Synthesis and Characterization of Zero-Valent Fe-Cu and Fe-Ni Bimetals for the Dehalogenation of Trichloroethylene Vapors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
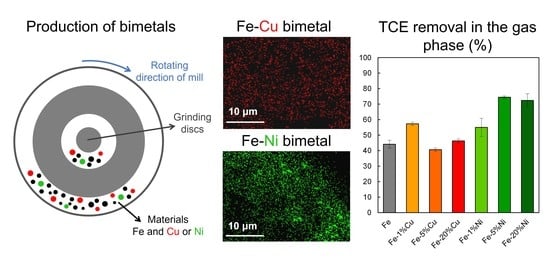
2.2. Preparation of Fe-Cu and Fe-Ni Bimetals
2.3. Materials Characterization
2.4. TCE Removal Experiments
3. Results and Discussion
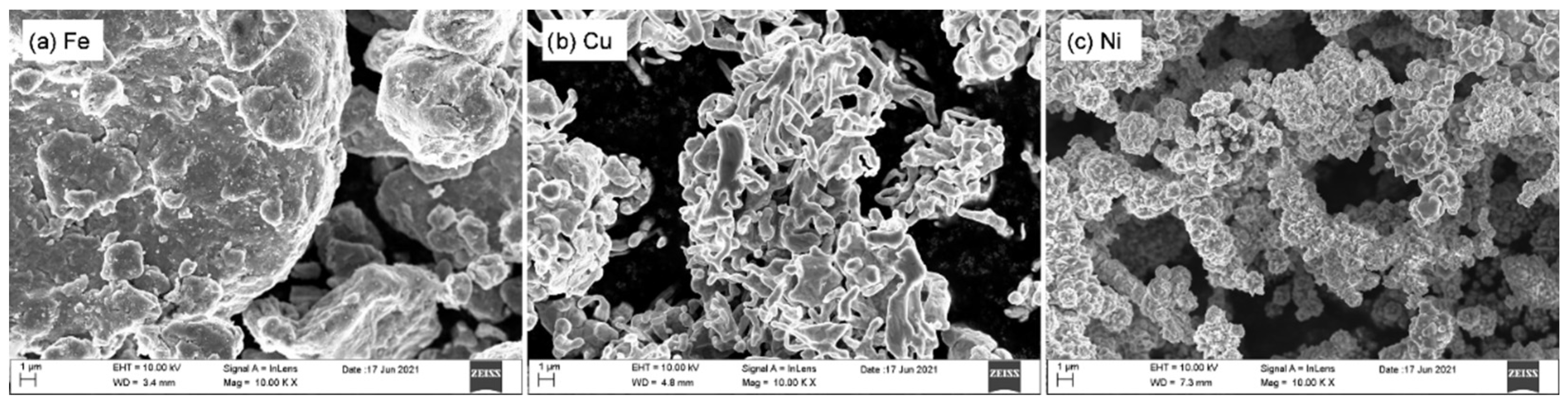
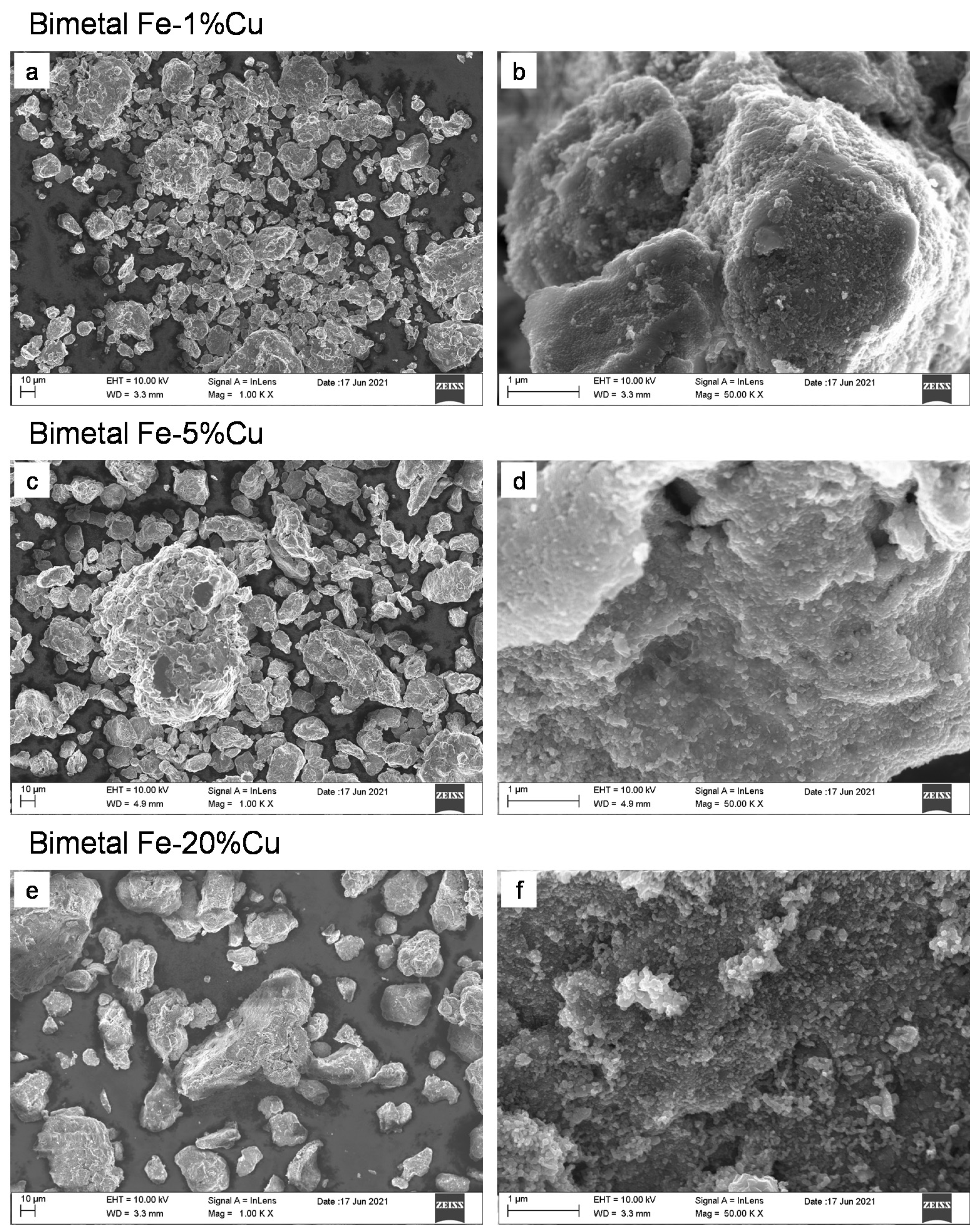
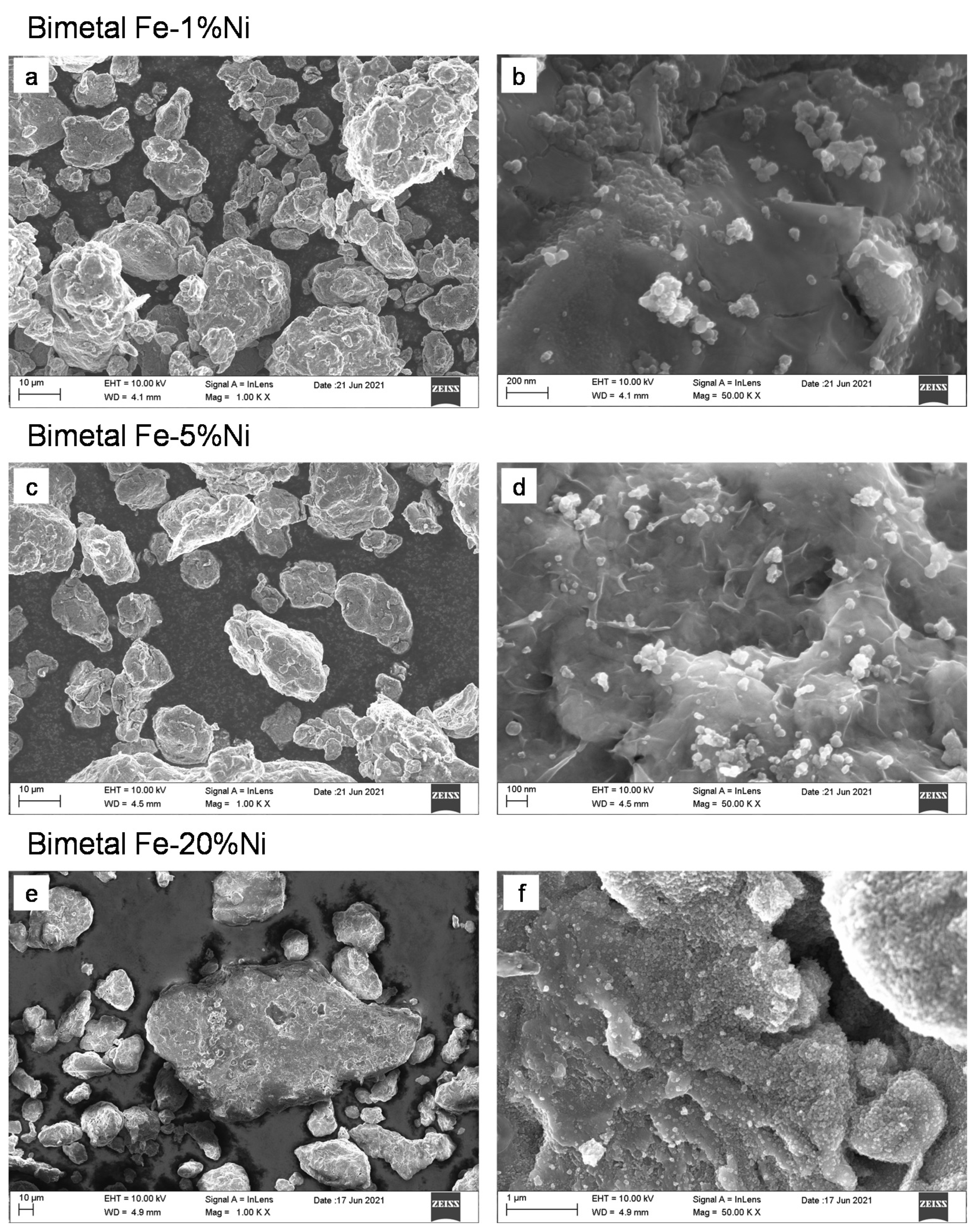
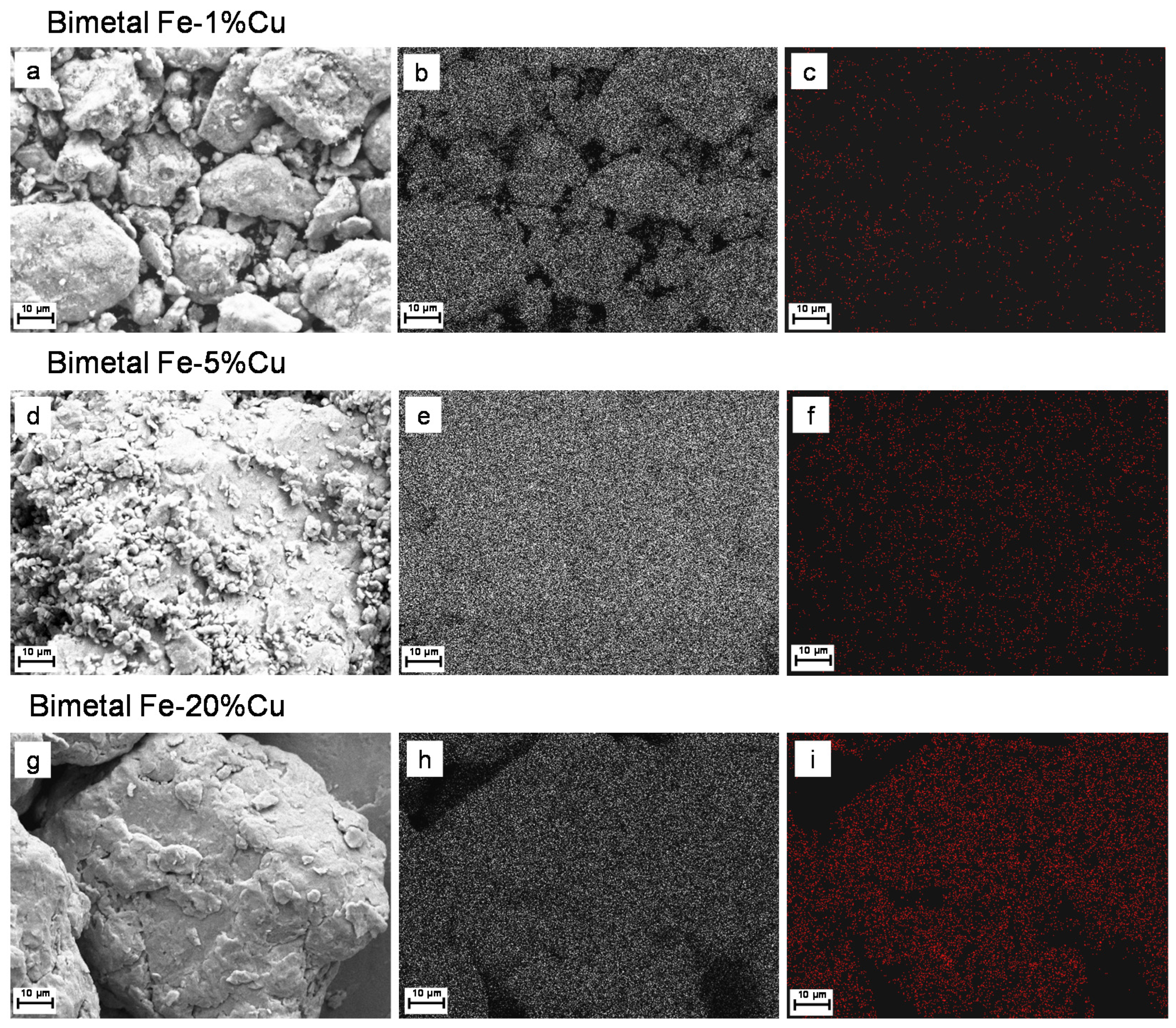
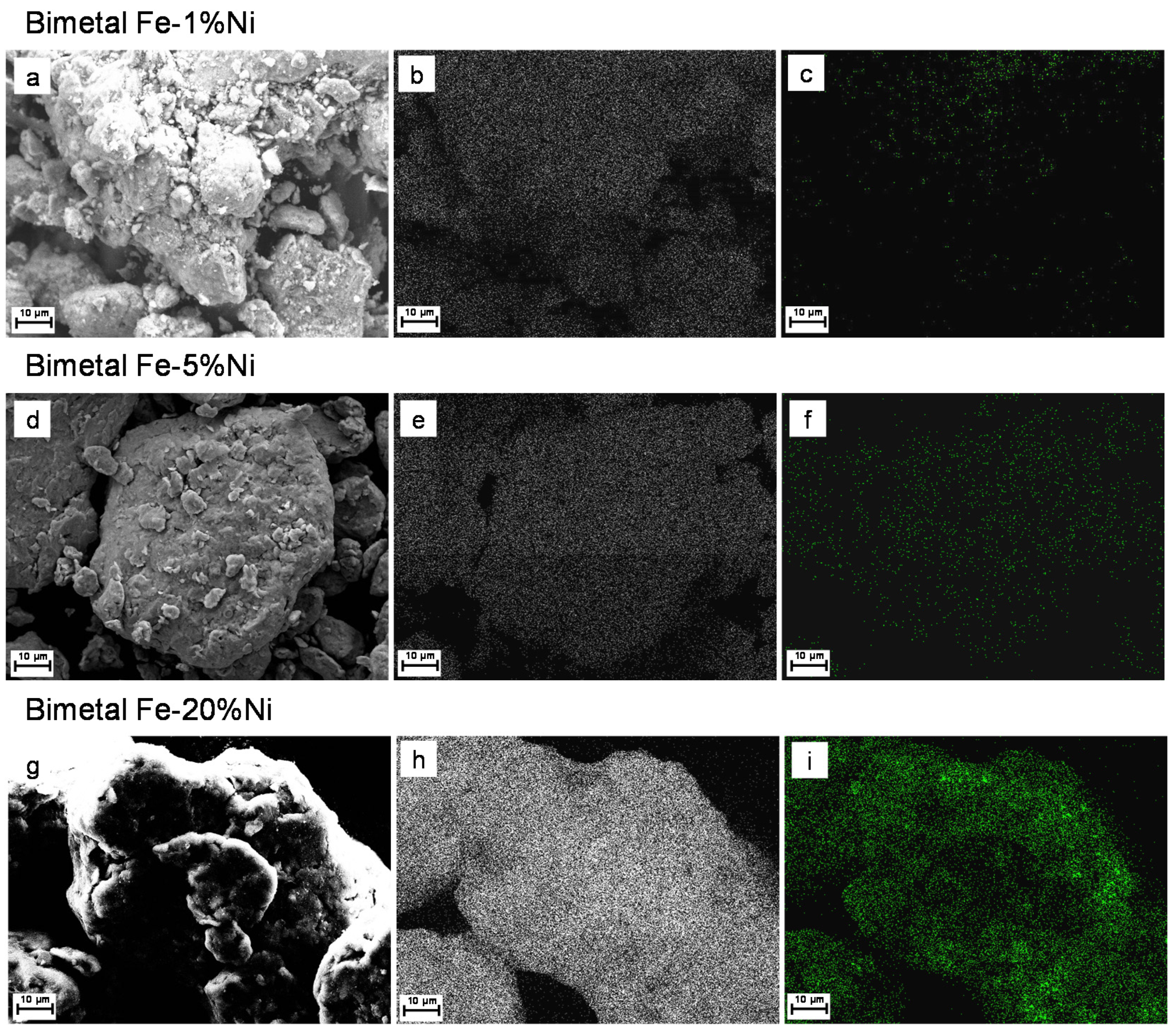
3.1. Microstructure and Morphology
3.2. Elemental Mapping
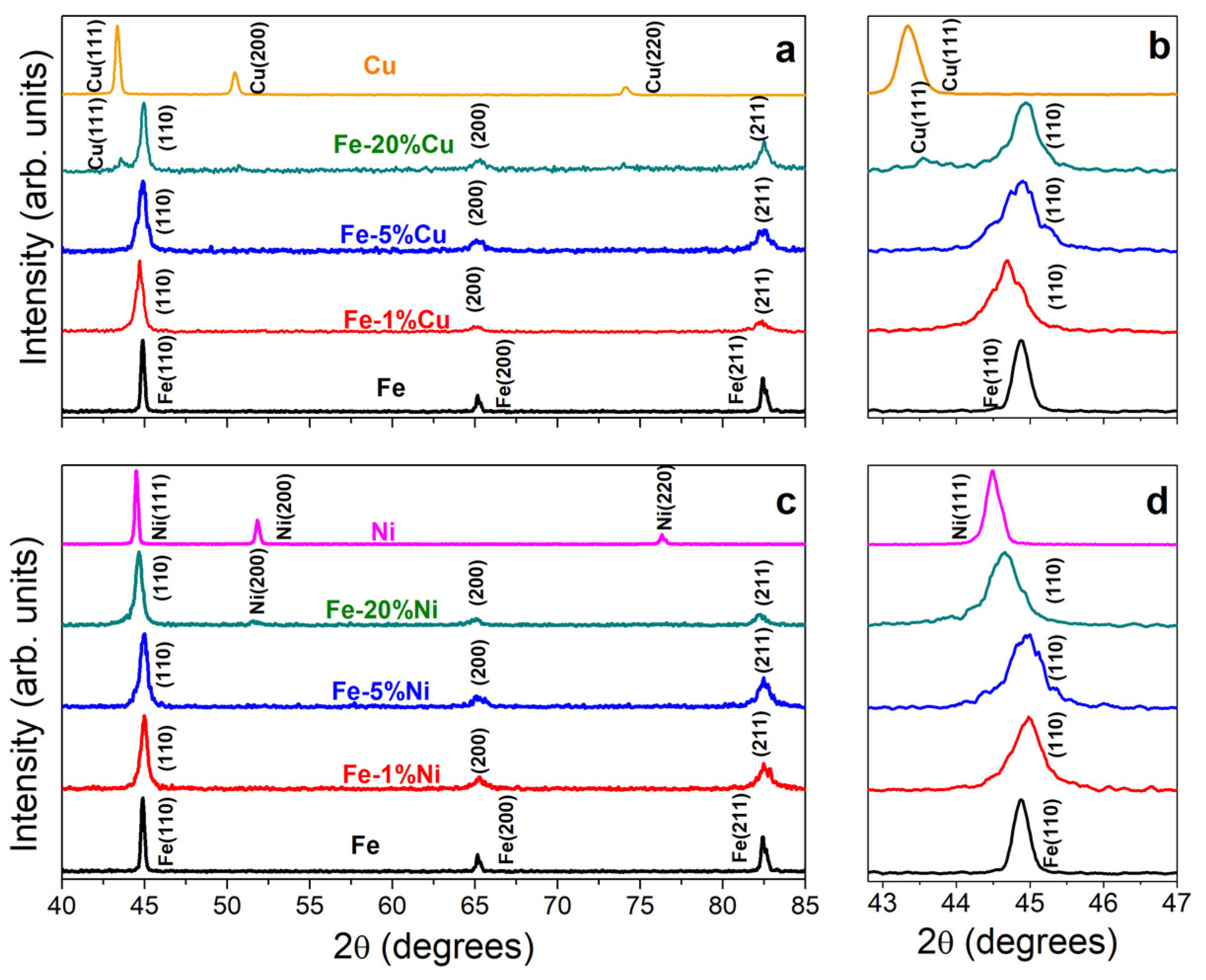
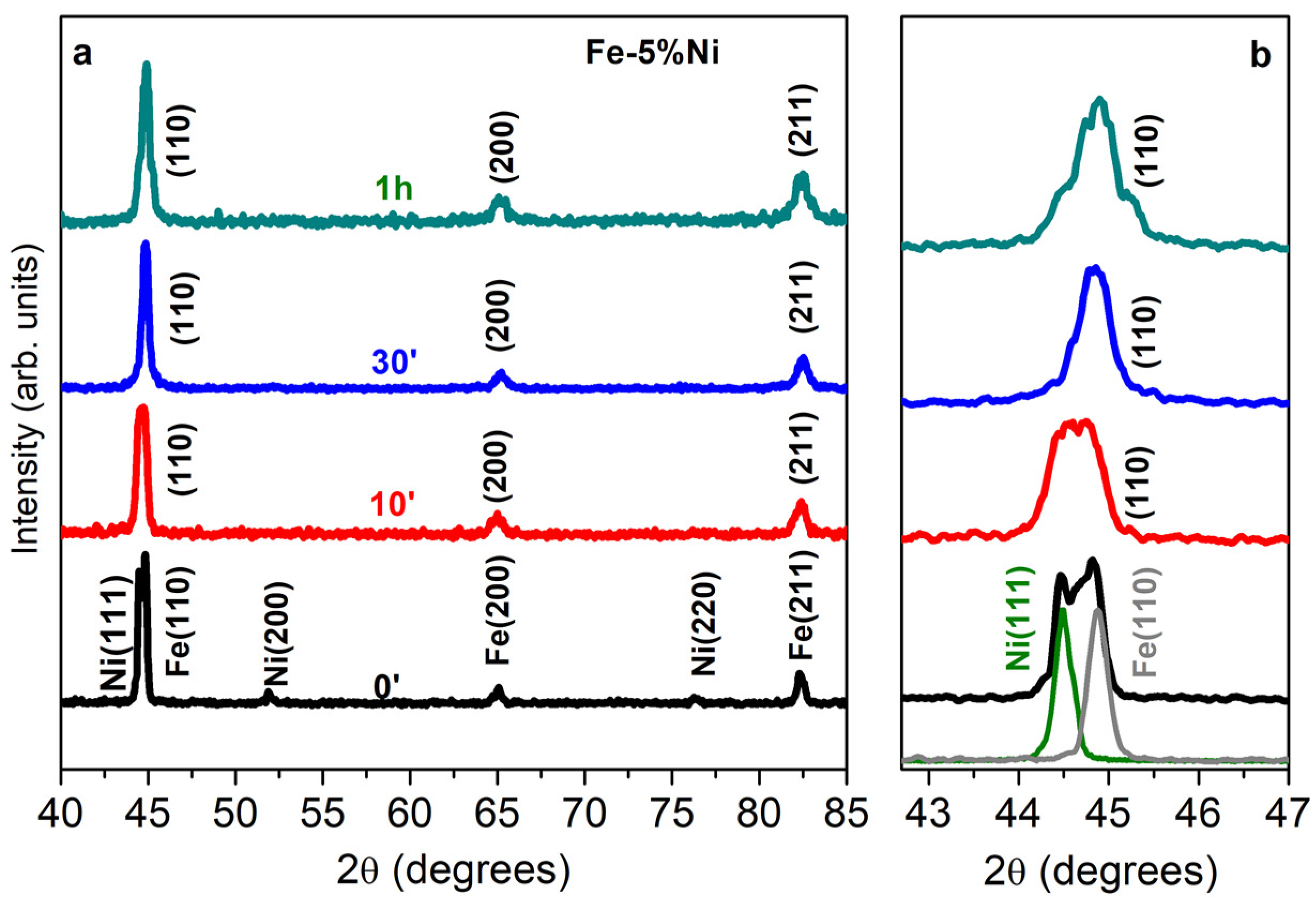
3.3. XRD Characterization
3.4. Specific Surface Area
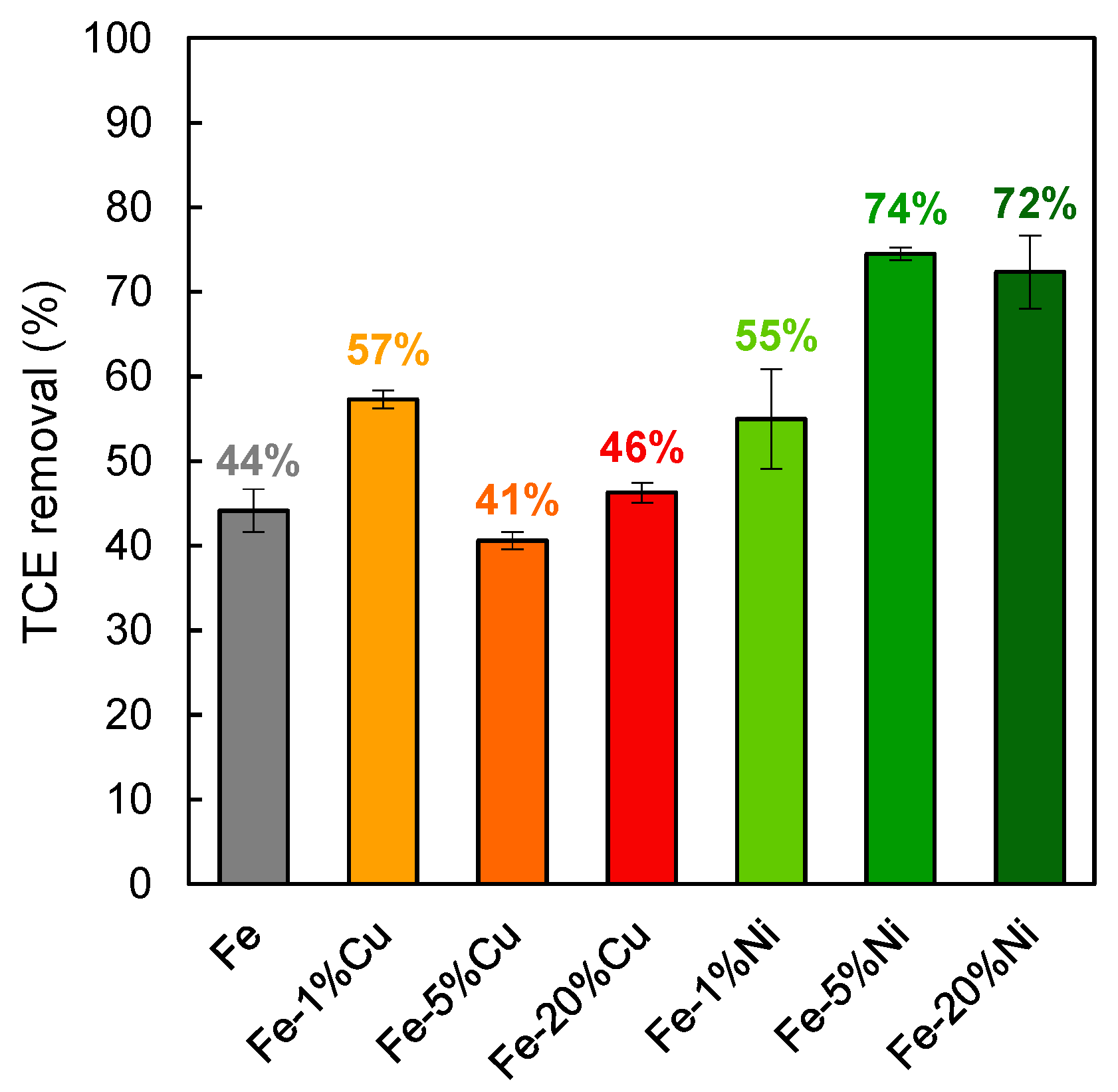
3.5. TCE Removal Using Different Fe-Cu and Fe-Ni Bimetals
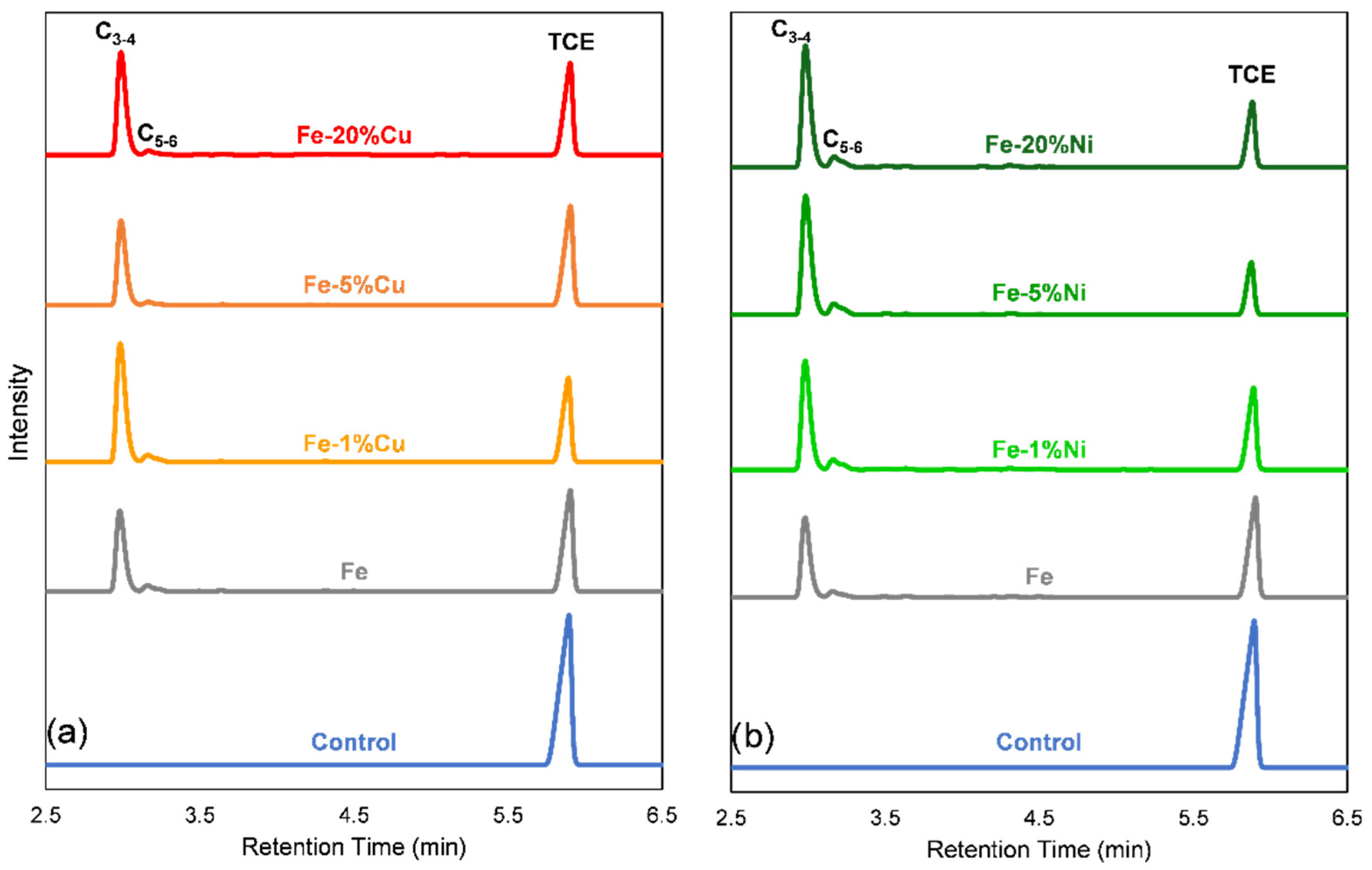
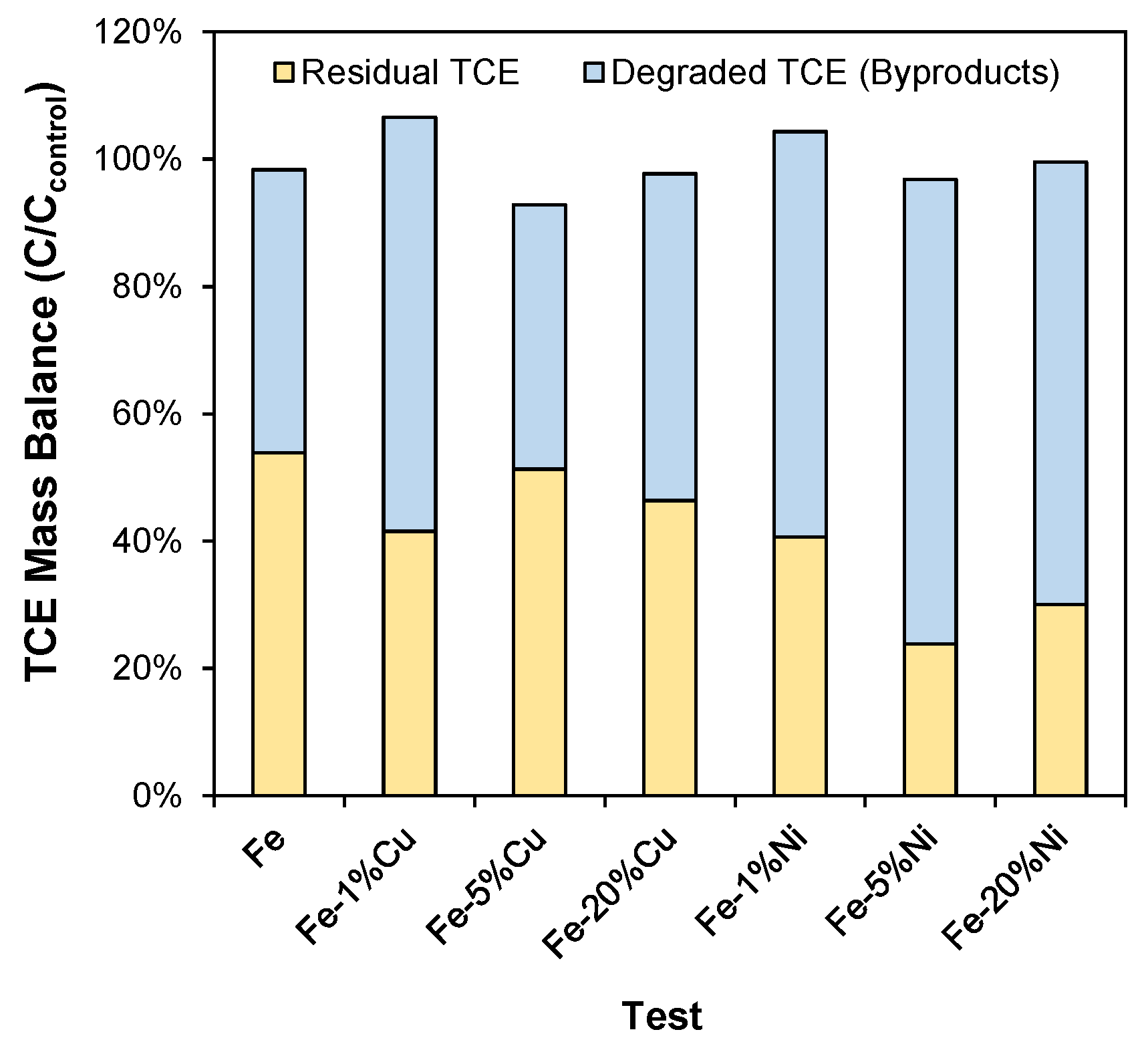
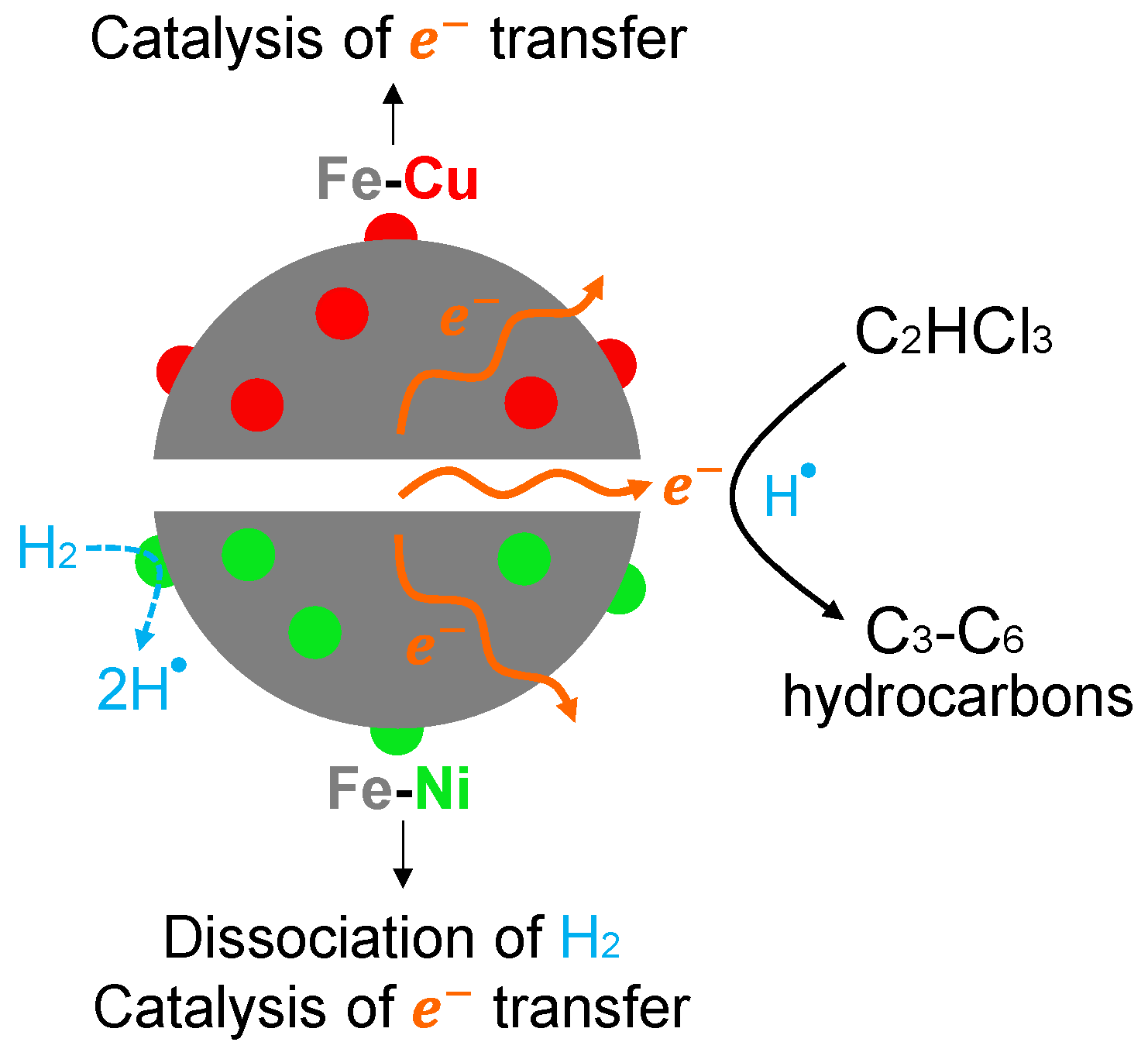
3.6. TCE Degradation Pathway, Byproducts, and Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ITRC. Permeable Reactive Barrier: Technology Update; Interstate Technology and Regulatory Council, PRB: Technology Update Team: Washington, DC, USA, 2011; Available online: http://www.itrcweb.org (accessed on 15 May 2022).
- Kim, Y.H.; Carraway, E.R. Reductive dechlorination of TCE by zero valent bimetals. Environ. Technol. 2003, 24, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Lo, S.L.; Liou, Y.H. Dechlorination of trichloroethylene in aqueous solution by noble metal-modified iron. J. Hazard. Mater. 2004, 116, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Song, H.; Li, Q.; Li, A. Fe/Cu bimetallic catalysis for reductive degradation of nitrobenzene under oxic conditions. Chem. Eng. J. 2016, 283, 366–374. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, H.; Xu, F.; Zhao, J. Enhanced hydrodechlorination of 4-chlorophenol by Cu/Fe bimetallic system via ball-milling. Desalin. Water Treat. 2017, 64, 157–164. [Google Scholar] [CrossRef] [Green Version]
- McCarty, P.L. Groundwater contamination by chlorinated solvents: History, remediation technologies and strategies. In In Situ Remediation of Chlorinated Solvent Plumes; Springer: New York, NY, USA, 2010; pp. 1–28. [Google Scholar]
- Phillips, D.H.; Nooten, T.V.; Bastiaens, L.; Russell, M.I.; Dickson, K.; Plant, S.; Ahad, J.M.E.; Newton, T.; Elliot, T.; Kalin, R.M. Ten year performance evaluation of a field-scale zero-valent iron permeable reactive barrier installed to remediate trichloroethene contaminated groundwater. Environ. Sci. Technol. 2010, 44, 3861–3869. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef]
- Zingaretti, D.; Verginelli, I.; Baciocchi, R. Dehalogenation of trichloroethylene vapors by partially saturated zero-valent iron. Sci. Total Environ. 2019, 647, 682–689. [Google Scholar] [CrossRef]
- Zingaretti, D.; Verginelli, I.; Luisetto, I.; Baciocchi, R. Horizontal permeable reactive barriers with zero-valent iron for preventing upward diffusion of chlorinated solvent vapors in the unsaturated zone. J. Contam. Hydrol. 2020, 234, 103687. [Google Scholar] [CrossRef]
- Powell, R.M.; Blowes, D.W.; Gillham, R.W.; Schultz, D.; Sivavec, T.; Puls, R.W.; Vogan, H.L.; Powell, P.D.; Landis, R. Permeable reactive barrier technologies for contaminant remediation. In US EPA; 1998; Volume 600, pp. 1–94. [Google Scholar]
- Duan, J.; Zhu, H.; Xu, F.; Zhao, J. A new approach to 4-chlorophenol dechlorination on monometallic copper compared to its Cu/Fe bimetallic system. Chem. Eng. J. 2016, 304, 282–288. [Google Scholar] [CrossRef]
- Rodrigues, R.; Betelu, S.; Colombano, S.; Tzedakis, T.; Masselot, G.; Ignatiadis, I. In Situ Chemical Reduction of Chlorinated Organic Compounds. In Environmental Soil Remediation and Rehabilitation; Springer International Publishing: Cham, Switzerland, 2020; pp. 283–398. [Google Scholar]
- Noubactep, C. On the operating mode of bimetallic systems for environmental remediation. J. Hazard. Mater. 2009, 164, 394–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkateshaiah, A.; Silvestri, D.; Wacławek, S.; Ramakrishnan, R.K.; Krawczyk, K.; Saravanan, P.; Pawlyta, M.; Padil, V.V.T.; Černik, M.; Dionysiou, D.D. A comparative study of the degradation efficiency of chlorinated organic compounds by bimetallic zero-valent iron nanoparticles. Environ. Sci. Water Res. Technol. 2022, 8, 162–172. [Google Scholar] [CrossRef]
- He, N.; Li, P.; Zhou, Y.; Ren, W.; Fan, S.; Verkhozina, V.A. Catalytic dechlorination of polychlorinated biphenyls in soil by palladium–iron bimetallic catalyst. J. Hazard. Mater. 2009, 164, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cwiertny, D.M.; Bransfield, S.J.; Livi, K.J.; Fairbrother, D.H.; Roberts, A.L. Exploring the influence of granular iron additives on 1, 1, 1-trichloroethane reduction. Environ. Sci. Technol. 2006, 40, 6837–6843. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, Z.; Shi, S.; Xu, W.; Sheng, H.; Gu, Y.; Jiang, Y.; Xi, B. Dechlorination of excess trichloroethene by bimetallic and sulfidated nanoscale zero-valent iron. Environ. Sci. Technol. 2018, 52, 8627–8637. [Google Scholar] [CrossRef]
- Lien, H.L.; Zhang, W.X. Nanoscale Pd/Fe bimetallic particles: Catalytic effects of palladium on hydrodechlorination. Appl. Catal. B Environ. 2007, 77, 110–116. [Google Scholar] [CrossRef]
- Chao, K.P.; Ong, S.K.; Fryzek, T.; Yuan, W.; Braida, W. Degradation of trichloroethylene using iron, bimetals and trimetals. J. Environ. Sci. Health Part A 2012, 47, 1536–1542. [Google Scholar] [CrossRef]
- Liu, X.; Wu, M.; Zhao, J. Removal of Trichloroethylene from Water by Bimetallic Ni/Fe Nanoparticles. Water 2022, 14, 1616. [Google Scholar] [CrossRef]
- Ko, S.O.; Lee, D.H.; Kim, Y.H. Kinetic studies of reductive dechlorination of chlorophenols with Ni/Fe bimetallic particles. Environ. Technol. 2007, 28, 583–594. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Liu, F.; Li, H.D. Degradation of tetrachloromethane and tetrachloroethene by Ni/Fe bimetallic nanoparticles. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2009; Volume 188, p. 012014. [Google Scholar]
- Xu, F.; Deng, S.; Xu, J.; Zhang, W.; Wu, M.; Wang, B.; Huang, J.; Yu, G. Highly active and stable Ni–Fe bimetal prepared by ball milling for catalytic hydrodechlorination of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 4576–4582. [Google Scholar] [CrossRef]
- Schrick, B.; Blough, J.L.; Jones, A.D.; Mallouk, T.E. Hydrodechlorination of trichloroethylene to hydrocarbons using bimetallic nickel−iron nanoparticles. Chem. Mater. 2002, 14, 5140–5147. [Google Scholar] [CrossRef]
- Zhuang, M.; Shi, W.; Wang, H.; Cui, L.; Quan, G.; Yan, J. Carbothermal Synthesis of Ni/Fe Bimetallic Nanoparticles Embedded into Graphitized Carbon for Efficient Removal of Chlorophenol. Nanomaterials 2021, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Liu, H.J.; Yang, F.L.; Xue, D.M.; Zhao, Y.Z. Dechlorination of three polychlorinated hydrocarbons in water using bemetallic systems. China Environ. Sci. 1998, 18, 333–336. [Google Scholar]
- Sui, H.; Rong, Y.; Song, J.; Zhang, D.; Li, H.; Wu, P.; Shen, Y.; Huang, Y. Mechanochemical destruction of DDTs with Fe-Zn bimetal in a high-energy planetary ball mill. J. Hazard. Mater. 2018, 342, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Liu, H.; Wang, J.; Zhao, D.; Fan, X. A new insight into the main mechanism of 2, 4-dichlorophenol dechlorination by Fe/Ni nanoparticles. Sci. Total Environ. 2019, 697, 133996. [Google Scholar] [CrossRef] [PubMed]
- Scaria, J.; Nidheesh, P.V.; Kumar, M.S. Synthesis and applications of various bimetallic nanomaterials in water and wastewater treatment. J. Environ. Manag. 2020, 259, 110011. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M. Assessing the Use and Application of Zero-Valent Iron Nanoparticle Technology for Remediation at Contaminated Sites. Jackson State University. 2009. Available online: https://clu-in.org/download/studentpapers/zero-valent-iron-cook.pdf (accessed on 15 May 2022).
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Gunawardana, B.; Singhal, N.; Swedlund, P. Degradation of chlorinated phenols by zero valent iron and bimetals of iron: A review. Environ. Eng. Res. 2011, 16, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Doong, R.A.; Lai, Y.L. Effect of metal ions and humic acid on the dechlorination of tetrachloroethylene by zerovalent iron. Chemosphere 2006, 64, 371–378. [Google Scholar] [CrossRef]
- Xiong, Z.; Lai, B.; Yang, P.; Zhou, Y.; Wang, J.; Fang, S. Comparative study on the reactivity of Fe/Cu bimetallic particles and zero valent iron (ZVI) under different conditions of N2, air or without aeration. J. Hazard. Mater. 2015, 297, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Qian, T.T.; Jiang, H. Bimetallic Fe nanoparticles: Recent advances in synthesis and application in catalytic elimination of environmental pollutants. Chem. Eng. J. 2014, 236, 448–463. [Google Scholar] [CrossRef]
- Zhang, S.S.; Yang, N.; Ni, S.Q.; Natarajan, V.; Ahmad, H.A.; Xu, S.; Fang, X.; Zhan, J. One-pot synthesis of highly active Ni/Fe nano-bimetal by simultaneous ball milling and in situ chemical deposition. RSC Adv. 2018, 8, 26469–26475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azabou, M.; Gharsallah, H.I.; Escoda, L.; Suñol, J.J.; Kolsi, A.W.; Khitouni, M. Mechanochemical reactions in nanocrystalline Cu–Fe system induced by mechanical alloying in air atmosphere. Powder Technol. 2012, 224, 338–344. [Google Scholar] [CrossRef]
- Moumeni, H.; Alleg, S.; Greneche, J.M. Structural properties of Fe50Co50 nanostructured powder prepared by mechanical alloying. J. Alloys Compd. 2005, 386, 12–19. [Google Scholar] [CrossRef]
- Wille, C.G.; Kirchheim, R. Time evolution of morphology in mechanically alloyed Fe–Cu. Ultramicroscopy 2011, 111, 730–737. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Elkedim, O. Magnetic properties of nanocrystalline Fe–10% Ni alloy obtained by planetary ball mills. J. Alloys Compd. 2013, 573, 157–162. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, F.; Zhao, J.; Jia, L.; Wu, K. Catalytic hydrodechlorination of monochloroacetic acid in wastewater using Ni-Fe bimetal prepared by ball milling. Environ. Sci. Pollut. Res. 2015, 22, 14299–14306. [Google Scholar] [CrossRef]
- Gheisari, K.; Javadpour, S.; Oh, J.T.; Ghaffari, M. The effect of milling speed on the structural properties of mechanically alloyed Fe–45% Ni powders. J. Alloys Compd. 2009, 472, 416–420. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Tee, Y.H.; Bachas, L.; Bhattacharyya, D. Degradation of trichloroethylene by iron-based bimetallic nanoparticles. J. Phys. Chem. C 2009, 113, 9454–9464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alami, A.H.; Hawili, A.A. Synthesis, characterization and applications of FeCu alloys. Appl. Surf. Sci. Adv. 2020, 1, 100027. [Google Scholar] [CrossRef]
- Eckert, J.; Holzer, J.C.; Krill, C.E., III; Johnson, W.L. Mechanically driven alloying and grain size changes in nanocrystalline Fe-Cu powders. J. Appl. Phys. 1993, 73, 2794–2802. [Google Scholar] [CrossRef] [Green Version]
- Eckert, J.; Holzer, J.C.; Johnson, W.L. Thermal stability and grain growth behavior of mechanically alloyed nanocrystalline Fe-Cu alloys. J. Appl. Phys. 1993, 73, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, J.; Yu, L.; Jia, G.; Jing, C.; Cao, S. Magnetic and frequency properties for nanocrystalline Fe–Ni alloys prepared by high-energy milling method. J. Magn. Magn. Mater. 2005, 285, 138–144. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Gong, L.; Fan, D.; Tratnyek, P.G.; Lowry, G.V. Quantifying the efficiency and selectivity of organohalide dechlorination by zerovalent iron. Environ. Sci. Process. Impacts 2020, 22, 528–542. [Google Scholar] [CrossRef]
- Thermo Environmental Instruments. TVA 1000 response factors. P/N 50039. 2000. Available online: https://tools.thermofisher.com/content/sfs/manuals/EPM-manual-TVA2020.pdf (accessed on 15 May 2022).
- Campbell, T.J.; Burris, D.R.; Roberts, A.L.; Wells, J.R. Trichloroethylene and tetrachloroethylene reduction in a metallic iron–water-vapor batch system. Environ. Toxicol. Chem. Int. J. 1997, 16, 625–630. [Google Scholar]

| Material | Treatment | SSA (m2/g) | Fe (%) | Cu (%) | Ni (%) |
|---|---|---|---|---|---|
| Cu | - | 0.5 | - | - | - |
| Ni | - | 0.5 | - | - | - |
| Fe | Milling | 0.3 | - | - | - |
| Fe-1%Cu | Milling | 0.7 | 98.4 | 1.6 | - |
| Fe-5%Cu | Milling | <LOQ | 95.3 | 4.7 | - |
| Fe-20%Cu | Milling | 0.5 | 81.9 | 18.1 | - |
| Fe-1%Ni | Milling | 0.3 | 99.0 | - | 1.0 |
| Fe-5%Ni | Milling | 0.3 | 94.8 | - | 5.2 |
| Fe-20%Ni | Milling | 0.2 | 85.3 | - | 14.7 |
| Fe-5%Cu* | - | - | 97.4 | 2.6 | - |
| Fe-5%Ni* | - | - | 89.1 | - | 10.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Settimi, C.; Zingaretti, D.; Sanna, S.; Verginelli, I.; Luisetto, I.; Tebano, A.; Baciocchi, R. Synthesis and Characterization of Zero-Valent Fe-Cu and Fe-Ni Bimetals for the Dehalogenation of Trichloroethylene Vapors. Sustainability 2022, 14, 7760. https://doi.org/10.3390/su14137760
Settimi C, Zingaretti D, Sanna S, Verginelli I, Luisetto I, Tebano A, Baciocchi R. Synthesis and Characterization of Zero-Valent Fe-Cu and Fe-Ni Bimetals for the Dehalogenation of Trichloroethylene Vapors. Sustainability. 2022; 14(13):7760. https://doi.org/10.3390/su14137760
Chicago/Turabian StyleSettimi, Clarissa, Daniela Zingaretti, Simone Sanna, Iason Verginelli, Igor Luisetto, Antonello Tebano, and Renato Baciocchi. 2022. "Synthesis and Characterization of Zero-Valent Fe-Cu and Fe-Ni Bimetals for the Dehalogenation of Trichloroethylene Vapors" Sustainability 14, no. 13: 7760. https://doi.org/10.3390/su14137760