Fertilization Regulates Grape Yield and Quality in by Altering Soil Nutrients and the Microbial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Sample Collection
2.4. Sample Analysis
2.5. Data Analyses
3. Results
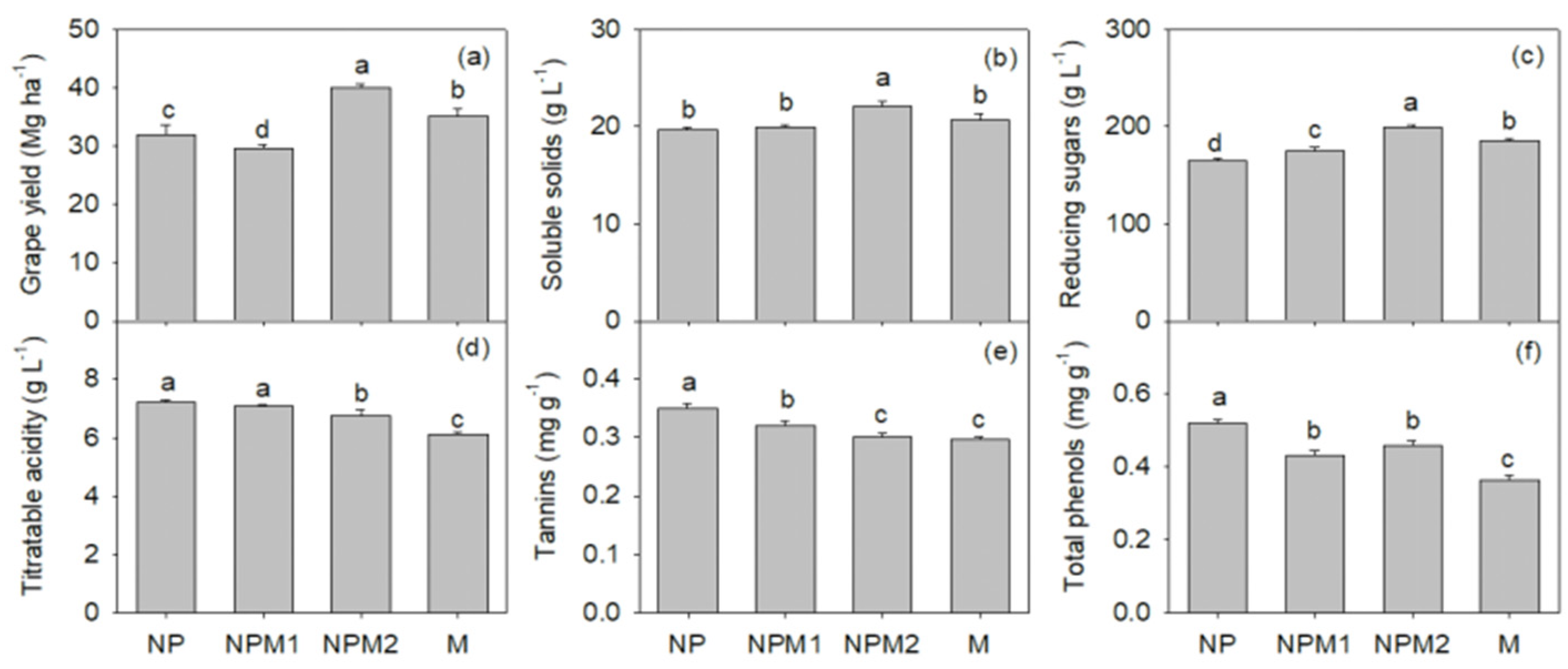
3.1. Grape Yield and Nutritional Ingredient Responses to Long-Term Fertilization
3.2. Changes in Soil Nutrients under Long-Term Fertilization
3.3. Changes in the Microbial Communities with Long-Term Fertilization
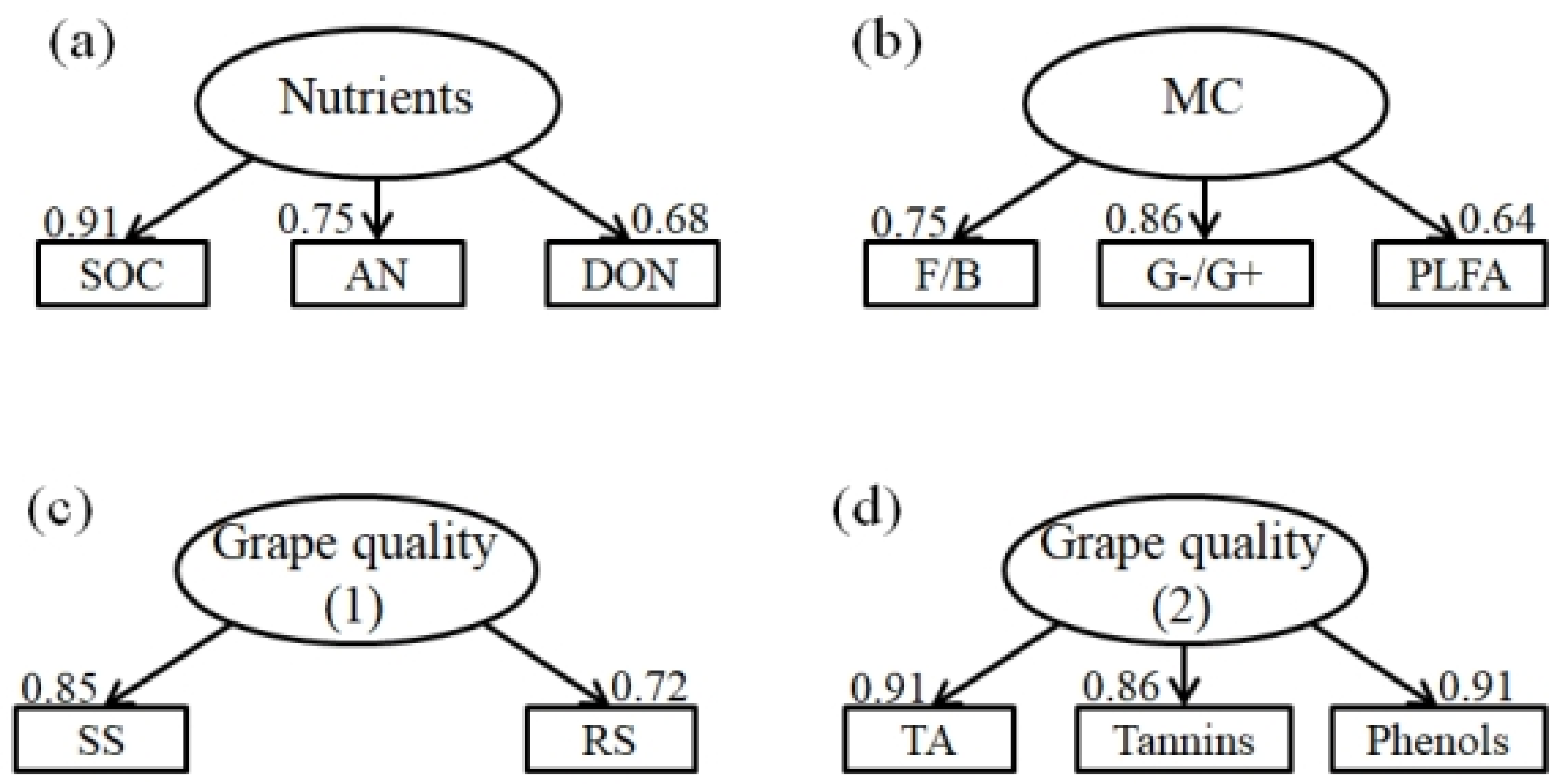
3.4. Mechanisms Driving Grape Yield and Quality
4. Discussion
4.1. Effect of Long-Term Fertilization on Grape Yield and Quality
4.2. Effects of Long-Term Fertilization on Soil Nutrients and Microbial Communities
4.3. Grape Yield and Quality Responses to Soil Nutrient, Microbial Community and Soil Fertility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Data. 2019. Available online: http://www.fao.org/faostat/en/#data/EP/visualize (accessed on 1 January 2022).
- Kubyshkin, A.V.; Avidzba, A.M.; Borisyuk, V.S.; Stoyanov, V.S.; Shramko, Y.I. Polyphenols of red grapes in wine and concentrates for use in rehabilitation technologies. Agrobiology 2017, 52, 622–630. [Google Scholar] [CrossRef]
- Copetti, C.; Franco, F.W.; Machado, E.d.R.; Soquetta, M.B.; Quatrin, A.; Ramos, V.d.M.; Moreira, J.C.F.; Emanuelli, T.; Sautter, C.K.; Penna, N.G. Acute consumption of bordo grape juice and wine improves serum antioxidant status in healthy individuals and inhibits reactive qxygen species production in human neuron-like cells. J. Nutr. Metab. 2018, 2018, 4384012. [Google Scholar] [CrossRef]
- Wang, R.; Sun, Q.; Chang, Q. Soil Types Effect on Grape and Wine Composition in Helan Mountain Area of Ningxia. PLoS ONE 2015, 10, e0116690. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L. Study on the Delineation of Ecological Protection Red Line in Ecological Fragile Region of the West. Meteorol. Environ. Res. 2018, 9, 19–23,29. [Google Scholar]
- Aldanondo-Ochoa, A.M.; Casasnovas-Oliva, V.L.; Arandia-Miura, A. Environmental efficiency and the impact of regulation in dryland organic vine production. Land Use Policy 2014, 36, 275–284. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Tillage Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Martínez, M.M.; Ortega, R.; Janssens, M.; Fincheira, P. Use of organic amendments in table grape: Effect on plant root system and soil quality indicators. J. Soil Sci. Plant Nutr. 2018, 18, 100–112. [Google Scholar] [CrossRef]
- Brunetto, G.; Ceretta, C.A.; de Melo, G.W.B.; Miotto, A.; Ferreira, P.A.A.; Couto, R.D.R.; da Silva, L.O.S.; Garlet, L.P.; Somavilla, L.M.; Cancian, A.; et al. Grape yield and must composition of ‘Cabernet Sauvignon’ grapevines with organic compost and urea fertilization. Rev. De Ciências Agroveterinárias 2018, 17, 212–218. [Google Scholar] [CrossRef]
- Gatti, M.; Squeri, C.; Garavani, A.; Frioni, T.; Dosso, P.; Diti, I.; Poni, S. Effects of Variable Rate Nitrogen Application on cv. Barbera Performance: Yield and Grape Composition. Am. J. Enol. Vitic. 2019, 70, 188–200. [Google Scholar] [CrossRef]
- Cai, A.; Zhang, W.; Xu, M.; Wang, B.; Wen, S.; Shah, S.A.A. Soil fertility and crop yield after manure addition to acidic soils in South China. Nutr. Cycl. Agroecosystems 2018, 111, 61–72. [Google Scholar] [CrossRef]
- Liang, G.; Cai, A.; Wu, H.; Wu, X.; Houssou, A.A.; Ren, C.; Wang, Z.; Gao, L.; Wang, B.; Li, S.; et al. Soil biochemical parameters in the rhizosphere contribute more to changes in soil respiration and its components than those in the bulk soil under nitrogen application in croplands. Plant Soil 2018, 435, 111–125. [Google Scholar] [CrossRef]
- Yang, J.; Gao, W.; Ren, S. Long-term effects of combined application of chemical nitrogen with organic materials on crop yields, soil organic carbon and total nitrogen in fluvo-aquic soil. Soil Tillage Res. 2015, 151, 67–74. [Google Scholar] [CrossRef]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Montiel-Rozas, M.M.; Dominguez, M.T.; Madejón, E.; Madejón, P.; Pastorelli, R.; Renella, G. Long-term effects of organic amendments on bacterial and fungal communities in a degraded Mediterranean soil. Geoderma 2018, 332, 20–28. [Google Scholar] [CrossRef]
- Yang, L.; Tan, L.; Zhang, F.; Gale, W.J.; Cheng, Z.; Sang, W. Duration of continuous cropping with straw return affects the composition and structure of soil bacterial communities in cotton fields. Can. J. Microbiol. 2018, 64, 167–181. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, S.; Jiang, H.; Hu, Y.; Lu, X. Influences of litter diversity and soil moisture on soil microbial communities in decomposing mixed litter of alpine steppe species. Geoderma 2020, 377, 114577. [Google Scholar] [CrossRef]
- Yao, R.J.; Yang, J.S.; Zhu, W. Impact of crop cultivation, nitrogen and fulvic acid on soil fungal community structure in salt-affected alluvial fluvo-aquic soil. Plant Soil 2021, 464, 539–558. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Palmer, A.S.; Winsley, T.; Lamb, E.; Bissett, A.; Brown, M.V.; van Dorst, J.; Ji, M.; Ferrari, B.C.; Grogan, P.; et al. Soil fertility is associated with fungal and bacterial richness, whereas pH is associated with community composition in polar soil microbial communities. Soil Biol. Biochem. 2014, 78, 10–20. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Report 2014; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Volume 106, pp. 12–21. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. Methods Soil Anal. Part 3—Chem. Methods 1996, 9, 961–1010. [Google Scholar]
- Gallaher, R.N.; Weldon, C.O.; Boswell, F.C. A Semiautomated Procedure for Total Nitrogen in Plant and Soil Samples. Soil Sci. Soc. Am. J. 1976, 40, 887–889. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods of Soil Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2000. (in Chinese) [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: New York, NY, USA, 2003. [Google Scholar]
- Page, A.L.; Miller, R.H.; Dennis, R.K. Methods of Soil Analysis: Part 2. Chemical and Microbiological Properties, 2nd ed.; ASA, SSSA Publishing: Madison, WI, USA, 1982; Volume 9, p. 1159. [Google Scholar]
- Jones, D.; Willett, V. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Frostegard, A.; Baath, E. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fert. Soils 1996, 22, 59–65. [Google Scholar] [CrossRef]
- Olsson, P.A.; Bååth, E.; Jakobsen, I.; Söderström, B. The use of phospholipid and neutral lipid fatty acids to estimate biomass of arbuscular mycorrhizal fungi in soil. Mycol. Res. 1995, 99, 623–629. [Google Scholar] [CrossRef]
- Zelles, L. Phospholipid fatty acid profiles in selected members of soil microbial communities. Chemosphere 1997, 35, 275–294. [Google Scholar] [CrossRef]
- Thomidis, T.; Zioziou, E.; Koundouras, S.; Karagiannidis, C.; Navrozidis, I.; Nikolaou, N. Effects of nitrogen and irrigation on the quality of grapes and the susceptibility to Botrytis bunch rot. Sci. Hortic. 2016, 212, 60–68. [Google Scholar] [CrossRef]
- Ozdemir, G.; Kitir, N.; Turan, M.; Ozlu, E. Impacts of organic and organo-mineral fertilizers on total phenolic, flavonoid, anthocyanin and antiradical activity of okuzgozu (Vitis vinifera L.) grapes. Acta Sci. Pol. Hortorum Cultus 2018, 17, 91–100. [Google Scholar] [CrossRef]
- Struijk, M.; Whitmore, A.P.; Mortimer, S.R.; Sizmur, T. Obtaining more benefits from crop residues as soil amendments by application as chemically heterogeneous mixtures. Soil 2020, 6, 467–481. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Kang, B.G.; Kim, W.T.; Yun, H.S.; Chang, S.C. Use of plant growth-promoting rhizobacteria to control stress responses of plant roots. Plant Biotechnol. Rep. 2010, 4, 179–183. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Zheng, M.; Jiang, L.; Luo, Y. Patterns and mechanisms of responses by soil microbial communities to nitrogen addition. Soil Biol. Biochem. 2017, 115, 433–441. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Penuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Jiang, G.; Xu, M.; He, X.; Zhang, W.; Huang, S.; Yang, X.; Liu, H.; Peng, C.; Shirato, Y.; Iizumi, T.; et al. Soil organic carbon sequestration in upland soils of northern China under variable fertilizer management and climate change scenarios. Glob. Biogeochem. Cycles 2014, 28, 319–333. [Google Scholar] [CrossRef]
- Peng, S.; He, Y.; Yang, S.; Xu, J. Effect of controlled irrigation and drainage on nitrogen leaching losses from paddy fields. Paddy Water Environ. 2014, 13, 303–312. [Google Scholar] [CrossRef]
- Yong-Zhong, S.; Xue-Fen, W.; Rong, Y.; Xiao, Y.; Wen-Jie, L. Soil Fertility, Salinity and Nematode Diversity Influenced by Tamarix ramosissima in Different Habitats in an Arid Desert Oasis. Environ. Manag. 2012, 50, 226–236. [Google Scholar] [CrossRef]
- Widdig, M.; Heintz-Buschart, A.; Schleuss, P.-M.; Guhr, A.; Borer, E.T.; Seabloom, E.W.; Spohn, M. Effects of nitrogen and phosphorus addition on microbial community composition and element cycling in a grassland soil. Soil Biol. Biochem. 2020, 151, 108041. [Google Scholar] [CrossRef]
- Shen, F.; Wu, J.; Fan, H.; Liu, W.; Guo, X.; Duan, H.; Hu, L.; Lei, X.; Wei, X. Soil N/P and C/P ratio regulate the responses of soil microbial community composition and enzyme activities in a long-term nitrogen loaded Chinese fir forest. Plant Soil 2018, 436, 91–107. [Google Scholar] [CrossRef]
- Spohn, M.; Klaus, K.; Wanek, W.; Richter, A. Microbial carbon use efficiency and biomass turnover times depending on soil depth—Implications for carbon cycling. Soil Biol. Biochem. 2016, 96, 74–81. [Google Scholar] [CrossRef]
- Gautam, A.; Sekaran, U.; Guzman, J.; Kovács, P.; Hernandez, J.L.G.; Kumar, S. Responses of soil microbial community structure and enzymatic activities to long-term application of mineral fertilizer and beef manure. Environ. Sustain. Indic. 2020, 8, 100073. [Google Scholar] [CrossRef]




| Treatment | Reclaimed Year | Synthetic Fertilizer (kg ha−1 Year−1) | Sheep Manure (kg ha−1 Year−1) | Total Nutrients (kg ha−1 Year−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Sy-N | Sy-P | Manure | Or-N | Or-P | N | P | ||
| CK0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NP | 25 | 540 | 300 | 0 | 0 | 0 | 540 | 300 |
| NPM1 | 20 | 135 | 75 | 12,000 | 240 | 60 | 375 | 135 |
| M | 16 | 0 | 0 | 36,000 | 624 | 180 | 624 | 180 |
| NPM2 | 15 | 540 | 300 | 15,000 | 302 | 72 | 842 | 372 |
| Soil Depth (cm) | Treatment | SOC | TN | AN | AP | AK | DOC | DON |
|---|---|---|---|---|---|---|---|---|
| 0–20 | CK0 | 4.34 | 0.36 | 61 | 15 | 448 | 207 | 36 |
| NP | 9.50 b | 20.05 b | 6.06 a | 12.11 a | −1.89 a | 12.31 c | 6.75 b | |
| NPM1 | 4.36 c | 14.21 c | 1.03 b | 10.66 a | −3.24 a | −0.82 d | −1.73 c | |
| NPM2 | 11.21 b | 31.01 a | 4.64 a | 11.14 a | −3.59 a | 16.02 b | 11.40 a | |
| M | 18.05 a | 31.48 a | 3.91 a | 7.06 a | −3.33 a | 19.06 a | 12.03 a | |
| 20–40 | CK0 | 4.11 | 0.36 | 59 | 23 | 366 | 217 | 35 |
| NP | 3.64 b | 5.25 b | 2.64 a | 4.85 a | −1.15 a | −0.34 c | −0.14 c | |
| NPM1 | 0.68 c | 0.73 c | 0.58 b | 4.86 a | −2.50 b | −2.12 d | −1.94 d | |
| NPM2 | 5.39 a | 12.04 a | 3.06 a | 2.25 b | −3.79 c | 2.86 b | 2.29 b | |
| M | 8.60 a | 11.01 a | 3.55 a | 0.45 c | −3.03 bc | 6.97 a | 4.29 a |
| Soil Depth (cm) | Treatment | MBC (mg kg−1) | MBN (mg kg−1) | F (nmol g−1) | B (nmol g−1) | F/B | AMF (nmol g−1) | ACT (nmol g−1) | G+ (nmol g−1) | G− (nmol g−1) | G−/G+ | PLFAs (nmol g−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–20 | CK0 | 297 | 38 | 13 | 87 | 0.15 | 5.91 | 25 | 35 | 33 | 0.93 | 160 |
| NP | 3.42 b | 3.13 b | 0.90 a | 0.44 a | 0.44 b | 1.04 a | 0.07 b | 0.06 b | 0.51 a | 0.46 a | 0.86 bc | |
| NPM1 | 0.31 c | 0.27 c | 0.85 a | 0.57 a | 0.25 c | 0.56 bc | 0.16 a | 0.58 a | 0.75 a | 0.07 c | 1.05 b | |
| NPM2 | 9.70 a | 9.10 a | 1.05 a | 0.30 a | 0.71 a | 0.81 b | 0.04 b | 0.14 b | 0.68 a | 0.53 a | 1.34 a | |
| M | 8.97 a | 8.45 a | 0.38 b | −0.25 b | 0.64 a | 0.32 c | −0.83 d | −0.33 c | −0.15 b | 0.18 b | 0.49 c | |
| 20–40 | CK0 | 256 | 34 | 10 | 60 | 0.18 | 5.16 | 20 | 27 | 26 | 0.97 | 137 |
| NP | 0.29 b | 0.17 b | 1.23 ab | 2.07 a | −0.47 b | 1.22 a | 0.68 a | 1.01 a | 1.09 ab | 0.05 b | 1.12 a | |
| NPM1 | −2.46 b | −2.63 b | 2.01 a | 2.08 a | −0.20 a | 1.36 a | 0.63 a | 1.33 a | 1.73 a | 0.20 a | 1.50 a | |
| NPM2 | 0.20 b | −0.01 b | 0.78 b | 2.13 a | −0.37 ab | 0.03 b | −0.17 ab | 0.82 a | 0.62 b | −0.15 c | 1.17 a | |
| M | 6.19 a | 5.41 a | −0.31 c | 0.54 b | −0.29 a | −0.86 c | −0.97 b | −0.43 b | −0.62 c | −0.04 b | −0.08 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Q.; Xie, X.; Xu, Y. Fertilization Regulates Grape Yield and Quality in by Altering Soil Nutrients and the Microbial Community. Sustainability 2022, 14, 10857. https://doi.org/10.3390/su141710857
Zhu Q, Xie X, Xu Y. Fertilization Regulates Grape Yield and Quality in by Altering Soil Nutrients and the Microbial Community. Sustainability. 2022; 14(17):10857. https://doi.org/10.3390/su141710857
Chicago/Turabian StyleZhu, Qianqian, Xiangwen Xie, and Yongmei Xu. 2022. "Fertilization Regulates Grape Yield and Quality in by Altering Soil Nutrients and the Microbial Community" Sustainability 14, no. 17: 10857. https://doi.org/10.3390/su141710857
APA StyleZhu, Q., Xie, X., & Xu, Y. (2022). Fertilization Regulates Grape Yield and Quality in by Altering Soil Nutrients and the Microbial Community. Sustainability, 14(17), 10857. https://doi.org/10.3390/su141710857





