Abstract
Supplementation of the substrate of mushrooms with calcium silicate and other minerals is usually used as a preventive measure against pests and other contaminants during the production of oyster mushrooms. Little is known of the effects of this supplementation on the quality of the mushrooms produced. In the work described herein, the supplementation of oyster mushrooms was performed with 5 supplementation levels (0%, 0.5%, 1%, 2% and 4%) on mushrooms from two different locations in Brazil, the two flushes of mushrooms produced were analysed in terms of phenolic compounds, organic acids, and the antioxidant, antibacterial and antifungal activities, and finally the data was subjected to a linear discriminant analysis to understand the discrimination of the supplementation percentages. Overall, intermediate supplementation until 1% seemed to have a positive effect on the mushrooms from Mogi-das-Cruzes region, while high supplementation favoured the mushrooms from the region of Presidente Prudente. Supplementation showed positive effects on the mushrooms by increasing the production of some secondary metabolites while not showing any negative cytotoxic effects.
1. Introduction
Bioactive compounds are found in many foods and are compounds capable of modulating metabolic processes and promoting health improvements, and have a biological value that goes beyond the nutritional value of the food. Examples of these compounds are polyphenols, carotenoids, tocopherols, organic acids, phytosterols, carbohydrates, fibres, vitamins C, A and E, and minerals, among others, which have antioxidant, antibacterial, and other health improving effects [1].
Mushrooms are very appreciated for having excellent nutritional and medicinal properties, being widely appreciated for their taste. Of all edible species of mushrooms, Pleurotus ostreatus (Jacq.) P. Kumm (oyster pearl mushroom) is one of the most cultivated and consumed, while also showing beneficial health properties [2,3]. Due to its broad importance to the human diet, there is a need to deepen the study of its composition in bioactive compounds to better understand its health benefits, but also understand how to increase these bioactive compounds through sustainable methods.
Silicon and calcium are beneficial for plants and mushroom growth, they contribute towards the wall of the cells, thus helping to maintain textural integrity, while also stimulating the development of mushroom fruiting bodies. Supplementation with calcium in the form of calcium silicate improves post-harvest storage conditions, thus improving the quality of mushrooms and increasing their shelf life [4,5,6,7].
Calcium silicate bases have many applications and are used in different areas. They are used in the biomedical field, where they have shown great biocompatibility, antibacterial activity, and bioactivity; the latter plays a fundamental role in the regeneration and healing of tissues, and the performance of calcium silicate materials is largely attributable to their bioactivity [8,9,10]. It has also been widely used in the production and cultivation of different plant crops [11,12,13,14,15]. The high compatibility of calcium silicate and mushrooms when compared to other supplementation materials has attracted attention towards its supplementation in several crops.
There is currently no information on the possible effects of calcium silicate supplementation during cultivation on bioactive molecules of oyster mushrooms. A previous study showed the impact of calcium silicate on the agronomical yields and chemical composition of oyster mushrooms, pointing out that very slight changes were sought in terms of nutritional quality, but an increase in bioactive compounds like tocopherols and vitamin D2 [3]. Following the results of that study, the effects of varying concentrations of calcium on the substrates of P. ostreatus were sought for the antioxidant, cytotoxic and antimicrobial activity, as well as the effects in polyphenols and organic acids.
2. Materials and Methods
2.1. Standards and Reagents
High Performance Liquid Chromatography (HPLC) grade acetonitrile (99.9%), n-hexane (95%) and ethyl acetate (99.8%) were purchased from Fisher Scientific (Lisbon, Portugal). Organic acid standards and 2,2′-azobis(2-methylpropionamidine) dihydrochloride (AAPH) were obtained from Sigma-Aldrich (St. Louis, MO, USA) and phenolic standards were acquired from Extrasynthèse (Genay, France). Potato dextrose agar (PDA) and potato dextrose broth (PDB) were acquired from Oxoid microbiology products (Hampshire, United Kingdom). p-Iodonitrotetrazolium chloride (INT) from Panreac Applichem (Barcelona, Spain), Tryptic soy broth (TSB), and Mueller-Hinton (MH) from Biolab® (Hungary). All other reagents and solvents were of analytical grade and obtained from common sources. Water was treated in a Milli-Q water purification system (TGI Pure Water Systems, Greenville, SC, USA).
2.2. Samples
One strain of P. ostreatus var. florida (Jacq.) P. Kumm was bought from producers of Mogi-das-Cruzes (coordinates: −23.52327, −46.2659766, company—Funghi & Flora) henceforth described as “MC”, and another in Presidente Prudente (−21.9629448, −51.6337427, company—Brasmicel), described as PP, both in the state of São Paulo, in Brazil. The inoculum was prepared by subculturing the mushroom, following the steps of production of subculture (petri dish with potato dextrose agar), production of mother spawn, and production of grain spawn (sorghum with lime and gypsum) for compost inoculation, as described by [16]. Two flushes of mushrooms were harvested for each inoculum.
2.3. Bioactive Molecules
2.3.1. Organic Acids
Organic acids were identified and quantified following the procedures described and optimized previously by Barros, et al. (2013) [17] after extraction with metaphosphoric acid. The analysis was done by means of ultrafast liquid chromatograph (UFLC, Shimadzu 20A series, Shimadzu Corporation, Kyoto, Japan) coupled to photodiode array detector (PDA), using 215 nm as the preferred wavelength. 3.6 mM of sulphuric acid was used as the mobile phase, at a flow rate of 0.8 mL/min. Quantification of organic acids was done by comparing the area of their peaks with calibration curves obtained from the commercial standards of each compound, using LabSolutions Multi LC-PDA software (Shimadzu Corporation, Kyoto, Japan), with the results being displayed in g per 100 g of dry weight (DW).
2.3.2. Phenolic Compounds
For the analysis of phenolic compounds, each sample was placed in a beaker (~1 g) and was extracted by magnetic stirring with ethanol:water (30 mL, 80:20, v/v) at room temperature and 150 rpm, for 1h. The extract was separated from the residue by filtration through Whatman No. 4 paper in a round flask. The residue was re-extracted again under the same conditions and the filtrates were evaporated at 40 °C to remove ethanol (rotary evaporator, Büchi, Flawil Switzerland). Subsequently, the aqueous phase of each sample was frozen and lyophilized (freeze 4.5 FreeZone model 7750031, Labconco, Kansas, USA) to obtain the respective extracts. The hydroethanolic extracts obtained were dissolved in ethanol: water (80:20, v/v at 10 mg/mL) and filtered through a 0.22-μm disposable LC filter disk.
Phenolic compounds were determined in a Dionex Ultimate 3000 HPLC (Thermo Scientific, San Jose, CA, USA), coupled to a diode array detector (DAD, set at 280, 330, and 370 nm) and a mass detector (MS), and also equipped with a quaternary pump, an automated injector (5 °C), a degasser, and a temperature adjusted column chamber (35 °C) [18]. The detection of compounds was achieved through a diode array detector (DAD) set at 280 nm of wavelength and coupled to a mass detector. The separation of the compounds relied on a C18 columns (Waters Spherisorb S3 ODS-2 (3 µm, 4.6 mm × 150 mm, Waters, Milford, MA, USA) operating at 35 °C and the mobile phase consisted of 0,1% of formic acid in water and acetonitrile, functioning in gradient mode. The gradient varied from 15% of acetonitrile for 5 min to 20% during another 5 min, then 10 min at 25%, another 10 min to 35% and finally another 10 min from 35% to 50%, being the column rebalanced from 10 min at a flux of 0.5 mL/min. The detection of masses was achieved using a Liner LTQ XL ion trap mass spectrometer (Thermo Finnigan, San Jose, Can, USA) equipped with an electrospray ionization source using nitrogen as the carrier gas [18]. The data was analyzed using Xcalibur software and the results were in µg per 100 g of DW.
2.4. Bioactivities
2.4.1. Antioxidant Activity
The thiobarbituric acid reactive substances (TBARS) inhibition and the oxidative hemolysis inhibition (OxHLIA) assays were performed for antioxidant activity evaluation. Trolox was used as a positive control in both in vitro assays. For the TBARS assay, the lyophilized extracts were dissolved in ethanol/water (80:20, v/v), and subjected to dilutions between 20 and 0.078 mg/mL. The inhibition of lipid peroxidation in porcine brain homogenates (Sus scrofa) was evaluated by the decrease in TBARS; the colour intensity of malondialdehyde-thiobarbituric acid (MDA-TBA) was measured by its absorbance at 515 nm. The inhibition rate (%) was calculated according to Spréa, et al. (2020) [19], and the results were expressed in EC50 values (mg/mL, sample concentration providing 50% of antioxidant activity).
The antihemolytic activity used the OxHLIA assay, as described and optimized by Lockowandt, et al. (2019) [20]. An erythrocyte solution (2.8%, v/v; 200 µL) prepared in PBS (pH 7.4) was mixed with 400 µL of: (i) extract solution (6–500 μg/mL in PBS); (ii) PBS (control); (iii) water (for complete haemolysis); or (iv) trolox (7.81–250 µg/mL PBS). After pre-incubation at 37 °C for 10 min with shaking, 200 μL of AAPH (160 mM in PBS) were added and the optical density was measured at 690 nm every ~10 min in a microplate reader (Bio-Tek Instruments, ELX800) until complete hemolysis. The results were expressed in IC50 values (µg/mL), meaning the concentration of extract capable of promoting a Δt delay in hemolysis of 60 and 120 min.
2.4.2. Antimicrobial Activity
The antimicrobial activity was analysed according to the procedure described previously [21]. The following Gram (+) bacteria, Staphylococcus aureus (ATCC 11632), Bacillus cereus (food isolate) and Listeria monocytogenes (NCTC 7973), as well as Gram (-) bacteria Escherichia coli (ATCC 25922), Enterobacter cloacae (ATCC 35030) and Salmonella Typhimurium (ATCC 13311), were tested; as for the tested micromycetes, the following were used: Aspergillus fumigatus (ATCC 2011), Aspergillus ochraceus (ATCC 12066), Aspergillus niger (ATCC 6275), Penicillium funiculosum (ATCC 36839), Penicillium ochrochloron from the American Type Culture Collection (ATCC 9112) and Penicillium verrucosum var. cyclopium (food isolate). The microorganisms are deposited at Mycological laboratory, Department of Plant Physiology, Institute for Biological research “Sinisa Stanković”, University of Belgrade, Serbia.
A microdilution method was implemented to perform the antimicrobial assay [22]. Bacterial/fungal suspensions were adjusted with sterile saline to a concentration of 1.0 × 106 CFU/mL. The mushroom extracts were dissolved in 30% ethanol, mixed with nutrient media for bacteria (Tryptic Soy Broth) or micromycetes (Malt medium) containing bacterial/fungal inoculum (1.0 × 105 CFU per well) in a final volume of 100 µL.
Minimum inhibitory concentrations (MIC) and minimum bactericidal/fungicidal concentrations (MBC/MFC) were defined as described previously [21]. Ampicillin and streptomycin (Panfarma, Belgrade, Serbia) were used as positive controls for the antibacterial activity test, while the commercial antifungals bifonazole and ketoconazole (Srbolek, Belgrade, Serbia) were used as positive controls for antifungal assay. Thirty percent ethanol was used as a negative control.
2.4.3. Cytotoxicity
The cytotoxicity of the mushrooms was analysed by means of the sulforhodamine B assay, as previously described by Barros, et al. (2013) [17]. Methanol:water extracts (80:20, v/v) were prepared for the cytotoxicity analyses, after that, they were redissolved in water to obtain stock solutions of 8 mg/mL and then subjected to further dilutions. Four human tumor cell lines were tested: NCI-H460 (non-small cell lung carcinoma), MCF-7 (breast adenocarcinoma), HepG2 (hepatocellular carcinoma), and HeLa (cervical carcinoma) of DSMZ (Leibniz-Institut DSMZ- Deutsche SammLung von Mikroorganismen und Zellkulturen GmbH). Each cell line was plated at a suitable density (7.5 × 103 cells/well for MCF-7 and NCI-H460 or 1.010 cells/well for HeLa and HepG2) in 96-well plates and allowed to bind for 24 h.
To evaluate cytotoxicity in non-tumor cells, a culture of cells (called PLP2) was prepared from a freshly harvested porcine liver obtained from a local slaughterhouse, according to a procedure established by Tsukatani et al. 2011 [23]. The absorbance was measured at 540 nm using an ELX800 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) [24]. Ellipticine was used as a positive control and the results were displayed at GI50 values (µg/mL) (concentration which inhibited 50% of net cell growth).
2.5. Statistical Analysis
Throughout the manuscript, all data are expressed as mean ± standard deviation. Samples were analysed through two-way analysis of variance (ANOVA) with type III sums of squares, after verifying homoscedasticity through a Levene’s test. The post-hoc test used was either a Tukey’s multiple test (homoscedastic sample) or Tamhane T2 test (non-homoscedastic samples) for the different Calcium Silicate supplementation and a Student’s T test for the two harvest periods. Using a two-way ANOVA, it is possible to verify the influence of the two factors, Harvest number (HN) and calcium silicate supplementation (CS), independently from each other. If a significant interaction was detected among the two factors (HN × CS p < 0.05), they were evaluated simultaneously, and some tendencies can be extracted from the estimated marginal means (EMM) plot. Inversely, if there was no significant interaction recorded among the two factors (HN × CS p > 0.05), they were analysed and classified independently using the post-hoc tests described above. Thus, the standard deviations were calculated from results obtained under different operational conditions, and should, therefore not be regarded as a measure of precision, rather as the range of the recorded values. All statistical analysis was performed using a p-value of 0.05 and using SPSS (version 25). A linear discriminant analysis (LDA) was performed to discriminate the different two harvests and percentage of supplementation. The LDA used the Wilk’s λ test with and F-value of 3.84 for entering and 2.71 for removal, using the leave-one-out cross validation procedure.
3. Results and Discussion
This work focused on the effects of different percentages of calcium silicate supplementation on the different bioactivities and individual bioactive molecules of P. ostreatus mushrooms.
3.1. Organic Acids and Phenolic Compounds
Table 1 shows the profile in organic acids and phenolic compounds of both mushrooms with the supplementation of CS during the two harvest periods (1st and 2nd flushes).

Table 1.
Organic and phenolic acids detected through ultrafast liquid chromatograph (UFLC)-diode array detector (DAD) and HPLC-DAD-ESI/MS (electro-spray ionization coupled to a mass spectrometer), respectively, of the different mushroom provenances across the two harvest periods in DW.
Table 1 is divided into two sections, each one pertaining to the locations of the strains, namely MC and PP. Each section is further divided into an upper and lower section, referring to the two factors, namely HN and CS. This sectioning of the tables derives from the two-way ANOVA employed to understand the influence of each factor individually, and thus, in the upper section of Table 1, within the ranges of the means are all both the values of HN, and in the lower sections are all the values from CS, namely 0%, 0.5%, 1%, 2% and 4%. This type of representation of the results allows for a much more trustworthy interpretation of the results, for each factor that caused change can be analysed independently of the influence of the other. The existence of an interaction between both factors is demonstrated by HN × CS p < 0.05, and thus only allows for general conclusions which can be extracted from the estimated marginal means (EMM) plots, whilst HN × CS p > 0.05 shows that each factor can be classified independently using Tukey’s HSD test to classify each factor individually.
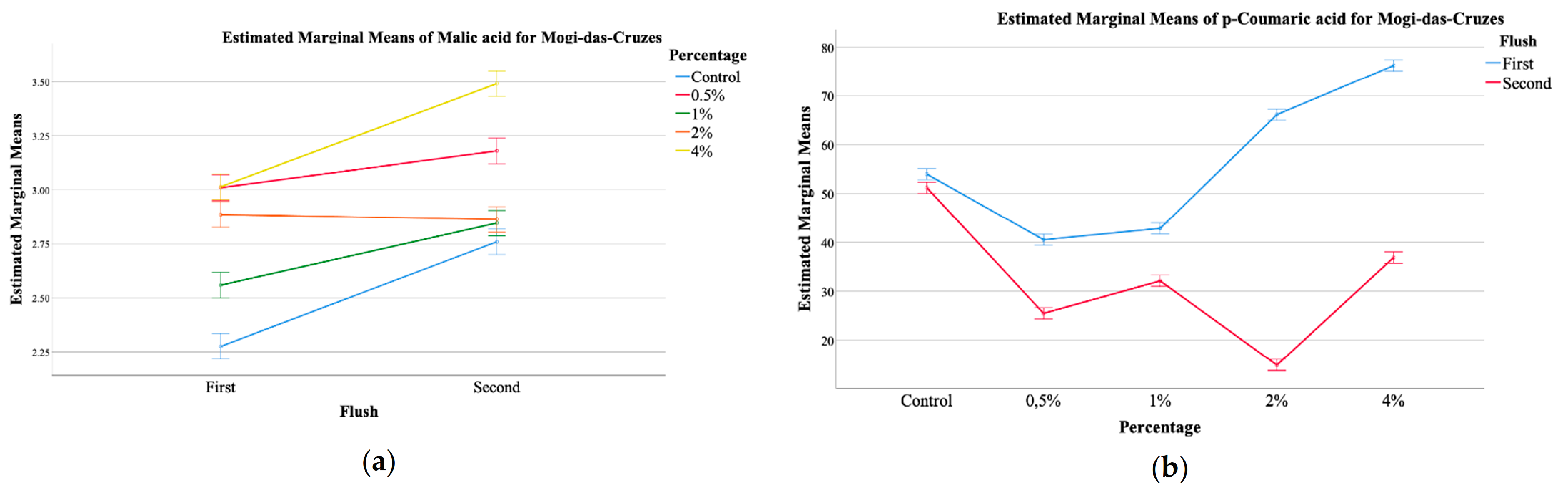
Regarding organic acids, three were detected, namely oxalic acid, malic acid and fumaric acids, with malic acid being the most abundant one. Overall, a significant interaction was sought for all three organic acids for both mushroom provenances, as well as their total amount, and thus only some general conclusions could be extracted from the EMM plots, present in Figure 1.
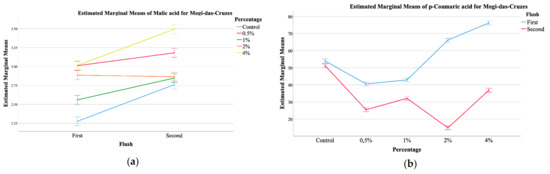
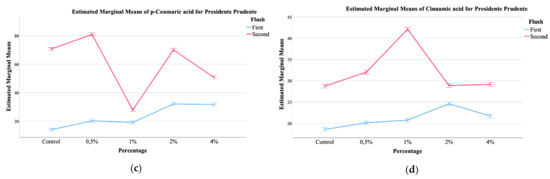
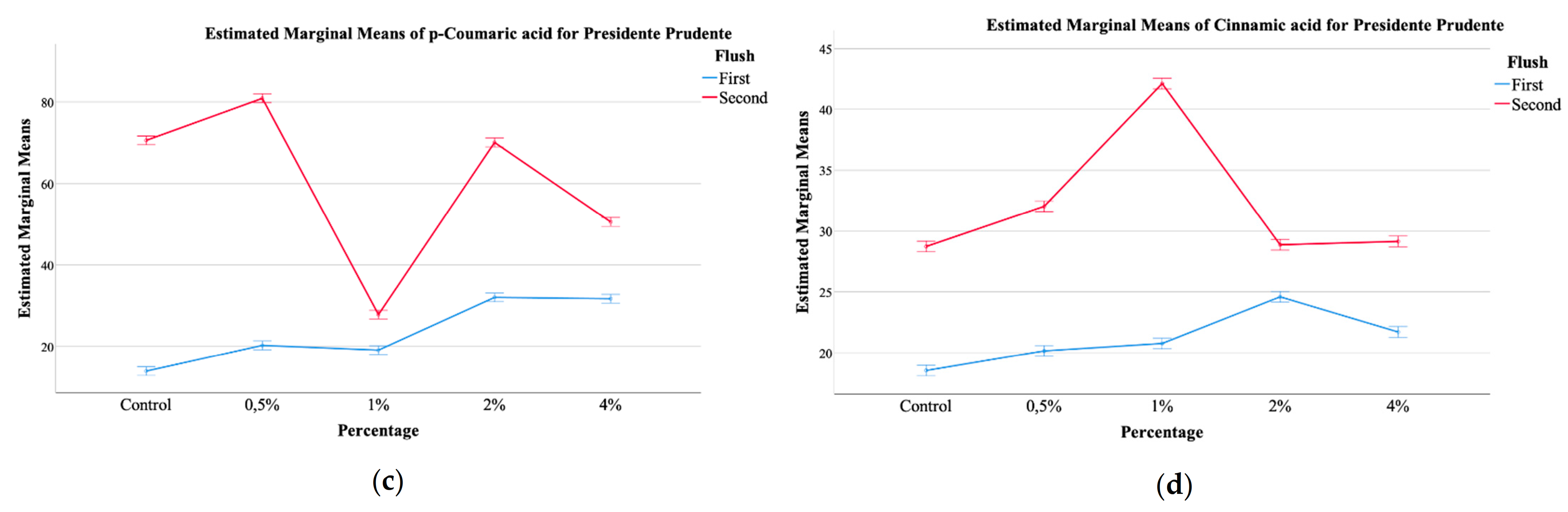
Figure 1.
EMM plots of mushrooms from Mogi das Cruzes (a,b), (a) malic acid, (b) p-coumaric acid, and Presidente Prudente (c,d), (c) p-coumaric acid, (d) cinnamic acid, mushrooms at the two different harvest periods.
The plots showed that malic acid from MC mushrooms supplemented with calcium silicate (Figure 1a) showed higher values of this organic acid, and a further increase from the first to the second flush, which was positively correlated with the increasing percentage of calcium silicate. Malic acid is an important carboxylic acid with beneficial effects on health [12,25]. Its values in the mushrooms increased with the increase of calcium silicate supplementation. It seems that a supplementation with 0.5% of calcium silicate favors the production of malic acid, especially in the first flush. Still, supplementation of 4% does not seem to increase the amount of malic acid in the first flush when compared to supplementation with 0.5%, but greatly stimulates its production in the second flush. With regards to the phenolic compounds, once again, a significant interaction was detected for all, both for MC and PP mushrooms. Of the main compounds detected, protocatechuic acid was the most abundant, followed by p-coumaric acid and finally cinnamic acid, which was the least abundant. The EMM plots in Figure 1 allow for some general tendencies, namely that for MC, supplementation with 4% shoed higher amounts of malic acid, while the control sample showed the least (Figure 1a), while the first flush showed higher values of p-coumaric acid with an increase related to the highest supplementation percentages, while for the second harvest this tendency was inverted (Figure 1b). Still, for PP, the second harvest showed higher values of p-coumaric acid, being this increase correlated with higher supplementation of calcium silicate (Figure 1c). Cinnamic acid, the least abundant phenolic compound showed and EMM plot for PP in which the highest amount was sought for the second harvest, that showed a maximum of this phenolic compound at 1% of supplementation with calcium silicate (Figure 1d).
3.2. Antioxidant Activity and Cytotoxicity in Non-Tumour Cell Line
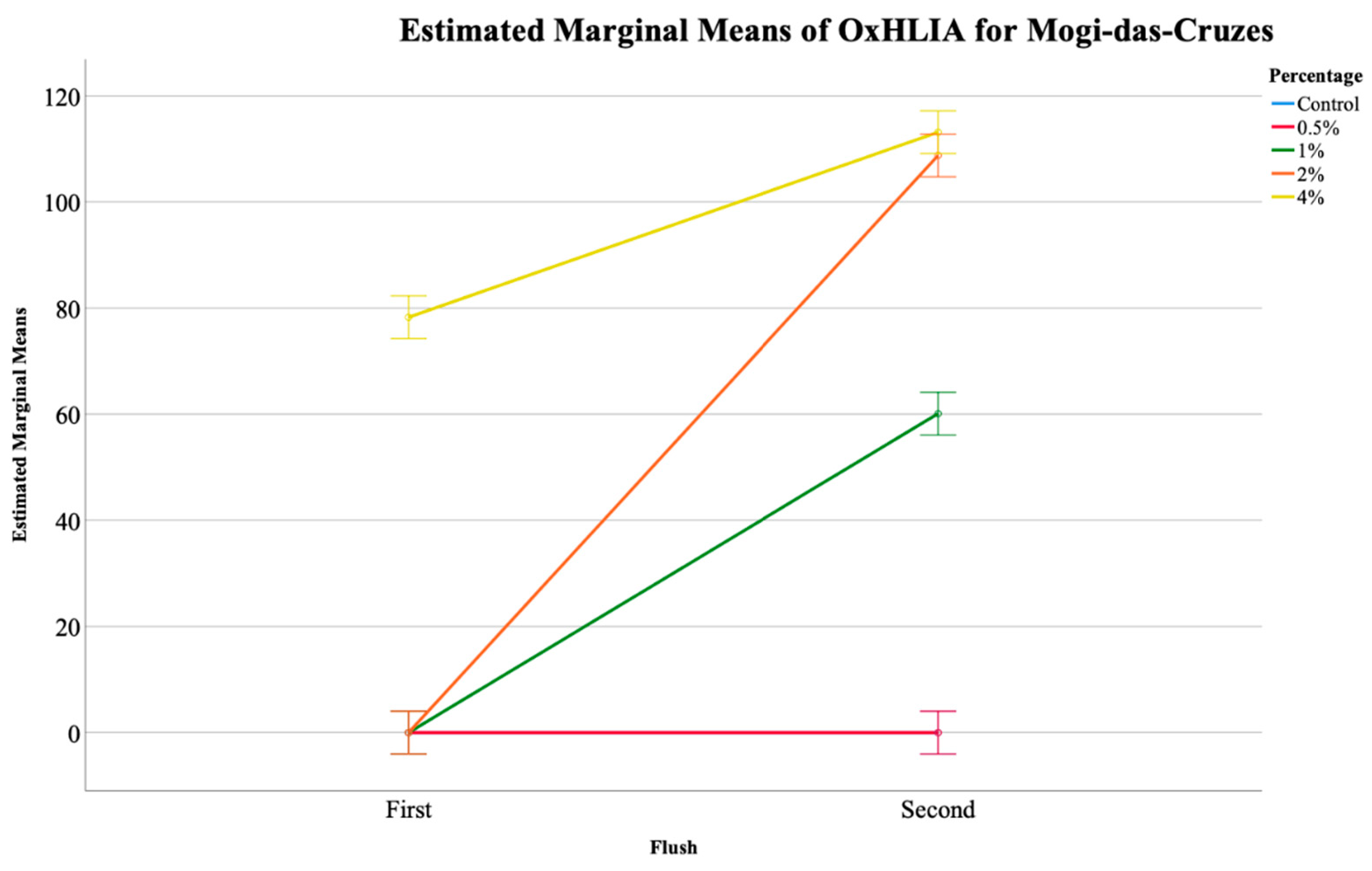
Table 2 shows the results of the two antioxidant activity assays carried out for the mushroom extracts, namely TBARS and OxHLIA, and once again a significant interaction was detected for both. The EMM plots show that for MC, in the OxHLIA assay, only the 4% supplementation of calcium silicate showed activity, although it reduced from the first to the second flush (Figure 2). For PP mushrooms, the supplementation with calcium silicate did not show any activity in the first flush, only showing activity in the second flush. In terms of TBARS assay, no EMM plots could be shown, although Table 2 shows a significant interaction between the flushes and the calcium silicate concentration implying that the slight changes found for this assay was due to both of the factors.

Table 2.
Antioxidant activity of the different mushroom provenances across the two harvest periods.
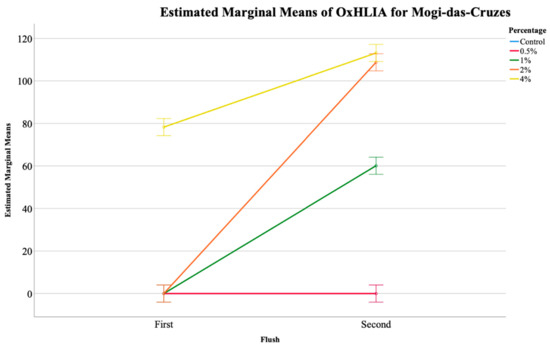
Figure 2.
Estimated marginal means (EMM) plot of the oxidative hemolysis inhibition (OxHLIA) analysis of the Mogi-das-Cruzes mushrooms.
Both samples were tested against a porcine liver primary cell line, showing no cytotoxicity at the tested concentrations, which indicates that the supplementation of calcium silicate in the mushroom substrate does not induce any cytotoxic effect. This result highlights the use of calcium silicate as a stimulant for bioactive molecules in certain crops without the drawbacks of other substrate supplementations.
3.3. Antimicrobial Activities
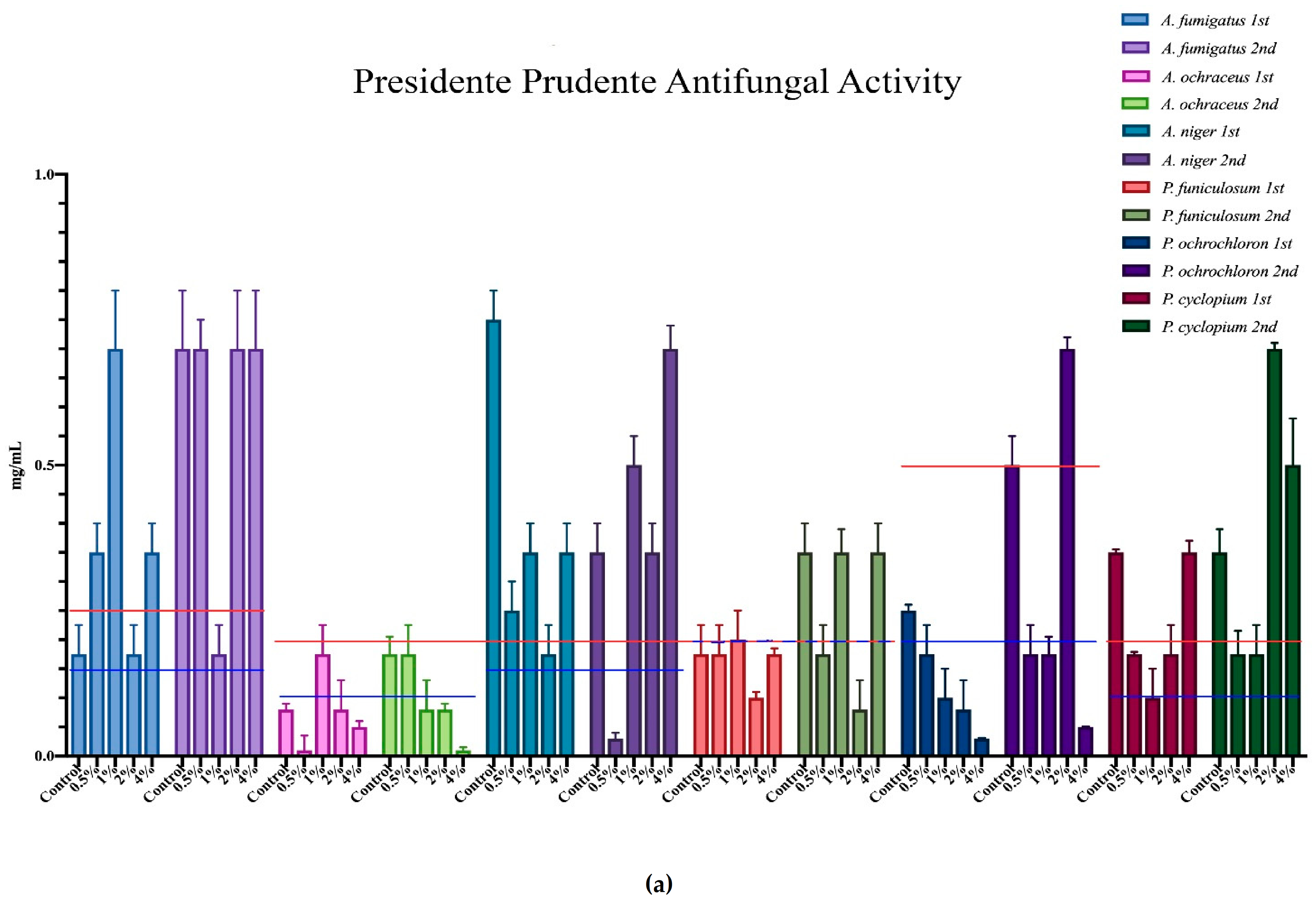
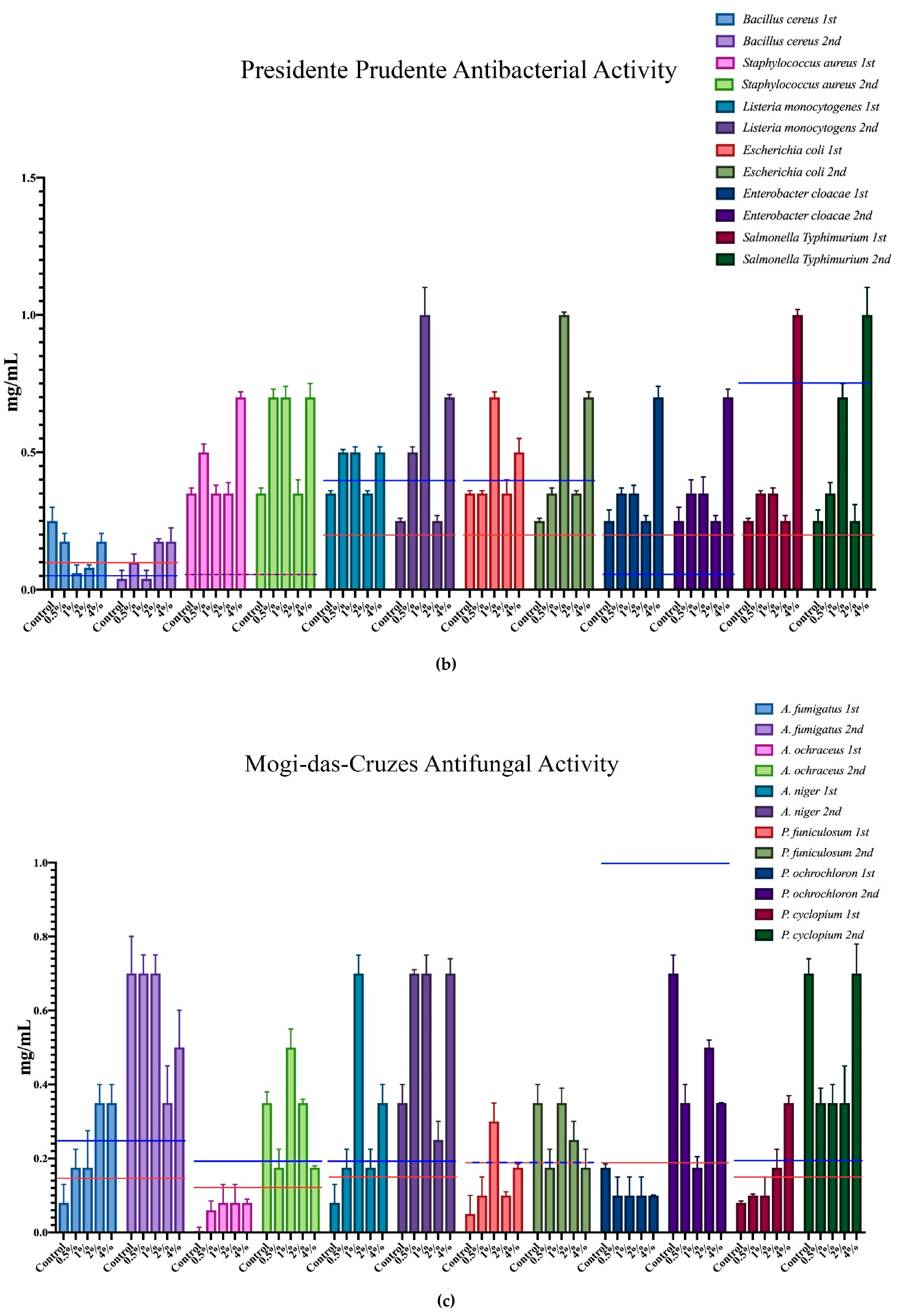
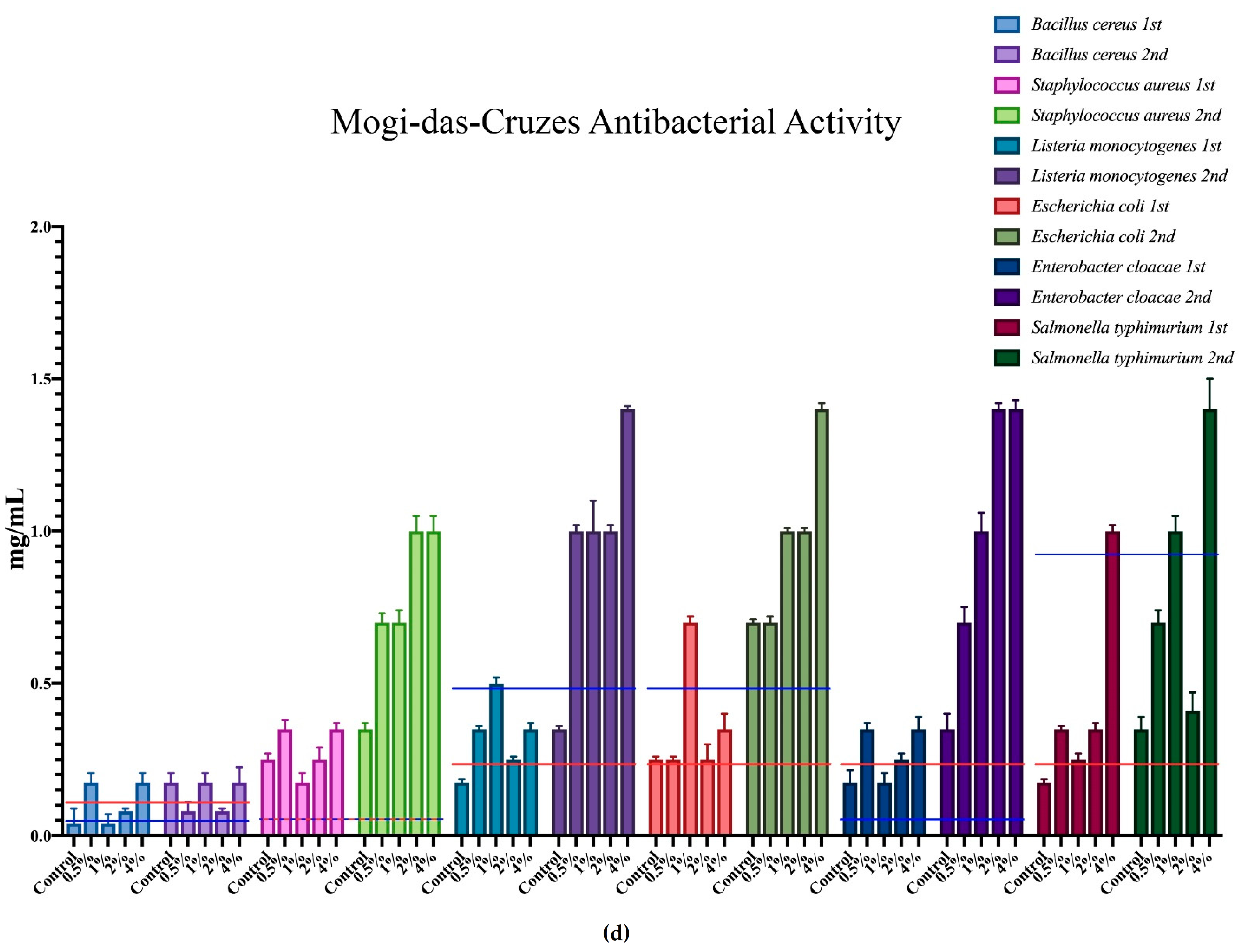
The minimum inhibition concentration (MIC) for the antimicrobial activity of the different mushrooms is represented in Figure 3, where each microorganism corresponds to a colour, and all of the different supplementation results are expressed within that same colour. Furthermore, the minimum inhibition concentrations of the positive controls are represented in each case through the red and blue horizontal lines.
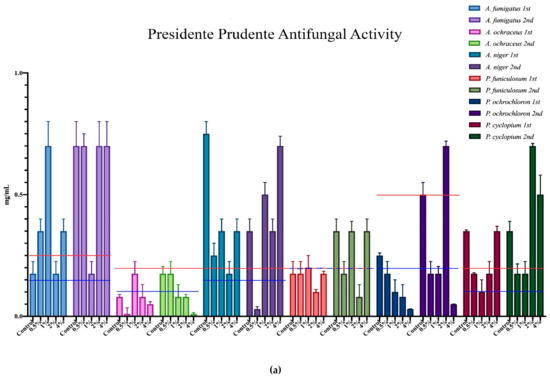
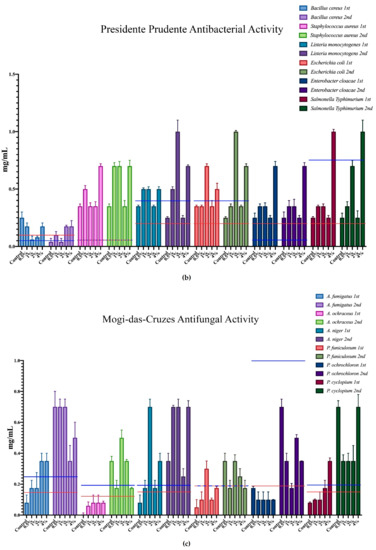
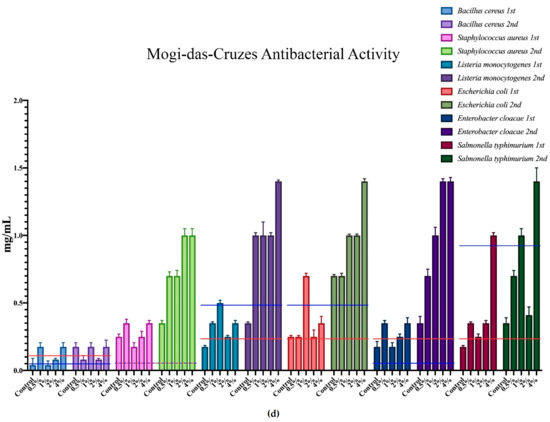
Figure 3.
Antimicrobial activity of the mushrooms from both flushes and different supplementation percentages compared to positive controls. For the antibacterial activity, the blue line represents the ampicillin minimum inhibitory concentrations (MIC) while the red line represents the MIC of streptomycin. In the antifungal charts, the red lines represent the MIC of ketoconazole and the blue the MIC of bifonazole. (a) antifungal activity of the mushrooms from Presidente Prudente; (b) antibacterial activity of the mushrooms from Presidente Prudente; (c) antifungal activity of the mushrooms from Mogi-das-Cruzes; (d) antibacterial activity of the mushrooms from Mogi-das-Cruzes.
The red line represents ketoconazole for the antifungal activity and streptomycin for the antibacterial activity, while the blue line represents bifonazole for the antifungal activity and ampicillin for the antibacterial activity. The mushrooms from PP showed very uniform values and low variations from the first flush to the second (Figure 3a). The best result was sought against B. cereus, in which some percentages of supplementation displayed better activity than both positive controls. Furthermore, most samples of MC showed better results against S. typhimurium when compared to ampicillin. Interestingly, the antifungal activity for the mushrooms of PP (Figure 3b) showed different results, in which the higher supplementation showed better results than the positive controls in both flushes for the same fungi, A. ochraceus and P. ochlorochloron in the second flush. The antibacterial activity for mushrooms from MC, represented in Figure 3c, showed that the mushrooms in the second flush had lower antibacterial activity and that supplementation did not have much influence on the activity, although for B. cereus, L. monocytogenes and E. coli, the values of the mushrooms were very close to the activity of one of the positive controls. Regarding Figure 3d, the antifungal activity for the mushrooms from MC, the supplementation did not seem to have an influence on the antifungal activity, and while there seemed to be a decrease from the first to the second flush, very good results were sought against P. ochrochloron, in which the mushroom seemed be have higher activity in the first flush than both positive controls. Furthermore, the first flush was also between both positive controls for A. ochraceus and P. funiculosum.
Overall, P. ostreatus mushrooms seem to have a higher antibacterial than antifungal activity and, in some cases, supplementation with calcium silicate increase this activity, especially in PP against fungi and MC against bacteria.
3.4. Linear Discriminant Analysis
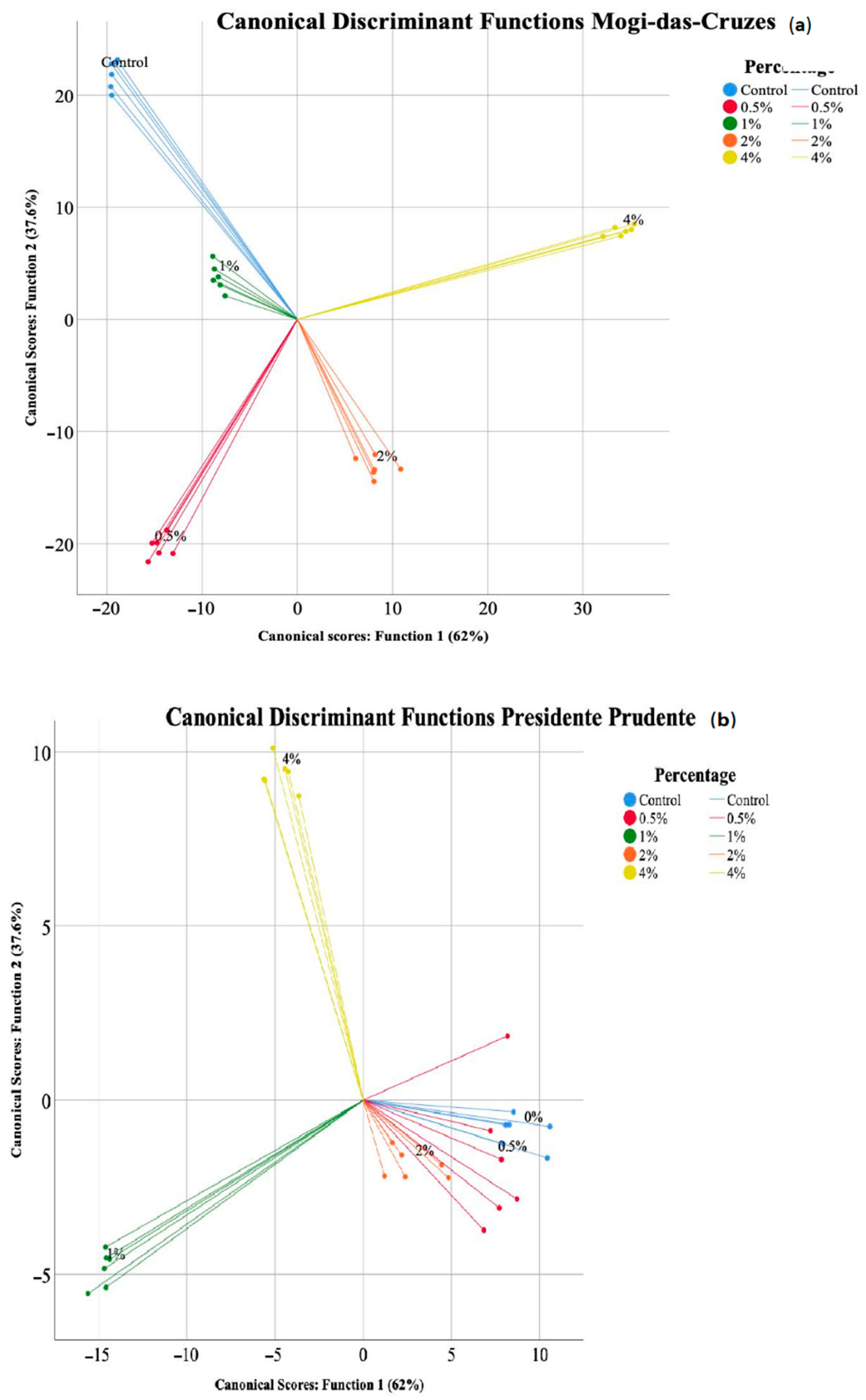
A linear discriminant analysis (LDA) was used to further discriminate the differences induced by the supplementation with calcium silicate to the mushrooms from the two regions. The LDA discriminated the supplementation percentages (Figure 4) for both mushrooms.
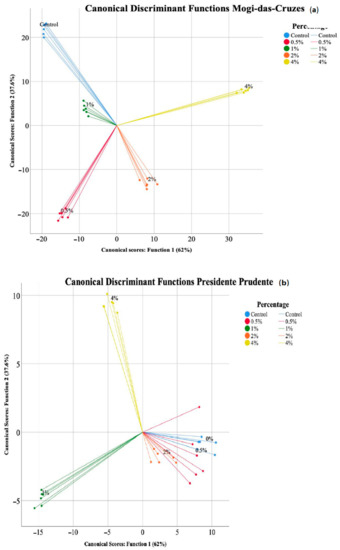
Figure 4.
Linear discriminant analysis (LDA) of the different calcium silicate supplementation percentages for each mushroom, (a) Mogi-das-Cruzes and (b) Presidente Prudente.
Regarding the mushrooms from MC (Figure 4a), the model defined 4 functions that accounted for 100% of the variance, although the first two included 99.6% (function 1—62%, function 2—37.6%). Among the 10 parameters analysed, 8 had discriminating ability, namely p-coumaric acid, OxHLIA assay, malic acid, protocatechuic acid, TBARS assay, oxalic acid, cinnamic acid and fumaric acid. The ones most correlated with function 1 were TBARS and OxHLIA assay as well as p-coumaric acid, while p-coumaric acid, protocatechuic acid and oxalic acid were highly correlated to function 2. By analysing Figure 4a a clear discrimination between the different supplemented mushrooms is visible, with the 4% calcium silicate supplemented mushrooms showing greater difference from the other samples, which was based on the antioxidant activity and p-coumaric acid values. Regarding the LDA for PP, shown in Figure 4b, the model also defined 4 functions that accounted for 100% of the variance, and the two first ones together accounted for 94.6% (function 1—72.5%, function 2—22.1%). Eight parameters showed discriminant ability, namely OxHLIA assay, cinnamic acid, protocatechuic acid, total organic acids, TBARS assay, p-coumaric acid, fumaric acid and oxalic acid. The parameters that best correlated to function 1 were p-coumaric acid, cinnamic acid and total organic acids, while p-coumaric acid, total organic acids and oxalic acid were best correlated with function 2. Figure 4b shows that contrary to Figure 4a, not all supplementation percentages were clustered, namely the control (0%) 0.5% and 2% supplementation were discriminated from 1 and 4% according to function 1, while the latter were separated according to function 2. Overall, the parameters were better at discriminating the supplementation from the mushroom from Mogi-das-Cruzes. Supplementation at 4% stands out as the most distinct from the other percentages and the control sample, revealing that an increase in supplementation probably over 4% might induce higher changes. Further studies with increasing supplementation percentages are needed to understand if they can improve on the chemical parameters of the mushrooms or have damaging effects.
4. Conclusions
Supplementation of mushroom substrate with minerals and other components has been carried out with relative success, aiming at controlling pests or increasing crop yields. Previous work has been carried out with calcium silicate studying the effects on biological yield and individual bioactive molecules [6]. In this work, the analysis of supplementation of calcium silicate was tested in terms of the potential bioactive properties that could arise or decrease from this supplementation. Overall, very slight changes were sought for the parameters analysed, implying that supplementation can have a beneficial effect on the antioxidant and antimicrobial activity of the mushrooms without drastically changing their chemical composition. For the mushrooms from MC, 1% of supplementation of calcium silicate seemed to promote an increase of cinnamic acid, although 0.5% of supplementation increased p-coumaric acid revealing that lower concentrations of calcium silicate are favoured in terms of secondary metabolites. The antimicrobial activity did not seem affected by the varying percentages of supplementation, although the mushrooms revealed some antimicrobial activity against A. ocharaceus and P. ochrochloron. The mushrooms from PP showed that higher supplementation favoured the increase of p-coumaric acid and malic acid and the antioxidant activity while also increasing the antimicrobial capacity against A. ochraceus and P. ochrochloron. The parameters used in the LDA had higher discriminating ability for the supplementation of the mushrooms from MC than from PP. Overall, supplementation of calcium silicate does not seem to negatively affect the mushrooms in terms of their bioactivities, organic, phenolic compounds or induce cytotoxicity, and might even have beneficial effects, namely their increase. From a sustainability point of view, the use of calcium silicate is moderately used in agriculture to help increase resistance to certain pests without the drawbacks of pesticides. Furthermore, it is a source of silicon for plants and mushrooms and using it as a stimulant for bioactive compounds in mushrooms stands as a valuable tool for their production. Thus, the use of calcium silicate can stand as an important supplement in agriculture, although further studies on vegetable crops and other mushrooms are needed to widen knowledge.
Author Contributions
R.V.C.C.—Investigation, Writing—Original draft; M.C.—Formal analysis, Writing—Original draft, Visualization; Â.F.—Formal analysis; J.P.—Formal analysis; D.S.—Formal analysis; M.S.—Writing—Review and Editing; D.C.Z.—Conceptualization, Supervision; J.D.V.C.—Supervision; A.M.G.-P.—Supervision, I.C.F.R.F.—Supervision; L.B.—Supervision, Writing—Review and Editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to CIMO (UIDB/00690/2020), and R.V.C. Cardoso’s PhD grant (SFRH/BD/137436/2018). L. Barros and A. Fernandes also thank the national funding by FCT, through the institutional scientific employment program-contract, and M. Carocho and J. Pinela (CEECIND/00831/2018; CEECIND/01011/2018) thank FCT, through the individual scientific employment program-contract. This work is funded by the European Regional Development Fund (ERDF) through the Regional Operational Program North 2020, within the scope of Project Mobilizador Norte-01-0247-FEDER-024479: ValorNatural®; and to European Agricultural Fund for Rural Development (EAFRD), through the Rural Development Program (PDR2020), within the scope of Project MicoCoating (PDR2020-101-031472). This work has been supported by the Ministry of Education, Science and Technological Development of Republic of Serbia (451-03-9/2021-14/200007).
Institutional Review Board Statement
Not suitable.
Informed Consent Statement
Not suitable.
Data Availability Statement
Not suitable.
Conflicts of Interest
The authors state no conflict of interest.
References
- Angiolillo, L.; Del Nobile, M.A.; Conte, A. The extraction of bioactive compounds from food residues using microwaves. Curr. Opin. Food Sci. 2015, 5, 93–98. [Google Scholar] [CrossRef]
- Naim, L.; Alsanad, M.A.; El Sebaaly, Z.; Shaban, N.; Fayssal, S.A.; Sassine, Y.N. Variation of Pleurotus ostreatus (Jacq. Ex Fr.) P. Kumm. (1871) performance subjected to differentdoses and timings of nano-urea. Saudi J. Biol. Sci. 2020, 27, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Nölle, N.; Argyropoulos, D.; Ambacher, S.; Müller, J.; Biesalski, H.K. Vitamin D2 enrichment in mushrooms by natural or artificial UV-light during drying. LWT Food Sci. Technol. 2017, 85, 400–404. [Google Scholar] [CrossRef]
- Thongsook, T.; Kongbangkerd, T. Influence of calcium and silicon supplementation into Pleurotus ostreatus substrates on quality of fresh and canned mushrooms. Food Sci. Technol. Int. 2011, 17, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Pozza, A.A.A.; Carvalho, J.G.; Guimares, P.T.G.; Figueiredo, F.C.; Araújo, A.R. Suprimento do silicato de cálcio e a eficiência nutricional de variedades de cafeeiro. Rev. Bras. Cienc. Solo 2009, 33, 1705–1714. [Google Scholar] [CrossRef][Green Version]
- Cardoso, R.V.C.; Carocho, M.; Fernandes, Â.; Zied, D.C.; Cobos, J.D.V.; González-Paramás, A.M.; Ferreira, I.C.F.R.; Barros, L. Influence of Calcium Silicate on the Chemical Properties of Pleurotus ostreatus var. florida (Jacq.) P. Kumm. J. Fungi 2020, 6, 299. [Google Scholar] [CrossRef] [PubMed]
- Tubana, B.S.; Babu, T.; Datnoff, L.E. A review of silicon in soils and plants and its role in US agriculture: History and future perspectives. Soil Sci. 2016, 181, 393–411. [Google Scholar] [CrossRef]
- Talabani, R.M.; Garib, B.T.; Masaeli, R. Bioactivity and physicochemical properties of three calcium silicate-based cements: An in vitro study. BioMed Res. Int. 2020, 2020, 9576930. [Google Scholar] [CrossRef]
- Jo, S.B.; Kim, H.K.; Lee, H.N.; Kim, Y.J.; Patel, K.D.; Knowles, J.C.; Lee, J.H.; Song, M. Physical properties and biofunctionalities of bioactive root canal sealers in vitro. Nanomaterials 2020, 10, 1750. [Google Scholar] [CrossRef]
- Venkatraman, S.K.; Swamiappan, S. Review on calcium- and magnesium-based silicates for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2020, 108, 1546–1562. [Google Scholar] [CrossRef]
- Patil, A.; Durgude, A.; Pharande, A.; Kadlag, A.; Nimbalkar, C. Effect of calcium silicate as a silicon source on growth and yield of rice in different acid soils of Karnataka, southern India Socioeconomics. Int. J. Chem. Stud. 2017, 5, 545–549. [Google Scholar]
- Marques, D.J.; Ferreira, M.M.; da Silva Lobato, A.K.; De Freitas, W.A.; Carvalho, J.D.A.; Ferreira, E.D.; Broetto, F. Potential of calcium silicate to mitigate water deficiency in maize. Bragantia 2016, 75, 275–285. [Google Scholar] [CrossRef][Green Version]
- Guntzer, F.; Keller, C.; Meunier, J.D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Parthiban, P.; Chinniah, C.; Baskaran, K.M.; Suresh, K.; Karthick, S. Influence of Calcium Silicate Application on the Population of Sucking Pests of Groundnut (Arachis hypogaea L.). Silicon 2019, 11, 1687–1692. [Google Scholar] [CrossRef]
- Kumara, B.H.; Yogendra, N.D.; Prakash, N.B.; Kumar, A. Effect of calcium silicate and need based nitrogen on pests management in aerobic rice (Oryza sativa L.). Int. J. Plant Prot. 2016, 9, 133–136. [Google Scholar] [CrossRef]
- de Andrade, M.C.N.; Kopytowski Filho, J.; Minhoni, M.T.D.A.; Coutinho, L.N.; Figueiredo, M.B. Productivity, biological efficiency, and number of Agaricus blazei mushrooms grown in compost in the presence of Trichoderma sp. and Chaetomium olivacearum contaminants. Braz. J. Microbiol. 2007, 38, 243–247. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized Analysis of Organic Acids in Edible Mushrooms from Portugal by Ultra-Fast Liquid Chromatography and Photodiode Array Detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb.f.: An underexploited and highly disseminated species. Ind. Crops Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef]
- Spréa, R.M.; Fernandes, Â.; Calhelha, R.C.; Pereira, C.; Pires, T.C.S.P.; Alves, M.J.; Canan, C.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Chemical and bioactive characterization of the aromatic plant: Levisticum officinale W.D.J. Koch: A comprehensive study. Food Funct. 2020, 11, 1292–1303. [Google Scholar] [CrossRef]
- Lockowandt, L.; Pinela, J.; Roriz, C.L.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Bredol, M.; Ferreira, I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019, 128, 496–503. [Google Scholar] [CrossRef]
- Glamočlija, J.; Stojković, D.; Nikolić, M.; Ćirić, A.; Reis, F.S.; Barros, L.; Ferreira, I.C.F.R.; Soković, M. A comparative study on edible Agaricus mushrooms as functional foods. Food Funct. 2015, 6, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Tsukatani, T.; Suenaga, H.; Shiga, M.; Noguchi, K.; Ishiyama, M.; Ezoe, T.; Matsumoto, K. Comparison of the WST-8 colorimetric method and the CLSI broth microdilution method for susceptibility testing against drug-resistant bacteria. J. Microbiol. Methods 2012, 90, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Abreu, R.M.V.; Ferreira, I.C.F.R.; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno [3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Characterisation of phenolic compounds in wild fruits from Northeastern Portugal. Food Chem. 2013, 141, 3721–3730. [Google Scholar] [CrossRef]
- Carocho, M.; Barros, L.; Antonio, A.L.; Barreira, J.C.M.; Bento, A.; Kaluska, I.; Ferreira, I.C.F.R. Analysis of organic acids in electron beam irradiated chestnuts (Castanea sativa Mill.): Effects of radiation dose and storage time. Food Chem. Toxicol. 2013, 55, 348–352. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).