Contribution of Rhizobium–Legume Symbiosis in Salt Stress Tolerance in Medicago truncatula Evaluated through Photosynthesis, Antioxidant Enzymes, and Compatible Solutes Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Plant Growth and Nodule Characterization
2.3. Estimation of Physiological Parameters and Measuring Survival Rate
2.4. Analysis of Enzymatic and Nonenzymatic Antioxidant Enzymes
2.5. Profiling Soluble Sugars and Compatible Solutes
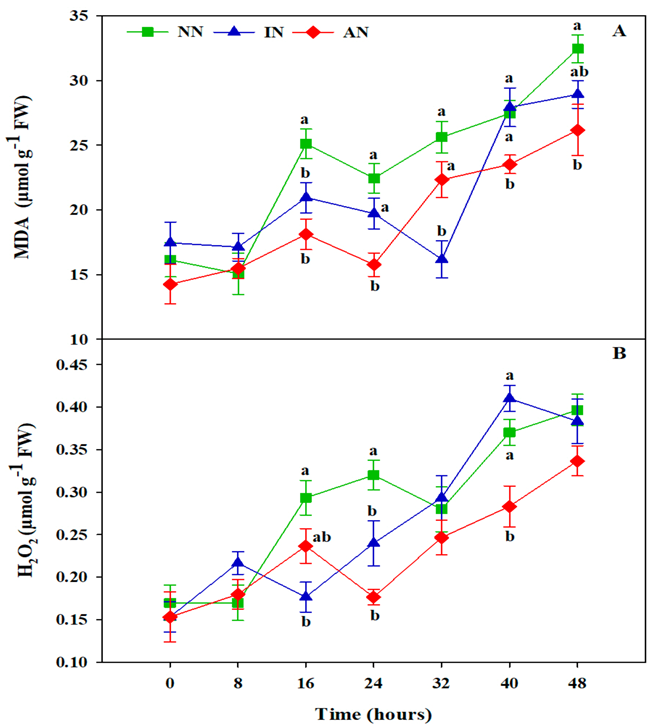
2.6. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Content
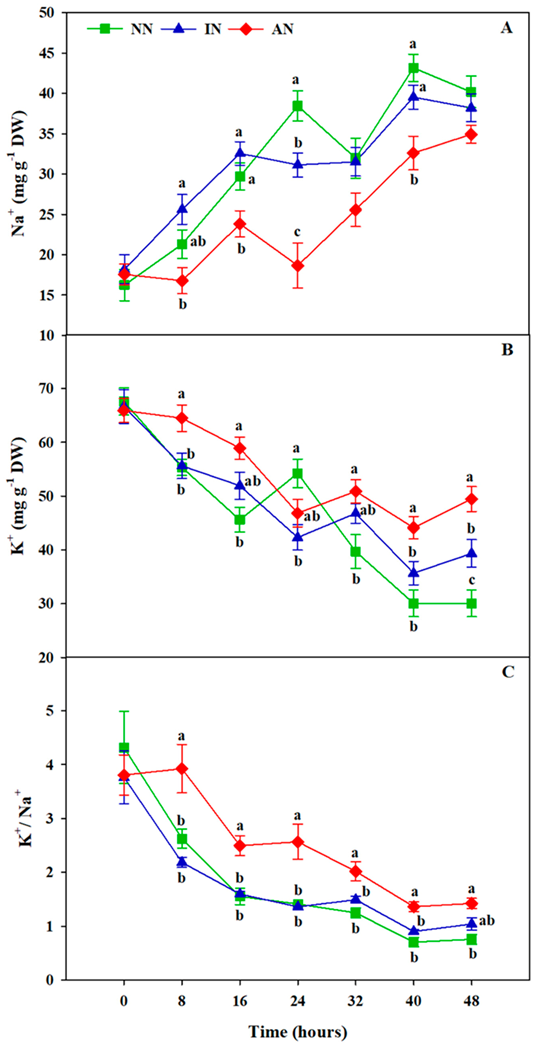
2.7. Determination of Na+ and K+
2.8. Stress Response Models and Salinity-Tolerance Index
2.9. Statistical Analyses
3. Results
3.1. Effect of Rhizobium Symbiosis on Plant Growth and Nodule Characterization
3.2. Effect of Rhizobium Symbiosis on Survival Rate, Relative Water Content, Electrolyte Leakage, Photosynthetic Pigments, and Gas Exchange Parameters
3.3. Rhizobium Symbiosis Reduced Oxidative Damage
3.4. Rhizobium Symbiosis Up-Regulates the Activities of Antioxidant Enzymes
3.5. Effects of Rhizobium Symbiosis on Osmolyte Contents and Nitric Oxide Quantification
3.6. Effects of Rhizobium Symbiosis on Ascorbate and Reduced Glutathione Content
3.7. Rhizobium Symbiosis Reduced Na+ Accumulation and Improved K+ Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. Status World’s Soil Resources; FAO: Rome, Italy, 2015; 650p, ISBN 978-92-5-109004-6. [Google Scholar]
- Mantri, N.; Patade, V.; Penna, S.; Ford, R.; Pang, E. Abiotic stress responses in plants: Present and future. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 1–19. [Google Scholar]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N.; Xu, M. Calcium-Enriched Animal Manure Alleviates the Adverse Effects of Salt Stress on Growth, Physiology and Nutrients Homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Bargaz, A.; Zaman-Allah, M.; Farissi, M.; Lazali, M.; Drevon, J.J.; Maougal, R.T.; Georg, C. Physiological and molecular aspects of tolerance to environmental constraints in grain and forage legumes. Int. J. Mol. Sci. 2015, 16, 18976–19008. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Sajid, H.; Mustafa, A.; Niamat, B.; Ahmad, Z.; Yaseen, M.; Kamran, M.; Rafique, M.; Ahmar, S.; Chen, J.T. Alleviation of salinity-induced oxidative stress, improvement in growth, physiology and mineral nutrition of canola (Brassica napus L.) through calcium-fortified composted animal manure. Sustainability 2020, 12, 846. [Google Scholar] [CrossRef] [Green Version]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [Green Version]
- Evelin, H.; Kapoor, R. Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza 2014, 24, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Chaoxing, H. Does inoculation with Glomus mosseae improve salt tolerance in pepper plants? J. Plant Growth Regul. 2014, 33, 644–653. [Google Scholar] [CrossRef]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Umar, W.; Ayub, M.A.; ur Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and Phosphorus Use Efficiency in Agroecosystems. In Resources Use Efficiency in Agriculture; Springer: Singapore, 2020; pp. 213–257. [Google Scholar]
- Rafique, M.; Naveed, M.; Mustafa, A.; Akhtar, S.; Munawar, M.; Kaukab, S.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z. The Combined Effects of Gibberellic Acid and Rhizobium on Growth, Yield and Nutritional Status in Chickpea (Cicer arietinum L.). Agronomy 2021, 11, 105. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhang, P.; Cao, Y.; Hu, T.; Yang, P. Rhizobium symbiosis contribution to short-term salt stress tolerance in alfalfa (Medicago sativa L.). Plant Soil 2016, 402, 247–261. [Google Scholar] [CrossRef]
- Ali, M.A.; Naveed, M.; Mustafa, A.; Abbas, A. The good, the bad, and the ugly of rhizosphere microbiome. In Probiotics and Plant Health; Springer: Singapore, 2017; pp. 253–290. [Google Scholar]
- Peoples, M.B.; Craswell, E.T. Biological nitrogen fixation: Investments, expectations and actual contributions to agriculture. Plant Soil 1992, 141, 13–39. [Google Scholar] [CrossRef]
- Aranjuelo, I.; Arrese-Igor, C.; Molero, G. Nodule performance within a changing environmental context. J. Plant Physiol. 2014, 171, 1076–1090. [Google Scholar] [CrossRef] [Green Version]
- Mhadhbi, H.; Aouani, M.E. Growth and nitrogen-fixing performances of Medicago truncatula-Sinorhizobium meliloti symbioses under salt (NaCl) stress: Micro-and macro-symbiont contribution into symbiosis tolerance. In Biosaline Agriculture and High Salinity Tolerance; Birkhäuser: Basel, Switzerland, 2012; pp. 91–98. [Google Scholar]
- López, M.; Herrera-Cervera, J.A.; Iribarne, C.; Tejera, N.A.; Lluch, C. Growth and nitrogen fixation in Lotus japonicus and Medicago truncatula under NaCl stress: Nodule carbon metabolism. J. Plant Physiol. 2008, 165, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Palma, F.; López-Gómez, M.; Tejera, N.A.; Lluch, C. Involvement of abscisic acid in the response of Medicago sativa plants in symbiosis with Sinorhizobium meliloti to salinity. Plant Sci. 2014, 223, 16–24. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water Culture Method for Growing Plants without Soil. Circular 347; University of California, College of Agriculture: Berkeley, CA, USA, 1950. [Google Scholar]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef]
- Ahmad, P.; Abdel Latef, A.A.; Hashem, A.; Abd_Allah, E.F.; Gucel, S.; Tran, L.S.P. Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front. Plant Sci. 2016, 7, 347. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, P.; Kalaji, M.H.; Baczewska, A.H.; Pawluśkiewicz, B.; Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Paunov, M.; Goltsev, V. Delayed chlorophyll a fluorescence, MR 820, and gas exchange changes in perennial ryegrass under salt stress. J. lumin. 2017, 183, 322–333. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Tripathi, D.K.; Yadav, S.; Kalaji, H.M.; Brestic, M. Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica 2019, 57, 209–216. [Google Scholar] [CrossRef]
- Liu, Y.S.; Geng, J.C.; Sha, X.Y.; Zhao, Y.X.; Hu, T.M.; Yang, P.Z. Effect of rhizobium symbiosis on low-temperature tolerance and antioxidant response in alfalfa (Medicago sativa L.). Front. Plant Sci. 2019, 10, 538. [Google Scholar] [CrossRef]
- Bianco, C.; Defez, R. Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J. Exp. Bot. 2009, 60, 3097–3107. [Google Scholar] [CrossRef]
- Fan, H.F.; Du, C.X.; Ding, L.; Xu, Y.L. Effects of nitric oxide on the germination of cucumber seeds and antioxidant enzymes under salinity stress. Acta Physiol. Plant. 2013, 35, 2707–2719. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef]
- Doganlar, Z.B.; Demir, K.; Basak, H.; Gul, I. Effects of salt stress on pigment and total soluble protein contents of three different tomato cultivars. Afr. J. Agric. Res. 2010, 5, 2056–2065. [Google Scholar]
- Hasanuzzaman, M.; Alam, M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. BioMed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef] [Green Version]
- Ahanger, M.A.; Qin, C.; Begum, N.; Maodong, Q.; Dong, X.X.; El-Esawi, M.; El-Sheikh, M.A.; Alatar, A.A.; Zhang, L. Nitrogen availability prevents oxidative effects of salinity on wheat growth and photosynthesis by up-regulating the antioxidants and osmolytes metabolism, and secondary metabolite accumulation. BMC Plant Biol. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Bioch. 2019, 137, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Theerawitaya, C.; Tisarum, R.; Samphumphuang, T.; Singh, H.P.; Cha-Um, S.; Kirdmanee, C.; Takabe, T. Physio-biochemical and morphological characters of halophyte legume shrub, Acacia ampliceps seedlings in response to salt stress under greenhouse. Front. Plant Sci. 2015, 6, 630. [Google Scholar] [CrossRef] [Green Version]
- Steppuhn, H.; Van Genuchten, M.T.; Grieve, C.M. Root-zone salinity: I. Selecting a product-yield index and response function for crop tolerance. Crop Sci. 2005, 45, 209–220. [Google Scholar] [CrossRef]
- Abrar, M.M.; Saqib, M.; Abbas, G.; Atiq-ur-Rahman, M.; Mustafa, A.; Shah, S.A.A.; Mehmood, K.; Maitlo, A.A.; Sun, N.; Xu, M. Evaluating the Contribution of Growth, Physiological, and Ionic Components Towards Salinity and Drought Stress Tolerance in Jatropha curcas. Plants 2020, 9, 1574. [Google Scholar] [CrossRef]
- Kiers, E.T.; Rousseau, R.A.; West, S.A.; Denison, R.F. Host sanctions and the legume-rhizobium mutualism. Nature 2003, 425, 78–81. [Google Scholar] [CrossRef]
- Kirova, E.; Tzvetkova, N.; Vaseva, I.; Ignatov, G. Photosynthetic responses of nitrate-fed and nitrogen-fixing soybeans to progressive water stress. J. Plant Nutr. 2008, 31, 445–458. [Google Scholar] [CrossRef]
- El-Akhal, M.R.; Rincón, A.; Coba de la Peña, T.; Lucas, M.M.; El Mourabit, N.; Barrijal, S.; Pueyo, J.J. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol. 2013, 15, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porcel, R.; Redondo-Gómez, S.; Mateos-Naranjo, E.; Aroca, R.; Garcia, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015, 185, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Ramzan, N.; Mustafa, A.; Samad, A.; Niamat, B.; Yaseen, M.; Ahmad, Z.; Hasanuzzaman, M.; Sun, N.; Shi, W.; et al. Alleviation of salinity induced oxidative stress in Chenopodium quinoa by Fe biofortification and biochar—endophyte interaction. Agronomy 2020, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- James, R.A.; Munns, R.; Von Caemmerer, S.; Trejo, C.; Miller, C.; Condon, T. Photosynthetic capacity is related to the cellular and subcellular partitioning of Na+, K+ and Cl− in salt-affected barley and durum wheat. Plant Cell Environ. 2006, 29, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of sodium transport in plants—Progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Y.; Li, Q.; Wang, H.; Zhang, J.; Du, J.; Feng, H.; Blumwald, E.; Yu, L.; Xu, G. Two NHX-type transporters from Helianthus tuberosus improve the tolerance of rice to salinity and nutrient deficiency stress. Plant Biotechnol. J. 2018, 16, 310–321. [Google Scholar] [CrossRef] [Green Version]
- Shabala, S.; Cuin, T.A. Potassium transport and plant salt tolerance. Physiol. Plantarum 2008, 133, 651–669. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Hattori, K.; Konomi, M.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar]
- Abdelaal, K.A.; Mazrou, Y.S.; Hafez, Y.M. Silicon foliar application mitigates salt stress in sweet pepper plants by enhancing water status, photosynthesis, antioxidant enzyme activity and fruit yield. Plants 2020, 9, 733. [Google Scholar] [CrossRef]
- Alzahrani, S.M.; Alaraidh, I.A.; Migdadi, H.; Alghamdi, S.; Khan, M.A.; Ahmad, P. Physiological, biochemical, and antioxidant properties of two genotypes of Vicia faba grown under salinity stress. Pak. J. Bot. 2019, 51, 786–798. [Google Scholar] [CrossRef] [Green Version]
- Serrano, R.; Mulet, J.M.; Rios, G.; Marquez, J.A.; De Larrinoa, I.F.; Leube, M.P.; Mendizabal, I.; Pascual-Ahuir, A.; Proft, M.; Ros, R.; et al. A glimpse of the mechanisms of ion homeostasis during salt stress. J. Exp. Bot. 1999, 50, 1023–1036. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B.; et al. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef]
- Matamoros, M.A.; Dalton, D.A.; Ramos, J.; Clemente, M.R.; Rubio, M.C.; Becana, M. Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol. 2003, 133, 499–509. [Google Scholar] [CrossRef] [Green Version]
- Kataria, S.; Baghel, L.; Jain, M.; Guruprasad, K.N. Magnetopriming regulates antioxidant defense system in soybean against salt stress. Biocatalysis Agric. Biotech. 2019, 18, 101090. [Google Scholar] [CrossRef]
- Frendo, P.; Harrison, J.; Norman, C.; Jiménez, M.J.H.; Van de Sype, G.; Gilabert, A.; Puppo, A. Glutathione and homoglutathione play a critical role in the nodulation process of Medicago truncatula. Mol. Plant Microbe Interact. 2005, 18, 254–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groten, K.; Dutilleul, C.; van Heerden, P.D.; Vanacker, H.; Bernard, S.; Finkemeier, I.; Dietz, K.J.; Foyer, C.H. Redox regulation of peroxiredoxin and proteinases by ascorbate and thiols during pea root nodule senescence. FEBS Lett. 2006, 580, 1269–1276. [Google Scholar] [CrossRef] [Green Version]
- El Msehli, S.; Lambert, A.; Baldacci-Cresp, F.; Hopkins, J.; Boncompagni, E.; Smiti, S.A.; Hérouart, D.; Frendo, P. Crucial role of (homo) glutathione in nitrogen fixation in Medicago truncatula nodules. New Phytol. 2011, 192, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.; Miyahara, K.; Tabata, K.; Esaka, M. Generation and properties of ascorbic acid-overproducing transgenic tobacco cells expressing sense RNA for L-galactono-1, 4-lactone dehydrogenase. Planta 2005, 220, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Latef, A.A.H.A.; Miransari, M. The role of arbuscular mycorrhizal fungi in alleviation of salt stress. In Use of Microbes for the Alleviation of Soil Stresses; Springer: New York, NY, USA, 2014; pp. 23–38. [Google Scholar]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Verdoy, D.; Coba de la Peña, T.; Redondo, F.J.; Lucas, M.M.; Pueyo, J.J. Transgenic Medicago truncatula plants that accumulate proline display nitrogen-fixing activity with enhanced tolerance to osmotic stress. Plant Cell Environ. 2006, 29, 1913–1923. [Google Scholar] [CrossRef]
- Singh, N.K.; Bracker, C.A.; Hasegawa, P.M.; Handa, A.K.; Buckel, S.; Hermodson, M.A.; Pfankoch, E.D.; Regnier, F.E.; Bressan, R.A. Characterization of osmotin: A thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiol. 1987, 85, 529–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Hakeem, K.R. Cell signaling during drought and salt stress. In Plant Signaling: Understanding the Molecular Crosstalk; Springer: New Delhi, India, 2014; pp. 227–239. [Google Scholar]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Tejera, N.A.; Lluch, C. Differential strategies of the model legumes Lotus japonicus and Medicago truncatula in the adaptation to salt stress: Photosynthetic and nutritional responses. Amer. J. Plant Physiol. 2010, 5, 153–162. [Google Scholar] [CrossRef]
- Webb, J.A.; Fletcher, R.A. Paclobutrazol protects wheat seedlings from injury due to waterlogging. Plant Growth Regul. 1996, 18, 201–206. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2014, 103, 93–99. [Google Scholar] [CrossRef]





| Treatments | Shoot Dry Weight (g) | Root Dry Weight (g) | Plant Dry Biomass (g) | Nodule Dry Weight (mg) | Nitrogen Content (g Kg−1) |
|---|---|---|---|---|---|
| NN | 0.67 ± 0.04 a | 1.06 ± 0.11 a | 1.73 ± 0.12 a | - | 19.21 ± 0.37 a |
| AN | 0.71 ± 0.07 a | 1.09 ± 0.01 a | 1.79 ± 0.09 a | 4.20 ± 0.23 a | 19.56 ± 0.23 a |
| IN | 0.68 ± 0.06 a | 1.08 ± 0.19 a | 1.75 ± 0.18 a | 2.35 ± 0.40 b | 19.83 ± 0.41 a |
| Osmolytes | Treatment | 0 h | 8 h | 16 h | 24 h | 32 h | 40 h | 48 h |
|---|---|---|---|---|---|---|---|---|
| Proline (µmol g−1 DW) | NN | 0.47 ± 0.07 a | 0.15 ± 0.02 c | 1.89 ± 0.1 b | 3.37 ± 0.2 a | 4.57 ± 0.1 a | 3.42 ± 0.4 a | 3.18 ± 0.1 b |
| IN | 0.73 ± 0.09 a | 0.95 ± 0.1 b | 3.95 ± 0.2 a | 2.53 ± 0.2 b | 1.44 ± 0.3 c | 2.92 ± 0.2 a | 4.73 ± 0.3 a | |
| AN | 0.69 ± 0.1 a | 1.59 ± 0.2 a | 2.39 ± 0.4 b | 3.23 ± 0.1 a | 2.9 ± 0.2 b | 3.64 ± 0.3 a | 4.83 ± 0.2 a | |
| Glycine Betaine (µg g−1 DW) | NN | 4.88 ± 0.2 c | 5.23 ± 0.3 b | 3.73 ± 0.6 c | 9.24 ± 0.6 a | 4.91 ± 0.4 b | 7.84 ± 0.6 b | 12.14 ± 0.8 ab |
| IN | 7.21 ± 0.3 b | 6.06 ± 0.2 b | 5.87 ± 0.4 b | 8.57 ± 0.7 a | 6.14 ± 0.5 b | 9.57 ± 0.5 ab | 11.37 ± 0.7 b | |
| AN | 11.08 ± 0.4 a | 10.29 ± 0.7 a | 9.37 ± 0.4 a | 9.9 ± 0.5 a | 10.57 ± 0.6 a | 11.28 ± 0.7a | 14.25 ± 0.8 a | |
| Amino Acids (mg g−1 DW) | NN | 0.13 ± 0.02 c | 0.1 ± 0.007 c | 0.08 ± 0.01 b | 1.61 ± 0.1 b | 2.46 ± 0.3 b | 2.77 ± 0.09 c | 3.62 ± 0.7 a |
| IN | 1.27 ± 0.06 b | 1.38 ± 0.06 b | 1.87 ± 0.08 a | 1.61 ± 0.1 b | 3.16 ± 0.09 a | 4.22 ± 0.2 a | 3.5 ± 0.3 a | |
| AN | 1.41 ± 0.07 a | 2.35 ± 0.2 a | 1.91 ± 0.1 a | 1.99 ± 0.08 a | 2.44 ± 0.08 b | 3.73 ± 0.1 b | 4.04 ± 0.2 a | |
| Soluble Sugars (mg g−1 DW) | NN | 3.92 ± 03 b | 7.31 ± 0.5 a | 4.85 ± 0.2 c | 5.15 ± 0.1 c | 13.02 ± 0.8 ab | 10.77 ± 0.9 b | 12.67 ± 0.8 b |
| IN | 5.13 ± 0.2 a | 5.17 ± 0.9 a | 10.53 ± 0.3 a | 9.61 ± 0.4 b | 15.93 ± 0.7 a | 17.66 ± 0.5 a | 16.45 ± 1 a | |
| AN | 5.03 ± 0.3 a | 4.84 ± 0.6 a | 7.86 ± 0.7 b | 13.95 ± 0.8 a | 10.68 ± 0.9 b | 15.43 ± 0.8 a | 19.49 ± 0.9 a | |
| Soluble Proteins (mg g−1 DW) | NN | 7.26 ± 0.3 b | 5.93 ± 0.9 b | 13.25 ± 0.4 a | 11.98 ± 0.3 a | 10.2 ± 0.5 b | 7.33 ± 0.7 b | 9.58 ± 0.6 b |
| IN | 8.57 ± 0.7 ab | 9.56 ± 0.8 a | 9.19 ± 0.5 b | 5.9 ± 0.4 c | 6.09 ± 0.6 c | 9.27 ± 0.4 b | 12.15 ± 0.5 a | |
| AN | 9.5 ± 0.8 a | 3.93 ± 0.9b | 14.71 ± 0.9 a | 7.73 ± 0.3 b | 22.41 ± 0.4 a | 18.04 ± 0.5 a | 13.94 ± 0.8 a | |
| Nitric Oxide (nmol g−1 FW) | NN | 0.13 ± 0.06 b | 0.49 ± 0.1 b | 0.65 ± 0.07 c | 1.7 ± 0.3 b | 0.86 ± 0.05 b | 1.11 ± 0.1 c | 1.18 ± 0.04 c |
| IN | 0.4 ± 0.04 ab | 1.64 ± 0.1 a | 1.22 ± 0.1 b | 1.89 ± 0.2 b | 2.37 ± 0.05 a | 2.67 ± 0.1 b | 2.98 ± 0.1 b | |
| AN | 0.72 ± 0.1 a | 1.26 ± 0.1 a | 1.95 ± 0.04 a | 2.87 ± 0.1 a | 1.76 ± 0.3 a | 3.29 ± 0.06 a | 3.61 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irshad, A.; Rehman, R.N.U.; Abrar, M.M.; Saeed, Q.; Sharif, R.; Hu, T. Contribution of Rhizobium–Legume Symbiosis in Salt Stress Tolerance in Medicago truncatula Evaluated through Photosynthesis, Antioxidant Enzymes, and Compatible Solutes Accumulation. Sustainability 2021, 13, 3369. https://doi.org/10.3390/su13063369
Irshad A, Rehman RNU, Abrar MM, Saeed Q, Sharif R, Hu T. Contribution of Rhizobium–Legume Symbiosis in Salt Stress Tolerance in Medicago truncatula Evaluated through Photosynthesis, Antioxidant Enzymes, and Compatible Solutes Accumulation. Sustainability. 2021; 13(6):3369. https://doi.org/10.3390/su13063369
Chicago/Turabian StyleIrshad, Annie, Rana Naveed Ur Rehman, Muhammad Mohsin Abrar, Qudsia Saeed, Rahat Sharif, and Tianming Hu. 2021. "Contribution of Rhizobium–Legume Symbiosis in Salt Stress Tolerance in Medicago truncatula Evaluated through Photosynthesis, Antioxidant Enzymes, and Compatible Solutes Accumulation" Sustainability 13, no. 6: 3369. https://doi.org/10.3390/su13063369








