MgO Nano-Catalyzed Biodiesel Production from Waste Coconut Oil and Fish Oil Using Response Surface Methodology and Grasshopper Optimization
Abstract
:1. Introduction
2. Materials and Methods
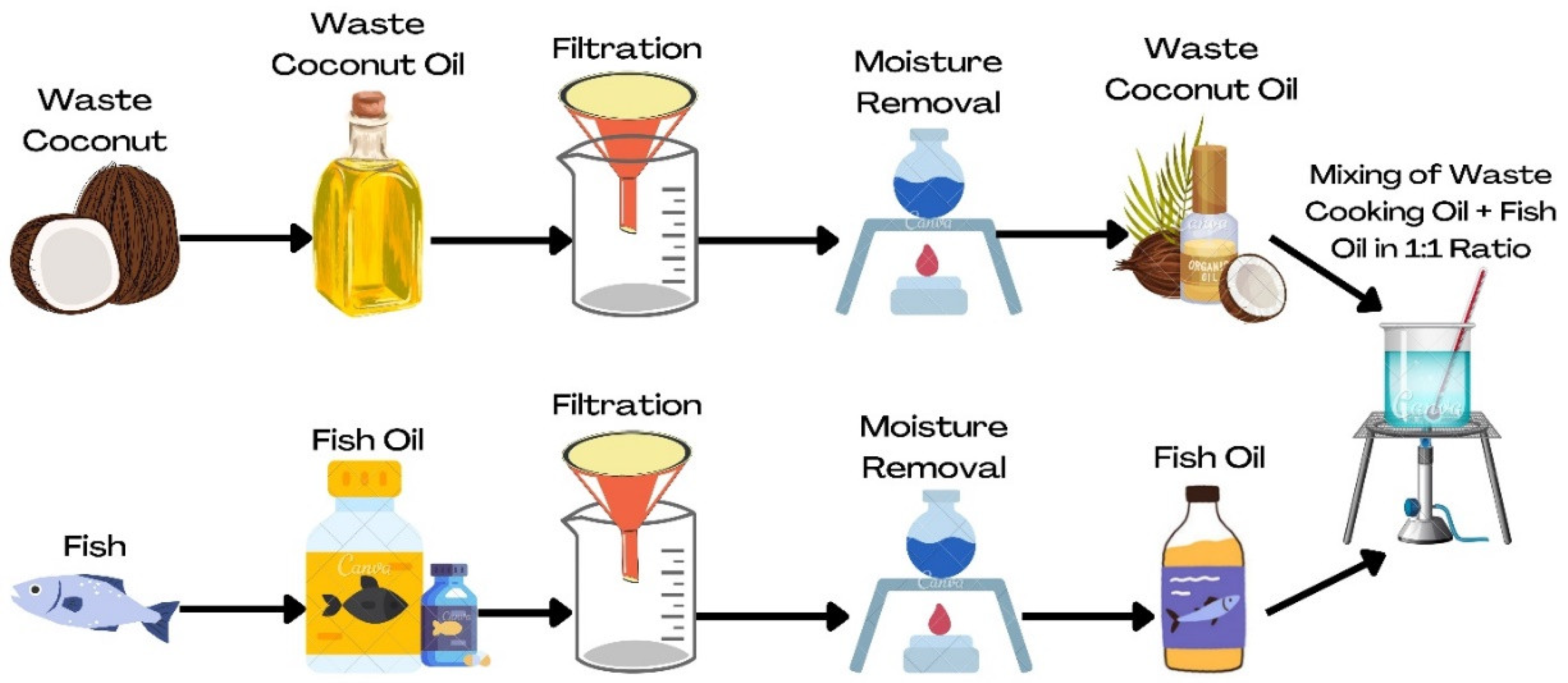
2.1. Sources of Waste Coconut Oil and Waste Fish Oil
2.2. Biodiesel Production Viz. Transesterification Process
Acid Catalyzed Esterification
2.3. Modeling and Analysis of Transesterification Process
2.4. Optimization of Transesterification Process
Grasshopper Optimization Algorithm
- Grasshopper searched for multidimensional space with a large-step size in the early phases that could ensure identifying global solutions in an unseen area;
- In the final phase, the grasshopper searched for solutions locally that could enhance the exploitation capabilities;
- During the optimal search, the comfort zone factor balances the exploration and exploitation capabilities that assist grasshoppers in preventing premature convergence and locating global solutions [69];
- Grasshoppers enhance the solutions from the initially generated random solutions over the progressive search as iteration progresses that could help to attain the global best fitness solutions.
3. Results & Discussions
3.1. Data Collection
3.2. Main Effect Factor Analysis
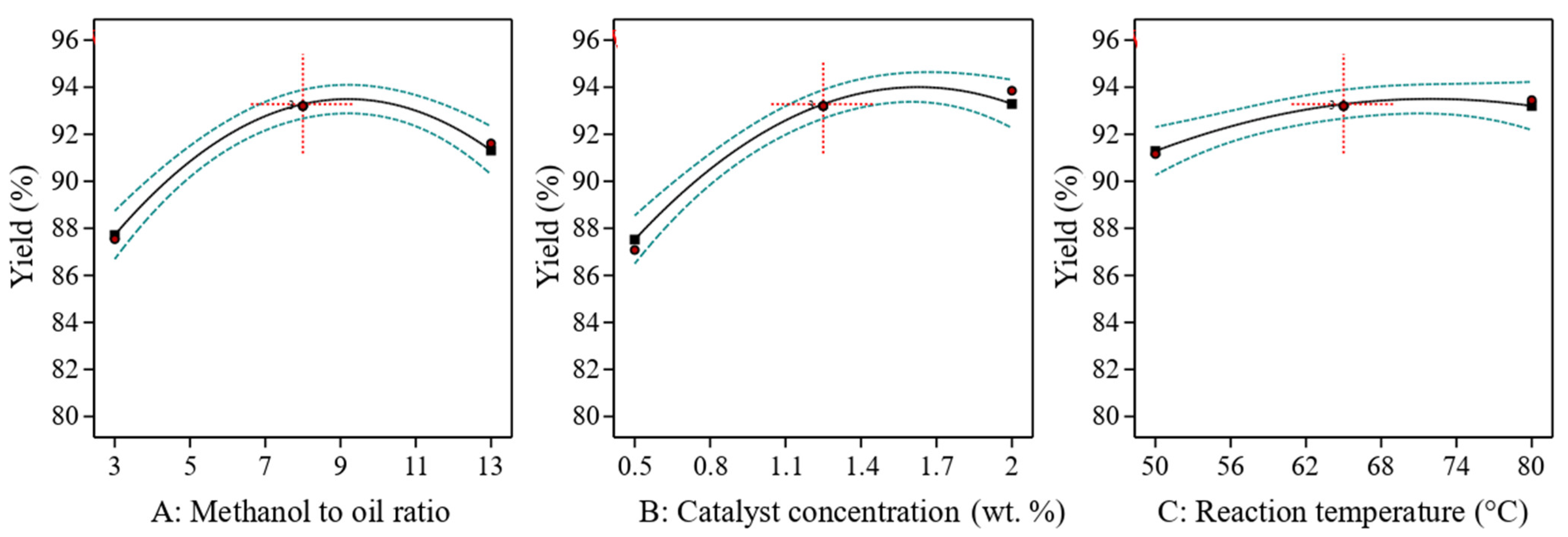
3.2.1. Effect of Methanol-To-Oil Molar Ratio
3.2.2. Effect of Catalyst Concentration
3.2.3. Effect of Reaction Temperature
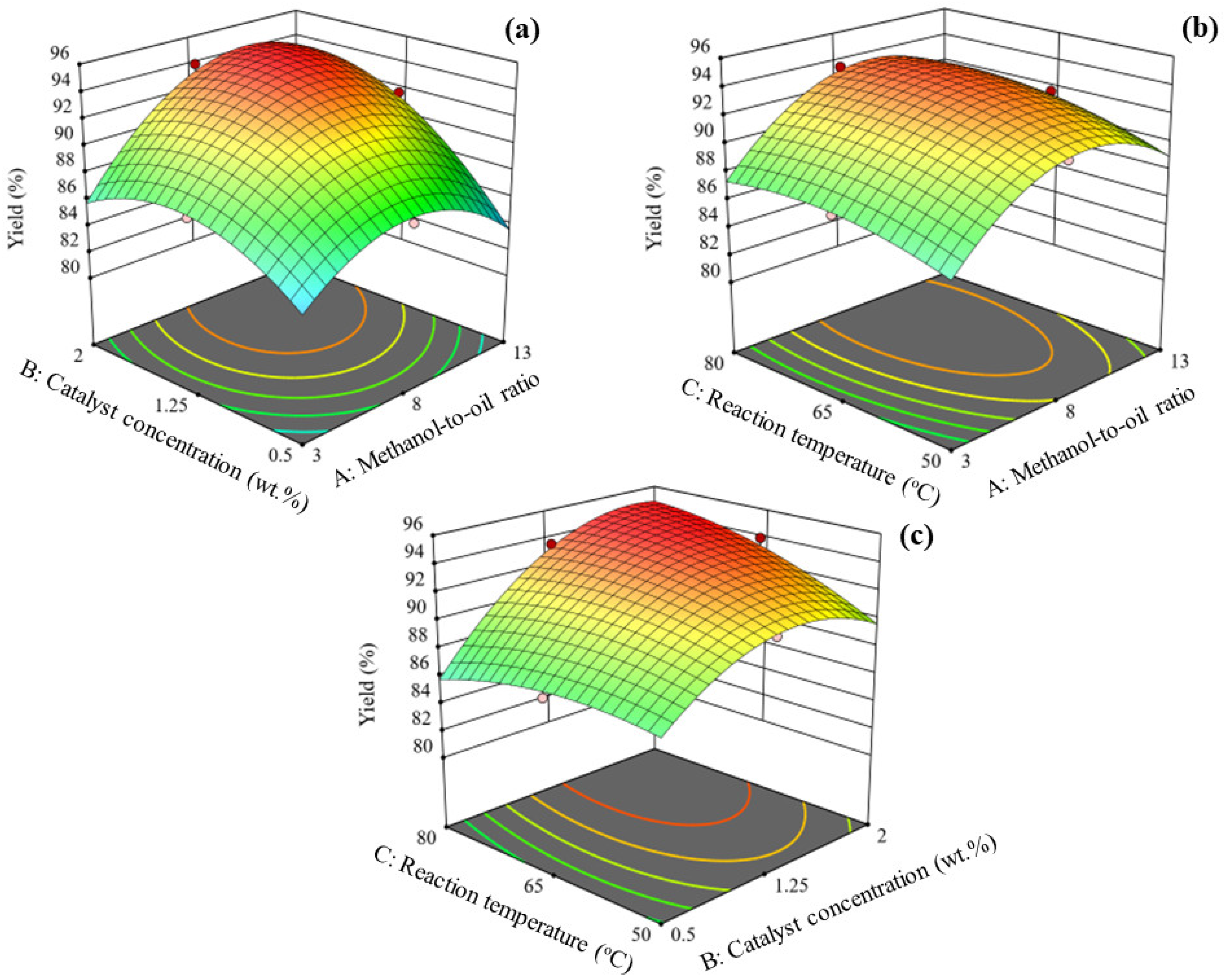
3.3. Interaction Factor Analysis: Surface Plot
- The biodiesel yield tends to improve with an increase in catalyst concentration and methanol-to-oil ratio up to the middle levels (refer to Figure 5a). The reaction temperature was kept fixed to 65 °C (the middle value of the operating range of RT) during the analysis. Catalyst concentration showed higher raise in biodiesel yield compared to the methanol-to-oil molar ratio. Increased concentration of MgO nanocatalyst increases the contact and availability of basic sites between catalyst and reactants, causing higher yield [77]. As the methanol-to-oil molar ratio was increased, the viscosity of the reaction mixture due to agitation involved in the transesterification process decreased (equilibrium shift towards the conversion side), resulting in increased biodiesel yield [24]. The combination of the highest catalyst concentration and methanol-to-oil ratio showed reduced biodiesel yield. The highest catalyst concentration with methanol to oil molar ratio could result in soap formation due to incomplete reaction. It is often difficult to separate the glycerol and biodiesel, resulting in reduced biodiesel yield [78].
- Figure 5b explains the effect of reaction temperature with methanol-to-oil molar ratio when the catalyst concentration of MgO is fixed to 1.25 wt.%. An increase in methanol-to-oil molar ratio up to ~10 increases the biodiesel yield. Beyond the critical value (i.e., after ~10), the conversion of biodiesel yield is reduced. The reduced biodiesel yield is beyond the required amount of methanol which tends to accumulate on the catalyst surface, reducing the reaction (due to the reduction of active sites) mixture and process efficacy in the conversion of higher yield [79]. Beyond the critical reaction temperature, the methanol transforming from the liquid phase to gas results in poor interaction between methanol oil and gases, decreasing biodiesel yield [80]. The resulted surface plot clearly shows that reaction temperature is less affected than the methanol-to-oil molar ratio towards converting oil to biodiesel yield.
- Figure 5c explains the interaction factor effects of reaction temperature and catalyst concentration tested for a fixed methanol-to-oil molar ratio equal to 8. The effect is seen to have a similar trend with more pronounced than the factors interaction of methanol-to-oil molar ratio and reaction temperature. The catalyst concentration up to the middle value of 1.25 wt.% increases the biodiesel yield and decreases later. Higher catalyst particles tend to accumulate and form a bulk mass, reducing the catalyst’s active surface area and increasing the mixture’s viscosity [81]. The effect of the reaction temperature is negligibly small compared to catalyst concentration. Increased values of reaction temperature decrease the biodiesel yield probably due to increased miscibility caused by a collision between the species; methanol vaporization ensures decreased availability of methanol in the reaction mixture. Similar observations are reported in the published literature [82].
3.4. Mathematical Regression Equation
3.5. Summary Results of GOA and RSM-DFA
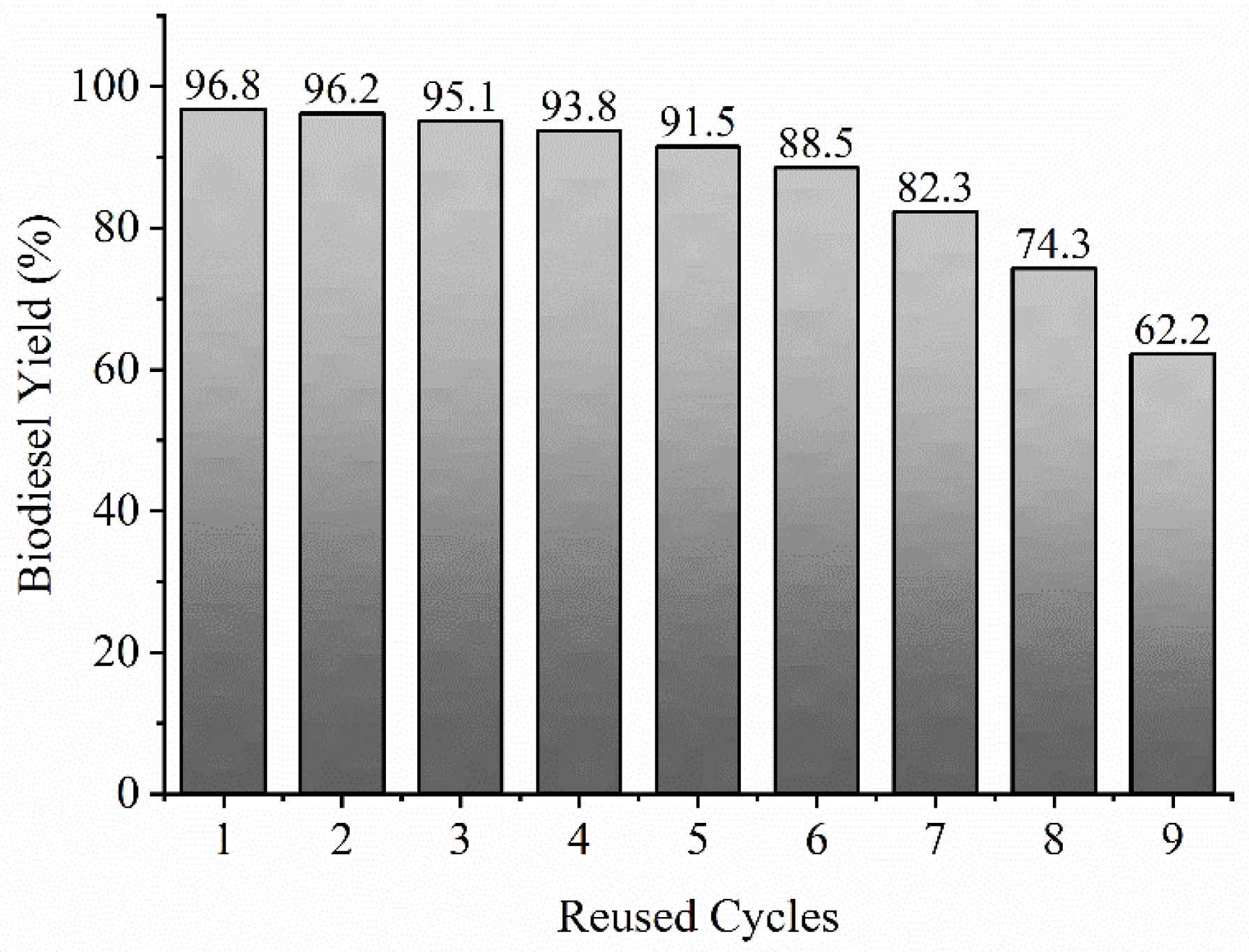
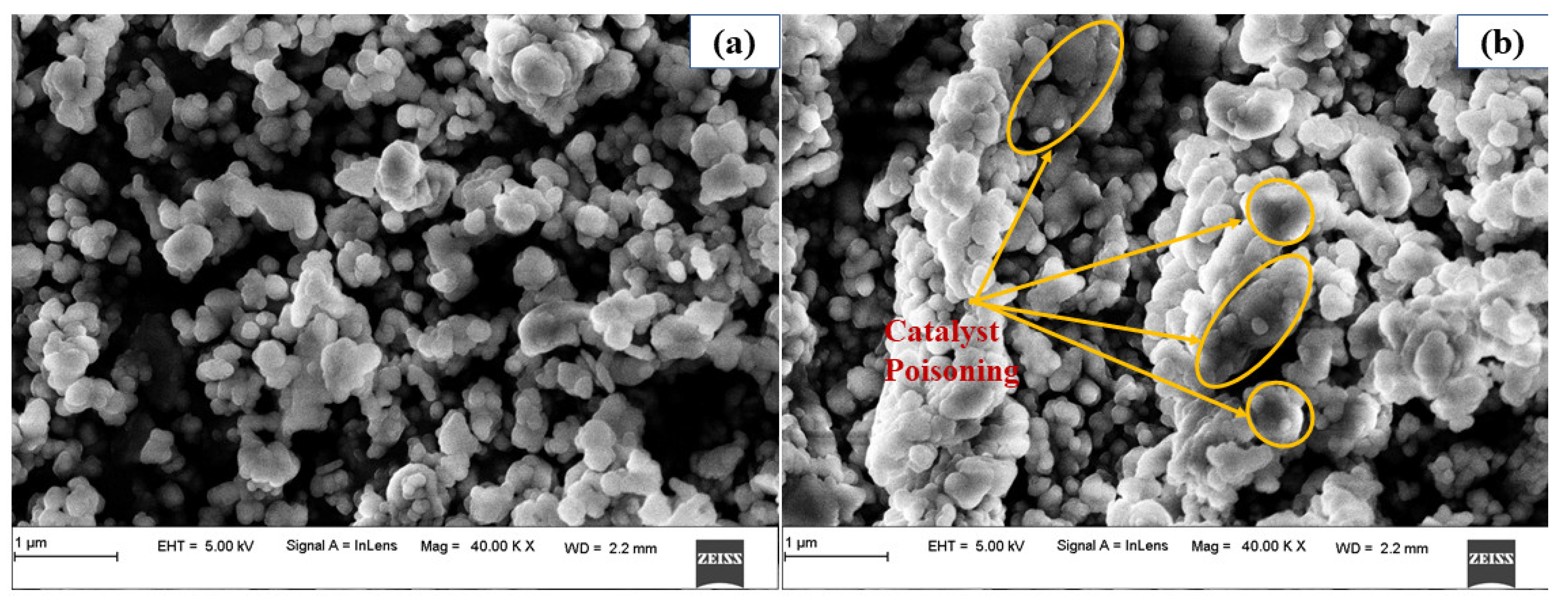
3.6. Reusability of MgO Nanocatalyst
3.7. Physicochemical Properties Evaluation: Biodiesel Yield, Hybrid Oil, WCO, WFO
4. Conclusions
- Using waste fish oil and coconut oil reduces feedstock cost (feedstock alone constitutes ~80% of total biodiesel conversion cost) and biodiesel conversion cost. CCD limits the large-scale experiments (reduces the material, time, equipment, energy, labor, and so on) to detail the process insights. GOA conducts optimal search (namely, regression equations derived with experimental data) to obtain global values (biodiesel yield) at reduced computation time of 30 s, without needing practical experiments;
- CCD-based experimental matrix was planned to study transesterification variables (M:O, MgO nanocatalyst, and RT) on biodiesel yield. All three linear (M:O, MgO nanocatalyst, and RT) parameters are found to make a significant contribution to biodiesel conversion. MgO CC contributions are more, followed by M:O and RT. The square terms of all three transesterification variables were significant, clearly defining non-linear relation with the biodiesel yield. All interaction terms (excluding M:O × RT) were found significant;
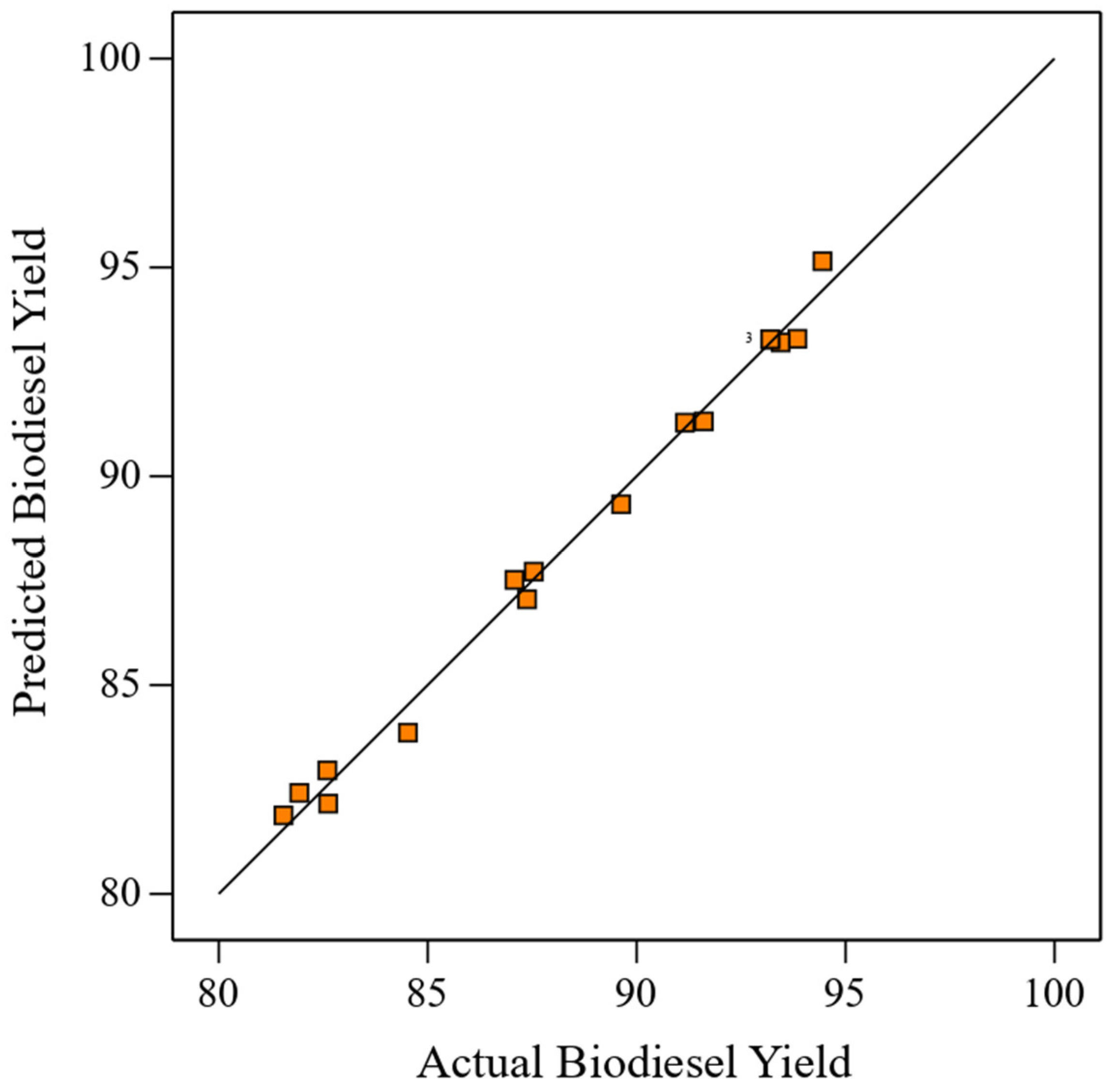
- The full quadratic model (linear + interaction + square terms) produces the best fit with an R2 value of 0.9869, resulting in prediction accuracy of 0.3829. The better prediction ensures model-derived empirical regression equations are statistically adequate and best suited for searching for optimal transesterification conditions that maximize biodiesel yield;
- GOA tuned parameters (Cmax: 1; Cmin: 0.00003; population size: 40; and maximum iteration: 100) resulted in maximum biodiesel conversion of 96.8% subjected to an optimal transesterification (M:O: 10.65, MgO CC: 2 wt.%, and RT: 80 °C) conditions;
- The MgO catalyst was recycled nine times to examine the stability and reusability under the optimal transesterification reaction conditions. The MgO nanocatalyst reused up to five cycles showed a negligible reduction (stable) in biodiesel yield from 96.8% to 91.5%, later reduced to 62.2% after nine cycles. Loss of crystallinity, agglomeration, and catalytic poisoning resulted in catalyst deactivation, and thereby reducing biodiesel conversion. However, stability and reusability of catalyst up to five cycles without catalyst deactivation ensures reduction of the catalyst cost in turn biodiesel production cost;
- The physicochemical properties of biodiesel fuel (conversion from hybrid oil: waste fish oil + waste coconut oil) are within the acceptable limit of biodiesel standard and are suitable to use in diesel engines.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Details of Specification of MgO Nano Powder Used as a Catalyst
| Form | Powder |
| Color | White |
| Odor | No Odor |
| Purity | 99.9% |
| APS | <100 nm |
| Molecular Formula | MgO |
| Molecular Weight | 40.304 g/mol |
| Density | 3.58 g/cm3 |
| Melting Point | 2852 °C |
| Boiling Point | 3600 °C |
| Stability | Completely Stable |
| Solubility | Insoluble in Water and Ethanol |
References
- IEA. Net Zero by 2050: A Roadmap for the Global Energy Sector; OECD Publishing: Paris, France, 2021. [Google Scholar] [CrossRef]
- Holechek, J.L.; Geli, H.M.; Sawalhah, M.N.; Valdez, R. A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050? Sustainability 2022, 14, 4792. [Google Scholar] [CrossRef]
- Shen, M.; Huang, W.; Chen, M.; Song, B.; Zeng, G.; Zhang, Y. (Micro) plastic crisis: Un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020, 254, 120138. [Google Scholar] [CrossRef]
- Khalili, S.; Rantanen, E.; Bogdanov, D.; Breyer, C. Global transportation demand development with impacts on the energy demand and greenhouse gas emissions in a climate-constrained world. Energies 2019, 12, 3870. [Google Scholar] [CrossRef]
- Haines, A.; Kovats, R.S.; Campbell-Lendrum, D.; Corvalán, C. Climate change and human health: Impacts, vulnerability and public health. Public Health 2006, 120, 585–596. [Google Scholar] [CrossRef]
- Fadda, J. Climate change: An overview of potential health impacts associated with climate change environmental driving forces. In Ali Singh Edited Book: Renewable Energy and Sustainable Buildings; Springer: Cham, Switzerland, 2020; pp. 77–119. [Google Scholar] [CrossRef]
- Bureika, G.; Matijošius, J.; Rimkus, A. Alternative Carbonless Fuels for Internal Combustion Engines of Vehicles. In A Sładkowski Edited Book: Ecology in Transport.: Problems and Solutions; Springer: Cham, Switzerland, 2020; pp. 1–49. [Google Scholar] [CrossRef]
- Ni, P.; Wang, X.; Li, H. A review on regulations, current status, effects and reduction strategies of emissions for marine diesel engines. Fuel 2020, 279, 118477. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Kumari, D. Chemical compositions, properties, and standards for different generation biodiesels: A review. Fuel 2019, 253, 60–71. [Google Scholar] [CrossRef]
- Salaheldeen, M.; Mariod, A.A.; Aroua, M.K.; Rahman, S.M.; Soudagar, M.E.M.; Fattah, I.M. Current state and perspectives on transesterification of triglycerides for biodiesel production. Catalysts 2021, 11, 1121. [Google Scholar] [CrossRef]
- Ma, X.; Liu, F.; Helian, Y.; Li, C.; Wu, Z.; Li, H.; Chu, H.; Wang, Y.; Lu, W.; Guo, M.; et al. Current application of MOFs based heterogeneous catalysts in catalyzing transesterification/esterification for biodiesel production: A review. Energy Convers. Manag. 2021, 229, 113760. [Google Scholar] [CrossRef]
- Maheswari, P.; Haider, M.B.; Yusuf, M.; Klemeš, J.J.; Bokhari, A.; Beg, M.; Al-Othman, A.; Kumar, R.; Jaiswal, A.K. A review on latest trends in cleaner biodiesel production: Role of feedstock, production methods, and catalysts. J. Clean. Prod. 2022, 355, 131588. [Google Scholar] [CrossRef]
- Yusoff, M.H.M.; Ayoub, M.; Hamza Nazir, M.; Zahid, I.; Ameen, M.; Abbas, W.; Shoparwe, N.F.; Abbas, N. Comprehensive review on biodiesel production from palm oil mill effluent. ChemBioEng Rev. 2021, 8, 439–462. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yilmaz, B.A.; Sargin, I. Effect of different animal fat and plant oil additives on physicochemical, mechanical, antimicrobial and antioxidant properties of chitosan films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef]
- Yahaya, W.M.A.W.; Dandan, M.A.; Samion, S.; Musa, M.N. A comprehensive review on palm oil and the challenges using vegetable oil as lubricant base-stock. J. Adv. Res. Fluid Mech. Therm. Sci. 2018, 52, 182–197. Available online: https://www.akademiabaru.com/submit/index.php/arfmts/article/view/2389l (accessed on 3 February 2022).
- Singh, D.; Sharma, D.; Soni, S.L.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel; Springer: London, UK, 2008; pp. 111–119. [Google Scholar] [CrossRef]
- Hong, I.K.; Park, J.W.; Lee, S.B. Optimization of fish-oil-based biodiesel synthesis. J. Ind. Eng. Chem. 2013, 19, 764–768. [Google Scholar] [CrossRef]
- Ravanipour, M.; Hamidi, A.; Mahvi, A.H. Microalgae biodiesel: A systematic review in Iran. Renew. Sustain. Energy Rev. 2021, 150, 111426. [Google Scholar] [CrossRef]
- Lin, C.Y.; Li, R.J. Fuel properties of biodiesel produced from the crude fish oil from the soapstock of marine fish. Fuel Process. Technol. 2009, 90, 130–136. [Google Scholar] [CrossRef]
- Behçet, R. Performance and emission study of waste anchovy fish biodiesel in a diesel engine. Fuel Process. Technol. 2011, 92, 1187–1194. [Google Scholar] [CrossRef]
- Prakash, S.; Prabhahar, M.; Sendilvelan, S.; Venkatesh, R.; Singh, S.; Bhaskar, K. Experimental studies on the performance and emission characteristics of an automobile engine fueled with fish oil methyl ester to reduce environmental pollution. Energy Procedia 2019, 160, 412–419. [Google Scholar] [CrossRef]
- Sharma, P.; Usman, M.; Salama, E.S.; Redina, M.; Thakur, N.; Li, X. Evaluation of various waste cooking oils for biodiesel production: A comprehensive analysis of feedstock. Waste Manag. 2021, 136, 219–229. [Google Scholar] [CrossRef]
- Borah, M.J.; Das, A.; Das, V.; Bhuyan, N.; Deka, D. Transesterification of waste cooking oil for biodiesel production catalyzed by Zn substituted waste egg shell derived CaO nanocatalyst. Fuel 2019, 242, 345–354. [Google Scholar] [CrossRef]
- Bolivar-Telleria, M.; Turbay, C.; Favarato, L.; Carneiro, T.; de Biasi, R.S.; Fernandes, A.A.R.; Santos, A.M.C.; Fernandes, P. Second-generation bioethanol from coconut husk. BioMed Res. Int. 2018, 2018, 4916497. [Google Scholar] [CrossRef]
- Sangkharak, K.; Chookhun, K.; Numreung, J.; Prasertsan, P. Utilization of coconut meal, a waste product of milk processing, as a novel substrate for biodiesel and bioethanol production. Biomass Convers. Biorefin. 2020, 10, 651–662. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.A.; Rahman, S.A.; Mofijur, M.; Mobarak, H.M.; Ashraful, A.M. Potential of biodiesel as a renewable energy source in Bangladesh. Renew. Sustain. Energy Rev. 2015, 50, 819–834. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Zulkurnain, N.; Rosid, S.J.M.; Azid, A.; Endut, A.; Toemen, S.; Ismail, S.; Abdullah, W.N.W.; Aziz, S.M.; Yusoff, N.M.; et al. Catalytic Transesterification of Coconut Oil in Biodiesel Production: A Review. Catal. Surv. Asia. 2022, 26, 129–143. [Google Scholar] [CrossRef]
- Kashyap, S.S.; Gogate, P.R.; Joshi, S.M. Ultrasound assisted synthesis of biodiesel from karanja oil by interesterification: Intensification studies and optimization using RSM. Ultrason. Sonochem. 2019, 50, 36–45. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Pham, M.; Deng, Y.; Nguyen, T.; Duy, V.; Le, D.; Zuo, W.; Peng, Q.; Zhang, Z. Effects of injection timing and injection pressure on performance and exhaust emissions of a common rail diesel engine fueled by various concentrations of fish-oil biodiesel blends. Energy 2018, 149, 979–989. [Google Scholar] [CrossRef]
- Yesilyurt, M.K. The effects of the fuel injection pressure on the performance and emission characteristics of a diesel engine fuelled with waste cooking oil biodiesel-diesel blends. Renew. Energy 2019, 132, 649–666. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.P.; Dwivedi, G. Impact of alcohol on biodiesel production and properties. Renew. Sustain. Energy Rev. 2016, 56, 319–333. [Google Scholar] [CrossRef]
- Zareh, P.; Zare, A.A.; Ghobadian, B. Comparative assessment of performance and emission characteristics of castor, coconut and waste cooking based biodiesel as fuel in a diesel engine. Energy 2017, 139, 883–894. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Hur, J.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Samuel, O.D.; Okwu, M.O. Comparison of Response Surface Methodology (RSM) and Artificial Neural Network (ANN) in modelling of waste coconut oil ethyl esters production. Energy Source Part A 2019, 41, 1049–1061. [Google Scholar] [CrossRef]
- Ong, M.Y.; Nomanbhay, S.; Kusumo, F.; Raja Shahruzzaman, R.M.H.; Shamsuddin, A.H. Modeling and optimization of microwave-based bio-jet fuel from coconut oil: Investigation of Response Surface Methodology (RSM) and Artificial Neural Network Methodology (ANN). Energies 2021, 14, 295. [Google Scholar] [CrossRef]
- Marso, T.M.M.; Kalpage, C.S.; Udugala-Ganehenege, M.Y. ZnO/CuO composite catalyst to pre-esterify waste coconut oil for producing biodiesel in high yield. React. Kinet. Mech. Catal. 2021, 132, 935–966. [Google Scholar] [CrossRef]
- Mahfud, M.; Suryanto, A.; Qadariyah, L.; Suprapto, S.; Kusuma, H.S. Production of methyl ester from coconut oil using microwave: Kinetic of transesterification reaction using heterogeneous CaO catalyst. Korean Chem. Eng. Res. 2018, 56, 275–280. [Google Scholar] [CrossRef]
- Abd Malek, M.N.F.; Pushparaja, L.; Hussin, N.M.; Embong, N.H.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Exploration of efficiency of nano calcium oxide (CaO) as catalyst for enhancement of biodiesel production. J. Micro. Biotech. Food Sci. 2021, 11, e3935. [Google Scholar] [CrossRef]
- Almeida, L.C.; Barbosa, M.S.; de Jesus, F.A.; Santos, R.M.; Fricks, A.T.; Freitas, L.S.; Pereira, M.M.; Lima, A.S.; Soares, C.M. Enzymatic transesterification of coconut oil by using immobilized lipase on biochar: An experimental and molecular docking study. Biotechnol. Appl. Biochem. 2021, 68, 801–808. [Google Scholar] [CrossRef]
- Kumar, S.A.; Sakthinathan, G.; Vignesh, R.; Banu, J.R.; Ala’a, H. Optimized transesterification reaction for efficient biodiesel production using Indian oil sardine fish as feedstock. Fuel 2019, 253, 921–929. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.; de Almeida, F.N.C.; Souza, G.K.; Asanome, I.Y.; Pereira, N.C. Synthesis and optimization of ethyl esters from fish oil waste for biodiesel production. Renew. Energy 2019, 133, 743–748. [Google Scholar] [CrossRef]
- Kumar, S.; Singhal, M.K.; Sharma, M.P. Utilization of mixed oils for biodiesel preparation: A review. Energy Sources Part A 2021, 43, 1–34. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Gupta, J.; Agarwal, M.; Dalai, A.K. Optimization of biodiesel production from mixture of edible and nonedible vegetable oils. Biocatal. Agric. Biotechnol. 2016, 8, 112–120. [Google Scholar] [CrossRef]
- da Costa Barbosa, D.; Serra, T.M.; Meneghetti, S.M.P.; Meneghetti, M.R. Biodiesel production by ethanolysis of mixed castor and soybean oils. Fuel 2010, 89, 3791–3794. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Razzaq, L.; Abbas, M.M.; Miran, S.; Asghar, S.; Nawaz, S.; Soudagar, M.E.M.; Shaukat, N.; Veza, I.; Khalil, S.; Abdelrahman, A.; et al. Response Surface Methodology and Artificial Neural Networks-Based Yield Optimization of Biodiesel Sourced from Mixture of Palm and Cotton Seed Oil. Sustainability 2022, 14, 6130. [Google Scholar] [CrossRef]
- Mukherjee, I.; Ray, P.K. A review of optimization techniques in metal cutting processes. Comput. Ind. Eng. 2006, 50, 15–34. [Google Scholar] [CrossRef]
- GC, M.P.; Krishna, P.; Parappagoudar, M.B. Squeeze casting process modeling by a conventional statistical regression analysis approach. Appl. Math. Model. 2016, 40, 6869–6888. [Google Scholar] [CrossRef]
- Shadidi, B.; Najafi, G.; Zolfigol, M.A. A Review of the Existing Potentials in Biodiesel Production in Iran. Sustainability 2022, 14, 3284. [Google Scholar] [CrossRef]
- Said, Z.; Nguyen, T.H.; Sharma, P.; Li, C.; Ali, H.M.; Ahmed, S.F.; Truong, T.H. Multi-attribute optimization of sustainable aviation fuel production-process from microalgae source. Fuel 2022, 324, 124759. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, Y.; Lu, S.; Zhang, Z.; Tan, D. A Comprehensive Review of the Properties, Performance, Combustion, and Emissions of the Diesel Engine Fueled with Different Generations of Biodiesel. Processes 2022, 10, 1178. [Google Scholar] [CrossRef]
- Sharma, P.; Sahoo, B.B. An ANFIS-RSM based modeling and multi-objective optimization of syngas powered dual-fuel engine. Int. J. Hydrog Energy 2022, 47, 19298–19318. [Google Scholar] [CrossRef]
- Soudagar, M.E.M.; Afzal, A.; Kareemullah, M. Waste coconut oil methyl ester with and without additives as an alternative fuel in diesel engine at two different injection pressures. Energy Sources Part A 2020, 42, 1–19. [Google Scholar] [CrossRef]
- Samuel, O.D.; Giwa, S.O.; El-Suleiman, A. Optimization of coconut oil ethyl esters reaction variables and prediction model of its blends with diesel fuel for density and kinematic viscosity. Biofuels 2016, 7, 723–733. [Google Scholar] [CrossRef]
- Amruth, E.; Sudev, L.J. Optimization of Transesterification Reaction Parameters for Fish Oil Biodiesel Production: A Response Surface Methodology Approach. J. Phys. Conf. Ser. 2019, 1240, 012140. [Google Scholar] [CrossRef]
- Shettigar, A.K.; Patel, G.C.M.; Chate, G.R.; Vundavilli, P.R.; Parappagoudar, M.B. Artificial bee colony, genetic, back propagation and recurrent neural networks for developing intelligent system of turning process. SN Appl. Sci. 2020, 2, 1–21. [Google Scholar] [CrossRef]
- Patel, G.C.M.; Jagadish. Experimental modeling and optimization of surface quality and thrust forces in drilling of high-strength Al 7075 alloy: CRITIC and meta-heuristic algorithms. J. Braz. Soc. Mech. Sci. Eng. 2021, 43, 1–21. [Google Scholar] [CrossRef]
- Venkataramana, S.H.; Shivalingaiah, K.; Davanageri, M.B.; Selvan, C.P.; Lakshmikanthan, A.; Chandrashekarappa, M.P.G.; Razak, A.; Anand, P.B.; Linul, E. Niger Seed Oil-Based Biodiesel Production Using Transesterification Process: Experimental Investigation and Optimization for Higher Biodiesel Yield Using Box–Behnken Design and Artificial Intelligence Tools. Appl. Sci. 2022, 12, 5987. [Google Scholar] [CrossRef]
- Rangappa, R.; Patel, G.C.M.; Chate, G.R.; Lokare, D.; Lakshmikanthan, A.; Giasin, K.; Pimenov, D.Y. Coaxiality error analysis and optimization of cylindrical parts of CNC turning process. Int. J. Adv. Manuf. Technol. 2022, 120, 6617–6634. [Google Scholar] [CrossRef]
- Jagadish; Patel, G.C.M.; Sibalija, T.V.; Mumtaz, J.; Li, Z. Abrasive water jet machining for a high-quality green composite: The soft computing strategy for modeling and optimization. J. Braz. Soc. Mech. Sci. Eng. 2022, 44, 1–20. [Google Scholar] [CrossRef]
- Soto-Mendoza, V.; García-Calvillo, I.; Ruiz-y-Ruiz, E.; Pérez-Terrazas, J. A hybrid grasshopper optimization algorithm applied to the open vehicle routing problem. Algorithms 2020, 13, 96. [Google Scholar] [CrossRef]
- Heidari, A.A.; Faris, H.; Aljarah, I.; Mirjalili, S. An efficient hybrid multilayer perceptron neural network with grasshopper optimization. Soft Comput. 2019, 23, 7941–7958. [Google Scholar] [CrossRef]
- Abualigah, L.; Diabat, A. A comprehensive survey of the Grasshopper optimization algorithm: Results, variants, and applications. Neural. Comput. Appl. 2020, 32, 15533–15556. [Google Scholar] [CrossRef]
- Embong, N.H.; Hindryawati, N.; Bhuyar, P.; Govindan, N.; Rahim, M.H.A.; Maniam, G.P. Enhanced biodiesel production via esterification of palm fatty acid distillate (PFAD) using rice husk ash (NiSO4)/SiO2 catalyst. Appl. Nanosci. 2021, 1–9. [Google Scholar] [CrossRef]
- Ma’arof, N.A.N.B.; Hindryawati, N.; Khazaai, S.N.M.; Bhuyar, P.; Rahim, M.H.A.; Maniam, G.P. Exploitation of cost-effective renewable heterogeneous base catalyst from banana (Musa paradisiaca) peel for effective methyl ester production from soybean oil. Appl. Nanosci. 2021, 1–12. [Google Scholar] [CrossRef]
- Khazaai, S.N.M.; Bhuyar, P.; Rahim, M.H.A.; Alwi, M.H.F.M.; Yiting, S.; Maniam, G.P. Rapid determination of diesel/biodiesel blend ratio using refractive index, density, and kinematic viscosity measurements. Biomass Convers. Biorefin. 2021, 1–7. [Google Scholar] [CrossRef]
- Mirjalili, S.Z.; Mirjalili, S.; Saremi, S.; Faris, H.; Aljarah, I. Grasshopper optimization algorithm for multi-objective optimization problems. Appl. Intell. 2018, 48, 805–820. [Google Scholar] [CrossRef]
- Saremi, S.; Mirjalili, S.; Lewis, A. Grasshopper optimisation algorithm: Theory and application. Adv. Eng. Softw. 2017, 105, 30–47. [Google Scholar] [CrossRef]
- Ashok, A.; Kennedy, L.J.; Vijaya, J.J.; Aruldoss, U. Optimization of biodiesel production from waste cooking oil by magnesium oxide nanocatalyst synthesized using coprecipitation method. Clean Technol. Environ. Policy 2018, 20, 1219–1231. [Google Scholar] [CrossRef]
- Abdullah, R.F.; Rashid, U.; Ibrahim, M.L.; Hazmi, B.; Alharthi, F.A.; Nehdi, I.A. Bifunctional nano-catalyst produced from palm kernel shell via hydrothermal-assisted carbonization for biodiesel production from waste cooking oil. Renew. Sustain. Energy Rev. 2021, 137, 110638. [Google Scholar] [CrossRef]
- Abusweireh, R.S.; Rajamohan, N.; Vasseghian, Y. Enhanced production of biodiesel using nanomaterials: A detailed review on the mechanism and influencing factors. Fuel 2022, 319, 123862. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Subbarao, D. Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod. 2013, 59, 131–140. [Google Scholar] [CrossRef]
- Chen, G.Y.; Shan, R.; Yan, B.B. Remarkably enhancing the biodiesel yield from palm oil upon abalone shell-derived CaO catalysts treated by ethanol. Fuel Process. Technol. 2016, 143, 110–117. [Google Scholar] [CrossRef]
- Wen, Z.; Yu, X.; Tu, S.T.; Yan, J.; Dahlquist, E. Synthesis of biodiesel from vegetable oil with methanol catalyzed by Li-doped magnesium oxide catalysts. Appl. Energy 2010, 87, 743–748. [Google Scholar] [CrossRef]
- Hebbar, H.H.; Math, M.C.; Yatish, K.V. Optimization and kinetic study of CaO nano-particles catalyzed biodiesel production from Bombax ceiba oil. Energy 2018, 143, 25–34. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Mubashir, M.; Asif, S.; Waseem, A.; Mukhtar, A.; Saqib, S.; Munawaroh, H.S.H.; Lam, M.K.; Khoo, K.S.; et al. A practical approach for synthesis of biodiesel via non-edible seeds oils using trimetallic based montmorillonite nano-catalyst. Bioresour. Technol. 2021, 328, 124859. [Google Scholar] [CrossRef]
- Foroutan, R.; Mohammadi, R.; Esmaeili, H.; Bektashi, F.M.; Tamjidi, S. Transesterification of waste edible oils to biodiesel using calcium oxide@ magnesium oxide nanocatalyst. Waste Manag. 2020, 105, 373–383. [Google Scholar] [CrossRef]
- Baskar, G.; Selvakumari, I.A.E.; Aiswarya, R.J.B.T. Biodiesel production from castor oil using heterogeneous Ni doped ZnO nanocatalyst. Bioresour. Technol. 2018, 250, 793–798. [Google Scholar] [CrossRef]
- Rasouli, H.; Esmaeili, H. Characterization of MgO nanocatalyst to produce biodiesel from goat fat using transesterification process. 3 Biotech 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Changmai, A.; Rano, R.; Vanlaveni, C.; Rokhum, L. A novel Citrus sinensis peel ash coated magnetic nanoparticles as an easily recoverable solid catalyst for biodiesel production. Fuel 2021, 286, 119447. [Google Scholar] [CrossRef]
- Patel, G.C.M.; Shettigar, A.K.; Parappagoudar, M.B. A systematic approach to model and optimize wear behaviour of castings produced by squeeze casting process. J. Manuf. Process. 2018, 32, 199–212. [Google Scholar] [CrossRef]
- Patel, G.C.M.; Krishna, P.; Parappagoudar, M.B.; Vundavilli, P.R. Multi-objective optimization of squeeze casting process using evolutionary algorithms. Int. J. Swarm Intell. Res. 2016, 7, 55–74. [Google Scholar] [CrossRef]
- Saffari, A.; Zahiri, S.H.; Khishe, M. Fuzzy grasshopper optimization algorithm: A hybrid technique for tuning the control parameters of GOA using fuzzy system for big data sonar classification. Iran. J. Electr. Electron. Eng. 2022, 18, 2131. [Google Scholar] [CrossRef]
- Malek, M.N.F.A.; Hussin, N.M.; Embong, N.H.; Bhuyar, P.; Rahim, M.H.A.; Govindan, N.; Maniam, G.P. Ultrasonication: A process intensification tool for methyl ester synthesis: A mini review. Biomass Convers. Biorefin. 2020, 1–11. [Google Scholar] [CrossRef]
- Jung, S.; Kim, M.; Lin, K.Y.A.; Park, Y.K.; Kwon, E.E. Biodiesel synthesis from bio-heavy oil through thermally induced transesterification. J. Clean. Prod. 2021, 294, 126347. [Google Scholar] [CrossRef]
- da Silva Dutra, L.; Pinto, M.C.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Manoel, E.A.; Greco-Duarte, J.; Freiere, D.M.G.; Pinto, J.C. How the biodiesel from immobilized enzymes production is going on: An advanced bibliometric evaluation of global research. Renew. Sustain. Energy Rev. 2022, 153, 111765. [Google Scholar] [CrossRef]
- Linganiso, E.C.; Tlhaole, B.; Magagula, L.P.; Dziike, S.; Linganiso, L.Z.; Motaung, T.E.; Moloto, N.; Tetana, Z.N. Biodiesel production from waste oils: A South African outlook. Sustainability 2022, 14, 1983. [Google Scholar] [CrossRef]
- Changmai, B.; Sudarsanam, P.; Rokhum, L. Biodiesel production using a renewable mesoporous solid catalyst. Ind. Crops Prod. 2020, 145, 111911. [Google Scholar] [CrossRef]
- Yahyaee, R.; Ghobadian, B.; Najafi, G. Waste fish oil biodiesel as a source of renewable fuel in Iran. Renew. Sustain. Energy Rev. 2013, 17, 312–319. [Google Scholar] [CrossRef]
- Keera, S.T.; El Sabagh, S.M.; Taman, A.R. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Abed, K.A.; El Morsi, A.K.; Sayed, M.M.; El Shaib, A.A.; Gad, M.S. Effect of waste cooking-oil biodiesel on performance and exhaust emissions of a diesel engine. Egypt. J. Pet. 2018, 27, 985–989. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Morken, J.; Lekang, O.I.; Yigezu, Z.D. Factors affecting the potential of Jatropha curcas for sustainable biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2021, 137, 110500. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef] [Green Version]


| Feedstock Type | Type of Catalyst | Process Parameters | Optimization | Major Results | Ref. |
|---|---|---|---|---|---|
| WCO | KOH | CC: 0.75–1.25 wt.%, RT: 25–75 °C, EOMR: 6–12 | CCD and RSM, ANN | 96.7% yield | [35] |
| WCO | KOH | OEMR: 1:6–1:12, Rt: 5–15 min, MWP: 100–500 W | BBD and ANN | 74.45% yield | [36] |
| WCO | ZnO/CuO oxide composite | CC: 0.005–2.665 wt.%, Rt: 15–235 min; RT: 5–105 °C, MOMR: 0–20 | CCRD and RSM | 90.3% yield | [37] |
| WCO | CaO | CC: 1–3 wt.%, MWP: 100–600 W; Rt: 1–3.5 min; RT: 5–110 °C, MOMR: 0–20 | OFAT | 33.84% yield | [38] |
| WCO | BCL | Rt: 96 h, RT: 40 °C, MOMR: 1:7 | OFAT | 48% yield | [40] |
| WFO (Sardine fish) | KOH | MQ: 20–30 vol.%, CC: 0.75–1.75 wt.%, Rt: 5–25 min | BBD | 96.57% yield | [41] |
| WFO | KOH | CC: 0.5–1.5 wt.%, RT: 30–60 °C, EOMR: 7–13 | CCRD | 96.41% yield | [42] |
| WCO | H2SO4 | CC: 8–12 mL/L, RT: 60–65 °C, Rt: 100–120 min | OFAT | NR | [55] |
| WCO | KOH | CC: 0.5–2.25 wt.%, RT: 30–80 °C, Rt: 30–70 min, EOMR: 3–12 | OFAT | 97.2% yield | [56] |
| WFO | NaOH and Na2HPO | MOMR: 3.48–8.52, Rt: 34–86 min, RT: 43–77 °C | CCRD | 94.6% yield | [57] |
| Levels | Methanol to Oil Molar Ratio, A | Catalyst Concentration (wt.%), B | Reaction Temperature (°C), C |
|---|---|---|---|
| Low | 3 | 0.5 | 50 |
| Medium | 8 | 1.25 | 65 |
| High | 13 | 2.0 | 80 |
| Run | A: Methanol to Oil Molar Ratio | B: Catalyst Concentration (Wt.%) | C: Reaction Temperature (°C) | Experimental Biodiesel Yield (%) | Predicted Biodiesel Yield (%) | Absolute Percent Deviation |
|---|---|---|---|---|---|---|
| 1 | 03 | 0.50 | 50 | 84.53 | 83.86 ± 1.2 | 0.793 |
| 2 | 13 | 2.00 | 80 | 94.45 | 95.14 ± 0.3 | 0.731 |
| 3 | 08 | 0.50 | 65 | 87.08 | 87.52 ± 1.1 | 0.505 |
| 4 | 03 | 2.00 | 50 | 81.93 | 82.42 ± 0.8 | 0.598 |
| 5 | 08 | 1.25 | 65 | 93.2 | 93.28 ± 0.6 | 0.086 |
| 6 | 03 | 0.50 | 80 | 81.55 | 81.88 ± 0.8 | 0.405 |
| 7 | 08 | 2.00 | 65 | 93.85 | 93.29 ± 0.9 | 0.597 |
| 8 | 13 | 0.50 | 80 | 82.62 | 82.16 ± 1.3 | 0.557 |
| 9 | 13 | 1.25 | 65 | 91.61 | 91.31 ± 0.5 | 0.327 |
| 10 | 08 | 1.25 | 80 | 93.45 | 93.20 ± 0.3 | 0.268 |
| 11 | 08 | 1.25 | 65 | 93.2 | 93.28 ± 0.7 | 0.086 |
| 12 | 13 | 0.50 | 50 | 82.60 | 82.95 ± 0.9 | 0.424 |
| 13 | 08 | 1.25 | 65 | 93.20 | 93.28 ± 0.5 | 0.086 |
| 14 | 13 | 2.00 | 50 | 89.63 | 89.33 ± 0.6 | 0.335 |
| 15 | 03 | 2.00 | 80 | 87.38 | 87.05 ± 0.7 | 0.378 |
| 16 | 08 | 1.25 | 50 | 91.16 | 91.28 ± 0.4 | 0.132 |
| 17 | 03 | 1.25 | 65 | 87.54 | 87.72 ± 0.7 | 0.206 |
| Model | Regression Equation | Regression Coefficient |
|---|---|---|
| Linear | Yield = 76.92 + 0.36 A + 3.848 B + 0.064 C | R2 = 0.3542, and Adj. R2 = 0.2052 |
| Linear + Interaction | Yield = 96.12448 − 0.5484A − 9.875B − 0.1514C + 0.521AB + 0.004 AC + 0.147 AC | R2 = 0.5049, and Adj. R2 = 0.2078 |
| Linear + Interaction + Square | Yield = 63.54662 + 1.86215 A + 2.90935 B + 0.447504 C + 0.521 AB + 0.00395 AC + 0.147 BC − 0.150659 A2 − 5.11374 B2 − 0.004607 C2 | R2 = 0.9869, and Adj. R2 = 0.9737 |
| Source | Sum of Squares | DF | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 349.91 | 9 | 38.88 | 107.18 | <0.0001 |
| A: M:O | 32.33 | 1 | 32.33 | 89.12 | <0.0001 |
| B: MgO CC | 83.29 | 1 | 83.29 | 229.62 | <0.0001 |
| C: RT | 9.22 | 1 | 9.22 | 25.41 | 0.0015 |
| AB | 30.54 | 1 | 30.54 | 84.19 | <0.0001 |
| AC | 0.7021 | 1 | 0.7021 | 1.94 | 0.2068 |
| BC | 21.88 | 1 | 21.88 | 60.32 | 0.0001 |
| A2 | 38.01 | 1 | 38.01 | 104.79 | <0.0001 |
| B2 | 22.17 | 1 | 22.17 | 61.12 | 0.0001 |
| C2 | 2.88 | 1 | 2.88 | 7.94 | 0.0259 |
| Residual | 2.54 | 7 | 0.3627 | ||
| Lack of Fit | 2.54 | 5 | 0.5078 | ||
| Pure Error | 0.0000 | 2 | 0.0000 | ||
| Cor Total | 352.45 | 16 |
| Optimization Method | Algorithm Specific Parameters | Fitness Value (Yield %) | Computation Time (Seconds) | Transesterification Condition |
|---|---|---|---|---|
| GOA | Cmax: 1 Cmin: 0.00003 Population size: 40 Maximum iteration: 100 | 95.95% | 30 | M:O = 10.65; CC = 1.977 wt.%; RT = 80 °C |
| RSM-DFA | NA | 94.96% | NA | M:O = 11.19; CC = 1.72 wt.%; RT = 69.92 °C |
| Properties | WFO | WCO | WFO + WCO | Biodiesel | Diesel | Test Standard: ASTM D6751-15C |
|---|---|---|---|---|---|---|
| Specific gravity, kg/m3 | 0.91 | 0.93 | 0.92 | 0.88 | 0.87 | 0.85 to 0.9 |
| Kinematic Viscosity at 40 °C, cSt (mm2/s) | 24.20 | 28.42 | 26.43 | 4.67 | 4.1 | 1.4 to 1.6 |
| Density, kg/m3 | 893 | 923 | 905 | 840 | 800 | 880 max. |
| Flash Point, °C | 224 | 266 | 253 | 131 | 58 | 100 min. |
| Fire Point, °C | 233 | 275 | 237 | 146 | 64 | - |
| Calorific Value, MJ/kg | 36.2 | 37.4 | 36.9 | 40.8 | 43.8 | - |
| Cloud Point, °C | 19 | 5 | 11 | 6 | −7 | −3 to 12 |
| Cetane index | 56 | 52 | 54.3 | 63.7 | 50.3 | 47 min. |
| Pour Point, °C | 6 | 3 | 5 | 4 | −8 | −15 to 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dharmegowda, I.Y.; Muniyappa, L.M.; Siddalingaiah, P.; Suresh, A.B.; Gowdru Chandrashekarappa, M.P.; Prakash, C. MgO Nano-Catalyzed Biodiesel Production from Waste Coconut Oil and Fish Oil Using Response Surface Methodology and Grasshopper Optimization. Sustainability 2022, 14, 11132. https://doi.org/10.3390/su141811132
Dharmegowda IY, Muniyappa LM, Siddalingaiah P, Suresh AB, Gowdru Chandrashekarappa MP, Prakash C. MgO Nano-Catalyzed Biodiesel Production from Waste Coconut Oil and Fish Oil Using Response Surface Methodology and Grasshopper Optimization. Sustainability. 2022; 14(18):11132. https://doi.org/10.3390/su141811132
Chicago/Turabian StyleDharmegowda, Impha Yalagudige, Lakshmidevamma Madarakallu Muniyappa, Parameshwara Siddalingaiah, Ajith Bintravalli Suresh, Manjunath Patel Gowdru Chandrashekarappa, and Chander Prakash. 2022. "MgO Nano-Catalyzed Biodiesel Production from Waste Coconut Oil and Fish Oil Using Response Surface Methodology and Grasshopper Optimization" Sustainability 14, no. 18: 11132. https://doi.org/10.3390/su141811132
APA StyleDharmegowda, I. Y., Muniyappa, L. M., Siddalingaiah, P., Suresh, A. B., Gowdru Chandrashekarappa, M. P., & Prakash, C. (2022). MgO Nano-Catalyzed Biodiesel Production from Waste Coconut Oil and Fish Oil Using Response Surface Methodology and Grasshopper Optimization. Sustainability, 14(18), 11132. https://doi.org/10.3390/su141811132









