The Influence of the Configuration of Two Electrochemical Reactors on the Process of Removing Atrazine from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Synthetic Solution
2.2. Experimental Reactors
2.3. Samples and Measurements
2.4. Experimental Design
3. Results
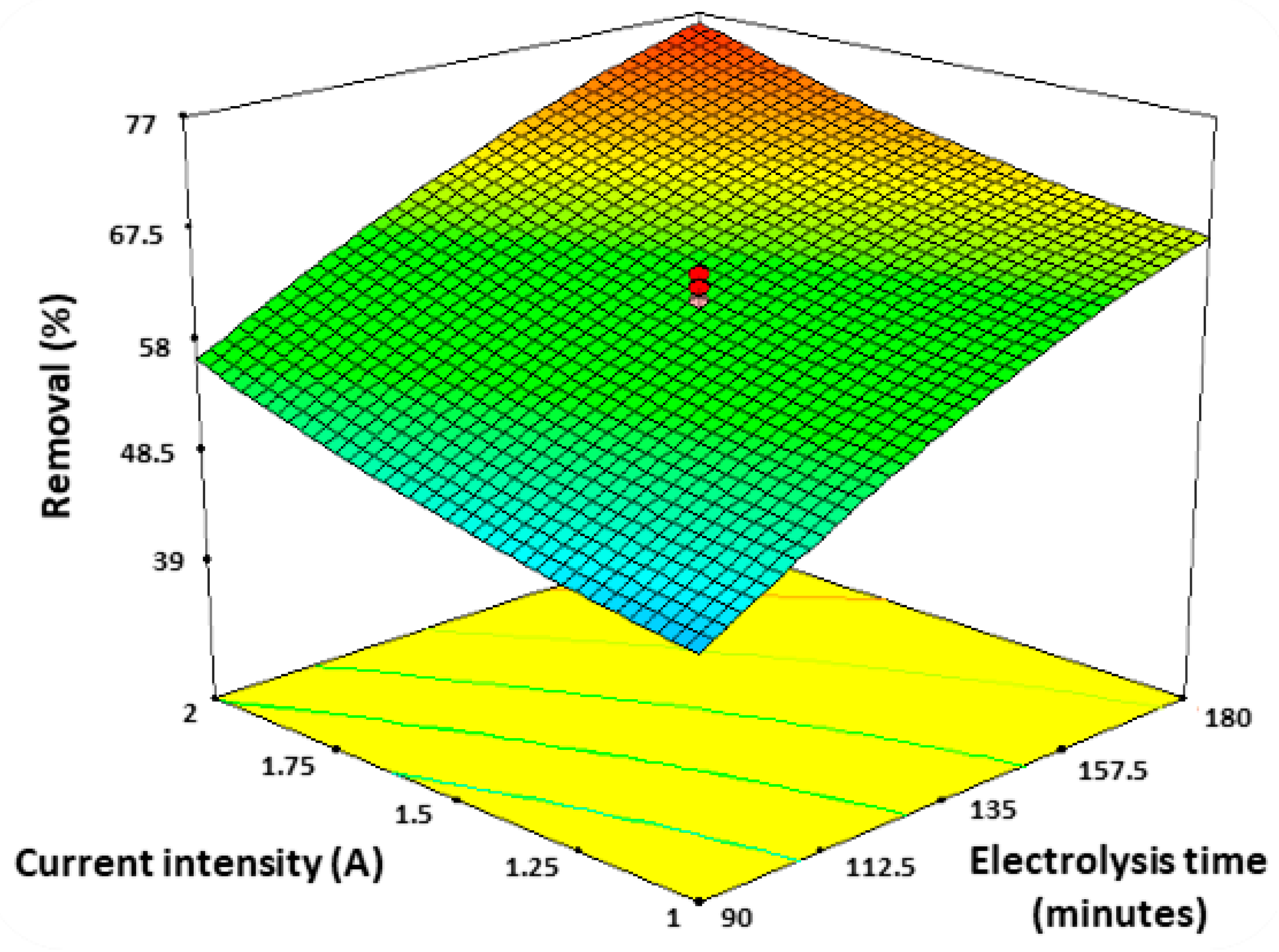
3.1. Atrazine Removal
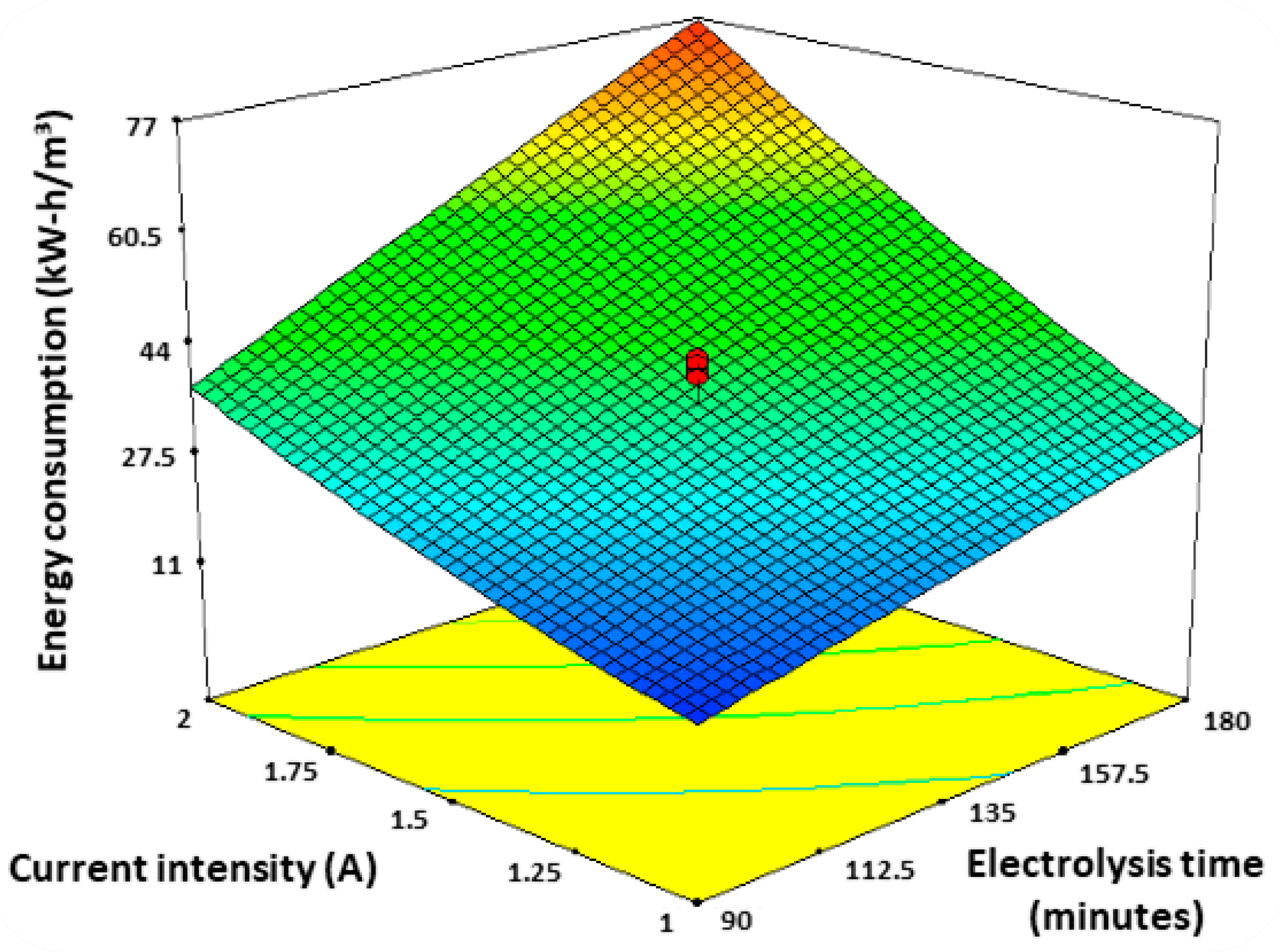
3.2. Energy Consumption
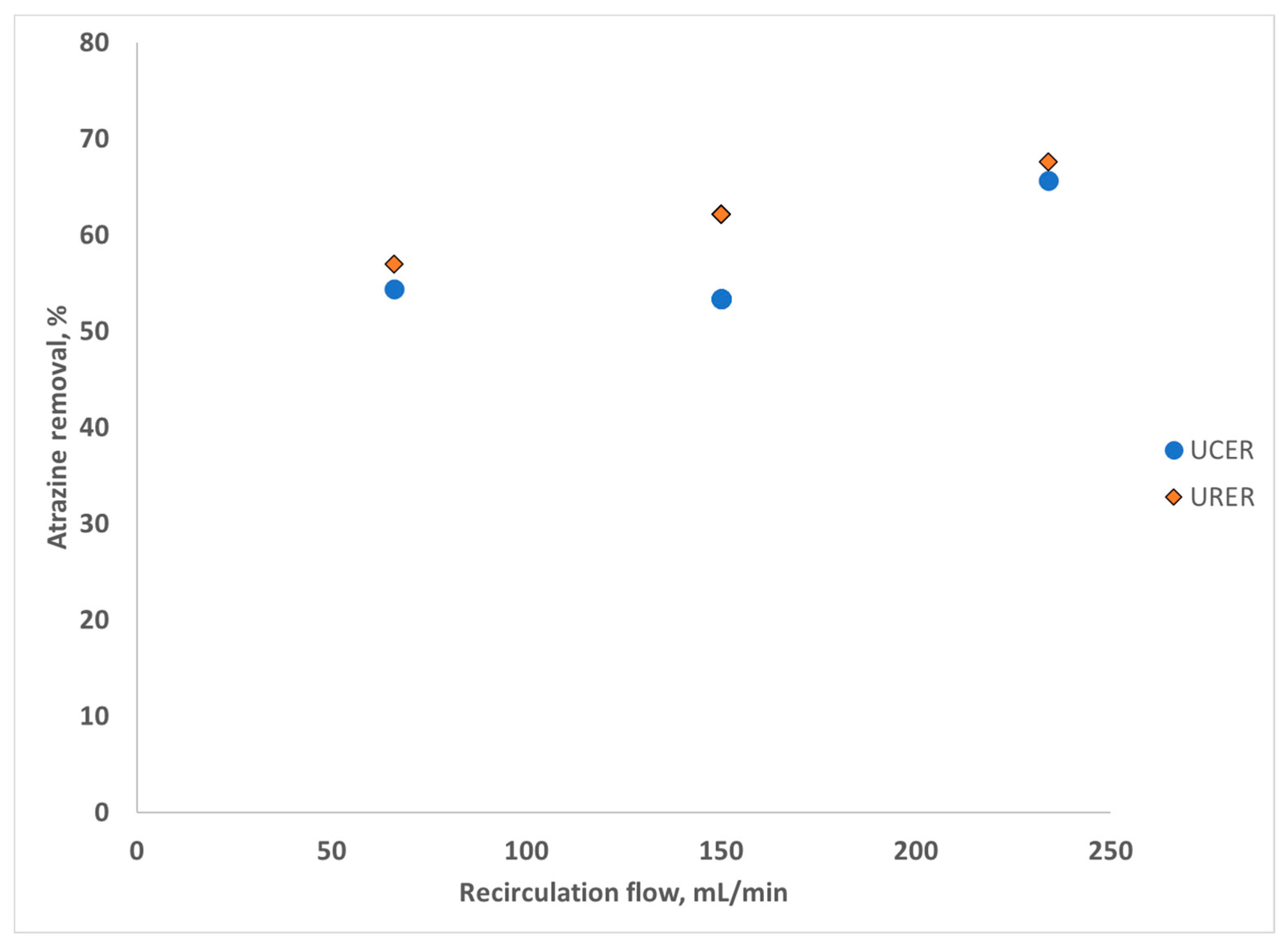
3.3. Recirculation Rate Effect
3.4. Process Efficiency
3.5. Reactors Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C0 | Initial atrazine concentration [mg/L] |
| Cf | Final atrazine concentration [mg/L] |
| E | Energy consumption [kWh/m3] |
| I | Current intensity, [A] |
| N | Relative removal ratio [%.m3/kWh] |
| Q | Recirculation flow rate, [mL/min] |
| R | Atrazine removal percentage [%] |
| t | Time, [min] |
| UCER | Up-flow cylindrical electro-chemical reactor |
| URER | Up-flow rectangular electro-chemical reactor |
References
- Balderrama-Carmona, A.Y.; Silva-Beltrán, P.S.; Ayala-Parra, P.A. Estimating the Health Risk Assessment of the Consumption of Oreochromis Niloticus, Tap Water, Surface Water and Prey Sediments, Contaminated with Heavy Metals in Communities Close to a Copper Mine and to Adolfo Ruiz Cortines Dam, in Sonora, Mexico. Rev. Bio Cienc. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-Wide Monitoring Survey on Emerging Polar Organic Contaminants in Wastewater Treatment Plant Effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef]
- Diamantis, B.P.; Stamatis, A.G. Incorporating Available Micro Gas Turbines and Fuel Cell: Matching Considerations and Performance Evaluation. Appl. Energy 2013, 103, 607–617. [Google Scholar] [CrossRef]
- Amadori, M.F.; Rodrigues, M.B.; Rebouças, C.C.; Peralta-Zamora, P.G.; Grassi, M.T.; Abate, G. Behavior of Atrazine and Its Degradation Products Deethylatrazine and Deisopropylatrazine in Oxisol Samples. Water Air Soil Pollut. 2016, 227, 1–13. [Google Scholar] [CrossRef]
- Hansen, A.M.; Treviño-Quintanilla, L.G.; Márquez-Pacheco, H.; Villada-Canela, M.; González-Márquez, L.C.; Guillén-Garcés, R.A.; Hernández-Antonio, A. Atrazina: Un Herbicida Polémico. Rev. Int. Contam. Ambient. 2013, 29, 65–84. Available online: https://www.revistascca.unam.mx/rica/index.php/rica/article/view/41420 (accessed on 20 January 2021).
- González-Márquez, L.C.; Hansen, A.M. Efecto de la salinidad en la adsorción de un herbicida en suelos agrícolas. Rev. Int. Contam. Ambient. 2014, 30, 191–199. Available online: http://www.scielo.org.mx/scielo.php?pid=S0188-49992014000200006&script=sci_abstract&tlng=pt (accessed on 20 January 2021).
- Góngora-Echeverría, V.R.; Martin-Laurent, F.; Quintal-Franco, C.; Lorenzo-Flores, A.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Dissipation and Adsorption of 2,4-D, Atrazine, Diazinon, and Glyphosate in an Agricultural Soil from Yucatan State, Mexico. Water Air Soil Pollut. 2019, 230, 1–15. [Google Scholar] [CrossRef]
- Tindall, J.; Friedel, M.J. Transport of Atrazine Versus Bromide and δO 18 in Sand. Water Air Soil Pollut. 2016, 227, 1–11. [Google Scholar] [CrossRef]
- Ding, X.; Wang, S.; Shen, W.; Mu, Y.; Wang, L.; Chen, H.; Zhang, L. Fe@Fe2O3 promoted electrochemical mineralization of atrazine via a triazinon ring opening mechanism. Water Res. 2017, 112, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Cao, X.; Long, X.; Li, X. Enhanced Degradation of Atrazine by Soil Microbial Fuel Cells and Analysis of Bacterial Community Structure. Water Air Soil Pollut. 2017, 228, 1–10. [Google Scholar] [CrossRef]
- Schwab, A.P.; Splichal, P.A.; Banks, M.K. Persistence of Atrazine and Alachlor in Ground Water Aquifers and Soil. Water Air Soil Pollut. 2006, 171, 203–235. [Google Scholar] [CrossRef]
- Jin, Y.; Davarpanah, A. Using Photo-Fenton and Floatation Techniques for the Sustainable Management of Flow-Back Produced Water Reuse in Shale Reservoirs Exploration. Water Air Soil Pollut. 2020, 231, 1–8. [Google Scholar] [CrossRef]
- Ghamsari, M.; Madrakian, T. Highly Fast and Efficient Removal of Some Cationic Dyes from Aqueous Solutions Using Sulfonated-Oxidized Activated Carbon. Anal. Bioanal. Chem. Res. 2019, 6, 157–169. [Google Scholar] [CrossRef]
- Almeida, F.B.P.S.; Meili, L.; Soletti, J.I.; Esquerre, K.P.S.O.R.; Ribeiro, L.M.O.; de Farias Silva, C.E. Oil produced water treatment using sugarcane solid residue as biosorbent. Rev. Mex. Ing. Química 2019, 18, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Castro-Gutiérrez, V.; Masís-Mora, M.; Carazo-Rojas, E.; Mora-López, M.; Rodríguez-Rodríguez, C.E. Fungal and Bacterial Co-Bioaugmentation of a Pesticide-Degrading Biomixture: Pesticide Removal and Community Structure Variations during Different Treatments. Water Air Soil Pollut. 2019, 230, 1–13. [Google Scholar] [CrossRef]
- Zaroual, Z.; Chaair, H.; Essadki, A.H.; El Ass, K.; Azzi, M. Optimizing the Removal of Trivalent Chromium by Electrocoagulation Using Experimental Design. Chem. Eng. J. 2009, 148, 488–495. [Google Scholar] [CrossRef]
- Sandoval-Reyes, J.L.; Ramírez-Zamora, R.M. Simultaneous removal of dissolved organic matter, microcystis aeruginosa, and microcystin-lr by pre-oxidation and coagulation-flocculation processes. Rev. Mex. Ing. Química 2019, 18, 889–900. [Google Scholar] [CrossRef]
- Valizadeh, K.; Davarpanah, A. Design and construction of a micro-photo bioreactor in order to dairy wastewater treatment by micro-algae: Parametric study. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 42, 611–624. [Google Scholar] [CrossRef]
- Farhadi, S.; Aminzadeh, B.; Torabian, A.; Khatibikamal, V.; Fard, M.A. Comparison of COD removal from pharmaceutical wastewater by electrocoagulation, photoelectrocoagulation, peroxi-electrocoagulation and peroxi-photoelectrocoagulation processes. J. Hazard. Mater. 2012, 219–220, 35–42. [Google Scholar] [CrossRef]
- García-Gómez, C.; Drogui, P.; Zaviska, F.; Seyhi, B.; Gortáres-Moroyoqui, P.; Buelna, G.; Neira-Sáenz, C.; Estrada-Alvarado, M.; Ulloa-Mercado, R.G. Experimental Design Methodology Applied to Electrochemical Oxidation of Carbamazepine Using Ti/PbO2 and Ti/BDD Electrodes. J. Electroanal. Chem. 2014, 732, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Romero-Soto, I.C.; Dia, O.; Leyva-Soto, L.A.; Drogui, P.; Buelna, G.; Díaz-Tenorio, L.M.; Ulloa-Mercado, R.G.; Gortáres-Moroyoqui, P. Degradation of Chloramphenicol in Synthetic and Aquaculture Wastewater Using Electrooxidation. J. Environ. Qual. 2018, 47, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zadaka, D.; Nir, S.; Radian, A.; Mishael, Y.G. Atrazine removal from water by polycation-clay composites: Effect of dissolved organic matter and comparison to activated carbon. Water Res. 2009, 43, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.H.; Abdel Azzem, M. Dissolved Organic Matter-Capped Silver Nanoparticles for Electrochemical Aggregation Sensing of Atrazine in Aqueous Systems. ACS Appl. Nano Mater. 2020, 3, 3868–3875. [Google Scholar] [CrossRef]
- Turan, N.B.; Sari Erkan, H.; Çaglak, A.; Bakırdere, S.; Engin, G.O. Optimization of atrazine removal from synthetic groundwater by electrooxidation process using titanium dioxide and graphite electrodes. Sep. Sci. Technol. 2020, 55, 3036–3045. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Blais, J.F.; Mercier, G.; Lafrance, P. Experimental Design Methodology Applied to Electrochemical Oxidation of the Herbicide Atrazine Using Ti/IrO2 and Ti/SnO2 Circular Anode Electrodes. J. Hazard. Mater. 2011, 185, 1499–1507. [Google Scholar] [CrossRef] [PubMed]

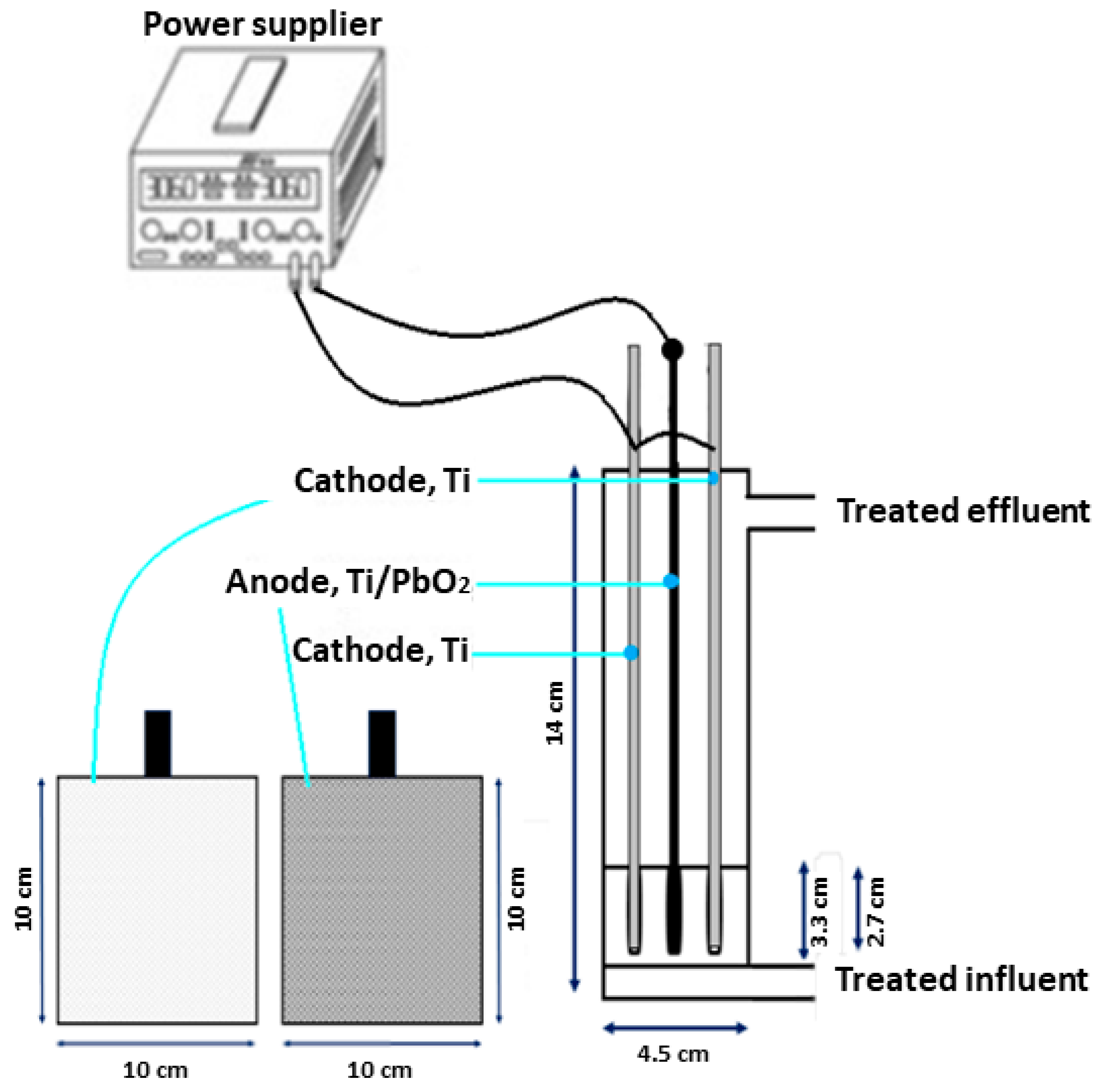


| Variables | Bottom Compound | Low Level | Central | High Level | Upper Compound |
|---|---|---|---|---|---|
| I (A) | 0.66 | 1 | 1.5 | 2 | 2.34 |
| t (min) | 59.32 | 90 | 135 | 180 | 210.68 |
| Q (mL/min) | 65.91 | 100 | 150 | 200 | 234.09 |
| Treatment | I (A) | t (min) | Q (mL/min) |
|---|---|---|---|
| 1 | 1 | 90 | 100 |
| 2 | 1.5 | 210.68 | 150 |
| 3 | 1.5 | 135 | 150 |
| 4 | 1.5 | 59.32 | 150 |
| 5 | 1.5 | 135 | 65.91 |
| 6 | 1 | 180 | 100 |
| 7 | 1.5 | 135 | 150 |
| 8 | 1 | 90 | 200 |
| 9 | 2 | 180 | 100 |
| 10 | 2 | 180 | 200 |
| 11 | 2 | 90 | 200 |
| 12 | 1.5 | 135 | 234.09 |
| 13 | 1 | 180 | 200 |
| 14 | 2 | 90 | 100 |
| 15 | 1.5 | 135 | 150 |
| 16 | 1.5 | 135 | 150 |
| 17 | 0.66 | 135 | 150 |
| 18 | 1.5 | 135 | 150 |
| 19 | 1.5 | 135 | 150 |
| 20 | 2.34 | 135 | 150 |
| Treatment | I (A) | t (min) | Q (mL/min) | R Removal (%) | E Energy Consumption (kWh/m3) |
|---|---|---|---|---|---|
| 1 | 1 | 90 | 100 | 31.30 | 15.95 |
| 2 | 1.5 | 210.68 | 150 | 71.23 | 61.77 |
| 3 | 1.5 | 135 | 150 | 53.48 | 39.89 |
| 4 | 1.5 | 59.32 | 150 | 34.97 | 11.14 |
| 5 | 1.5 | 135 | 65.91 | 54.40 | 25.62 |
| 6 | 1 | 180 | 100 | 53.06 | 31.09 |
| 7 | 1.5 | 135 | 150 | 53.38 | 25.80 |
| 8 | 1 | 90 | 200 | 31.68 | 15.68 |
| 9 | 2 | 180 | 100 | 59.54 | 78.55 |
| 10 | 2 | 180 | 200 | 76.89 | 73.64 |
| 11 | 2 | 90 | 200 | 61.05 | 37.91 |
| 12 | 1.5 | 135 | 234.09 | 65.68 | 42.95 |
| 13 | 1 | 180 | 200 | 34.49 | 31.09 |
| 14 | 2 | 90 | 100 | 40.54 | 39.55 |
| 15 | 1.5 | 135 | 150 | 53.45 | 41.42 |
| 16 | 1.5 | 135 | 150 | 51.81 | 40.81 |
| 17 | 0.66 | 135 | 150 | 36.67 | 12.83 |
| 18 | 1.5 | 135 | 150 | 55.25 | 39.27 |
| 19 | 1.5 | 135 | 150 | 53.05 | 42.34 |
| 20 | 2.34 | 135 | 150 | 67.49 | 71.80 |
| Treatment | I (A) | t (min) | Q (mL/min) | R Removal (%) | E Energy consumption (kWh/m3) |
|---|---|---|---|---|---|
| 1 | 1 | 90 | 100 | 46.95 | 22.77 |
| 2 | 1.5 | 210.68 | 150 | 76.44 | 132.63 |
| 3 | 1.5 | 135 | 150 | 62.68 | 81.92 |
| 4 | 1.5 | 59.32 | 150 | 39.04 | 33.57 |
| 5 | 1.5 | 135 | 65.91 | 56.94 | 71.80 |
| 6 | 1 | 180 | 100 | 60.61 | 47.73 |
| 7 | 1.5 | 135 | 150 | 61.25 | 64.43 |
| 8 | 1 | 90 | 200 | 44.06 | 26.59 |
| 9 | 2 | 180 | 100 | 73.62 | 133.64 |
| 10 | 2 | 180 | 200 | 77.45 | 137.45 |
| 11 | 2 | 90 | 200 | 62.03 | 69.82 |
| 12 | 1.5 | 135 | 234.09 | 67.56 | 65.05 |
| 13 | 1 | 180 | 200 | 68.28 | 50.73 |
| 14 | 2 | 90 | 100 | 52.80 | 68.18 |
| 15 | 1.5 | 135 | 150 | 62.70 | 79.77 |
| 16 | 1.5 | 135 | 150 | 61.14 | 72.10 |
| 17 | 0.66 | 135 | 150 | 60.49 | 20.12 |
| 18 | 1.5 | 135 | 150 | 61.52 | 79.47 |
| 19 | 1.5 | 135 | 150 | 63.77 | 80.08 |
| 20 | 2.34 | 135 | 150 | 72.23 | 150.29 |
| Treatment | R Atrazine Removal (%) | E Energy Consumption (kWh/m3) | N Relative Removal Ratio |
|---|---|---|---|
| 4 | 34.97 | 11.14 | 3.14 |
| 17 | 36.68 | 12.83 | 2.86 |
| 7 | 60.11 | 25.80 | 2.33 |
| 5 | 54.41 | 25.62 | 2.12 |
| 1 | 31.30 | 15.95 | 1.96 |
| 6 | 53.07 | 31.09 | 1.71 |
| 11 | 61.06 | 37.91 | 1.61 |
| 18 | 56.13 | 35.90 | 1.56 |
| 12 | 65.69 | 42.95 | 1.53 |
| 15 | 60.13 | 41.42 | 1.45 |
| 3 | 53.84 | 39.89 | 1.35 |
| 16 | 49.54 | 40.81 | 1.21 |
| 19 | 50.56 | 42.34 | 1.19 |
| 2 | 71.23 | 61.77 | 1.15 |
| 13 | 34.50 | 31.09 | 1.11 |
| 10 | 76.89 | 73.64 | 1.04 |
| 14 | 40.55 | 39.55 | 1.03 |
| 20 | 67.49 | 71.80 | 0.94 |
| 9 | 59.54 | 78.55 | 0.76 |
| 8 | 31.69 | 80.73 | 0.39 |
| Treatment | R Atrazine Removal (%) | E Energy Consumption (kWh/m3) | N Relative Removal Ratio |
|---|---|---|---|
| 17 | 60.4900 | 20.12 | 3.01 |
| 1 | 46.9500 | 22.77 | 2.06 |
| 8 | 44.0600 | 26.59 | 1.66 |
| 13 | 68.2800 | 50.73 | 1.35 |
| 6 | 60.6100 | 47.73 | 1.27 |
| 4 | 39.0400 | 33.57 | 1.16 |
| 12 | 67.5600 | 65.05 | 1.04 |
| 7 | 61.2500 | 64.43 | 0.95 |
| 11 | 62.0300 | 69.82 | 0.89 |
| 16 | 61.1400 | 72.10 | 0.85 |
| 19 | 63.7700 | 80.08 | 0.80 |
| 5 | 56.9400 | 71.80 | 0.79 |
| 15 | 62.7000 | 79.77 | 0.79 |
| 14 | 52.8000 | 68.18 | 0.77 |
| 18 | 61.5200 | 79.47 | 0.77 |
| 3 | 62.6800 | 81.92 | 0.77 |
| 2 | 76.4400 | 132.63 | 0.58 |
| 10 | 77.4500 | 137.45 | 0.56 |
| 9 | 73.6200 | 133.64 | 0.55 |
| 20 | 72.2300 | 150.29 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nápoles-Armenta, J.; Vidales-Contreras, J.A.; Leyva-Soto, L.A.; Meza-Escalante, E.R.; Díaz-Tenorio, L.M.; García-Gómez, C.; Martínez-Orozco, E.; De La Mora-Orozco, C.; Gortáres-Moroyoqui, P.; Salcedo-Gastelum, L.A. The Influence of the Configuration of Two Electrochemical Reactors on the Process of Removing Atrazine from Water. Sustainability 2021, 13, 5267. https://doi.org/10.3390/su13095267
Nápoles-Armenta J, Vidales-Contreras JA, Leyva-Soto LA, Meza-Escalante ER, Díaz-Tenorio LM, García-Gómez C, Martínez-Orozco E, De La Mora-Orozco C, Gortáres-Moroyoqui P, Salcedo-Gastelum LA. The Influence of the Configuration of Two Electrochemical Reactors on the Process of Removing Atrazine from Water. Sustainability. 2021; 13(9):5267. https://doi.org/10.3390/su13095267
Chicago/Turabian StyleNápoles-Armenta, Juan, Juan Antonio Vidales-Contreras, Luis Alonso Leyva-Soto, Edna Rosalba Meza-Escalante, Lourdes Mariana Díaz-Tenorio, Celestino García-Gómez, Edgardo Martínez-Orozco, Celia De La Mora-Orozco, Pablo Gortáres-Moroyoqui, and Lilian Alejandra Salcedo-Gastelum. 2021. "The Influence of the Configuration of Two Electrochemical Reactors on the Process of Removing Atrazine from Water" Sustainability 13, no. 9: 5267. https://doi.org/10.3390/su13095267
APA StyleNápoles-Armenta, J., Vidales-Contreras, J. A., Leyva-Soto, L. A., Meza-Escalante, E. R., Díaz-Tenorio, L. M., García-Gómez, C., Martínez-Orozco, E., De La Mora-Orozco, C., Gortáres-Moroyoqui, P., & Salcedo-Gastelum, L. A. (2021). The Influence of the Configuration of Two Electrochemical Reactors on the Process of Removing Atrazine from Water. Sustainability, 13(9), 5267. https://doi.org/10.3390/su13095267







