Cadmium Uptake and Growth Responses of Seven Urban Flowering Plants: Hyperaccumulator or Bioindicator?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Culture and Cd Exposure
2.2. Plant and Soil Analysis
2.3. Data Analysis
2.4. Statistical Analyses
3. Results and Discussion
3.1. Growth Responses of the Seven Flowering Plants
3.1.1. Differences in Biomass among the Seven Flowering Plants
3.1.2. Differences in Height among the Seven Flowering Plants
3.2. Accumulation Characteristics of the Seven Flowering Plants
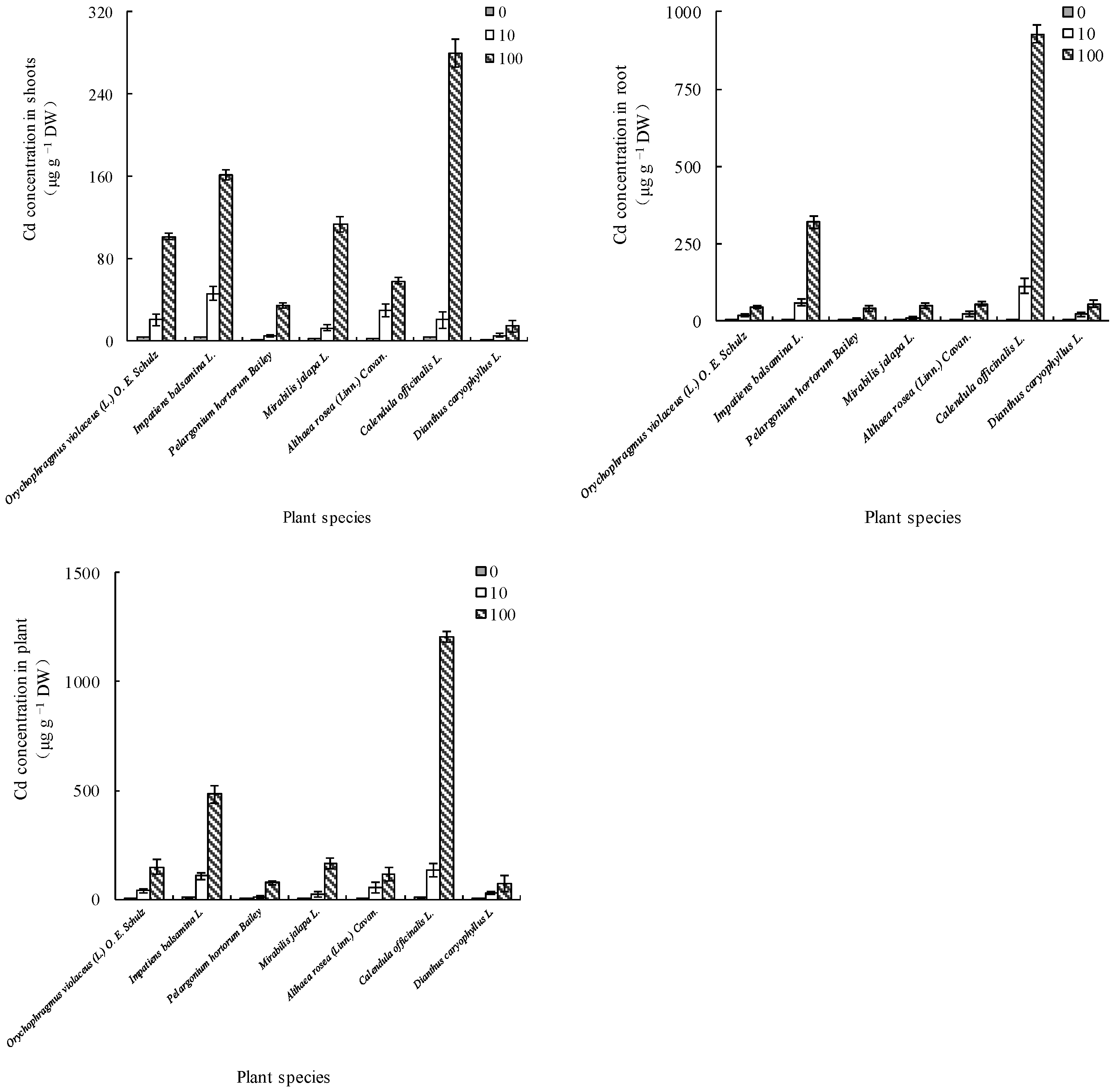
3.2.1. Differences in Cd Accumulation among the Seven Flowering Plants
3.2.2. Differences in Cd Uptake and BCF among the Seven Flowering Plants
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carrus, G.; Scopelliti, M.; Lafortezza, R.; Colangelo, G.; Ferrini, F.; Salbitano, F.; Agrimi, M.; Portoghesi, L.; Semenzato, P.; Sanesi, G. Go greener, feel better? The positive effects of biodiversity on the well-being of individuals visiting urban and peri-urban green areas. Landsc. Urban Plan. 2015, 134, 221–228. [Google Scholar] [CrossRef]
- Boim, A.G.F.; Melo, L.C.A.; Moreno, F.N.; Alleoni, L.R.F. Bioconcentration factors and the risk concentrations of potentially toxic elements in garden soils. J. Environ. Manag. 2016, 170, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-M.; Fiala, M.J.; Park, D.; Wade, T.L. Review of pollutants in urban road dust and stormwater runoff: Part 1. Heavy metals released from vehicles. Int. J. Urban Sci. 2016, 20, 334–360. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Bai, Y.R.; Wang, J.Y. Distribution of urban soil heavy metal and pollution evaluation in different functional zones of Yinchuan city. Environ. Sci. 2016, 37, 710–716. [Google Scholar]
- Midhat, L.; Ouazzani, N.; Esshaimi, M.; Ouhammou, A.; Mandi, L. Assessment of heavy metals accumulation by spontaneous vegetation: Screening for new accumulator plant species grown in Kettara mine-Marrakech, Southern Morocco. Int. J. Phytoremediat. 2017, 19, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Wang, X.; Shafifi, M.; Penttinen, P.; Xu, W.; Ma, J.; Zhong, B.; Guo, J.; Xu, M.; Ye, Z.; et al. Remediation efficacy of Sedum plumbizincicola as affected by intercropping of landscape plants and oxalic acid in urban cadmium contaminated soil. J. Soils Sediments 2019, 19, 3512–3520. [Google Scholar] [CrossRef]
- Men, C.; Liu, R.; Xu, L.; Wang, Q.; Guo, L.; Miao, Y.; Shen, Z. Source-specific ecological risk analysis and critical source identification of heavy metals in road dust in Beijing, China. J. Hazard. Mater. 2020, 388, 121763. [Google Scholar] [CrossRef]
- Zeng, Z.; Luo, W.-G.; Yi, F.-C.; Huang, F.-Y.; Wang, C.-X.; Zhang, Y.-P.; Cheng, Q.-Q.; Wang, Z. Horizontal Distribution of Cadmium in Urban Constructed Wetlands: A Case Study. Sustainability 2021, 13, 5381. [Google Scholar] [CrossRef]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef] [Green Version]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of Heavy Metals on Plants: An Overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotox. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska-Malina, J. Functions of organic matter in polluted soils: The effect of organic amendments on phytoavailability of heavy metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A review of soil heavy metal pollution from industrial and agricultural regions in China: Pollution and risk assessment. Sci. Total Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Clarke, J.M.; Kochian, L.V. Zinc effects on cadmium accumulation and partitioning innear-isogenic lines of durum wheat that differ in grain cadmium concentration. New Phytol. 2005, 167, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Järup, L.; Åkesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P. Mechanisms of cadmium carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 272–279. [Google Scholar] [CrossRef]
- Nordberg, G.F. Historical perspectives on cadmium toxicology. Toxicol. Appl. Pharmacol. 2009, 238, 192–200. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; He, X. Influence of Cd2+ on growth and chlorophyll fluorescence in a hyperaccumulator—Lonicera japonica Thunb. J. Plant Growth Regul. 2015, 34, 672–676. [Google Scholar] [CrossRef]
- Mahajan, P.; Kaushal, J. Role of phytoremediation in reducing cadmium toxicity in soil and water. J. Toxicol. 2018, 2018, 4864365. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Sun, Q.; Wang, R.; Cao, L. Cadmium accumulation and main rhizosphere characteristics of seven French marigold (Tagetes patula L.) cultivars. Int. J. Phytoremediat. 2018, 20, 1171–1178. [Google Scholar] [CrossRef]
- Chowardhara, B.; Borgohain, P.; Saha, B.; Awasthi, J.P.; Moulick, D.; Panda, S.K. Phytotoxicity of Cd and Zn on three popular Indian mustard varieties during germination and early seedling growth. Biocatal. Agric. Biotechnol. 2019, 21, 101349. [Google Scholar] [CrossRef]
- Fattahi, B.; Arzani, K.; Souri, M.K.; Barzegar, M. Effects of cadmium and lead on seed germination, morphological traits, and essential oil composition of sweet basil (Ocimum basilicum L.). Ind. Crop. Prod. 2019, 138, 111584. [Google Scholar] [CrossRef]
- Jiang, M.; Jiang, J.; Li, S.; Li, M.; Tan, Y.; Song, S.; Shu, Q.; Huang, J. Glutamate alleviates cadmium toxicity in rice via suppressing cadmium uptake and translocation. J. Hazard. Mater. 2020, 384, 121319. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yin, G.C.; Xu, Q.Y.; Yan, J.H.; Hseu, Z.Y.; Zhu, L.W.; Lin, Q.T. Influence of aged biochar modified by Cd2+ on soil properties and microbial community. Sustainability 2020, 12, 4868. [Google Scholar] [CrossRef]
- Fujimaki, S.; Suzui, N.; Ishioka, N.S.; Kawachi, N.; Ito, S.; Chino, M.; Nakamura, S.I. Tracing cadmium from culture to spikelet: Noninvasive imaging and quantitative characterization of absorption, transport, and accumulation of cadmium in an intact rice plant. Plant Physiol. 2010, 152, 1796–1806. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Castagna, A.; Ranieri, A.; di Toppi, L.S. Cadmium tolerance in Brassica juncea roots and shoots is affected by antioxidant status and phytochelatin biosynthesis. Plant Physiol. Biochem. 2012, 57, 15–22. [Google Scholar] [CrossRef]
- Borges, K.L.R.; Salvato, F.; Alcântara, B.K.; Nalin, R.S.; Piotto, F.Â.; Azevedo, R.A. Temporal dynamic responses of roots in contrasting tomato genotypes to cadmium tolerance. Ecotoxicology 2018, 27, 245–258. [Google Scholar] [CrossRef]
- Cai, K.; Li, C.; Song, Z.; Gao, X.; Wu, M. Pollution and health risk assessment of carcinogenic elements As, Cd, and Cr in multiple media—A case of a sustainable farming area in China. Sustainability 2019, 11, 5208. [Google Scholar] [CrossRef] [Green Version]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Singh, O.V.; Labana, S.; Pandey, G.; Budhiraja, R.; Jain, R.K. Phytoremediation: An overview of metallic ion decontamination from soil. Appl. Microbiol. Biotechnol. 2003, 61, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nirola, R.; Megharaj, M.; Palanisami, T.; Aryal, R.; Venkateswarlu, K.; Naidu, R. Evaluation of metal uptake factors of native trees colonizing an abandoned copper mine—A quest for phytostabilization. J. Sustain. Min. 2015, 14, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, D.; Pitre, F.E.; Nissim, W.G.; Labrecque, M. Differential uptake of silver, copper and zinc suggests complementary species-specific phytoextraction potential. Int. J. Phytoremediat. 2016, 18, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.B.; Rahman, M.M.; Islam, M.R.; Mondal, D.; Naidu, R. Response of Iron and Cadmium on Yield and Yield Components of Rice and Translocation in Grain: Health Risk Estimation. Front. Environ. Sci. 2021, 9, 716770. [Google Scholar] [CrossRef]
- Seo, B.-H.; Kim, H.S.; Kuppusamy, S.; Kim, K.-H.; Kim, K.-R. Enhanced nitrogen and phosphorus removal by woody plants with deep-planting technique for the potential environmental management of carcass burial sites. Sustainability 2017, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, H.; Pourmajidian, M.R.; Hodjati, S.M.; Fallah, A.; Nath, S. Effect of soil-applied lead on mineral contents and biomass in Acer cappadocicum, Fraxinus excelsior and Platycladus orientalis seedlings. iForest 2017, 10, 722–728. [Google Scholar] [CrossRef] [Green Version]
- Li, S.S.; Wang, M.; Zhao, Z.Q.; Ma, C.B.; Chen, S.B. Adsorption and desorption of Cd by soil amendment: Mechanisms and environmental implications in field-soil remediation. Sustainability 2018, 10, 2337. [Google Scholar] [CrossRef] [Green Version]
- Gerhardt, K.; Gerwing, P.; Greenberg, B. Opinion: Taking phytoremediation from proven technology to accepted practice. Plant Sci. 2017, 256, 170–185. [Google Scholar] [CrossRef]
- Hussain, S.; Akram, M.; Abbas, G.; Murtaza, B.; Shahid, M.; Shah, N.S.; Bibi, I.; Niazi, N.K. Arsenic tolerance and phytoremediation potential of Conocarpus erectus L. and Populus deltoides L. Int. J. Phytoremediat. 2017, 19, 985–991. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, C.; Ni, X.; Ma, W.; Wei, H. Assessing soil metal levels in an industrial environment of northwest china and the phytoremediation potential of its native plants. Sustainability 2018, 10, 2686. [Google Scholar] [CrossRef] [Green Version]
- Whiting, S.N.; Leake, J.R.; McGrath, S.P.; Baker, A.J. Positive responses to Zn and Cd by roots of the Zn and Cd hyperac-cumulator Thlaspi caerulescens. New Phytol. 2000, 145, 199–210. [Google Scholar] [CrossRef] [Green Version]
- McGrath, S.P.; Lombi, E.; Gray, C.W.; Caille, N.; Dunham, S.J.; Zhao, F.J. Field evaluation of Cd and Zn phytoextraction potential by the hyperaccumulators Thlaspi caerulescens and Arabidopsis halleri. Environ. Pollut. 2006, 141, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.B.; Zhou, Q.X.; Wang, L.; Liu, W.T. Cadmium tolerance and accumulation characteristics of Bidens pilosa L. as a potential Cd-hyperaccumulator. J. Hazard. Mater. 2008, 161, 808–814. [Google Scholar] [CrossRef]
- Jaffré, T.; Pillon, Y.; Thomine, S.; Merlot, S. The metal hyperaccumulators from new caledonia can broaden our understanding of nickel accumulation in plants. Front. Plant Sci. 2013, 4, 279. [Google Scholar] [CrossRef] [Green Version]
- Shah, V.; Daverey, A. Phytoremediation: A multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. Innov. 2020, 18, 100774. [Google Scholar] [CrossRef]
- Roosens, N.; Verbruggen, N.; Meerts, P.; Ximenez-Embun, P.; Smith, J. Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ. 2003, 10, 1657–1672. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U. Metal hyperaccumulation in plants. Annu. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Ent, A.V.D.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar]
- Forte, J.; Mutiti, S. Phytoremediation potential of Helianthus annuus and Hydrangea paniculata in copper and lead-contaminated soil. Water Air Soil Pollut. 2017, 228, 77. [Google Scholar] [CrossRef]
- Yadav, K.K.; Gupta, N.; Kumar, A.; Reece, L.M.; Singh, N.; Rezania, S.; Khan, S.A. Mechanistic understanding and holistic approach of phytoremediation: A review on application and future prospects. Ecol. Eng. 2018, 120, 274–298. [Google Scholar] [CrossRef]
- Navarro-León, E.; Oviedo-Silva, J.; Ruiz, J.M.; Blasco, B. Possible role of HMA4a TILLING mutants of Brassica rapa in cadmium phytoremediation programs. Ecotoxicol. Environ. Saf. 2019, 180, 88–94. [Google Scholar] [CrossRef]
- Coakley, S.; Cahill, G.; Enright, A.-M.; O’Rourke, B.; Petti, C. Cadmium Hyperaccumulation and Translocation in Impatiens Glandulifera: From Foe to Friend? Sustainability 2019, 11, 5018. [Google Scholar] [CrossRef] [Green Version]
- Dai, H.; Wei, S.; Skuza, L.; Zhang, Q. Phytoremediation of two ecotypes cadmium hyperaccumulator Bidens pilosa L. sourced from clean soils. Chemosphere 2021, 273, 129652. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of phytoremediation potential of five Cd (hyper) accumulators in two Cd contaminated soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Stärk, H.J.; Wennrich, R.; Levizou, E.; Merbach, I.; Rinklebe, J. Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ. Int. 2021, 146, 106233. [Google Scholar] [CrossRef]
- Wilson, A.; Kendal, D.; Moore, J.L. Humans and ornamental plants: A mutualism? Ecopsychology 2016, 8, 257–263. [Google Scholar] [CrossRef]
- Scholz, T.; Hof, A.; Schmitt, T. Cooling effects and regulating ecosystem services provided by urban trees novel analysis approaches using urban tree cadastre data. Sustainability 2018, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Ciftcioglu, G.C.; Ebedi, S.; Abak, K. Evaluation of the relationship between ornamental plants-based ecosystem services and human wellbeing: A case study from Lefke Region of North Cyprus. Ecol. Indic. 2019, 102, 278–288. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Hu, S.; Wang, J.; Qian, J. A new type of ecological floating bed based on ornamental plants experimented in an artificially made eutrophic water body in the laboratory for nutrient removal. Bull. Environ. Contam. Toxicol. 2021, 106, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, Y.; Cai, Z.; Liu, J.; Yu, B.; Zhou, Q. Phytoremediation of petroleum hydrocarbon-contaminated saline-alkali soil by wild ornamental Iridaceae species. Int. J. Phytoremediat. 2017, 19, 300–308. [Google Scholar] [CrossRef]
- Chandanshive, V.V.; Kadam, S.K.; Khandare, R.V.; Kurade, M.B.; Jeon, B.H.; Jadhav, J.P.; Govindwar, S.P. In situ phytoremediation of dyes from textile wastewater using garden ornamental plants, effect on soil quality and plant growth. Chemosphere 2018, 210, 968–976. [Google Scholar] [CrossRef]
- Mukherjee, A.; Agrawal, M. Use of GLM approach to assess the responses of tropical trees to urban air pollution in relation to leaf functional traits and tree characteristics. Ecotoxicol. Environ. Saf. 2018, 152, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from indoor air by ornamental potted plants: A pilot study using a palm species, under the controlled environment. Chemosphere 2018, 197, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, M.; Gul, I.; Ahmed, I.; Zeeshan, M.; Hashmi, I.; Amin, B.A.Z.; Kallerhoff, J.; Arshad, M. Metal tolerant bacteria enhanced phytoextraction of lead by two accumulator ornamental species. Chemosphere 2019, 227, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Kotsia, D.; Deligianni, A.; Fyllas, N.M.; Stasinakis, A.S.; Fountoulakis, M.S. Converting treatment wetlands into “treatment gardens”: Use of ornamental plants for greywater treatment. Sci. Total Environ. 2020, 744, 140889. [Google Scholar] [CrossRef] [PubMed]
- Rydlová, J.; Püschel, D. Arbuscular mycorrhiza, but not hydrogel, alleviates drought stress of ornamental plants in peat-based substrate. Appl. Soil Ecol. 2020, 146, 103394. [Google Scholar] [CrossRef]
- Saxena, P.; Sonwani, S. Remediation of ozone pollution by ornamental plants in indoor environment. Glob. J. Environ. Sci. Manag. 2020, 6, 497–508. [Google Scholar]
- Gladkov, E.A.; Gladkova, O.V. Ornamental plants adapted to urban ecosystem pollution: Lawn grasses tolerating deicing reagents. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef]
- Taemthong, W. Air quality improvement using ornamental plants in classrooms. J. Green Build. 2021, 16, 201–216. [Google Scholar] [CrossRef]
- Traversari, S.; Cacini, S.; Galieni, A.; Nesi, B.; Nicastro, N.; Pane, C. Precision Agriculture Digital Technologies for Sustainable Fungal Disease Management of Ornamental Plants. Sustainability 2021, 13, 3707. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soils and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Liu, A.; Hamel, C.; Elmi, A.; Costa, C.; Ma, B.; Smith, D.L. Concentrations of K, Ca and Mg in maize colonized by arbuscular mycorrhizal fungi under field conditions. Can. J. Soil Sci. 2002, 82, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Chaudhary, H.J.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium spiked soil modulates root organic acids exudation and ionic contents of two differentially Cd tolerant maize (Zea mays L.) cultivars. Ecotoxicol. Environ. Saf. 2017, 141, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Toxicology rethinks its central belief: Hormesis demands a reappraisal of the way risks are assessed. Nature 2003, 421, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J. Hormesis: Why it is important to toxicology and toxicologists. Environ. Toxicol. Chem. 2008, 27, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R.B. Hormesis and plant biology. Environ. Pollut. 2009, 157, 42–48. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, W.; He, X.; Jia, L.; Yu, S.; Zhao, M. Hormetic responses of Lonicera Japonica Thunb. to cadmium stress. Dose-Response 2015, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bayçu, G.; Moustaka, J.; Gevrek, N.; Moustakas, M. Chlorophyll fluorescence imaging analysis for elucidating the mechanism of photosystem II acclimation to cadmium exposure in the hyperaccumulating plant Noccaea caerulescens. Materials 2018, 11, 2580. [Google Scholar] [CrossRef] [Green Version]
- Zeng, P.; Guo, G.; Xiao, X.; Peng, C.; Feng, W. Phytoextraction potential of Pteris vittata L. co-planted with woody species for As, Cd, Pb and Zn in contaminated soil. Sci. Total Environ. 2019, 650, 594–603. [Google Scholar] [CrossRef]
- Martin, R.J.; Deo, B. Effect of plant population on calendula (Calendula officinalis L.) flower production. N. Z. J. Crop Hort. Sci. 2000, 28, 37–44. [Google Scholar] [CrossRef]
- Gesch, R.W. Growth and yield response of calendula (Calendula officinalis) to sowing date in the northern U.S. Ind. Crop Prod. 2013, 45, 248–252. [Google Scholar] [CrossRef]
- Król, B.; Paszko, T. Harvest date as a factor affecting crop yield, oil content and fatty acid composition of the seeds of calendula (Calendula officinalis L.) cultivars. Ind. Crop Prod. 2017, 97, 242–251. [Google Scholar] [CrossRef]
- Biermann, U.; Butte, W.; Holtgrefe, R.; Feder, W.; Metzger, J.O. Esters of calendula oil and tung oil as reactive diluents for alkyd resins. Eur. J. Lipid Sci. Technol. 2010, 112, 103–109. [Google Scholar] [CrossRef]


| Parameters | Soil |
|---|---|
| Type | Meadow |
| pH | 7.29 ± 0.02 |
| Cation exchange capacity (CEC) (cmol + kg−1) | 19.17 ± 0.06 |
| Organic matter (g kg−1) | 20.65 ± 0.08 |
| Total N (g kg−1) | 0.89 ± 0.01 |
| Available P (mg kg−1) | 8.83 ± 0.07 |
| Available K (mg kg−1) | 75.52 ± 0.05 |
| Clay/% | 32.09 ± 0.11 |
| Sand/% | 21.75 ± 0.06 |
| Silt/% | 44.12 ± 0.05 |
| Available Ca (g kg−1) | 2.38 ± 0.04 |
| Available Mg (g kg−1) | 0.61 ± 0.02 |
| Cd (mg kg−1) | 0.16 ± 0.03 |
| Plant Species | Families and Genera | Life Form | Plant Images |
|---|---|---|---|
| Orychophragmus violaceus (L.) O. E. Schulz | Cruciferae, Orychophragmus | Annual or biennial |  |
| Impatiens balsamina L. | Balsaminaceae, Impatiens | Annual |  |
| Pelargonium hortorum Bailey | Geraniaceae, Pelargonium | Perennial |  |
| Mirabilis jalapa L. | Nyctaginaceae, Zinnia | Annual |  |
| Althaea rosea (Linn.) Cavan. | Malvaceae, Althaea | Biennial |  |
| Calendula officinalis L. | Asteraceae, Calendula | Annual |  |
| Dianthus caryophyllus L. | Caryophyllaceae, Dianthus | Perennial |  |
| Plant Species | Cd Uptake (μg plant−1d−1) | BCF in Plants | ||||
|---|---|---|---|---|---|---|
| 0 | 10 | 100 | 0 | 10 | 100 | |
| Orychophragmus violaceus (L.) O. E. Schulz | 0.10 ± 0.01 | 0.71 ± 0.03 | 2.40 ± 0.05 | 3.94 | 1.47 | |
| Impatiens balsamina L. | 0.22 ± 0.03 | 3.30 ± 0.07 | 13.77 ± 0.16 | 10.69 | 4.82 | |
| Pelargonium hortorum Bailey | 0.08 ± 0.01 | 1.68 ± 0.05 | 6.35 ± 0.13 | 1.10 | 0.76 | |
| Mirabilis jalapa L. | 0.31 ± 0.01 | 1.26 ± 0.06 | 8.97 ± 0.13 | 2.29 | 1.64 | |
| Althaea rosea (Linn.) Cavan. | 0.08 ± 0.01 | 0.86 ± 0.03 | 0.73 ± 0.08 | 5.36 | 1.14 | |
| Calendula officinalis L. | 0.39 ± 0.05 | 8.13 ± 0.08 | 80.77 ± 0.19 | 13.49 | 12.06 | |
| Dianthus caryophyllus L. | 0.16 ± 0.03 | 0.69 ± 0.03 | 0.43 ± 0.02 | 2.72 | 0.71 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Chen, M.; Lin, M.; Chen, Q.; Lu, Q.; Yao, J.; He, X. Cadmium Uptake and Growth Responses of Seven Urban Flowering Plants: Hyperaccumulator or Bioindicator? Sustainability 2022, 14, 619. https://doi.org/10.3390/su14020619
Liu Z, Chen M, Lin M, Chen Q, Lu Q, Yao J, He X. Cadmium Uptake and Growth Responses of Seven Urban Flowering Plants: Hyperaccumulator or Bioindicator? Sustainability. 2022; 14(2):619. https://doi.org/10.3390/su14020619
Chicago/Turabian StyleLiu, Zhouli, Mengdi Chen, Maosen Lin, Qinglin Chen, Qingxuan Lu, Jing Yao, and Xingyuan He. 2022. "Cadmium Uptake and Growth Responses of Seven Urban Flowering Plants: Hyperaccumulator or Bioindicator?" Sustainability 14, no. 2: 619. https://doi.org/10.3390/su14020619
APA StyleLiu, Z., Chen, M., Lin, M., Chen, Q., Lu, Q., Yao, J., & He, X. (2022). Cadmium Uptake and Growth Responses of Seven Urban Flowering Plants: Hyperaccumulator or Bioindicator? Sustainability, 14(2), 619. https://doi.org/10.3390/su14020619








