Application of Trichoderma viride and Pseudomonas fluorescens to Cabbage (Brassica oleracea L.) Improves Both Its Seedling Quality and Field Performance
Abstract
:1. Introduction
2. Material and Methods
Statistical Analysis
3. Results
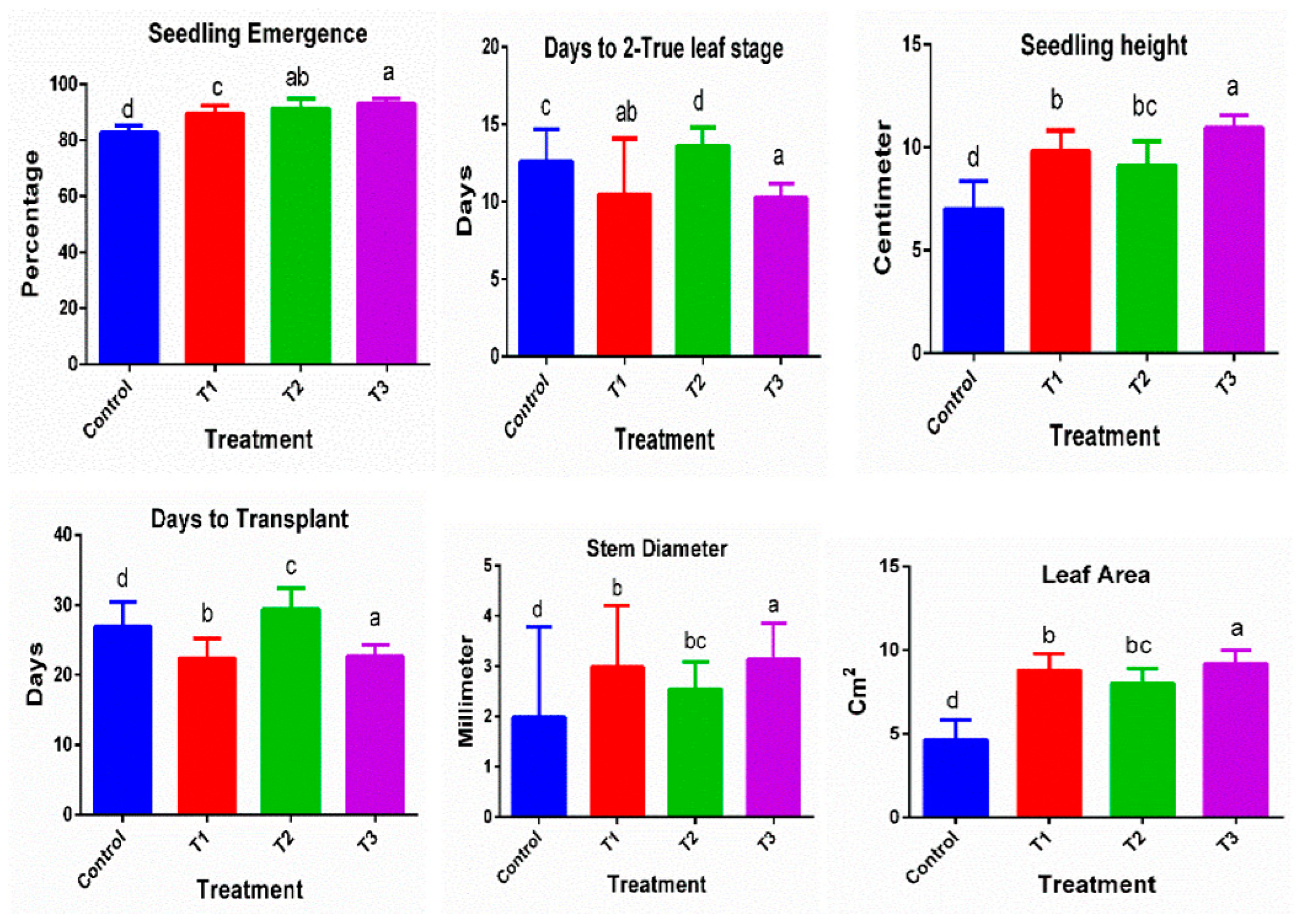
3.1. Effect on Seedlings’ Morphological Quality Characteristics
3.2. Effect on the Field Performance of Cabbage
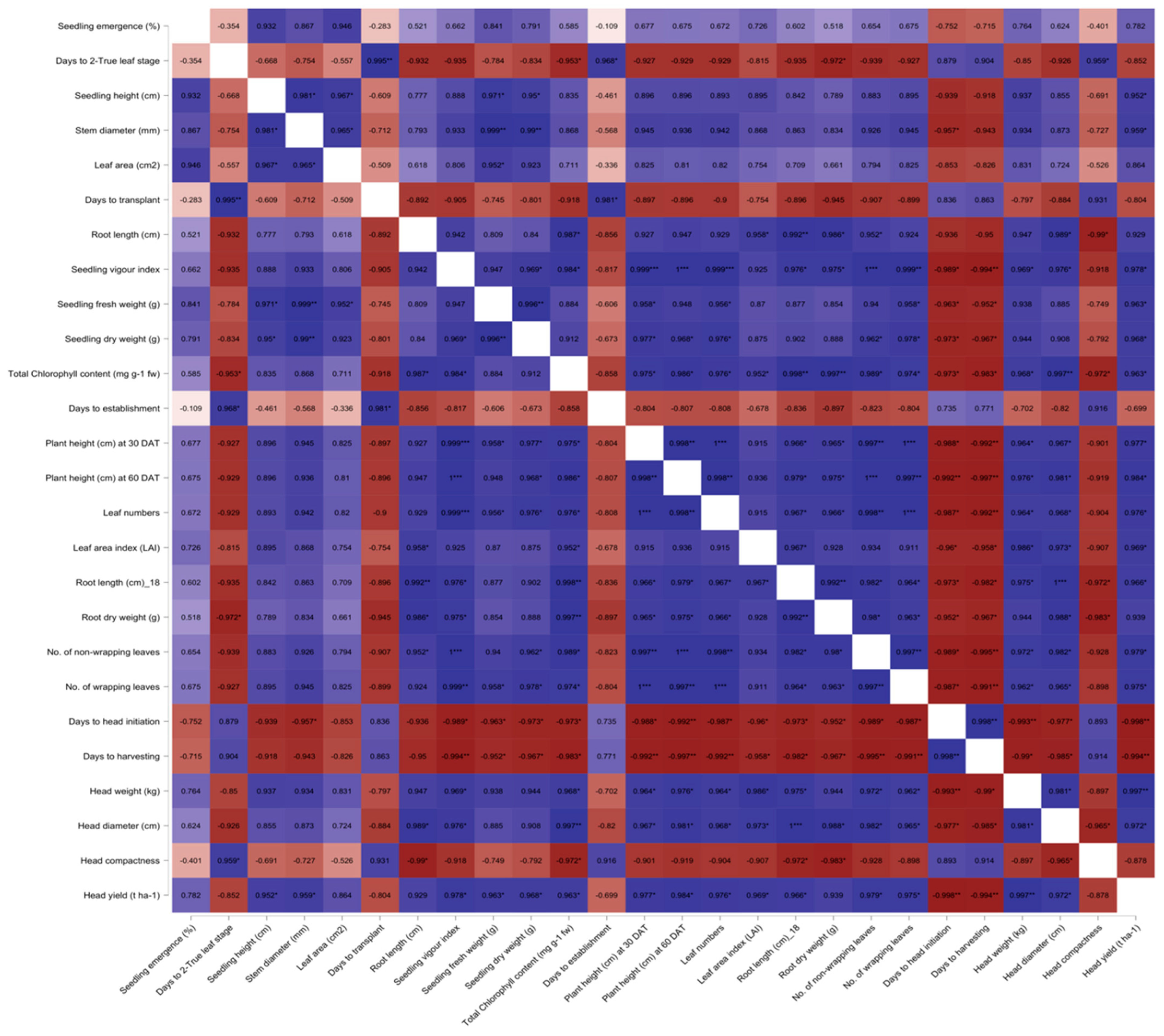
3.3. Correlations among the Traits
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhandari, S.R.; Choi, C.S.; Rhee, J.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Lee, J.G. Seasonal Variation in Agronomic Characteristics and Sugar Content of Cabbage Genotypes. Chil. J. Agric. Res. 2021, 81, 80–91. [Google Scholar] [CrossRef]
- Zhao, Y.; Yue, Z.; Zhong, X.; Lei, J.; Tao, P.; Li, B. Distribution of Primary and Secondary Metabolites among the Leaf Layers of Headed Cabbage (Brassica oleracea Var. capitata). Food Chem. 2020, 312, 126028. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Lee, J.G. Profiling of Individual Desulfo-Glucosinolate Content in Cabbage Head (Brassica oleracea Var. capitata) Germplasm. Molecules 2020, 25, 1860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, R.; Arif, S.; Sikander, M.A.; Memon, A.R. Effect of Using Bio-Control Agents on Growth, Yield, Head Quality and Root Rot Control in Broccoli Plants. Int. J. Acad. Res. 2011, 3, 71–80. [Google Scholar]
- Emmanuel, O.C.; Babalola, O.O. Productivity and Quality of Horticultural Crops through Co-Inoculation of Arbuscular Mycorrhizal Fungi and Plant Growth Promoting Bacteria. Microbiol. Res. 2020, 239, 126569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cook, J.; Nearing, J.T.; Zhang, J.; Raudonis, R.; Glick, B.R.; Langille, M.G.; Cheng, Z. Harnessing the Plant Microbiome to Promote the Growth of Agricultural Crops. Microbiol. Res. 2021, 245, 126690. [Google Scholar] [CrossRef]
- Verma, R.; Maurya, B.R.; Meena, V.S. Integrated Effect of Bio-Organics with Chemical Fertilizer on Growth, Yield and Quality of Cabbage (Brassica oleracea Var capitata). Indian J. Agric. Sci. 2014, 84, 914–919. [Google Scholar]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural Sustainability: Microbial Biofertilizers in Rhizosphere Management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Yildirim, E.; Karlidag, H.; Turan, M.; Dursun, A.; Goktepe, F. Growth, Nutrient Uptake, and Yield Promotion of Broccoli by Plant Growth Promoting Rhizobacteria with Manure. HortScience 2011, 46, 932–936. [Google Scholar] [CrossRef] [Green Version]
- Hafeez, F.Y.; Yasmin, S.; Ariani, D.; Renseigné, N.; Zafar, Y.; Malik, K.A. Plant Growth-Promoting Bacteria as Biofertilizer. Agron. Sustain. Dev. 2006, 26, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Rakshit, A. Impact Assessment of Bio Priming Mediated Nutrient Use Efficiency for Climate Resilient Agriculture. In Climate Change and Agriculture in India: Impact and Adaptation; Springer: Berlin/Heidelberg, Germany, 2019; pp. 57–68. [Google Scholar]
- Jha, M.K.; Urraiya, P.; Jha, B.; Sahu, M.R. Effect of Organic, Inrganic & Biofertilizers on Growth Attributes of Cabbage (Brassica oleracea Var. capitata). Under CG Plain Zone. J. Pharmacogn. Phytochem. 2017, 6, 2298–2300. [Google Scholar]
- Sarkar, D.; Ray, S.; Singh, N.K.; Rakshit, A.; Singh, H.B. Seed Priming with Bio-Inoculants Triggers Nutritional Enrichment in Vegetables: A Review. Int. J. Agric. Environ. Biotechnol. 2018, 736, 727–735. [Google Scholar]
- Meena, S.K.; Rakshit, A.; Meena, V.S. Effect of Seed Bio-Priming and N Doses under Varied Soil Type on Nitrogen Use Efficiency (NUE) of Wheat (Triticum aestivum L.) under Greenhouse Conditions. Biocatal. Agric. Biotechnol. 2016, 6, 68–75. [Google Scholar] [CrossRef]
- Ferjani, R.; Gharsa, H.; Estepa-Pérez, V.; Gómez-Sanz, E.; Cherni, M.; Mahjoubi, M.; Boudabous, A.; Torres, C.; Ouzari, H.-I. Plant Growth-Promoting Rhizopseudomonas: Expanded Biotechnological Purposes and Antimicrobial Resistance Concern. Ann. Microbiol. 2019, 69, 51–59. [Google Scholar] [CrossRef]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-Tolerant Pseudomonas Spp. Improve the Growth Performance of Finger Millet (Eleusine coracana (L.) Gaertn.) under Non-Stressed and Drought-Stressed Conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Karungi, J.; Kyamanywa, S.; Ekbom, B. Organic Soil Fertility Amendments and Tritrophic Relationships on Cabbage in Uganda: Experiences from on-Station and on-Farm Trials. Afr. J. Agric. Res. 2010, 5, 2862–2867. [Google Scholar]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-C.; Glick, B.R.; Bashan, Y.; Ryu, C.-M. Enhancement of Plant Drought Tolerance by Microbes. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 383–413. [Google Scholar]
- Tanwar, A.; Aggarwal, A.; Kaushish, S.; Chauhan, S. Interactive Effect of AM Fungi with Trichoderma viride and Pseudomonas fluorescens on Growth and Yield of Broccoli. Plant Prot. Sci. 2013, 49, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Delta, A.K.; Kaushik, P. Glomus Mosseae and Pseudomonas fluorescens Application Sustains Yield and Promote Tolerance to Water Stress in Helianthus annuus L. Stresses 2021, 1, 305–316. [Google Scholar] [CrossRef]
- Saini, I.; Aggarwal, A.; Kaushik, P. Inoculation with Mycorrhizal Fungi and Other Microbes to Improve the Morpho-Physiological and Floral Traits of Gazania Rigens (L.) Gaertn. Agriculture 2019, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- El-Sawah, A.; El-Keblawy, A.; Ali, D.; Ibrahim, H.; El-Sheikh, M.; Sharma, A.; Alhaj Hamoud, Y.; Shaghaleh, H.; Brestic, M.; Skalicky, M.; et al. Arbuscular Mycorrhizal Fungi and Plant Growth-Promoting Rhizobacteria Enhance Soil Key Enzymes, Plant Growth, Seed Yield, and Qualitative Attributes of Guar. Agriculture 2021, 11, 194. [Google Scholar] [CrossRef]
- Delfine, S.; Alvino, A.; Loreto, F.; Centritto, M.; Santarelli, G. Effects of Water Stress on the Yield and Photosynthesis of Field-Grown Sweet Pepper (Capsicum annuum L.). In Proceedings of the III International Symposium on Irrigation of Horticultural Crops 537, Lisbon, Portugal, 28 June–2 July 1999; pp. 223–229. [Google Scholar]
- Abdul, B.A.; Anderson, J.D. Vigour Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Panse, V.G.; Sukhatme, P.V. Statistical Methods for Agricultural Workers. Stat. Methods Agric. Work. 1954, 48, 323–361. [Google Scholar]
- David, B.V.; Chandrasehar, G.; Selvam, P.N. Pseudomonas fluorescens: A Plant-Growth-Promoting Rhizobacterium (PGPR) With Potential Role in Biocontrol of Pests of Crops. In Crop Improvement through Microbial Biotechnology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 221–243. ISBN 978-0-444-63987-5. [Google Scholar]
- Mishra, V.; Gupta, A.; Kaur, P.; Singh, S.; Singh, N.; Gehlot, P.; Singh, J. Synergistic Effects of Arbuscular Mycorrhizal Fungi and Plant Growth Promoting Rhizobacteria in Bioremediation of Iron Contaminated Soils. Int. J. Phytoremediat. 2016, 18, 697–703. [Google Scholar] [CrossRef]
- Behera, B.C.; Singdevsachan, S.K.; Mishra, R.R.; Dutta, S.K.; Thatoi, H.N. Diversity, Mechanism and Biotechnology of Phosphate Solubilising Microorganism in Mangrove—A Review. Biocatal. Agric. Biotechnol. 2014, 3, 97–110. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Aggarwal, A. Impact of Arbuscular Mycorrhizal Fungi with Trichoderma viride and Pseudomonas fluorescens on Growth, Yield and Oil Content in Helianthus Annuus L. J. Essent. Oil Bear. Plants 2015, 18, 444–454. [Google Scholar] [CrossRef]
- Sarkar, D.; Sankar, A.; Devika, O.S.; Singh, S.; Shikha; Parihar, M.; Rakshit, A.; Sayyed, R.Z.; Gafur, A.; Ansari, M.J.; et al. Optimizing Nutrient Use Efficiency, Productivity, Energetics, and Economics of Red Cabbage Following Mineral Fertilization and Biopriming with Compatible Rhizosphere Microbes. 2021, 11, 15680. Sci. Rep. 2021, 11, 15680. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant Growth-Promoting Rhizobacteria (PGPR): Emergence in Agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Turan, M.; Esitken, A.; Sahin, F. Plant Growth Promoting Rhizobacteria as Alleviators for Soil Degradation. In Bacteria in Agrobiology: Stress Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 41–63. ISBN 978-3-642-23464-4. [Google Scholar]
- Faruk, M. Efficacy of Different Substrates to Formulate Trichoderma Harzianum against Seedling Disease of Cabbage. Bangladesh J. Agric. Res. 2019, 44, 127–138. [Google Scholar] [CrossRef]
- Yadav, L.P.; Kavita, A. Yield and Quality Response of Cabbage (Brassica oleracea) Var. Pride of India to Nitrogen and Biofertilizers. Curr. Hortic. 2016, 4, 7–10. [Google Scholar]
- Jha, M.K.; Patel, D.; Verma, S.; Sao, Y. Effect of Organic, Inorganic and Biofertilizers on Head Yield of Cabbage (Brassica oleracea Var. capitata). Trends Biosci. 2017, 10, 7052–7054. [Google Scholar]
- Marulanda, A.; Barea, J.-M.; Azcón, R. Stimulation of Plant Growth and Drought Tolerance by Native Microorganisms (AM Fungi and Bacteria) from Dry Environments: Mechanisms Related to Bacterial Effectiveness. J. Plant Growth Regul. 2009, 28, 115–124. [Google Scholar] [CrossRef]
- Samancioglu, A.; Yildirim, E.; Turan, M.; Kotan, R.; Sahin, U.; Kul, R. Amelioration of Drought Stress Adverse Effect and Mediating Biochemical Content of Cabbage Seedlings by Plant Growth Promoting Rhizobacteria. IJAB 2016, 18, 948–956. [Google Scholar] [CrossRef]
- Nahar, M.; Rahman, M.; Kibria, M.; Karim, A.R.; Miller, S. Use of Tricho-Compost and Tricho-Leachate for Management of Soil-Borne Pathogens and Production of Healthy Cabbage Seedlings. Bangladesh J. Agric. Res. 2013, 37, 653–664. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.M.; Akhtar, N.; Islam, M.T.; Faruq, A.N. Effect of Trichoderma Harzianum and Some Selected Soil Amendments on Damping-Off Disease of Cabbage and Cauliflower. J. Exp. Biosci. 2011, 2, 37–42. [Google Scholar]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, J.; Yang, X.; Cui, Y.; Chen, L.; Ran, W.; Shen, Q. Putative Trichoderma Harzianum Mutant Promotes Cucumber Growth by Enhanced Production of Indole Acetic Acid and Plant Colonization. Plant Soil 2013, 368, 433–444. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant Growth Promoting Rhizobia: Challenges and Opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef] [Green Version]
- Khalil, M.E. Efficiency of Trichoderma viride and Bacillus Subtilis as Biocontrol Agents against Root Rot Caused by Fusarium Solani in Tomato. Egypt. J. Agric. Res. 2019, 97, 507–516. [Google Scholar]
- Duc, N. Combined Inoculation of Arbuscular Mycorrhizal Fungi, Pseudomonas fluorescens and Trichoderma Spp. for Enhancing Defense Enzymes and Yield of Three Pepper Cultivars. Appl. Ecol. Environ. Res. 2017, 15, 1815–1829. [Google Scholar] [CrossRef]
- Ekinci, M.; Turan, M.; Yildirim, E.; Güneş, A.; Kotan, R.; Dursun, A. Effect of Plant Growth Promoting Rhizobacteria on Growth, Nutrient, Organic Acid, Amino Acid and Hormone Content of Cauliflower (Brassica oleracea L. Var. botrytis) Transplants. Acta Sci. Pol. Hortorum Cultus 2014, 13, 71–85. [Google Scholar]
- Mia, M.B.; Shamsuddin, Z.H.; Mahmood, M. Effects of Rhizobia and Plant Growth Promoting Bacteria Inoculation on Germination and Seedling Vigor of Lowland Rice. Afr. J. Biotechnol. 2012, 11, 3758–3765. [Google Scholar]
- Cheah, L.H.; Veerakone, S.; Kent, G. Biological Control of Clubroot on Cauliflower with Trichoderma and Streptomyces spp. N. Z. Plant Prot. 2000, 53, 18–21. [Google Scholar] [CrossRef] [Green Version]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of Phosphates and Micronutrients by the Plant-Growth-Promoting and Biocontrol Fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vij, S.; Sharma, N.; Sharma, M.; Mohanta, T.K.; Kaushik, P. Application of Trichoderma viride and Pseudomonas fluorescens to Cabbage (Brassica oleracea L.) Improves Both Its Seedling Quality and Field Performance. Sustainability 2022, 14, 7583. https://doi.org/10.3390/su14137583
Vij S, Sharma N, Sharma M, Mohanta TK, Kaushik P. Application of Trichoderma viride and Pseudomonas fluorescens to Cabbage (Brassica oleracea L.) Improves Both Its Seedling Quality and Field Performance. Sustainability. 2022; 14(13):7583. https://doi.org/10.3390/su14137583
Chicago/Turabian StyleVij, Shilpa, Neha Sharma, Meenakshi Sharma, Tapan Kumar Mohanta, and Prashant Kaushik. 2022. "Application of Trichoderma viride and Pseudomonas fluorescens to Cabbage (Brassica oleracea L.) Improves Both Its Seedling Quality and Field Performance" Sustainability 14, no. 13: 7583. https://doi.org/10.3390/su14137583






