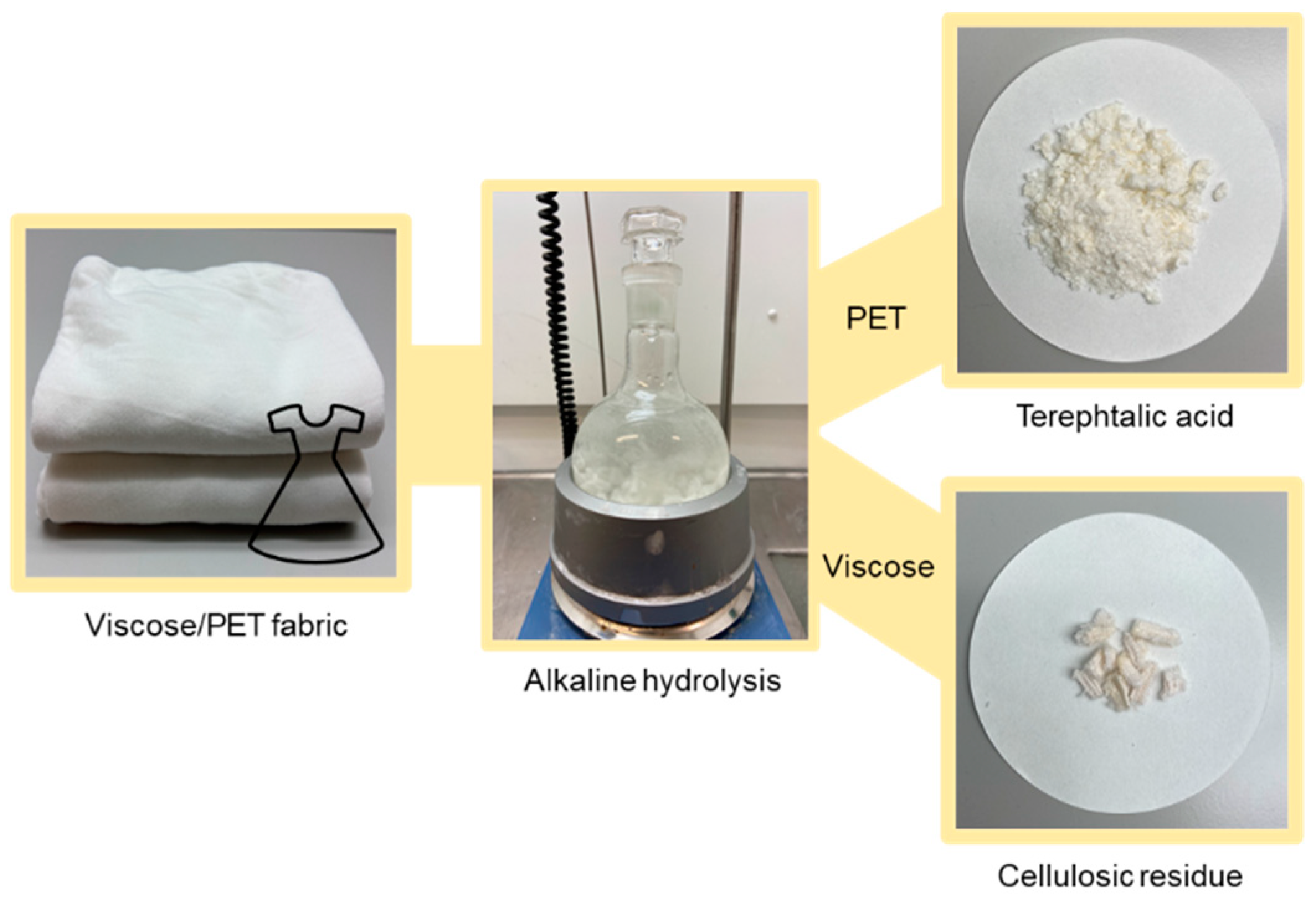
Abstract
Material recycling requires solutions that are technically, as well as economically and ecologically, viable. In this work, the technical feasibility to separate textile blends of viscose and polyester using alkaline hydrolysis is demonstrated. Polyester is depolymerized into the monomer terephthalic acid at high yields, while viscose is recovered in a polymeric form. After the alkaline treatment, the intrinsic viscosity of cellulose is decreased by up to 35%, which means it may not be suitable for conventional fiber-to-fiber recycling; however, it might be attractive in other technologies, such as emerging fiber processes, or as raw material for sugar platforms. Further, we present an upscaled industrial process layout, which is used to pinpoint the areas of the proposed process that require further optimization. The NaOH economy is identified as the key to an economically viable process, and several recommendations are given to decrease the consumption of NaOH. To further enhance the ecological end economic feasibility of the process, an increased hydrolysis rate and integration with a pulp mill are suggested.
1. Introduction
World fiber production has seen a continuous increase over the last decades, and the current world fiber market reaches well above 100 million tons of produced fibers per year [1], where the majority is used for apparel and home textiles. Consequently, the amount of disposed textiles is increasing, and there is an urgent need for improved handling of end-of-life textiles and textile recycling [2]. One major challenge considering the recycling of textiles is the abundance of multi-material textiles, i.e., fabrics composed of two or more different types of polymers. Mechanical recycling of such multi-material textiles produces materials of inferior properties, due to the distinct properties and processing needs of the different fiber types. There is currently a lack of recycling options for these textiles, which results in them being predisposed for landfill or incineration.
The mixture of polyester (PET) and cellulose fibers, especially cotton, is common, due to the beneficial effect of combining PET, which is durable and inexpensive, with cellulose fibers, which have good water absorbency, thereby contributing to the comfort of the fabric. However, to recycle this type of material, a separation is required for further valorization of the two components. Various routes of cellulose/PET separation have been investigated, i.e., through degradation, depolymerization, or dissolution of at least one of the two components.
PET can be maintained in its polymeric form through selective dissolution [3,4,5] or hydrolysis [6,7] of cotton. However, the molecular weight of PET is decreased in a recycled fabric compared to a virgin fabric due to laundering and wear. The lowered molecular weight of PET may result in recycled PET fibers with inferior mechanical properties, or even make the melt–spinning process impossible. In contrast, if PET is depolymerized, PET monomers are obtained, which, after suitable purification, may be used in the production of new PET [8]. Cotton from cellulose/PET textiles is currently recycled into dissolving pulp on a commercial or demo scale by actors such as OnceMore, Ambercycle, and WornAgain.
Simultaneous depolymerization of PET and recovery of the cotton fibers through alkaline hydrolysis has previously been successfully demonstrated [9,10]. While the aqueous alkaline environment causes hydrolytic cleavage of the ester bond of PET, cotton is relatively resistant to alkali degradation [11]. In particular, in the presence of a phase transfer catalyst, the depolymerization of PET in 10% NaOH at 90 °C is fulfilled within less than 1 h, with limited degradation of the cotton fraction [9]. However, whether alkaline hydrolysis can be successfully applied to fabrics containing PET blended with other types of cellulose fibers, e.g., regenerated fibers such as viscose or Lyocell, remains to be investigated.
Cotton and regenerated cellulosic fibers differ in several aspects, which will dictate how the cellulose is affected under alkaline conditions. The first distinction is in the ultrastructure of fibers, with the presence of a primary cell wall in cotton fibers, a structural feature that regenerated fibers lack [12]. Compared to regenerated cellulose fibers, the cellulose in cotton is of much higher molecular weight. Cotton has a typical average degree of polymerization (DP) of 2000 [13], Lyocell in the range of 400–700 [14], and viscose even lower, between 250–400 [13]. Further, the crystallinity varies between fibers—for native cotton, it is around 70%, while for viscose about 25–30%. The crystallites in regenerated fibers are also much smaller compared to cotton, and have a lower degree of orientation [13].
At the relevant process conditions, a NaOH concentration of a maximum of 10 wt% and temperatures below 100 °C, cellulose is known to undergo peeling reactions. End-wise peeling results in the removal of anhydroglucose units from the reducing end of the cellulose chain. Peeling will continue until the competitive stopping reaction occurs, and, depending on the conditions, i.e., alkalinity and temperature, peeling may proceed for 50–60 units [15]. Regenerated cellulosic fibers, having a lower DP than cotton, are potentially more prone to these peeling reactions.
A recent life-cycle analysis study concluded that there are potential environmental benefits in using cotton rendered from separation of cotton/PET through alkaline hydrolysis as a raw material in viscose or Lyocell production [16]. For regenerated fibers, the DP might be too low after separation, and, consequently, the cellulose fibers would not be suitable as raw material for conventional fiber-to-fiber recycling, but may be recovered as other products [17]. One option could be to produce cellulose nanocrystals from the cellulose fraction, which has been demonstrated using both cotton and cotton/PET blends as a raw material [18,19]. Furthermore, the complete depolymerization of cellulose into glucose allows for the production of specialty chemicals or biofuels [20]. Such processes to valorize degraded cellulosic textiles have already been proposed, commonly through a pretreatment of the cellulose, followed by enzymatic hydrolysis. Alkaline pretreatment with bases such as sodium hydroxide [21] and sodium carbonate [22] has been described, and could favorably be combined with alkaline hydrolysis of cellulose/PET textile blends.
The properties of the cellulose fraction, after hydrolysis and separation of a viscose/PET textile blend, dictate which recycling pathways will be most promising. Thus, in this study, alkaline hydrolysis of viscose/PET blends was performed, and the cellulose fibers were evaluated with respect to yield, molecular weight, and fiber morphology. Furthermore, a process layout for an industrial upscaling of the process for cellulose/PET separation via alkaline hydrolysis was constructed based on the experimental results. The process layout was constructed in the form of a mass balance model using the process simulation tool WinGEMS, and can serve as a basis to indicate the economic and ecological potential of an upscaling of the proposed hydrolysis. We believe that applied studies on material recycling can benefit largely from a close collaboration between experimental work and techno–economical and life-cycle analysis. It allows the direction of the experimental work to better favor an economically and ecologically viable process, a factor that is often overlooked. Any recycling option must be better than the use of virgin materials, i.e., have a lower climate impact, and be economically feasible. In this work, an upscaled industrial process layout is presented, which identifies the areas of the proposed process that require further optimization. Moreover, it does not put hard numbers on the operations, a practice that might prematurely disregard a promising recycling option due to a lack of reliable data in the early stages of process development.
2. Experimental Section
2.1. Materials
For this study, three types of never-used viscose were used: a commercially available garment with a blended fabric containing 70% viscose and 30% polyester, a mixture of 70% viscose and 30% polyester filaments, and a pure viscose fabric supplied by a Swedish fashion company. The two different fabrics were either used as received or washed, and subsequently cut into approximately 1 × 1 cm pieces. Laundering was performed at 60 °C, according to Swedish standard SS-EN ISO 6330:2012. A portion of the laundered fabric was also shredded (New Shunxing, NSX-QT 310). Moreover, a neat polyester fabric was laundered and cut in the same manner as the neat viscose fabric. Sodium hydroxide (NaOH, 50%) and sulfuric acid (H2SO4, reagent grade, 95–97%) were obtained from Sigma-Aldrich, and acetic acid (glacial, 99%,) was obtained from Fisher Scientific, and used as received.
2.2. Methods
2.2.1. Hydrolysis
Alkaline hydrolysis of viscose/PET, neat viscose, and neat PET samples was performed at a solids-to-liquids ratio of 1:100 in aqueous NaOH. The NaOH concentration was 5 wt%. The aqueous NaOH was heated to 90 °C before adding the oven-dried (2 h, 105 °C) sample to the reaction vessel. Hydrolysis was performed for the selected time (60–1440 min). The reaction was quenched by immersing the reactor in an ice bath. Post-reaction, the solid residue was separated from the reaction solution via filtration, using a tight knit PET–wire fabric as the barrier. The solids were neutralized in 5% acetic acid, and thereafter washed with water and dried at 105 °C for 4 h to calculate the gravimetric yield. The filtrate was kept in the freezer until further analysis.
2.2.2. Composition
The composition of the mixed viscose/PET fabric was probed via selective dissolution of viscose in cupriethylenediamine (CED), whereafter the weight of the solid PET residue was determined.
2.2.3. Intrinsic Viscosity
Limiting viscosity of the cellulose samples was measured after dissolution in CED, according to ISO 5351. The degree of polymerization was calculated from the correlation formulated by Immergut et al. [23], as cited in SCAN-C 15:62 (DP0.905 = 0.75[η], η given in cm3/g). The correlation is known to be flawed, but is commonly used, which facilitates comparisons to other studies.
2.2.4. WAXS
The crystallinity of viscose was measured by wide angle X-ray scattering (WAXS) at Chalmers University of Technology (Mat:Nordic, SAXSLAB) with a 0.9 mm beam diameter, and a Rigaku 003+ high brilliance microfocus Cu-radiation source at 130 mm distance, with the sample irradiated over 20 min. The intensity was normalized with transmission.
2.2.5. FTIR Spectroscopy
Attenuated total reflectance Fourier transform infrared (ATR–FTIR) spectroscopy was performed on a Bruker Tensor 27 equipped with the Specac Golden Gate ATR accessory.
2.2.6. NMR Spectroscopy
The purity of terephtalic acid (TPA) obtained from PET depolymerization was determined via nuclear magnetic resonance (NMR) spectroscopy. A small portion of the filtrate from the sample of Fabric70/30 after hydrolysis for 360 min was acidified with H2SO4 (4M) to precipitate TPA. TPA was dissolved in 5% NaOH/D2O solution prior to analysis. The NMR measurements were conducted on a Varian 400-MR spectrometer operating at 9.4 T, equipped with a OneNMRProbe. The 1H-NMR spectrum parameters included a 5 μs 1H-detection pulse, 2.5 s acquisition time, 2 s recycle delay, and 32 scans. The samples were studied using D2O as a solvent, and the chemical shifts were referenced to the residual solvent signal.
2.2.7. Upscaled Process Layout
The proposed upscaled process layout was based on a mass and energy balance steady-state model constructed using the process simulation tool WinGEMS (Valmet®, Espoo, Finland). This modular simulation tool is specifically developed for the pulp and paper industry, and handles the modeling of suspensions with ease. The first step in creating the mass and energy balance model involves constructing a hypothetical process flowsheet, using the so-called blocks in the WinGEMS graphical environment. These are modular (drag and drop) and pre-coded for a vast set of process operations, spanning simple splitting and mixing to heat exchangers, evaporators, and washers. Moreover, the stream structure and its components need to be defined by the user, as do the necessary chemical reactions. The basic model for a chemical reaction is a simple stoichiometric conversion whose extent is also defined by the user, while the actual thermodynamic aspects are usually handled outside of the simulation environment using other, more rigorous tools. For the establishment of energy balances, WinGEMS has built-in expressions for estimating the specific enthalpies of the streams. For liquid streams, the enthalpy is a function of temperature, dissolved and suspended solids, and the specific heat of water and solids. The enthalpies of steam streams is determined by a built-in steam table (function of temperature and pressure). The amount of heat from reactions had to be manually calculated and used as an input for the heat exchanger blocks to estimate changes in the stream temperatures. In this study, the procedures employed within the experimental work, including the chemical additions, solids-to-liquids ratio, etc., as well as the experimental results (reaction yields, product purities, etc.), were used as a basic input for the model around the hydrolysis and precipitation stages. The remaining stages in the process layout were proposed based on the general engineering know-how regarding standard industrial processes (washing, heat exchangers, etc.), experiences from similar studies, reasonable simplifications and assumptions (discussed in detail in Section 3), as well as the basic principles of green chemistry. The results are hence to be seen as a first iteration of a possible upscaling of the process. The overall objective for the upscaling development was to obtain a hypothetical process layout which was as efficient as possible from the perspective of chemicals and energy use, and subsequently suggest improvements that would help to make it economically and environmentally sustainable.
3. Results and Discussion
3.1. Experimental Study
Alkaline hydrolysis of blends of viscose/PET fibers was studied for two different cases: a 70/30 mix of pure viscose and pure PET filaments (Filament70/30), and a knitted viscose/PET fabric with a 70/30 composition (Fabric70/30) (Table 1). The composition of the mixed fabric was confirmed via selective dissolution of viscose in CED and determination of the weight of the solid PET residue. The choice of samples allows to study the efficiency of the separation for samples with different accessibilities, i.e., a mix of filaments relative to a knit fabric from mixed yarns. Further, alkaline degradation of viscose in terms of mass loss, DP, and crystallinity was studied for a neat viscose fabric. The accessibility of neat viscose to alkaline degradation was studied for a 100% viscose fabric (Viscose100) prepared in three different ways: never laundered, cut to 1 × 1 cm pieces (Viscose100a); laundered, cut to 1 × 1 cm pieces (Viscose100b); and laundered, shredded to display the individual fibers (Viscose100c). Finally, the hydrolysis of a knitted PET fabric, which was laundered and cut to 1 × 1 cm pieces, was studied to produce the data used in the upscaled process model.

Table 1.
Description of viscose-containing samples.
Mild reaction conditions (90 °C, 5 wt% NaOH) and reaction times between 60–1440 min were chosen, as the DP of cellulose has been shown to decrease with increasing temperature and NaOH concentration during hydrolysis [9]. For closed loop-recycling, i.e., fiber-to-fiber recycling of regenerated fibers, it is of the utmost importance to preserve the molecular weight of the cellulose fraction in the mixed fabric. Simultaneously, to allow for separation of the blended textile, the reaction conditions should allow for full PET depolymerization. Furthermore, a reaction below the boiling point of the aqueous solution eliminates the need for a pressurized system and consumes less energy. The reaction yields a solid cellulose residue, and the PET monomers terephtalic acid (TPA) and ethylene glycol (EG) (Figure 1).

Figure 1.
Schematic of the reaction.
The mass losses of samples Filament70/30 and Fabric70/30 were comparable after 60 min of hydrolysis (Figure 2a), and were also in the same range as Viscose100 (Figure 2b). This implies that no major hydrolysis of PET occurs within the first 60 min of reaction, but the mass loss originates predominantly from viscose. We ascribe this to fast degradation of low molecular weight fragments and accessible regions of the cellulose in viscose. At longer reaction times, the mixed yarns and knitted structure of Fabric70/30 does decrease the hydrolysis rate and, consequently, the mass loss compared to Filament70/30 is also decreased. Even after 24 h of hydrolysis, PET removal is not complete in Fabric70/30, indicated by the <30% mass loss. This is confirmed by FTIR analysis, where the ester peak at 1705 cm−1, significant for PET, is still visible after 24 h of hydrolysis (Figure 3).
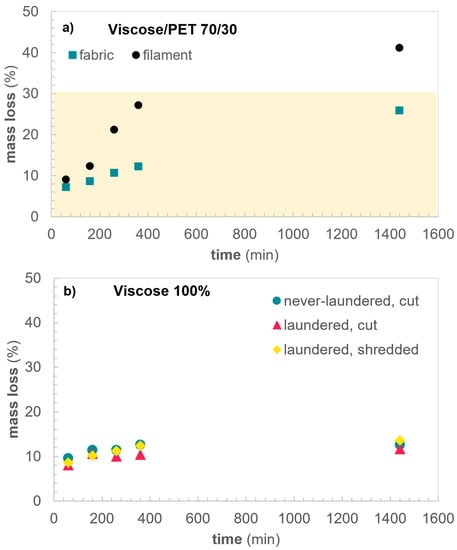
Figure 2.
Mass loss during hydrolysis of (a) blends of PET and viscose and (b) 100% viscose fabric. The yellow part in Figure 2a represents the wt% of PET in the samples.
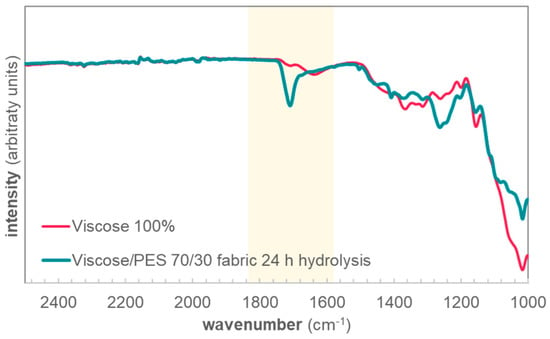
Figure 3.
FTIR spectra of the viscose/PET fabric after 24 h of hydrolysis, and a reference 100% viscose fabric. Highlighted is the peak at 1705 cm−1, significant for the ester bonds in PET.
In contrast, Filament70/30 shows a 40% mass loss, indicating full depolymerization of PET and an additional 10% mass loss from cellulose, consistent with the mass loss in a neat viscose sample after 24 h reaction. Textile construction of a blended fabric, hence, has a substantial impact on the hydrolysis rate of PET. Contrary, for Viscose100 there is no significant difference between the mass losses of samples of different accessibility. Laundering the sample at 60 °C removes surface finishing and other processing aids remnant on the fabric which increase the exposure to the alkaline environment during hydrolysis, while shredding increases the surface area of fabrics. Interestingly, most of the mass loss is seen during the first 60 min of reaction for all viscose samples (Figure 2b), where the never-laundered sample displays a 10% weight loss and the laundered samples, cut or shredded, 8 and 9%, respectively. After 24 h the weight loss has increased to 13% for the never-laundered sample and 12 and 14% for the laundered cut and shredded samples, respectively. Thus, more than 50% of the total mass loss occurs during the early stages of reaction.
Characterization of Reaction Products
The mass loss during the first 60 min of reaction is accompanied with a significant increase in the crystallinity of the Viscose100b sample (Figure 4). This is signified by a sharpening of the peaks centered around 2θ values of 12 and 20. These peaks have previously been assigned to cellulose II, the crystalline structure of regenerated cellulosic fibers [24]. The supramolecular structure of cellulose is decisive to the kinetics of all degradation reactions [25], and a higher rate of alkaline peeling is expected in disordered, compared to crystalline, regions. The increase in crystallinity is in line with our postulate that the mass loss during the first hour of hydrolysis is due to the removal of low molecular weight fragments and accessible regions of the viscose, by means of peeling reactions.
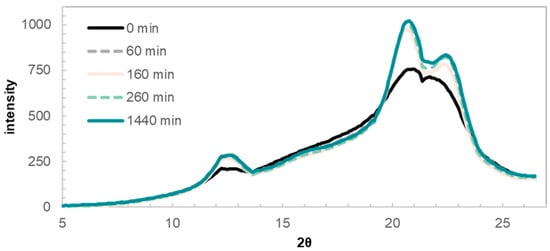
Figure 4.
WAXS 1D spectra of sample Viscose100b after 60, 160, 260, and 1440 min of reaction time, as well as the reference sample prior to immersion in hot alkali.
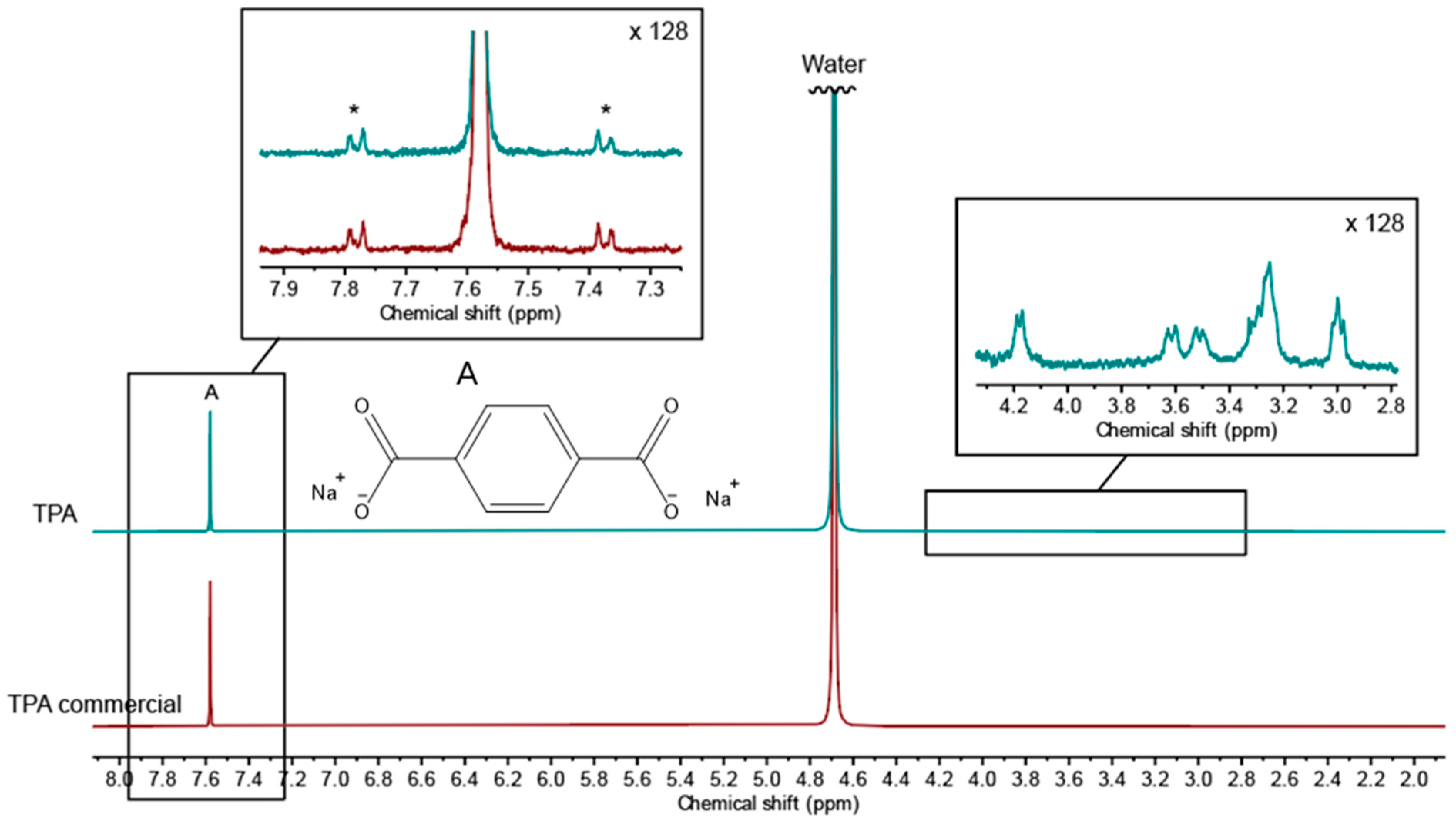
The purity of precipitated TPA from the viscose/PET fabric was determined via NMR spectroscopy, with a commercial TPA as the reference. The spectrum of the precipitate shows a distinct singlet at 7.58 ppm, which is assigned to pure TPA (Figure 5). A zoom-in of the baseline around the signal is shown as an insert. No other signals than the 13C-satellites from singlet A (in the insert marked with an *) can be seen in the zoom-in, proving that no residues of other reaction products stemming from incomplete depolymerization of PET are present in the precipitate. This is in accordance with the literature, where hydrolysis of PET is described as taking place at the external surface of the solid-state PET, with the major decomposition reaction occurring at the ends of the polymer chains [26].
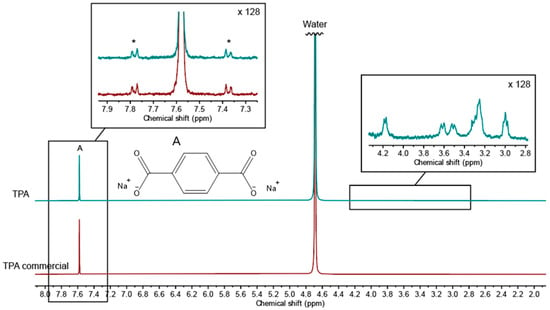
Figure 5.
1H-NMR spectra of TPA precipitated from the reaction filtrate, along with a commercial TPA reference. The singlet from TPA is marked with an A, and in the zoom-in of this signal, the 13C-satellites from A is marked with an *. Zoom-in around 3.4 ppm show residual viscose degradation products.
Around 3.4 ppm, the experimentally obtained TPA displays some additional small peaks, characteristic for protons in the vicinity of oxygen, as seen in the insert. These are assigned to traces of viscose degradation products, such as d-glucoisosaccharinic acids, the predominant product of peeling reactions during alkaline conditions [15].
Having confirmed the purity of TPA obtained from hydrolysis of PET from viscose/PET, further characterization of the cellulosic residue was undertaken. The molecular weight of cellulose is commonly approximated by the intrinsic viscosity (IV) of a cellulose solution in cupri–ethylene diamine (CED). Prior to alkaline treatment, the viscose filaments in sample Filament70/30 had an IV of 200 mL g−1, while the IV of Viscose100 was slightly lower, 170 mL g−1 (Table 1). The IV of Fabric70/30 could not be measured, due to degradation. Cellulose is known to degrade in CED, i.e., the solvent used for measuring IV. Even when finely dispersed in the solvent, complete dissolution of the cellulose part of the viscose/PET fabric could not be achieved before degradation occurred, hence the obtained IV values were not reliable, and are therefore not reported. However, data on PET depolymerization from Fabric 70/30 (Figure 3) indicate that a reaction time of at least 24 h is needed for full PET depolymerization under the present, mild reaction conditions. We assume that the degradation of viscose will not be significantly impacted by the presence of polyester in the blended fabric, and may be approximated by the IV of neat viscose samples. For all neat viscose samples, the IV decreased with reaction time, and measured between 130–150 mL g−1 after 24 h (Figure 6). The IV may be approximatively converted to DP, using the relationship formulated by Immergut [23] (c.f. Experimental section):
where [η] is the intrinsic viscosity in mL g−1. After 24-h reaction, the IV is decreased by between 20–50 mL g−1, which, using Equation (1), corresponds to 20–55 glucose units. The decrease in DP is in line with a peeling reaction, which typically proceeds for up to 50–60 units before a stopping reaction occurs [15]. The final IV of samples is too low for applications in commercial regenerated cellulose fibers or cellulose derivates, such as cellulose ethers, nitrates, and acetates, which typically lies in the range of 400–600 mL g−1 [27]. However, low molecular weight polysaccharides can find other uses, e.g., by hydrolysis into nanocrystalline cellulose [28] or in sugar platforms for further valorization into specialty chemicals and biofuels [29].
DP0.905 = 0.75[η]
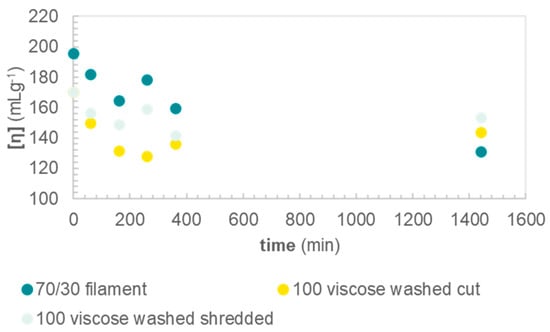
Figure 6.
Intrinsic viscosities of samples as a function of hydrolysis time.
3.2. Upscaled Process Layout
Based on the relevant experimental results in the section above, as well as a number of assumptions, a process layout for a greenfield standalone industrial upscaling of the viscose/PET separation process was constructed. The process layout aims at providing guidelines on the aspects of the proposed industrial process, which require more development and optimization for the whole process to become viable from an economic and environmental point of view. In essence, the process layout should be seen as a first step on a necessary path of various iterations between process development and experimental work to lift the process to higher technology readiness levels (TRLs). With respect to the early experimental results of the project, rather than conducting a conventional quantitative techno–economic evaluation, which may result in irrelevant results at this stage, this study emphasizes the qualitative measures to be implemented in future projects. The ultimate objective of the process is to completely hydrolyze PET and separate its monomers, TPA and EG, while keeping the viscose fraction as intact as possible. The PET monomers could be sold for production of PET, or used as a platform chemical. The IV of viscose afterhydrolysis (Figure 6) is likely too low for fiber-to-fiber recycling, and while other outputs are proposed, these are currently not available on an industrial scale, and their economic value is difficult to estimate.
3.2.1. Use of Experimental Data
The yields used in the hydrolysis stage in the model were based on experiments with pure fabric fractions of PET and viscose, respectively, as demonstrated in Table 2. The composition of the inlet textile in the model is 70/30 viscose/PET. The textile-to-solvent ratio and NaOH concentration were kept at 1:100 and 5 wt%, respectively, the same as in the experimental study.

Table 2.
Experimental values of mass loss from hydrolysis of neat viscose or neat PET fabric used in the model.
Since it was difficult to obtain a reliable alkali consumption from the experimental results, a theoretical consumption based on the respective mass losses of PET and viscose was assumed. The hydrolysis of PET into its monomers was modeled according to Equation (2). It was assumed that the PET fabric is composed of only poly(ethylene terephthalate) (PET), i.e., the hydrolysis of PET requires two moles of NaOH per repeating unit. While this is true for most textile PET, textile terminology does not distinguish between PET and other types of polyesters. Furthermore, according to the same textile terminology, up to 15% of non-polyester copolymers are allowed in the polymer chain [30]. For viscose, it was approximated that all of the degraded viscose would be recovered as glucose, and the formation of each glucose molecule would consume one OH− ion.
The consumption of sulfuric acid in precipitation of TPA was based on the stoichiometric reactions in Equations (3)–(5). TPA has a pKa of 3.5, and sulfuric acid is consumed in the acidification of the filtrate as well as the protonation of TPA. The consumption of sulfuric acid required to reach a pH of 3.5 was estimated by a simulated titration in OLI. It was assumed that the complete precipitation of TPA took place in the model.
3.2.2. Proposed Process Layout
With the exception of the experimental data presented in the previous section, all other process stages and values in the process layout originate from assumptions and estimates. Figure 7 displays the first proposed process layout for an upscaling of the viscose/PET separation process.
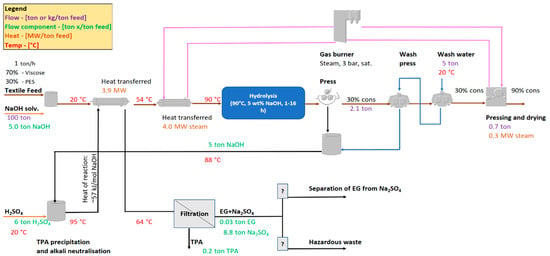
Figure 7.
The proposed process layout for the viscose/PET separation process via alkaline hydrolysis. Purple values indicate total flow, green indicates component flow in the stream, brown indicates heat transfer, and red indicates the temperature. The values are to be seen as indicative and, in this case, pertain to a hydrolysis residence time of 24 h.
The textile feed is first mixed with the alkaline solvent before it is heated via two indirect heat exchangers to the temperature of the hydrolysis reactor. The heat of reaction from the neutralization of alkali (~57 kJ/mol NaOH) is used to heat exchange the textile–solvent feed in the first heat exchanger. The heat of reaction was calculated from the standard enthalpies of formation of H2SO4 (aq), NaOH, Na2SO4, and H2O (l) [31]. External heating is required to ramp up the textile–solvent feed to the temperature of the hydrolysis reactor. A natural gas burner, operating at 3 bar, supplies the second heat exchanger with saturated steam. A gas burner was chosen above other technologies (e.g., biomass boiler, electric boiler) due to its simplicity, as well as the lower investment and operating costs. However, this means that the process must be placed in the proximity of a chemical cluster, with access to a natural gas infrastructure. The modeled residence time in the hydrolysis reactor, and, consequently, the yield, can be any of the ones presented in Table 2. Following the hydrolysis reactor is a simple dewatering press (without a wash liquor), from which the outlet consistency is 30%. It is assumed that the carryover, i.e., the liquid following the viscose fraction, has the same composition as the filtrate from the press. Next, two counter-current wash presses with the same discharge consistency purify the viscose fraction from residual alkali, dissolved PET monomers, etc. The assumed displacement ratio (washing efficiency) and dilution factor (mass of excess filtrate per mass of viscose) is ~0.5 and ~5 ton/ton viscose, respectively. Both the displacement ratio and the dilution factor are typical parameters applied in pulp mills during the washing of the pulp. The filtrate from the wash presses is subsequently mixed with the filtrate from the dewatering press. To make the viscose fraction easier to transport, a pressing and drying section increases the viscose consistency to 90%. The gas burner also supplies the drying section with the necessary heat.
The filtrate from the dewatering press is sent to a vessel for precipitation of TPA with sulfuric acid. As mentioned previously, the reaction of neutralizing alkali with sulfuric acid is heavily exothermic, and the excess heat is used to pre-heat the feed. Subsequent washing and filtering of the outlet stream is then performed to separate the TPA from the process. The remaining aqueous phase contains EG, degraded viscose, and Na2SO4, formed during the neutralization of the residual alkali. As indicated by Figure 7, the most suitable fate of this stream is still uncertain, and will be discussed further at a later stage.
Below is a list of additional assumptions in the process layout:
- There is no heat loss from the heat exchangers;
- The hydrolysis reactor is assumed to be well-insulated, so that the temperature of 90 °C can be held constantly during the reaction, with no need for supporting heat;
- The viscose fraction is still pumpable at a consistency of 30%;
- For the heating of water, the specific heat capacity is 4.2 kJ kg K−1.
3.2.3. Suggestions for Process Optimization
The proposed process layout in Figure 7 is, in essence, a direct translation of the laboratory procedures, and results in an upscaled process; consequently, certain areas and aspects of the proposed process need further elaboration to make them more efficient and applicable. Minimizing the basic operational expenditures (chemicals, energy etc.) is one of the most apparent and necessary actions to focus on. Investigation into the most suitable management of the residual EG/Na2SO4 fraction, as well as the best application for the viscose fraction, would also be required to further develop the process.
The basic operational expenditures of the process are NaOH, natural gas, and H2SO4. In this process, the use of NaOH has been identified as the weakest point. Since NaOH is a relatively expensive chemical (~550 EUR/ton [32]), due to the high power consumption during its production, and, given its prominent role in the proposed process, efficient use of NaOH is key to the process economy. 5 tons NaOH/ton of textile feed is fed to the current process to reach the pH at which the reaction is assumed to proceed most efficiently. However, even in the process case with the highest alkali consumption, i.e., 24 h of hydrolysis (Table 2), the amount of residual alkali leaving the reactor is still as much as ~4.85 ton/ton of textile feed, i.e., only a fraction of this valuable chemical is irreversibly consumed. In the proposed process, this means that a massive amount of unused alkali, a value of around 2670 EUR/ton textile, follows the filtrate to the TPA precipitation tank, and is neutralized into Na2SO4. Not only is this an inefficient use of NaOH, but it is also an inefficient use of H2SO4 which is used for neutralization. At least three robust process improvements, to be evaluated separately and subsequently in combination, to increase the NaOH economy have been identified:
- Lower the textile-to-solvent ratio during hydrolysis significantly from 1:100. Assuming the same alkali consumption for a textile-to-solvent ratio of 1:10 would mean an input of 0.50 ton NaOH/ton textile, a residual alkali of 0.35 ton/ton textile, and a value of ~193 EUR/ton textile that is neutralized. Initial experimental results show that wetting of the cut fabric is possible down to a textile-to-solvent ratio of 1:15, and that full depolymerization of neat PET textile is achieved after 24 h of hydrolysis at this ratio.
- Lower the concentration of NaOH in the solvent from 5 wt%. Similar to the reduction of the textile-to-solvent ratio, this process change must also be experimentally evaluated to determine the lowest possible NaOH concentration without the risk of decreased yields.
- Separate the TPA salt in the filtrate from the dewatering press before precipitation (Figure 7) with a nano filtration (NF) membrane and/or ion exchange technology. By doing so, the permeate, with most of the residual alkali, could be recycled, and mixed back in with the textile feed. There are pH-stable NF membranes that appear as promising candidates which are able to withstand the alkaline filtrate, with a cutoff down to 200 Dalton [33]. The TPA salt (TPA-Na2) has a molecular weight of 210 g/mol, and might possibly be effectively separated in the NF membrane, although this should also be verified experimentally.
For the third process improvement listed above, a new iteration of the process layout was created (Figure 8). In it, it is assumed that an effective recirculation of 90% of the residual alkali with the aid of a NF membrane is possible. It is assumed that 10% of the total flow follows the retentate with up-concentrated TPA. Compared to Figure 7, the required makeup of fresh NaOH decreases by 87%, and the charge of H2SO4 decreases by 88%. The energy consumption also decreases by 72%, since the recycling of the high temperature residual alkali stream, at 87 °C, drastically lowers the need for external heating for the ramp up to 90 °C.
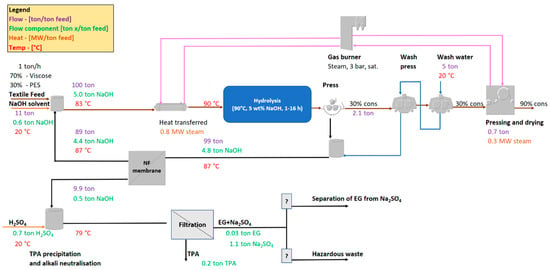
Figure 8.
The proposed process layout for the viscose/PET separation process with the improvement of separating and recycling residual alkali back to the textile feed. Purple values indicate total flow, green indicates component flow in the stream, brown indicates heat transfer, and red indicates the temperature. The values are to be seen as indicative and, in this case, refer to a hydrolysis residence time of 16 h.
As mentioned previously, it will be necessary to identify treatment options for the residual EG/Na2SO4 fraction after TPA precipitation. Within the frames of this work, it has not yet been clarified whether it is possible to separate EG from Na2SO4. Although commercially available technologies for glycol recovery do exist, preliminary discussions with one of the providers of such a technology (cleantech company Recyctec [34]) indicate that the EG/Na2SO4 stream in question might be difficult to process due to its high conductivity. Furthermore, the amount of EG in the stream is relatively small, and it might not be economically viable to separate it, but perhaps it could be for Na2SO4, which dominates the composition of the stream. If it is suspected that the fraction cannot be separated, the question remains whether to regard it as hazardous waste, or if it can simply be treated in a wastewater treatment plant. A recycling company [35], which was contacted, concluded that a detailed composition of the stream would need to be available for the proper classification.
It is of great importance to allow the PET hydrolysis to be completed to avoid contamination of the viscose fraction by the remaining PET. Experimental results indicate that up to 16 h are required for complete hydrolysis of neat PET and the Filament70/30 sample. However, for the blended fabric, Fabric70/30, complete removal of PET was not achieved, even after 24h of hydrolysis (Figure 2a). Phase transfer catalysts have previously been used in alkaline hydrolysis of both PET and cotton/PET blends, and their use might be advisable to speed up reaction, debottleneck the process, and minimize the volume of the reaction vessel. One potential alternative would be to conduct the hydrolysis in multiple step reactors, with a countercurrent flow of the alkaline solution.
The proposed process in this work depicts a standalone plant. It is well known that integration between chemical industrial processes might have various economic and environmental benefits, as compared to a standalone process. Integration of the viscose/PET separation process with a Kraft pulp mill would yield several potential benefits:
- Instead of having a gas burner, low pressure steam could be bought from the pulp mill. This removes the capital expenditure (gas burner) of the viscose/PET process, and, further, the pulp mill steam should be less expensive, as well as renewable, compared to natural gas.
- If it is not possible to separate the EG/Na2SO4 fraction, the existing infrastructure at the pulp mill may offer some alternatives for its disposal. If the EG/Na2SO4 stream can be treated as a normal effluent, it could simply be sent to the wastewater treatment plant. Otherwise, it could perhaps be evaporated, and subsequently incinerated in the recovery boiler; however, this would be at the expense of increased energy consumption in the mill’s evaporation plant. Furthermore, it must be investigated whether the amount of Na2SO4 in the stream will significantly alter the pulp mill’s Na/S balance, possibly increasing the NaOH makeup demand, as well as the associated costs.
- The chemicals used in the viscose/PET process, NaOH and H2SO4, are already used in the pulp mill. The viscose/PET process can potentially directly use the prepared solutions of these chemicals from the pulp mill.
- Other economic benefits related to infrastructure, such as a reduced need for land and ground preparation, shared buildings, and utilities infrastructure (e.g., power substation, fresh- and cooling water), as well as shipping and/or transportation.
4. Conclusions
Alkaline hydrolysis of PET from viscose/PET fabrics was shown to render pure terephthalic acid and a cellulose fraction with a decreased molecular weight and increased crystallinity. A complete depolymerization of PET, as well as the subsequent separation of the blended fabric, requires long reaction times, >24 h under the present, mild reaction conditions. The intrinsic viscosity of cellulose after hydrolysis is 130–150 mL g−1, too low for use in commercially regenerated fibers or traditional cellulose derivatives, such as cellulose ethers or nitrates. In fact, all samples show an initial IV, prior to hydrolysis, below the desired IV range for dissolving pulps aimed for use in various commercial uses, probably due to a reduction in molecular weight as a function of viscose processing. However, low molecular weight polysaccharides can find other uses, e.g., through hydrolysis into nanocrystalline cellulose, or in sugar platforms for further valorization into specialty chemicals and biofuels. An upscaled process layout was constructed, based on the proposed process, with the objective of optimizing chemical and energy use. From this model, a number of process developments were suggested. We believe that an iterative process between small-scale experimental work and techno–economic and life cycle analysis is highly beneficial in developing recycling processes which are economically and ecologically viable.
Future Suggestions
The inefficient use of NaOH was recognized as the weakest point in the proposed process, whose cost, at present, would very likely exceed any reasonable revenue from the sales of TPA, EG, and the cellulose fraction. Efforts must be dedicated to reducing NaOH consumption, e.g., by the use of an NF membrane or a lower textile-to-solvent ratio. These results should be used as a guide to further experimental work, to favor an economically and ecologically viable process.
Further, experimental work should focus on increasing the hydrolysis rate, as the slow kinetics of hydrolysis of the blended textile require large volume reactors. This may be accomplished using a phase transfer catalyst, or an increase in either temperature or NaOH concentration.
In future studies, proper characterization of the EG/Na2SO4 stream and identification of separation technologies and waste treatment alternatives should be included. Given the low content of EG in the stream, it is possibly more relevant to identify technologies which could be used to separate Na2SO4, which dominates the composition of the stream. Furthermore, the process would probably largely benefit from an integration with a pulp mill.
It is emphasized that the values, presented as output from the process layouts, should be seen as indicative for the overall process. Even though some aspects of the proposed process do not seem encouraging at this current stage, it is far too early to reject it. Instead, the suggested actions to take in future work should be seen as important steppingstones to close the loop for a fraction of the multi-material textiles.
Author Contributions
Conceptualization: J.B., M.B. and K.J.; Data curation, A.P., J.W. and A.I.; Funding acquisition, H.d.l.M.; Investigation, A.P., J.W. and A.I.; Writing—original draft, A.P., J.W. and A.I.; Writing—review and editing, J.B., M.B., K.J. and H.d.l.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Södra Skogsägarnas stiftelse för Forskning, Utveckling och Utbildning, grant number 2019-106.
Acknowledgments
Södras forskningsstiftelse is gratefully acknowledged for financial support, and Södra Innovation för fruitful discussions. Jehona Sjöberg, Angéle Cruz, Carina Berglund, and Elham Franzén are acknowledged for their invaluable experimental help. We would also like to acknowledge the use of the Chalmers Material Characterization Lab (CMAL), and the help from Michal Strach.
Conflicts of Interest
The authors declare no conflict of interest. The industrial reference group contributed to the formulation of the research question, but had no role in the design, execution, interpretation, or writing of the study.
References
- The Fiber Year GmbH. The Fiber Year 2019; The Fiber Year GmbH: Speicher, Switzerland, 2019. [Google Scholar]
- Piribauer, B.; Bartl, A. Textile recycling processes, state of the art and current developments: A mini review. Waste Manag. Res. 2019, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Haslinger, S.; Hummel, M.; Anghelescu-Hakala, A.; Määttänen, M.; Sixta, H. Upcycling of cotton polyester blended textile waste to new man-made cellulose fibers. Waste Manag. 2019, 97, 88–96. [Google Scholar] [CrossRef] [PubMed]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Baghaei, B.; Compiet, S.; Skrifvars, M. Mechanical properties of all-cellulose composites from end-of-life textiles. J. Polym. Res. 2020, 27, 260. [Google Scholar] [CrossRef]
- Ouchi, A.; Toida, T.; Kumaresan, S.; Ando, W.; Kato, J. A new methodology to recycle polyester from fabric blends with cellulose. Cellulose 2010, 17, 215–222. [Google Scholar] [CrossRef]
- Piribauer, B.; Bartl, A.; Ipsmiller, W. Enzymatic textile recycling—Best practices and outlook. Waste Manag. Res. 2021, 39, 1277–1290. [Google Scholar] [CrossRef]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef]
- Brelid, H.; Bogren, J. A Process for Separation of the Cellulosic Part from a Polyester and Cellulose Composition. U.S. Patent WO2020013755A1, 2 September 2021. [Google Scholar]
- Glaus, M.A.; Van Loon, L.R. Degradation of cellulose under alkaline conditions: New insights from a 12 years degradation study. Environ. Sci. Technol. 2008, 42, 2906–2911. [Google Scholar] [CrossRef]
- Rollins, M.L.; Tripp, V.W. Optical and electron microscopic studies of cotton fiber structure. Text. Res. J. 1954, 24, 345–357. [Google Scholar] [CrossRef]
- Broadbent, A.D. Artificially made fibres based on cellulose. In Basic Principles of Textile Coloration; Sociaty of Dyers and Colourists: Bradford, UK, 2001; pp. 92–106. [Google Scholar]
- Luo, M.; Roscelli, V.A.; Neogi, A.N.; Sealey, J.E., II; Jewell, R.A. Lyocell Fibers, and Compositions for Making the Same. U.S. Patent CA2323437C, 9 July 2001. [Google Scholar]
- Sixta, H.; Potthast, A.; Krotschek, A.W. Chemical pulping processes: Sections 4.1–4.2.5. In Handbook of Pulp; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; pp. 109–229. [Google Scholar]
- Sandin, G.; Peters, G.M. Environmental impact of textile reuse and recycling—A review. J. Clean. Prod. 2018, 184, 353–365. [Google Scholar] [CrossRef]
- Östlund, Å.; Wedin, H.; Bolin, L.; Berlin, J. Textilåtervinning—Tekniska Möjligheter och Utmaningar; Naturvårdsverket: Stockholm, Swedish, 2015. [Google Scholar]
- Vanzetto, A.B.; Beltrami, L.V.R.; Zattera, A.J. Textile waste as precursors in nanocrystalline cellulose synthesis. Cellulose 2021, 28, 6967–6981. [Google Scholar] [CrossRef]
- Ruiz-Caldas, M.X.; Carlsson, J.; Sadiktsis, I.; Jaworski, A.; Nilsson, U.; Mathew, A. Cellulose Nanocrystals from Postconsumer Cotton and Blended Fabrics: A Study on Their Properties, Chemical Composition, and Process Efficiency. ACS Sustain. Chem. Eng. 2022, 10, 3787–3798. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Ruuth, E.; Stigsson, L.; Galbe, M.; Wallberg, O. Novel sustainable alternatives for the fashion industry: A method of chemically recycling waste textiles via acid hydrolysis. Waste Manag. 2021, 121, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sofokleous, M.; Christofi, A.; Malamis, D.; Mai, S.; Barampouti, E.M. Bioethanol and biogas production: An alternative valorisation pathway for green waste. Chemosphere 2022, 296, 133970. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Novy, V.; Stigsson, L.; Galbe, M.; Wallberg, O. Towards circular fashion—Transforming pulp mills into hubs for textile recycling. RSC Adv. 2021, 11, 12321–12329. [Google Scholar] [CrossRef]
- Immergut, E.H.; Schurz, J.; Mark, H. Viskositätszahl-Molekulargewichts-Beziehung für Cellulose und Untersuchungen von Nitrocellulose in verschiedenen Lösungsmitteln. Mon. Chem. Verwandte Teile And. Wiss. 1953, 84, 219–249. [Google Scholar] [CrossRef]
- Borysiak, S.; Garbarczyk, J. Applying the WAXS method to estimate the supermolecular structure of cellulose fibres after mercerisation. Fibres Text. East. Eur. 2003, 11, 104–106. [Google Scholar]
- Knill, C.J.; Kennedy, J.F. Degradation of cellulose under alkaline conditions. Carbohydr. Polym. 2002, 51, 281–300. [Google Scholar] [CrossRef]
- Kumar, S.; Guria, C. Alkaline hydrolysis of waste poly(ethylene terephthalate): A modified shrinking core model. J. Macromol. Sci. Part A 2005, 42, 237–251. [Google Scholar] [CrossRef]
- Wennerström, M.; Bylund, S. Method for Controlling Viscosity in Dissolving Pulps. AI Patent WO 2017/105322, 22 June 2017. [Google Scholar]
- Prado, K.S.; Gonzales, D.; Spinacé, M.A.S. Recycling of viscose yarn waste through one-step extraction of nanocellulose. Int. J. Biol. Macromol. 2019, 136, 729–737. [Google Scholar] [CrossRef] [PubMed]
- European Commision. From the Sugar Platform to Biofuels and Biochemicals. 2015. Available online: https://ec.europa.eu/energy/sites/ener/files/documents/EC%20Sugar%20Platform%20final%20report.pdf (accessed on 23 January 2022).
- BISFA. Terminology of Man-Made Fibres. 2017. Available online: http://www.bisfa.org/wp-content/uploads/2018/06/2017-BISFA-Terminology-final.pdf (accessed on 19 April 2022).
- Wagman, D.D.; Evans, W.H.; Parker, V.B.; Schumm, R.H.; Halow, I.; Bailey, S.M.; Churney, K.L. The NBS tables of chemical thermodynamic properties. Selected values for inorganic and C1 and C2 organic substances in SI units. J. Phys. Chem. Ref. Data 1982, 11, 38, 58, 232–299. [Google Scholar]
- Ruiz, J.; Rincón, C.; Contreras, L.; Sidney, R.R.; Almarza, C. Sustainable and negative carbon footprint solid-based NaOH technology for CO2 capture. ACS Sustain. Chem. Eng. 2020, 8, 19003–19012. [Google Scholar] [CrossRef]
- Koch. Available online: Https://www.kochseparation.com/wp-content/uploads/2020/10/Selro-NF-MPS-34-2-5-and-4-inch-elements.pdf (accessed on 15 February 2022).
- Recyctec. Available online: https://www.recyctec.se/en/ (accessed on 15 February 2022).
- Stena Recycling. Available online: https://www.stenarecycling.se/en/ (accessed on 15 February 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).