The chemical composition (%) of the debarked sawdust mix (mean and standard deviation) is glucans 40.9 (±0.5), xylans 7.45 (±0.10), galactans 2.58 (±0.07), arabinans 0.77 (±0.02), mannans 14.8 (±0.2), acetyl groups 1.22 (±0.02), insoluble lignin 29.2 (±0.1), water extractives 0.73 (±0.01), ethanol extractives 1.54 (±0.03), and ashes 0.04 (±0.00).
3.1. Simple Factorial Design: Effect of Ethanol–Liquor Ratio and Alkaline Load
A simple factorial design with two factors (
Table 1) was used to understand the effect of the ethanol–water ratio (ethanol:water) and its interaction with the alkaline load (
AL, %odw). Results are summarized in
Table 4.
The extent of delignification is positively affected by both factors (mainly by the alkaline load), but not by their interaction, as shown in Equation (4) (R
2 = 87.3%, regression model in codified independent variables).
Increasing levels of ethanol in the cooking liquor ease the delignification process. This tendency has been previously reported by Shatalov et al. [
24]. The authors, working with soda–ethanol pulping of
Arundo donax L. reed, proved the beneficial effect of ethanol addition on lignin removal. In addition, they stated that the ethanol in the cooking liquor reduces its surface tension, improving the mass transfer of active chemicals (increasing the hydroxide ion concentration in the fiber) and degradation products. Moreover, the ethanol hinders the condensation reactions of lignin by the alkylation of the benzyl alcohol groups [
26]. Other authors found that the addition of a low alcohol content (4%) to the alkaline liquor increased the delignification speed in the soda pulping of Pinus sylvestris wood, which allows for the reduction of alkaline charge to achieve the same degree of delignification for the same conditions of time and temperature [
27].
The total pulp yield and the selectivity are not affected by the ethanol–water ratio, in contrast to the statements of the other authors. When working with ethanol–kraft pulping of aspen and spruce chips, Yoon et al. [
28] found a significant gain in pulp yield with the increasing ethanol content in cooking liquor.
Regarding the chemical composition, acid-insoluble lignin depends on both variables (R2 = 88.2%). The contents of glucans, xylans, and galactans in pulps are affected by the AL (R2 = 94.8, 78.8, and 70.3%, respectively), but not by the ethanol–liquor ratio.
3.2. 32 Design: Effect of the Alkaline Load and Time at Maximum Temperature
The effect of the alkaline load was studied in-depth, together with the effect of time, using a 3
2 design. The obtained results of each experiment are summarized in
Table 5. Experiment 9 (central point) is the mean value of five replicates.
All of the experiments render a well-delignified material. The total yield varied between 39.1% (Experiment 4) to 49.9% (Experiment 1), and the statistical analysis reveals a strong dependence on AL, tmax, and their interaction.
The information provided by statistical models is crucial for understanding the behavior of the individual components of the raw material, optimizing the operational variables, and shaping the characteristics of the final product.
The experimental values of the total yield were adjusted to a regression model in codified independent variables (Equation (5), R
2 = 96.7%). The curves estimated by the model are plotted in
Figure 1.
The influence of the AL on total yield is more noticeable at a higher time at maximum temperature. The difference in total yields for experiments carried out at 19.0 and 23.3% AL is slightly higher than the difference exhibited by experiments conducted at 23.3 and 27.6% AL.
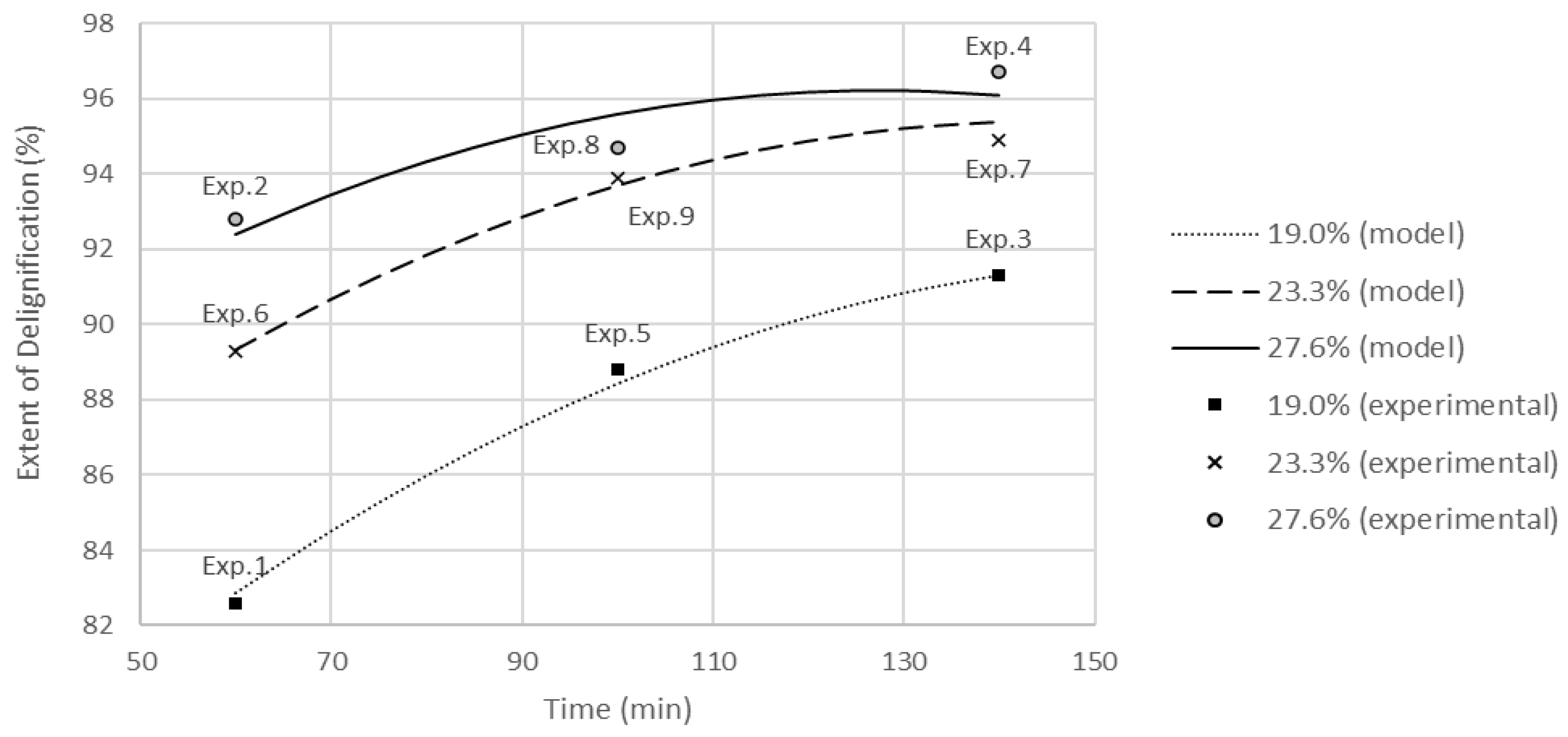
The delignification for each experiment was calculated in accordance with Equation (1). The maximum extent of delignification (96.7%) and the lowest yield (39.07%) were achieved using the most severe conditions (Experiment 4). All of the studied factors significantly affected the extent of delignification. The experimental results were adjusted to a second-order model using codified independent variables (Equation (6), R
2 = 98.6%). The curves estimated by the model are plotted in
Figure 2.
All of the studied factors proved to have a significant effect on the delignification extent. Time at maximum temperature has more impact on treatments with an alkaline load of 19.0%odw: Small changes in time rendered relevant changes in delignification. At higher AL, the impact of time weakens.
The model predicts an increase in barely of 1.26% on the extent of delignification for 140 min-treatments at 23.3 and 27.6 %odw AL, probing that the effect of AL decreases when cooking for long periods.
In accordance with the statistical model, the treatment with an alkaline load of 27.6 %odw reaches its maximum delignification at about 127 min.
The desirability function identifies the combination of factors that simultaneously optimize multiple answers. This function, expressed on a scale from 0 to 1, was used to maximize both the total yield and the extent of delignification (
Figure 3). The maximum desirability is 0.68 and is achieved at the conditions of Experiment 2, i.e., 60 min at maximum temperature and an alkaline load of 27.6 %odw.
Figure 4 shows the evolution of the total yield and the extent of delignification with the alkaline load and cooking time. The plotted curves are a powerful tool for adjusting the operational variables to obtain a target outcome.
The selectivity, defined herein as the ratio between the extent of delignification and the total carbohydrate loss (Equation (2)), is significantly affected by
AL,
tmax, and their interaction. The experimental selectivities are plotted in
Figure 5.
Selectivity is strongly affected by the AL-tmax interaction. When working at low AL (19.0%), increasing the time at maximum temperature produces a slight increase in selectivity. Moreover, this tendency applies to the degree of delignification, which implies that carbohydrates are better preserved at low AL, even for long periods at maximum temperature (140 min-treatment). Therefore, when using a low alkaline load, it is possible to obtain high levels of delignification by increasing the cooking time without selectivity loss. On the contrary, when using higher alkaline loads, tmax increase causes relevant selectivity loss. In severe conditions, delignification increases of approximately 6% and 4% for AL = 23.3% and AL = 27.6%, respectively, correspond to a selectivity loss of about 8%. This information is relevant when evaluating the economics and the environmental impact of the process regarding energy requirements and chemical consumption.
The pulps’ chemical composition was determined, and statistical analysis was performed for each component. During the soda−ethanol treatment of pine sawdust, the glucans, xylans, and galactans (expressed as %odw) decrease, when the AL and tmax increase. The mannans content, which ranged between 3.29 and 4.05 %odw, did not show a statistical dependency on the studied factors.
The experimental values of acid-insoluble lignin and total carbohydrates content of pulps, expressed as %odw, were adjusted to regression models (R
2 = 98.8% and 95.5%, respectively), as shown in Equations (7) and (8), respectively.
By expressing the chemical compositions as % over dry wood mass, it is possible to understand how the process conditions affect each component, regardless of the behavior of the others. Both parameters depend on the AL, tmax, the interaction AL-tmax, and the quadratic effect of both variables.
Figure 6 shows the total carbohydrate content (TCC) and the acid-insoluble lignin (AIL) behavior, in accordance with the regression models (Equations (7) and (8)). When working with low alkaline loads (19.0 %odw), AIL decreases sharply with time, while TCC exhibits a smooth variation (Region I): This explains selectivity values at 19.9 %odw
AL. However, in agreement with selectivity values for higher alkaline loads, AIL curves for 23.3 %odw and 26.7 %odw
AL flatten when time increases above 120 and 140 min, respectively, while TCC continues to decrease (Region II).
The kappa number is widely used in the pulp industry as a quick and reliable method to determine the residual lignin in pulp. This parameter was measured and adjusted to a regression model (R
2 = 99.7%), as shown in Equation (9).
Figure 7 is a helpful tool if cooking at a target kappa number, as it allows for the fixation of the operational variables that fulfill that requirement.
The ethanol–water process arose as an alternative to the kraft process, but ethanol pulping requires extreme conditions. For example, the Alcell process treats wood with 50% (weight–weight) ethanol–water at temperatures of 190–200 °C, corresponding to an operating pressure of 400–500 psig [
29]. On the contrary, this work presents an ethanol–soda system with a lower amount of ethanol (35% vol/vol) and the usual kraft pulping temperature (170 °C), resulting in a pressure of about 180 psig. It has the advantages of both systems, soda and ethanol. These treatment conditions are referred to as “mild conditions”.
Soda–ethanol pulping of pine sawdust results were compared to the kraft and soda–AQ pulping performed in the conditions of the central point of the 3
2 design (
Table 2) and using the same raw materials (
Table 6). In addition, the central point was performed on pine chips.
At the conditions of the central point (100 min-treatment and 23.3 %odw of alkaline load), similar selectivities were achieved by kraft pulping and soda–ethanol delignification of pine sawdust, suggesting that soda–ethanol at mild conditions and the kraft process are equally efficient. However, due to its sulfur-free nature, the soda–ethanol process is a more suitable candidate for the delignification stage in a biorefinery scheme for pine sawdust, where a minimum environmental impact is a pursuit. In addition, the soda–ethanol process preserves more hemicelluloses than kraft pulping, which may be desirable, for example, to produce microfibrillated cellulose since they facilitate mechanical action, reducing energy consumption [
30].
Compared with the soda–AQ treatment performed under the same conditions, the soda–ethanol process showed lower selectivity. However, it exhibited a lower delignification than soda–ethanol, which could probably be one reason for the higher carbohydrate preservation.