Antifungal, Antiviral, and HPLC Analysis of Phenolic and Flavonoid Compounds of Amphiroa anceps Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pathogen Isolation for Root Rot and Grey Mold
2.2. ITS Sequencing and Identification
2.3. Sampling Site and Algal Biomass Collection
2.4. Extraction of the Algal Biomass
2.5. Antifungal Activity of the Algal Extract
2.6. Source of the Virus, Inoculum Preparation, and Greenhouse Antiviral Activity Assays
2.7. Characterization of Phenolic and Flavonoid Compounds Using HPLC
2.8. Statistical Analysis
3. Results
3.1. Isolation and Preliminary Identification
3.2. ITS Characterization
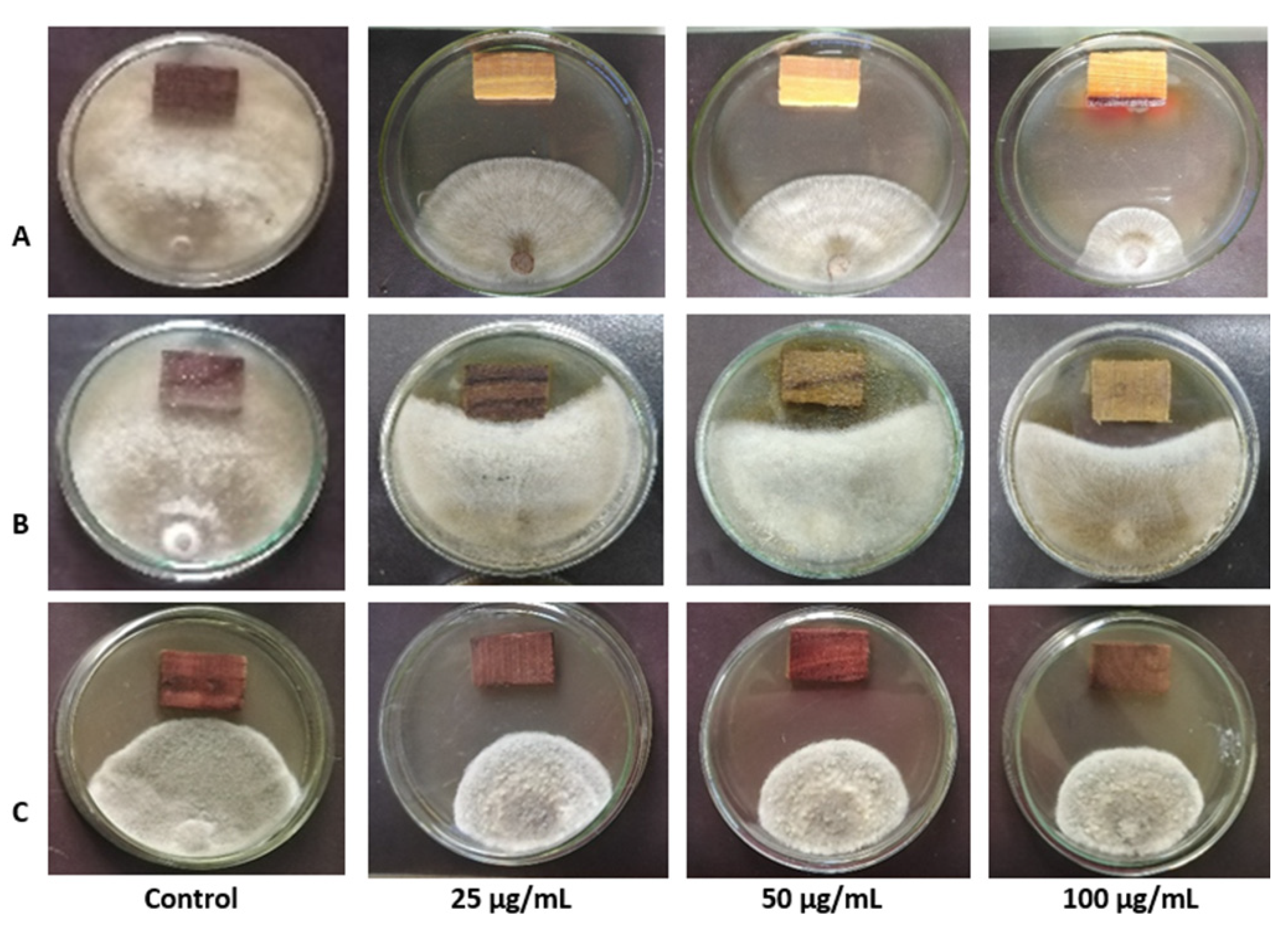
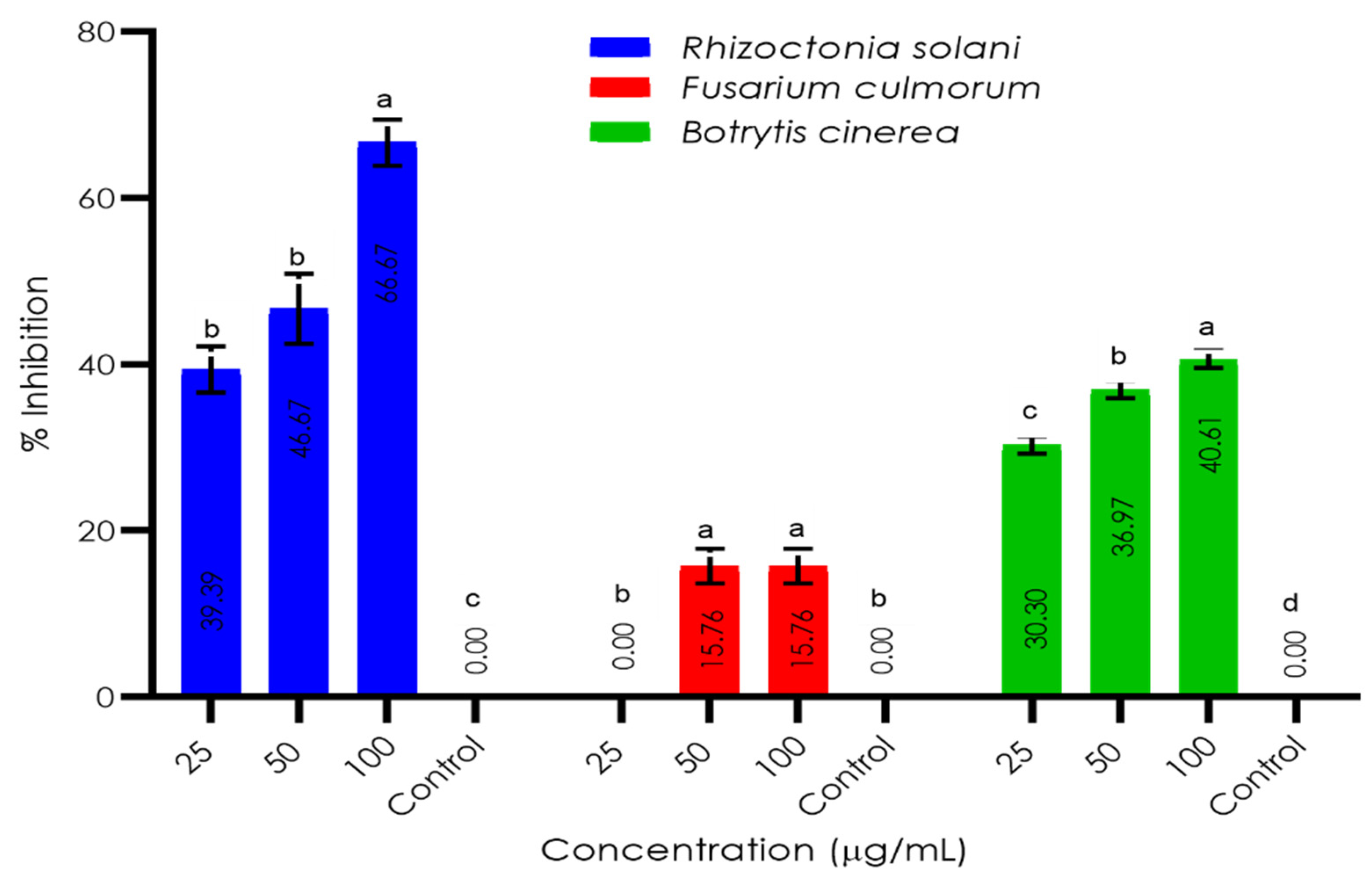
3.3. Invitro Antifungal Activity of the A. anceps Extract
3.4. Inhibitory Effects of A. anceps Extract against TMV
3.5. Phenolic and Flavonoid Compounds of the Algal Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raj, E.D.S. UV–VIS and HPLC studies on Amphiroa anceps (Lamarck) Decaisne. Arab. J. Chem. 2016, 9, S907–S913. [Google Scholar]
- Mofeed, J.; Deyab, M.; Mohamed, A.; Moustafa, M.; Negm, S.; El-Bilawy, E. Antimicrobial activities of three seaweeds extract against some human viral and bacterial pathogens. Biocell 2022, 46, 247. [Google Scholar] [CrossRef]
- El-Baroty, G.S.; Moussa, M.Y.; Shallan, M.A.; Ali, M.A.; Sabh, A.Z.; Shalaby, E.A. Contribution to the aroma, biological activities, minerals, protein, pigments and lipid contents of the red alga: Asparagopsis taxiformis (Delile) Trevisan. J. Appl. Sci. Res. 2007, 3, 1825–1834. [Google Scholar]
- Aslam, A.; Fazal, T.; uz Zaman, Q.; Shan, A.; Rehman, F.; Iqbal, J.; Rashid, N.; Rehman, M.S.U. Biorefinery of Microalgae for Nonfuel Products. In Microalgae Cultivation for Biofuels Production; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 197–209. [Google Scholar]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H. An overview on red algae bioactive compounds and their pharmaceutical applications. J. Complement. Integr. Med. 2020, 17. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Hernández, J.; Paseiro-Losada, P.; López-Cervantes, J. An HPLC method for the quantification of sterols in edible seaweeds. Biomed. Chromatogr. 2004, 18, 183–190. [Google Scholar] [CrossRef]
- König, G.M.; Wright, A.D.; Linden, A. Plocamium hamatum and its monoterpenes: Chemical and biological investigations of the tropical marine red alga. Phytochemistry 1999, 52, 1047–1053. [Google Scholar] [CrossRef]
- Roy, S.; Anantharaman, P. Biosynthesis of silver nanoparticle by Amphiroa anceps (Lamarck) decaisne and Its biomedical and ecological implications. J. Nanomed. Nanotechnol. 2018, 9. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Hafez, E. Plant Viral Diseases in Egypt and Their Control. In Cottage Industry of Biocontrol Agents and Their Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 403–421. [Google Scholar]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to Tobacco mosaic virus. JAPS J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- Tan, Q.-W.; Gao, F.-L.; Wang, F.-R.; Chen, Q.-J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef]
- Ahmed, M.F.A.; El-Fiki, I.A.I. Effect of biological control of root rot diseases of strawberry using Trichoderma spp. Middle East J. Appl. Sci. 2017, 7, 482–492. [Google Scholar]
- Abdelkhalek, A.; Al-Askar, A.A.; Elbeaino, T.; Moawad, H.; El-Gendi, H. Protective and Curative Activities of Paenibacillus polymyxa against Zucchini yellow mosaic virus Infestation in Squash Plants. Biology 2022, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.M.; El-Okkiah, S.; Elsadany, A.Y.; Bedier, M.Y.; Omara, R.I.; Behiry, S.I.; Hassan, S.; Abdelkhalek, A. Systemic resistance induction of tomato plants against tomato mosaic virus by microalgae. Egypt. J. Biol. Pest Control 2022, 32, 1–7. [Google Scholar] [CrossRef]
- Baloch, G.N.; Tariq, S.; Ehteshamul-Haque, S.; Athar, M.; Sultana, V.; Ara, J. Management of root diseases of eggplant and watermelon with the application of asafoetida and seaweeds. J. Appl. Bot. Food Qual. 2013, 86, 138–142. [Google Scholar]
- Khan, A.M.; Naz, S.; Abid, M. Evaluation of marine red alga Melanothamnus afaqhusainii against Meloidogyne incognita, fungus and as fertilizing potential on okra. Pak. J. Nematol. 2016, 34, 91–100. [Google Scholar]
- Jiménez, E.; Dorta, F.; Medina, C.; Ramírez, A.; Ramírez, I.; Peña-Cortés, H. Anti-phytopathogenic activities of macro-algae extracts. Mar. Drugs 2011, 9, 739–756. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, O.D.; Sinclair, J.B. Basic Plant Pathology Methods; CRC press: Boca Raton, FL, USA, 2017; ISBN 1315138131. [Google Scholar]
- Alexopoulos, C.J.; Mims, C.W.; Blackwell, M. Introductory Mycology; John Wiley and Sons: New York, NY, USA, 1996; ISBN 0471522295. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N. Social and Economic Dimensions of Carrageenan Seaweed Farming. 2013. Available online: https://tamug-ir.tdl.org/bitstream/handle/1969.3/29143/a-i3344e.pdf?sequence=1 (accessed on 17 August 2022).
- Londo, G. The decimal scale for relevés of permanent quadrats. In Sampling Methods and Taxon Analysis in Vegetation Science: Releve Surveys,’Vegetationsaufnahmen’, Floristic Analysis of Plant Communities; Handbook of vegetation sciences; The Hague (The Netherlands) Junk: The Hague, The Netherlands, 1984; pp. 45–49. [Google Scholar]
- Brodie, J.; Wilbraham, J.; Pottas, J.; Guiry, M.D. A revised check-list of the seaweeds of Britain. J. Mar. Biol. Assoc. UK 2016, 96, 1005. [Google Scholar] [CrossRef]
- De Clerck, O.; Coppejans, E. Marina Algae of the Jubail Marine Wildlife Sanctuary, Saudi Arabia. 1996. Available online: http://www.vliz.be/imisdocs/publications/239149.pdf (accessed on 17 August 2022).
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mansour, M.M.A.; Elansary, H.O. Evaluation of the effect of inner and outer bark extracts of Sugar Maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 2019, 39, 136–147. [Google Scholar] [CrossRef]
- Gooding, G.V., Jr.; Hebert, T.T. A simple technique for purification of Tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285. [Google Scholar] [PubMed]
- Abdelkhalek, A.; Ismail, I.A.; Dessoky, E.S.; El-Hallous, E.I.; Hafez, E. A tomato kinesin-like protein is associated with Tobacco mosaic virus infection. Biotechnol. Biotechnol. Equip. 2019, 33, 1424–1433. [Google Scholar] [CrossRef]
- Behiry, S.I.; Okla, M.K.; Alamri, S.A.; El-Hefny, M.; Salem, M.Z.M.; Alaraidh, I.A.; Ali, H.M.; Al-Ghtani, S.M.; Monroy, J.C.; Salem, A.Z.M. Antifungal and antibacterial activities of Musa paradisiaca L. peel extract: HPLC analysis of phenolic and flavonoid contents. Processes 2019, 7, 215. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Ibrahim, I.H.M.; Ali, H.M.; Helmy, H.M. Assessment of the use of natural extracted dyes and pancreatin enzyme for dyeing of four natural textiles: HPLC analysis of phytochemicals. Processes 2020, 8, 59. [Google Scholar] [CrossRef]
- Strange, R.N.; Scott, P.R. Plant disease: A threat to global food security. Annu. Rev. Phytopathol. 2005, 43. [Google Scholar] [CrossRef]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy Metals and Pesticides Toxicity in Agricultural Soil and Plants: Ecological Risks and Human Health Implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological control of plant pathogens: A global perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Deyab, M.; Mofeed, J. Evaluation of Biochemical composition and bioactivities of two Ulva species (Ulva lactuca and Ulva fasciata); a comparative study. Biosci. Res. 2019, 16, 3801–3811. [Google Scholar]
- Deyab, M.; Mofeed, J.; El-Bilawy, E.; Ward, F. Antiviral activity of five filamentous cyanobacteria against coxsackievirus B3 and rotavirus. Arch. Microbiol. 2020, 202, 213–223. [Google Scholar] [CrossRef]
- Selvin, J.; Lipton, A.P. Biopotentials of Ulva fasciata and Hypnea musciformis collected from the peninsular coast of India. J. Mar. Sci. Technol. 2004, 12, 1. [Google Scholar] [CrossRef]
- Lubobi, S.; Matunda, C.; Kumar, V.; Omboki, B. Isolation of bioactive secondary metabolites from seaweeds Amphiroa anceps against chicken meat associated pathogens. J. Antimicrob 2016, 2. [Google Scholar] [CrossRef]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Xiaojun, Y.; Xiancui, L.; Chengxu, Z.; Xiao, F. Prevention of fish oil rancidity by phlorotannins from Sargassum kjellmanianum. J. Appl. Phycol. 1996, 8, 201–203. [Google Scholar] [CrossRef]
- Nagai, T.; Yukimoto, T. Preparation and functional properties of beverages made from sea algae. Food Chem. 2003, 81, 327–332. [Google Scholar] [CrossRef]
- Kolanjinathan, K.; Stella, D. Antibacterial activity of marine macro algae against human pathogens. Recent Res. Sci. Technol. 2009, 1, 20–22. [Google Scholar]
- Omar, H.H.; Gumgumji, N.M.; Shiek, H.M.; El-Kazan, M.M.; El-Gendy, A.M. Inhibition of the development of pathogenic fungi by extracts of some marine algae from the red sea of Jeddah, Saudi Arabia. Afr. J. Biotechnol. 2012, 11, 13697–13704. [Google Scholar]
- Kumar, K.S.; Ganesan, K.; Rao, P.V.S. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty–An edible seaweed. Food Chem. 2008, 107, 289–295. [Google Scholar] [CrossRef]
- Robak, J.; Gryglewski, R.J. Bioactivity of flavonoids. Pol. J. Pharmacol. 1996, 48, 555–564. [Google Scholar]
- Pandian, P.; Selvamuthukumar, S.; Manavalan, R.; Parthasarathy, V. Screening of antibacterial and antifungal activities of red marine algae Acanthaphora spicifera (Rhodophyceae). J. Biomed. Sci. Res. 2011, 3, 444–448. [Google Scholar]
- Sultana, V.; Baloch, G.N.; Ara, J.; Ehteshamul-Haque, S.; Tariq, R.M.; Athar, M. Seaweeds as an alternative to chemical pesticides for the management of root diseases of sunflower and tomato. J. Appl. Bot. Food Qual. 2012, 84, 162. [Google Scholar]
- Soliman, A.S.; Ahmed, A.Y.; Abdel-Ghafour, S.E.; El-Sheekh, M.M.; Sobhy, H.M. Antifungal bio-efficacy of the red algae Gracilaria confervoides extracts against three pathogenic fungi of cucumber plant. Middle East J. Appl. Sci 2018, 8, 727–735. [Google Scholar]
- Karthick, M.; Balachandar, M.; Raja, M.; Raj, R.A. Antibacterial activity of red alga Amphiroa anceps (rhodophyceae: Lithophyllaceae) against selected human pathogens. Sci. Acta Xaver. 2019, 10, 15–19. [Google Scholar]
- Waziri, H.M.A. Plants as antiviral agents. J. Plant Pathol. Microbiol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Li, Z.; Guo, X.; Dawuti, G.; Aibai, S. Antifungal activity of ellagic acid in vitro and in vivo. Phyther. Res. 2015, 29, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.; Aibai, S. Antifungal activity of gallic acid in vitro and in vivo. Phyther. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Possamai Rossatto, F.C.; Tharmalingam, N.; Escobar, I.E.; d’Azevedo, P.A.; Zimmer, K.R.; Mylonakis, E. Antifungal Activity of the Phenolic Compounds Ellagic Acid (EA) and Caffeic Acid Phenethyl Ester (CAPE) against Drug-Resistant Candida auris. J. Fungi 2021, 7, 763. [Google Scholar] [CrossRef]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP 53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar] [CrossRef]
- Hirasawa, M.; Takada, K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J. Antimicrob. Chemother. 2004, 53, 225–229. [Google Scholar] [CrossRef]
- Davidson, P.M.; Taylor, T.M.; Schmidt, S.E. Chemical preservatives and natural antimicrobial compounds. Food Microbiol. Fundam. Front. 2012, 765–801. [Google Scholar] [CrossRef]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef] [PubMed]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A review study on Punica granatum L. J. Evid. Based. Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]


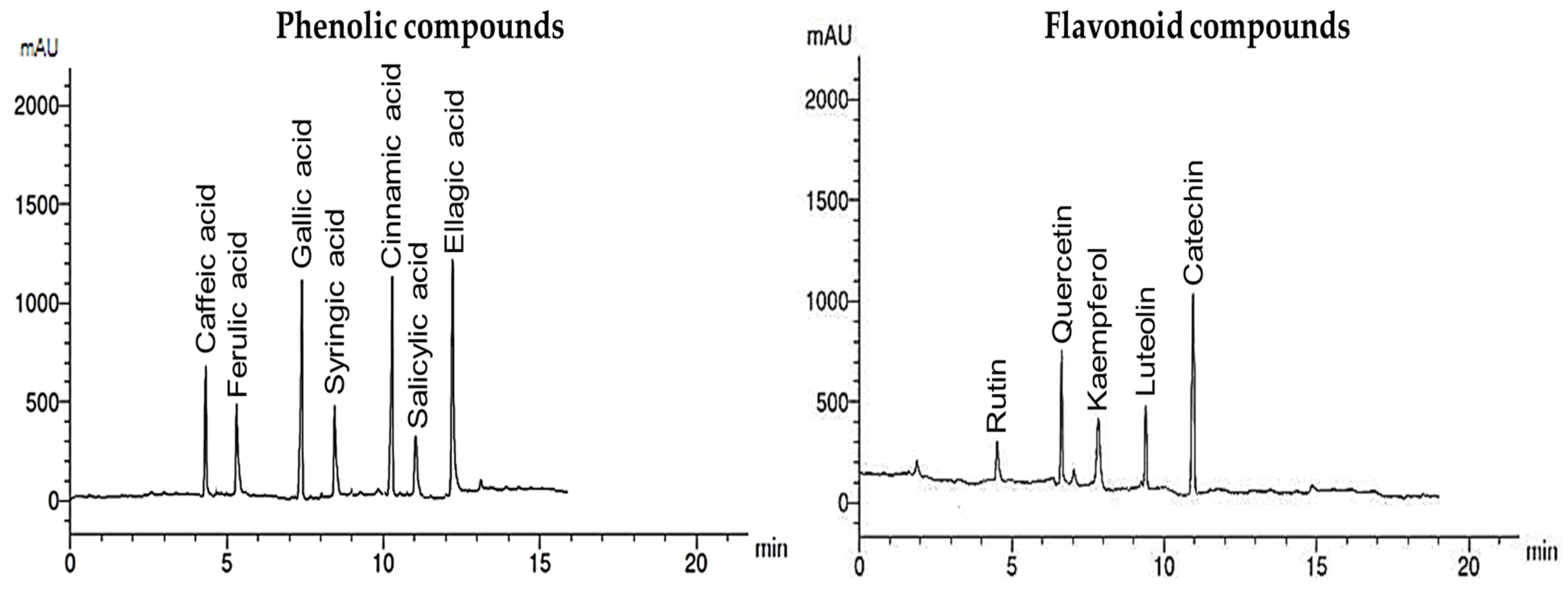
| Phenolic Compounds | Flavonoid Compounds | ||||
|---|---|---|---|---|---|
| Compound | RT | Concentration (µg/mL) | Compound | RT | Concentration (µg/mL) |
| Caffeic acid | 4.2 | 8.66 | Rutin | 4.8 | 0.59 |
| Ferulic acid | 5.1 | 6.24 | Quercetin | 6.8 | 4.86 |
| Gallic acid | 7.0 | 18.36 | Kaempferol | 8.0 | 3.87 |
| Syringic acid | 8.6 | 5.04 | Luteolin | 9.1 | 4.63 |
| Cinnamic acid | 10.0 | 17.64 | Catechin | 11.0 | 12.45 |
| Salicylic acid | 11.0 | 5.72 | |||
| Ellagic acid | 12.3 | 19.05 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Bilawy, E.H.; Al-Mansori, A.-N.A.; Soliman, S.A.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Sabry, A.E.-N.; Elsharkawy, M.M.; Heflish, A.A.; Behiry, S.I.; et al. Antifungal, Antiviral, and HPLC Analysis of Phenolic and Flavonoid Compounds of Amphiroa anceps Extract. Sustainability 2022, 14, 12253. https://doi.org/10.3390/su141912253
El-Bilawy EH, Al-Mansori A-NA, Soliman SA, Alotibi FO, Al-Askar AA, Arishi AA, Sabry AE-N, Elsharkawy MM, Heflish AA, Behiry SI, et al. Antifungal, Antiviral, and HPLC Analysis of Phenolic and Flavonoid Compounds of Amphiroa anceps Extract. Sustainability. 2022; 14(19):12253. https://doi.org/10.3390/su141912253
Chicago/Turabian StyleEl-Bilawy, Emad H., Al-Naji A. Al-Mansori, Seham A. Soliman, Fatimah O. Alotibi, Abdulaziz A. Al-Askar, Amr A. Arishi, Abd El-Naser Sabry, Mohsen Mohamed Elsharkawy, Ahmed A. Heflish, Said I. Behiry, and et al. 2022. "Antifungal, Antiviral, and HPLC Analysis of Phenolic and Flavonoid Compounds of Amphiroa anceps Extract" Sustainability 14, no. 19: 12253. https://doi.org/10.3390/su141912253
APA StyleEl-Bilawy, E. H., Al-Mansori, A.-N. A., Soliman, S. A., Alotibi, F. O., Al-Askar, A. A., Arishi, A. A., Sabry, A. E.-N., Elsharkawy, M. M., Heflish, A. A., Behiry, S. I., & Abdelkhalek, A. (2022). Antifungal, Antiviral, and HPLC Analysis of Phenolic and Flavonoid Compounds of Amphiroa anceps Extract. Sustainability, 14(19), 12253. https://doi.org/10.3390/su141912253









