Azoarcus indigens NAB04, and A. caulinodens NAB38 | Beet, sugarcane, and vegetables | Helps with nitrogen fixation and phosphorus solubilization and produces growth-promoting hormones |
| Symbion®-N, Symbion®-P, and Symbion®-K (T. Stanes & Company Ltd., India) | Rhizobium, Azotobacter, Azospirillum, Acetobacter; B. megaterium var. phosphaticum; and Frateuria aurantia | Promotion of plant growth, improved root and shoot weight, and a stronger root system | |
| Ceres® (Biovitis, France) | Pseudomonas fluorescens | Field and horticultural crops | Biocontrol agent against pathogens |
| Gmax® PGPR (Greenmax AgroTech, India) | P. fluorescens, Azotobacter, and phosphobacteria | Field crops | Nitrogen and phosphatic nutrition, disease prevention and helps in plant growth promotion. |
| PGPR Biostimulant | Crop | Beneficial Effects | Mode of Action | References |
|---|---|---|---|---|
| Bacillus sp. | Lettuce | Growth, biomass, and yield of plants | Increased production of phytohormones and availability of nutrients | [46] |
| Azospirillum brasilense, Gluconacetobacter diazotrophicus, Herbaspirillum seropedicae, and Burkholderia ambifaria | Onion | Plant growth, crop yield, and increased number of bulbs | Production of plant hormones and solubilization of nutrients that cause uptake of nutrients | [47] |
| Bacillus pumilus, B. mojavensis, B. Amyloliquefaciens, and P. putida. | Tomato | Growth and production and nutrient uptake | Synthesis of indole-3-acetic acid N2-fixation and P solubilization | [48] |
| PGPR (Bacillus subtilis) | Tomato | Improved fruit quality | Enhanced production of phenols, flavonoids, carotenoids, and antioxidants | [49] |
| Pseudomonas aeruginosa | Wheat | Nutrient uptake | N2 fixation involving many reactions and synthesis of organic acids | [50] |
| Azospirillum brasilense (Sp7b and Sp245b) | Cucumber, lettuce, and tomato | Enhanced germination, root length, and weight; vigor index of germinating seeds | Production of a substantial amount of phytohormones such as IAA | [51] |
| Bacillus pumilus and Pseudomonas pseudoalcaligenes | Rice | Stimulated growth and production | Phosphate solubilization and production of IAA, gibberellins, siderophores, and ACC utilization | [52] |
| Azospirillum brasilense | Maize, sorghum, wheat, barley, and legumes | Biostimulated growth and production | Synthesis of indoleacetic acid (IAA), nitric oxide, carotenoids, and numerous cell surface components | [53] |
| PGPR Biostimulants | Crop Plants | Type of Abiotic Stress | Mode of Action | References |
|---|---|---|---|---|
| Glutamicibacter sp. YD01 | Rice | Salt tolerance | Ethylene mediation, reacive oxygen species (ROS) accumulation, maintaining photosynthetic efficiency and ion homeostasis, increasing expression of stress-related genes, the activity of ACC oxidase, and acquisition of K+ | [90] |
| Bacillus sp., Azospirillum lipoferum, Azospirillum brasilense, and Pseudomonas stutzeri | Wheat | Salt stress | Production of phytohormones and osmoregulators, and enzyme (ROS scavenging) activation | [91] |
| Gluconacetobacter diazotrophicus Pal5 | Red rice | Drought stress alleviation | Increased production of Abscisic acid (ABA), osmoprotectants (proline and glycine betaine) and e AT-hook motif nuclear-localized (AHLs) | [92] |
| Gluconacetobacter diazotrophicus Pal5 | Red rice | Water stress alleviation | Increased ABA production, enhanced chlorophyll synthesis, and increased trehalose and α-tocopherol content in roots. | [93] |
| Azospirillum spp. (Az19) | Maize | Water/drought stress alleviation | Increased production of proline, trehalose (glutamate) and glycine-betaine | [94] |
| Bacillus spp XT13, XT38, and XT110 | Maize | Drought stress | Increased proline content accompanied by reduced Ascorbate Peroxidae (APX) and glutathione reductase (GR) activities, increased nutrient uptake | [95] |
| Pseudomonas entomophila (PE3) | Sunflower | Salinity stress alleviation | Exopolysaccharides, IAA, gibberellic acid, and siderophores | [96] |
| P. fragi, P. proteolytica, P. fluorescens, P. chloropaphis, and Brevibacterium frigoritolerans | Bean | Cold stress | Reduced chill injury, lipid peroxidation, and ice-nucleating activity corresponding to ROS level, and stimulation of apoplastic antioxidant enzyme activities | [97] |
| Pseudochrobactrum kiredjianiae | Wheat | Cold stress | Growth promotion and biocontrol | [98] |
| Pseudomonasfluorescens | Maize | Heavy metal stress | Production of IAA | [99] |
| Azotobacter chroococcum | Maize | Heavy metal stress | Production of siderophores, ammonia, and 1-aminocyclopropane-1-carboxylate deaminase (ACCD) | [100] |
| PGPR Biostimulants | Crop | Biotic Stress | Mode of Action | References |
|---|---|---|---|---|
| Bacillus cereus (PX35), Serratia sp. XY21, and Bacillus subtilis SM21 | Tomato | Root-knot nematodes | Synergistic biocontrol | [120] |
| Pseudomonas aeruginosa LV | Tomato | Bacterial stem rot | Extracellular-bioactive compounds (phytoalexins, flavonoids, defensins, proteins, and phenolics) | [121] |
| B. subtilis 26DCryChS | Potato | Late blight agent and damaged by Colorado potato beetle larvae | Production of Cry1Ia δ-endotoxin, stimulating transcription of jasmonate reliant genes promoting transcription of salicylate reliant gene (PR1) | [122] |
| Lactobacillus plantarum PM411 and Lactobacillus plantarum TC92 | Strawberry | Disease prevention in strawberry and kiwi fruit | Antimicrobial metabolites (lactic acid) production that disrupts pathogen’s cell membranes | [123] |
| B. subtilis BS2 | Tomato | Tomato wilt | Production of defense enzymes such as peroxidase, polyphenol oxidase, chitinase, and phenylalanine | [124] |
| Bacillus safensis and Bacillus altitudinis | Cabbage | Black rot | IAA production | [125] |
| B. velezensis, B. mojavensis, and B. safensis | Soybean | Phytophthora root rot | IAA production | [126] |
| Bacillus cereu, B. subtilis BSV, and B. subtilis BSP | Ginger | Blister blight | 1-aminocyclopropane,1, carboxylic acid production | [127] |
| B. cepacia GRB35 | Ginger | Soft rot in ginger | Fungicide production | [128] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamid, B.; Zaman, M.; Farooq, S.; Fatima, S.; Sayyed, R.Z.; Baba, Z.A.; Sheikh, T.A.; Reddy, M.S.; El Enshasy, H.; Gafur, A.; et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability 2021, 13, 2856. https://doi.org/10.3390/su13052856
Hamid B, Zaman M, Farooq S, Fatima S, Sayyed RZ, Baba ZA, Sheikh TA, Reddy MS, El Enshasy H, Gafur A, et al. Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability. 2021; 13(5):2856. https://doi.org/10.3390/su13052856
Chicago/Turabian StyleHamid, Basharat, Muzafar Zaman, Shabeena Farooq, Sabah Fatima, R. Z. Sayyed, Zahoor Ahmad Baba, Tahir Ahmad Sheikh, Munagala S. Reddy, Hesham El Enshasy, Abdul Gafur, and et al. 2021. "Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops" Sustainability 13, no. 5: 2856. https://doi.org/10.3390/su13052856
APA StyleHamid, B., Zaman, M., Farooq, S., Fatima, S., Sayyed, R. Z., Baba, Z. A., Sheikh, T. A., Reddy, M. S., El Enshasy, H., Gafur, A., & Suriani, N. L. (2021). Bacterial Plant Biostimulants: A Sustainable Way towards Improving Growth, Productivity, and Health of Crops. Sustainability, 13(5), 2856. https://doi.org/10.3390/su13052856














