Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Chemicals
2.2. Hydrothermal Carbonization Reaction
2.3. Composition of Metal Solution
2.4. Adsorption and Desorption Experiments for Metal Remediation with Hydrochar
2.5. Adsorption Kinetics and Langmuir Isotherm
2.6. Characterization of Algae, Hydrochar, and Spent Hydrochar
2.6.1. N2 Adsorption and Desorption Analysis
2.6.2. Fourier Transform Infra-Red (FTIR) Spectroscopy
2.6.3. Thermogravimetric Analysis (TGA)
2.6.4. Scanning Electron Microscopy (SEM)
2.6.5. Proximate and CHONS Analysis
2.7. Statistical Analysis
3. Results and Discussion
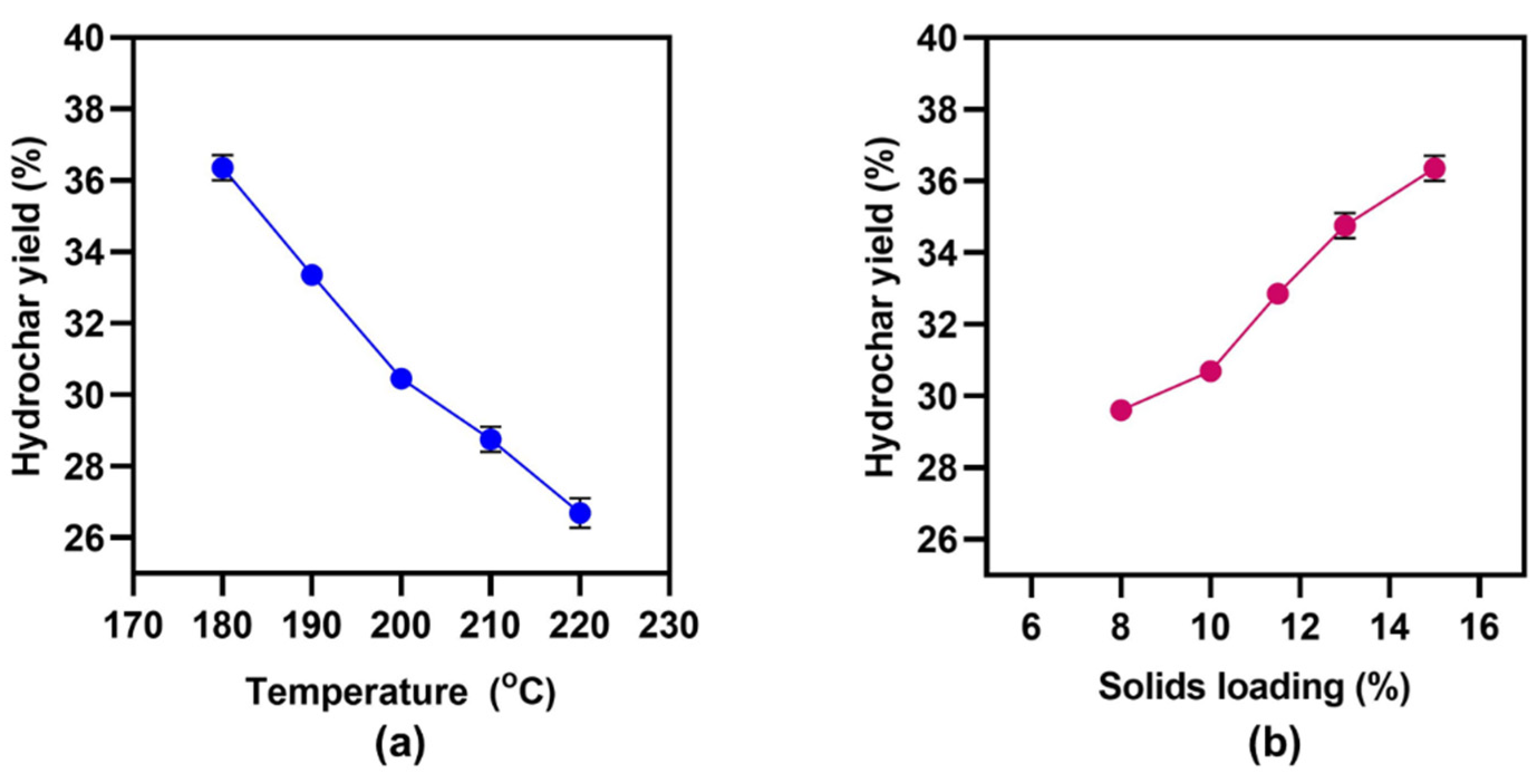
3.1. Effects of HTC Temperature and Solids Loading
3.2. Characteristics of P. oculatum LEA and Hydrochar
3.2.1. Surface Area and Pore Size
3.2.2. Surface Functional Groups
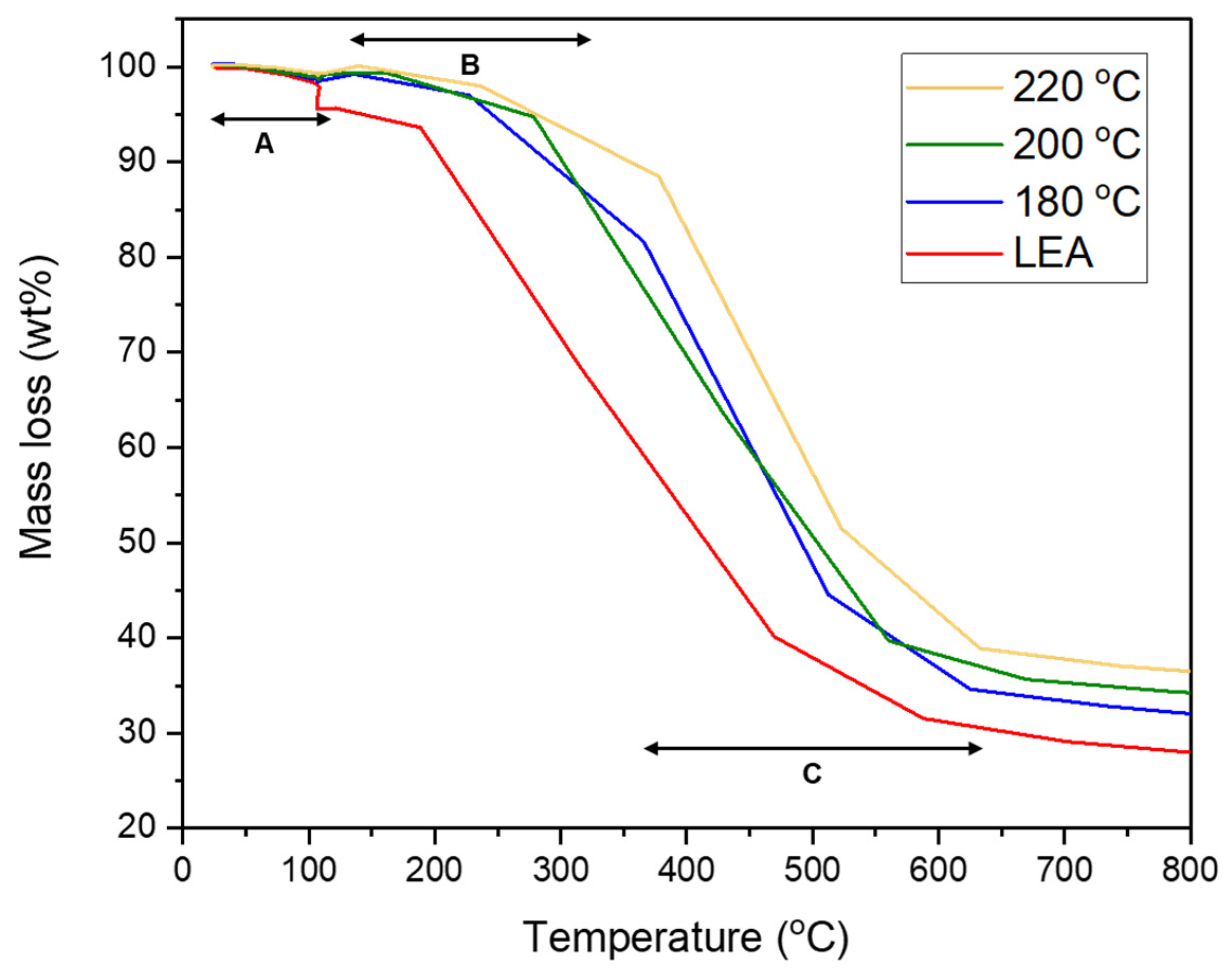
3.2.3. Combustion Behavior
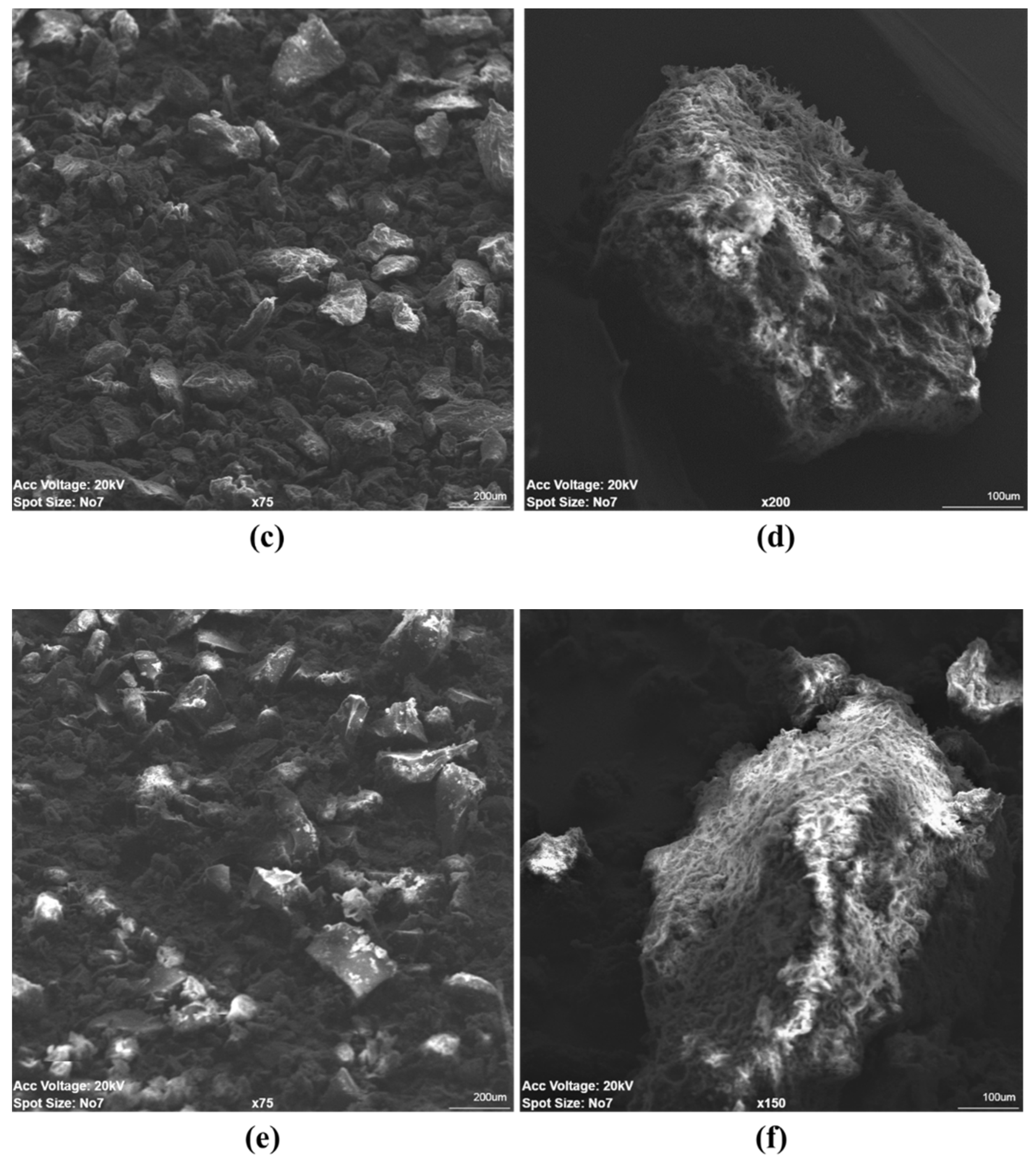
3.2.4. Scanning Electron Microscopy (SEM)
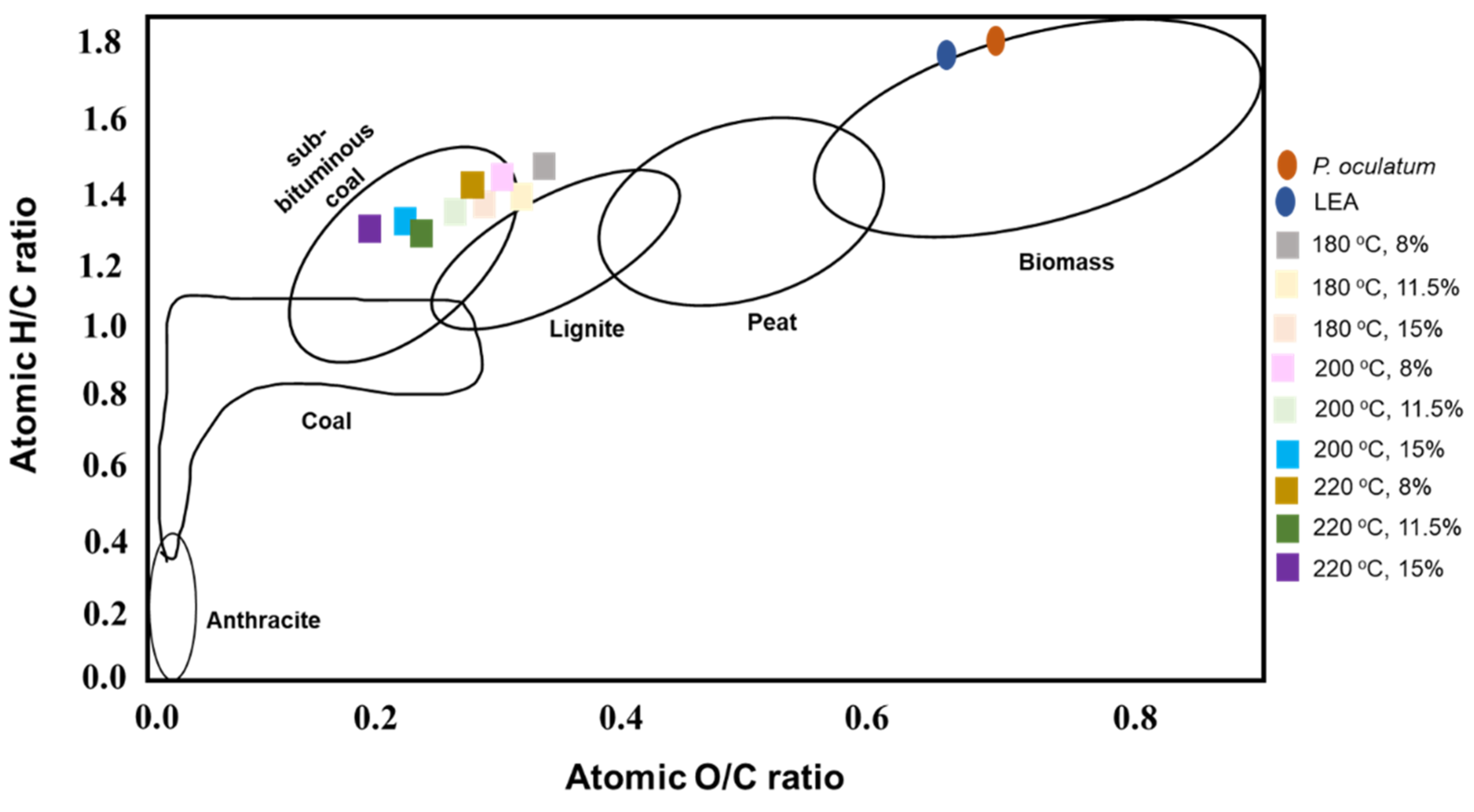
3.2.5. Proximate and Elemental Composition
3.3. Metal Removal from Solution by Algal Hydrochar
3.3.1. Selection of Adsorption Conditions
3.3.2. Metal Solution Treatment
3.3.3. Adsorption Kinetics
3.3.4. Langmuir Isotherm Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsarpali, M.; Arora, N.; Kuhn, J.N.; Philippidis, G.P. Lipid-extracted algae as a source of biomaterials for algae biorefineries. Algal Res. 2021, 57, 102354. [Google Scholar] [CrossRef]
- Broch, A.; Jena, U.; Hoekman, S.K.; Langford, J. Analysis of Solid and Aqueous Phase Products from Hydrothermal Carbonization of Whole and Lipid-Extracted Algae. Energies 2013, 7, 62–79. [Google Scholar] [CrossRef] [Green Version]
- Shi, N.; Liu, Q.; He, X.; Wang, G.; Chen, N.; Peng, J.; Ma, L. Molecular Structure and Formation Mechanism of Hydrochar from Hydrothermal Carbonization of Carbohydrates. Energy Fuels 2019, 33, 9904–9915. [Google Scholar] [CrossRef]
- Puccini, M.; Stefanelli, E.; Hiltz, M.; Seggiani, M.; Vitolo, S. Activated carbon from hydrochar produced by hydrothermal carbonization of wastes. Chem. Eng. Trans. 2017, 57, 169–174. [Google Scholar]
- Zhang, S.; Zhu, X.; Zhou, S.; Shang, H.; Luo, J.; Tsang, D.C. Chapter 15—Hydrothermal Carbonization for Hydrochar Production and Its Application. In Biochar from Biomass and Waste; Ok, Y.S., Tsang, D.C.W., Bolan, N., Novak, J.M., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 275–294. [Google Scholar]
- Zhang, X.; Wang, Y.; Cai, J.; Wilson, K.; Lee, A.F. Bio/hydrochar Sorbents for Environmental Remediation. Energy Environ. Mater. 2020, 3, 453–468. [Google Scholar] [CrossRef]
- Tsarpali, M.; Arora, N.; Kuhn, J.N.; Philippidis, G.P. Beneficial use of the aqueous phase generated during hydrothermal carbonization of algae as nutrient source for algae cultivation. Algal Res. 2021, 60, 102485. [Google Scholar] [CrossRef]
- Niinipuu, M.; Bergknut, M.; Boily, J.-F.; Rosenbaum, E.; Jansson, S. Influence of water matrix and hydrochar properties on removal of organic and inorganic contaminants. Environ. Sci. Pollut. Res. 2020, 27, 30333–30341. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Guo, J.-Z.; Liu, J.-L.; Fang, L.; Lv, J.-Q.; Lv, K. Removal of aqueous-phase lead ions by dithiocarbamate-modified hydrochar. Sci. Total Environ. 2020, 714, 136897. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.; Takahashi, F.; Yoshikawa, K. Characterization and application of microalgae hydrochar as a low-cost adsorbent for Cu(II) ion removal from aqueous solutions. Environ. Sci. Pollut. Res. 2018, 25, 32721–32734. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhang, S.; Li, G.; Du, Q.; Yang, F. Preparation of montmorillonite modified biochar with various temperatures and their mechanism for Zn ion removal. J. Hazard. Mater. 2020, 391, 121692. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb(II) onto modified hydrochar: Modeling and mechanism analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef] [PubMed]
- Barakat, M. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency Industrial Wastewater. 2021. Available online: https://www.epa.gov/npdes/industrial-wastewater (accessed on 18 October 2021).
- Shahedi, A.; Darban, A.K.; Taghipour, F.; Jamshidi-Zanjani, A. A review on industrial wastewater treatment via electrocoagulation processes. Curr. Opin. Electrochem. 2020, 22, 154–169. [Google Scholar] [CrossRef]
- Activated Carbon Market by Type, Application (Liquid Phase (Water Treatment, Foods & Beverages, Pharmaceutical & Medical), Gas Phase (Industrial, Automotive), and Region (APAC, North America, Europe, Middle East, South America)—Global Forecast to 2026. 2021. Available online: https://www.marketsandmarkets.com/Market-Reports/activated-carbon-362.html (accessed on 18 October 2021).
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment. In Green Adsorbents for Pollutant Removal; Environmental Chemistry for a Sustainable World; Crini, G., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 18. [Google Scholar] [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent advances on pollutants removal by rice husk as a bio-based adsorbent: A critical review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, R.; Pant, D.; Malaviya, P. Engineered algal biochar for contaminant remediation and electrochemical applications. Sci. Total Environ. 2021, 774, 145676. [Google Scholar] [CrossRef]
- Dogaris, I.; Loya, B.; Cox, J.; Philippidis, G. Study of landfill leachate as a sustainable source of water and nutrients for algal biofuels and bioproducts using the microalga Picochlorum oculatum in a novel scalable bioreactor. Bioresour. Technol. 2019, 282, 18–27. [Google Scholar] [CrossRef]
- Dogaris, I.; Welch, M.; Meiser, A.; Walmsley, L.; Philippidis, G. A novel horizontal photobioreactor for high-density cultivation of microalgae. Bioresour. Technol. 2015, 198, 316–324. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Gouda, N.; Panda, A.; Singh, R.K.; Ratha, S.K. Pyrolytic conversion of protein rich microalgae Arthrospira platensis to bio-oil. Res. J. Chem. Environ. 2018, 22, 54–65. [Google Scholar]
- Giachini, A.J.; Sulzbach, T.S.; Pinto, A.L.; Armas, R.D.; Cortez, D.H.; Silva, E.P.; Buzanello, E.B.; Soares, Á.G.; Soares, C.R.F.S.; Rossi, M.J. Microbially-enriched poultry litter-derived biochar for the treatment of acid mine drainage. Arch. Microbiol. 2018, 200, 1227–1237. [Google Scholar] [CrossRef]
- Gustafsson, J.P. Visual MINTEQ 3.0 User Guide; Department of Land and Water Resources Engineering, KTH Royal Institute of Technology: Stockholm, Sweden, 2005. [Google Scholar]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, S.; Wang, B.; Wang, Q.; Yang, G.; Chen, J. Effect of Residence Time on Hydrothermal Carbonization of Corn Cob Residual. Bioresour 2015, 10, 3979–3986. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Ross, A.B. The Influence of Residence Time during Hydrothermal Carbonisation of Miscanthus on Bio-Coal Combustion Chemistry. Energies 2019, 12, 523. [Google Scholar] [CrossRef] [Green Version]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, S.M.; Davis, H.T.; Jader, L.R.; Lefebvre, P.A.; Sadowsky, M.; Schendel, F.J.; Von Keitz, M.G.; Valentas, K.J. Hydrothermal carbonization of microalgae. Biomass Bioenergy 2010, 34, 875–882. [Google Scholar] [CrossRef]
- Biller, P.; Ross, A.B. Hydrothermal processing of algal biomass for the production of biofuels and chemicals. Biofuels 2012, 3, 603–623. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.; Mubarak, N.; Bhutto, A.W.; Abro, R.; Mazari, S.; Ali, B.S. An overview of effect of process parameters on hydrothermal carbonization of biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Kumar, V.; Verma, R.; Verma, M.; Kumar, A.; Vlaskin, M.S.; Nanda, M.; Kim, H. Graphitic bio-char and bio-oil synthesis via hydrothermal carbonization-co-liquefaction of microalgae biomass (oiled/de-oiled) and multiple heavy metals remediations. J. Hazard. Mater. 2021, 409, 124987. [Google Scholar] [CrossRef]
- Mumme, J.; Eckervogt, L.; Pielert, J.; Diakité, M.; Rupp, F.; Kern, J. Hydrothermal carbonization of anaerobically digested maize silage. Bioresour. Technol. 2011, 102, 9255–9260. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Chemical, structural and energy properties of hydrochars from microwave-assisted hydrothermal carbonization of glucose. Int. J. Ind. Chem. 2016, 7, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Balasubramanian, R.; Srinivasan, M. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Driver, T.; Bajhaiya, A.K.; Allwood, J.W.; Goodacre, R.; Pittman, J.K.; Dean, A.P. Metabolic responses of eukaryotic microalgae to environmental stress limit the ability of FT-IR spectroscopy for species identification. Algal Res. 2015, 11, 148–155. [Google Scholar] [CrossRef]
- Khoo, C.G.; Lam, M.K.; Mohamed, A.R.; Lee, K.T. Hydrochar production from high-ash low-lipid microalgal biomass via hydrothermal carbonization: Effects of operational parameters and products characterization. Environ. Res. 2020, 188, 109828. [Google Scholar] [CrossRef]
- Gai, C.; Zhang, Y.; Chen, W.-T.; Zhang, P.; Dong, Y. An investigation of reaction pathways of hydrothermal liquefaction using Chlorella pyrenoidosa and Spirulina platensis. Energy Convers. Manag. 2015, 96, 330–339. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Chen, H.; Cai, T.; Liu, Z. Hydrochar and pyrochar for sorption of pollutants in wastewater and exhaust gas: A critical review. Environ. Pollut. 2021, 268, 115910. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Peters, K.; Xia, X.; Pomerantz, A.; Mullins, O. Chapter 3—Geochemistry Applied to Evaluation of Unconventional Resources. In Unconventional Oil and Gas Resources Handbook; Ma, Y.Z., Holditch, S.A., Eds.; Elsevier BV: Amsterdam, The Netherlands, 2016; pp. 71–126. [Google Scholar]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Shrestha, A.; Acharya, B.; Farooque, A.A. Study of hydrochar and process water from hydrothermal carbonization of sea lettuce. Renew. Energy 2021, 163, 589–598. [Google Scholar] [CrossRef]
- Engineering ToolBox, Standard Grade Coal—Heat Values. 2010. Available online: https://www.engineeringtoolbox.com/coal-heating-values-d_1675.html (accessed on 18 October 2021).
- Arora, N.; Gulati, K.; Patel, A.; Pruthi, P.A.; Poluri, K.M.; Pruthi, V. A hybrid approach integrating arsenic detoxification with biodiesel production using oleaginous microalgae. Algal Res. 2017, 24, 29–39. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, C.; Zan, Y.; Miao, G.; Wang, H.; Kong, L. Hydrothermal Carbonization of Microalgae (Chlorococcum sp.) for Porous Carbons with High Cr(VI) Adsorption Performance. Appl. Biochem. Biotechnol. 2018, 186, 414–424. [Google Scholar] [CrossRef]
- Amin, M.; Chetpattananondh, P. Biochar from extracted marine Chlorella sp. residue for high efficiency adsorption with ultrasonication to remove Cr(VI), Zn(II) and Ni(II). Bioresour. Technol. 2019, 289, 121578. [Google Scholar] [CrossRef] [PubMed]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Tran, T.H.; Le, A.H.; Pham, T.H.; Nguyen, D.T.; Chang, S.W.; Chung, W.J.; Nguyen, D.D. Adsorption isotherms and kinetic modeling of methylene blue dye onto a carbonaceous hydrochar adsorbent derived from coffee husk waste. Sci. Total Environ. 2020, 725, 138325. [Google Scholar] [CrossRef] [PubMed]





| P. oculatum | LEA | 180 °C, 8% | 180 °C, 11.5% | 180 °C, 15% | 200 °C, 8% | 200 °C, 11.5% | 200 °C, 15% | 220 °C, 8% | 220 °C, 11.5% | 220 °C, 15% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture (wt.% wet) | 4.1 | 4.0 | 1.0 | 0.4 | 0.7 | 1.3 | 0.6 | 1.1 | 1.2 | 0.4 | 0.8 |
| Volatile (wt.% dry) | 74.6 | 74.3 | 69.8 | 72.1 | 70.8 | 64.0 | 63.2 | 70.1 | 64.8 | 62.8 | 67.3 |
| Fixed Carbon (%) | 11.9 | 12.3 | 20.0 | 18.9 | 18.8 | 24.9 | 26.4 | 20.8 | 20.8 | 24.3 | 21.8 |
| Ash (%) | 9.4 | 9.4 | 9.2 | 8.6 | 8.6 | 9.8 | 9.9 | 8.1 | 13.2 | 12.5 | 10.2 |
| C | 45.0 | 43.1 | 57.8 | 62.2 | 62.8 | 58.6 | 60.4 | 65.1 | 59.3 | 62.8 | 67.3 |
| H | 6.8 | 6.4 | 7.1 | 7.3 | 7.3 | 6.9 | 6.8 | 7.5 | 6.9 | 6.9 | 7.4 |
| O | 39.5 | 41.9 | 27.4 | 23.4 | 22.8 | 28.3 | 26.3 | 21.1 | 27.8 | 24.4 | 18.9 |
| N | 7.7 | 7.7 | 7.2 | 6.7 | 6.6 | 5.8 | 6.0 | 6.0 | 5.7 | 5.5 | 6.1 |
| S | 1.0 | 0.9 | 0.6 | 0.5 | 0.5 | 0.5 | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 |
| H/C | 1.80 | 1.77 | 1.46 | 1.40 | 1.39 | 1.40 | 1.34 | 1.37 | 1.39 | 1.31 | 1.31 |
| O/C | 0.66 | 0.73 | 0.36 | 0.28 | 0.27 | 0.36 | 0.33 | 0.24 | 0.35 | 0.29 | 0.21 |
| HHV (MJ/kg) | 17.9 | 16.3 | 24.8 | 27.2 | 27.6 | 24.6 | 25.5 | 28.9 | 24.9 | 26.7 | 29.9 |
| E (%) | - | - | 44.8 | 54.8 | 61.1 | 41.6 | 43.9 | 53.8 | 36.9 | 41.5 | 48.5 |
| Cycle 1 | |||||
| Pseudo Second-Order | Langmuir Isotherm | ||||
| qe exp (mg/g) | qe (mg/g) | k2 (g/mg min) | qe (mg/g) | KL (mL/mg) | |
| Al+3 | 8.90 | 9.13 | 0.0016 | 8.84 | 0.03 |
| Cu+2 | 1.61 | 1.74 | 0.0143 | 1.58 | 0.05 |
| Fe+2 | 7.50 | 7.72 | 0.0007 | 7.56 | 0.04 |
| Mg+2 | 8.50 | 9.03 | 0.0009 | 8.84 | 0.26 |
| Mn+2 | 1.20 | 1.43 | 0.0071 | 1.25 | 1.45 |
| Pb+2 | 0.59 | 0.61 | 0.0837 | 0.58 | 0.3 |
| Cycle 2 | |||||
| Pseudo Second-Order | Langmuir Isotherm | ||||
| qe exp (mg/g) | qe (mg/g) | k2 (g/mg min) | qe (mg/g) | KL (mL/mg) | |
| Al+3 | 6.32 | 6.76 | 0.0071 | 6.46 | 0.04 |
| Cu+2 | 1.50 | 1.58 | 0.0135 | 1.52 | 0.11 |
| Fe+2 | 1.20 | 1.25 | 0.0157 | 1.30 | 0.09 |
| Mg+2 | 6.00 | 6.11 | 0.0010 | 6.17 | 0.006 |
| Mn+2 | 0.23 | 0.27 | 0.0591 | 0.25 | 0.53 |
| Pb+2 | - | - | - | - | - |
| Cycle 3 | |||||
| Pseudo Second-Order | Langmuir Isotherm | ||||
| qe exp (mg/g) | qe (mg/g) | k2 (g/mg min) | qe (mg/g) | KL (mL/mg) | |
| Al+3 | 3.00 | 3.33 | 0.0011 | 3.16 | 0.02 |
| Cu+2 | 0.80 | 0.84 | 0.0291 | 0.81 | 0.36 |
| Fe+2 | 0.47 | 0.45 | 0.0190 | 0.52 | 0.05 |
| Mg+2 | 4.00 | 3.97 | 0.0030 | 3.98 | 0.01 |
| Mn+2 | 0.70 | 0.69 | 0.0161 | 0.73 | 0.21 |
| Pb+2 | - | - | - | - | - |
| Cycle 4 | |||||
| Pseudo Second-Order | Langmuir Isotherm | ||||
| qe exp (mg/g) | qe (mg/g) | k2 (g/mg min) | qe (mg/g) | KL (mL/mg) | |
| Al+3 | 8.90 | 9.32 | 0.0009 | 9.09 | 0.01 |
| Cu+2 | 1.85 | 1.88 | 0.0159 | 1.83 | 0.08 |
| Fe+2 | 6.42 | 6.57 | 0.0020 | 6.62 | 0.01 |
| Mg+2 | 8.70 | 8.43 | 0.0011 | 8.60 | 0.01 |
| Mn+2 | 1.39 | 1.43 | 0.0158 | 1.36 | 0.04 |
| Pb+2 | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsarpali, M.; Kuhn, J.N.; Philippidis, G.P. Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent. Sustainability 2022, 14, 455. https://doi.org/10.3390/su14010455
Tsarpali M, Kuhn JN, Philippidis GP. Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent. Sustainability. 2022; 14(1):455. https://doi.org/10.3390/su14010455
Chicago/Turabian StyleTsarpali, Magdalini, John N. Kuhn, and George P. Philippidis. 2022. "Hydrothermal Carbonization of Residual Algal Biomass for Production of Hydrochar as a Biobased Metal Adsorbent" Sustainability 14, no. 1: 455. https://doi.org/10.3390/su14010455






