Hydrochar Derived from Spent Mushroom Substrate Ameliorates Soil Properties and Nutrient Levels in Saline–Sodic Soil: An Incubation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sample Collection
2.2. Carbonization
2.3. Incubation Study
2.4. In Situ Rice Pot Seedling
2.5. Measurements
2.6. Statistical analysis
3. Results
3.1. SMSHC Analysis
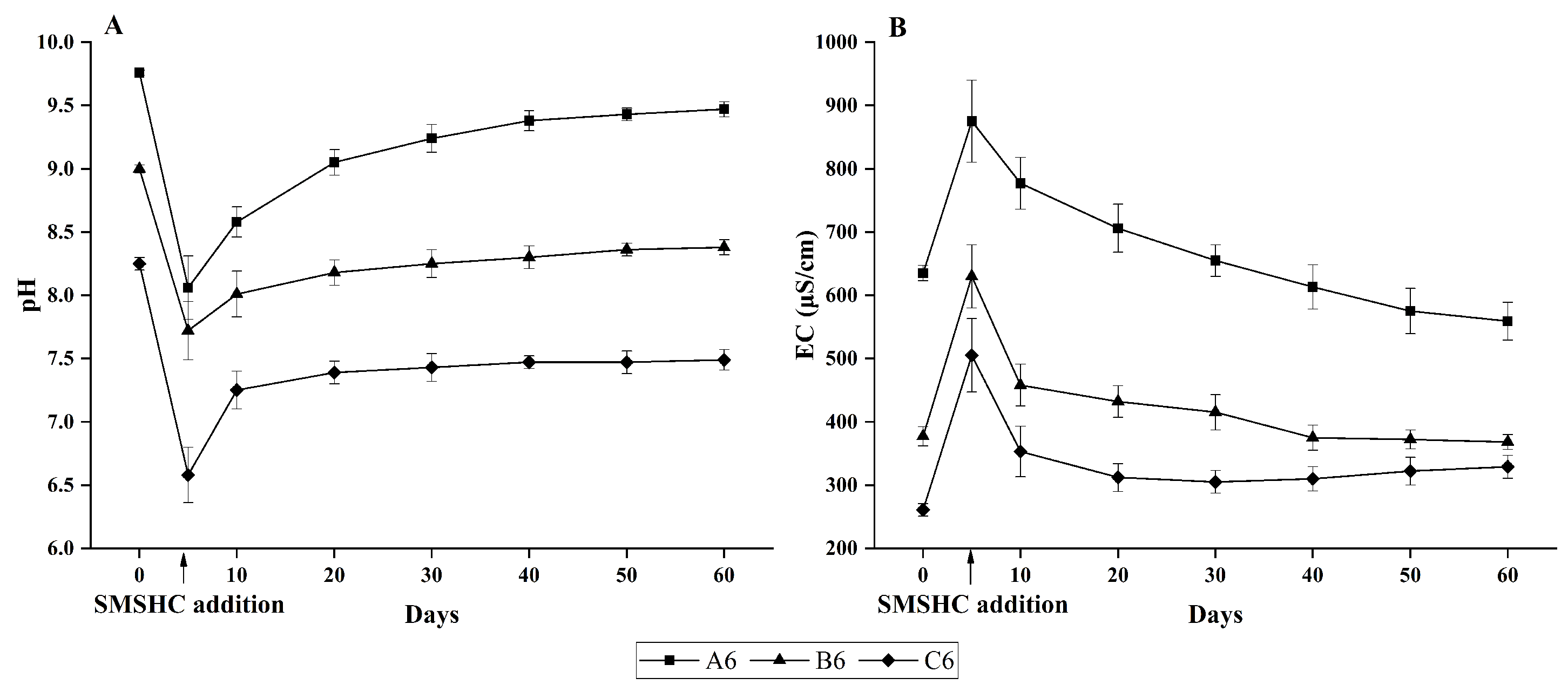
3.2. Soil Chemical Properties Ameliorated by SMSHC Addition
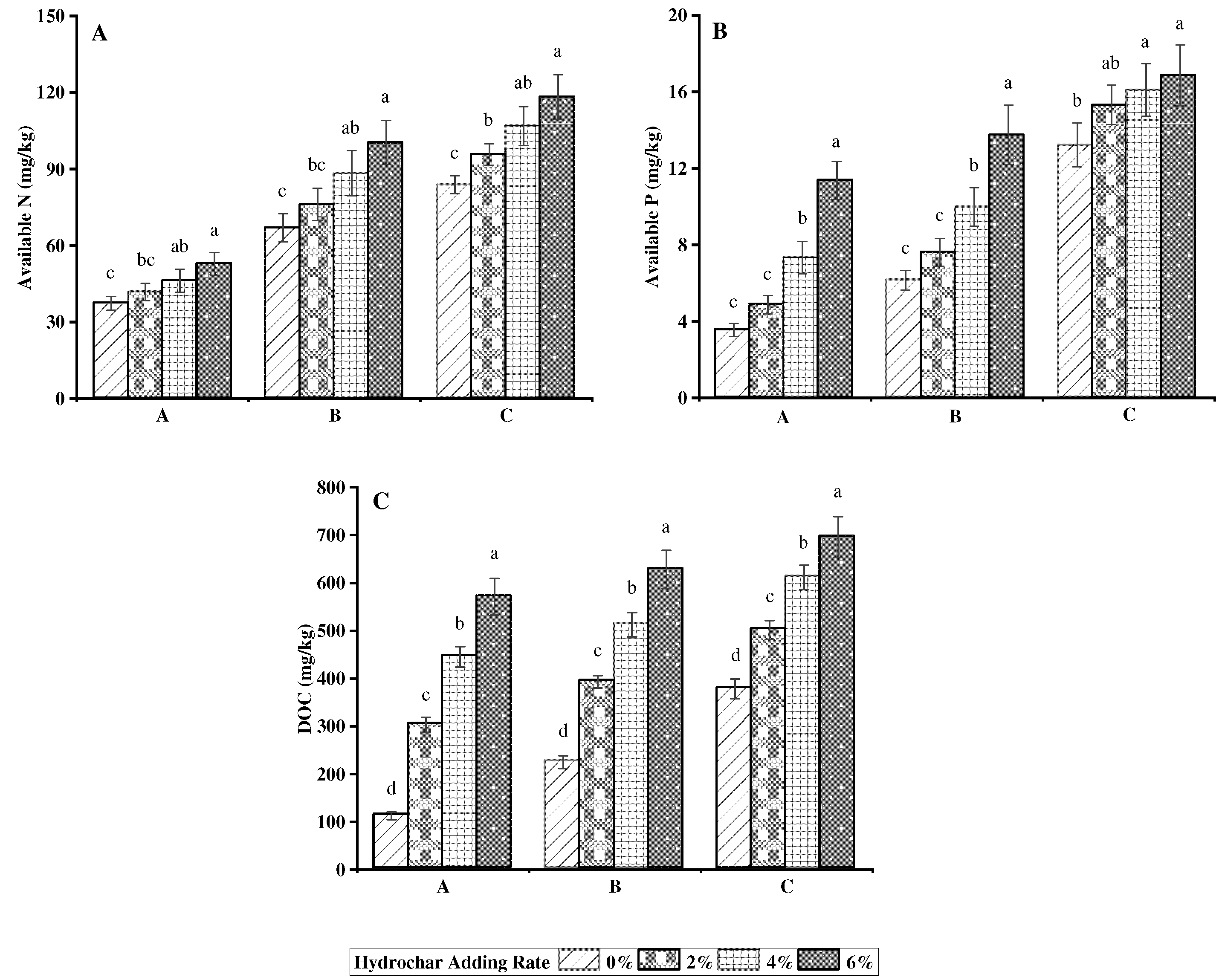
3.3. Soil Fertility Improved by SMSHC Addition
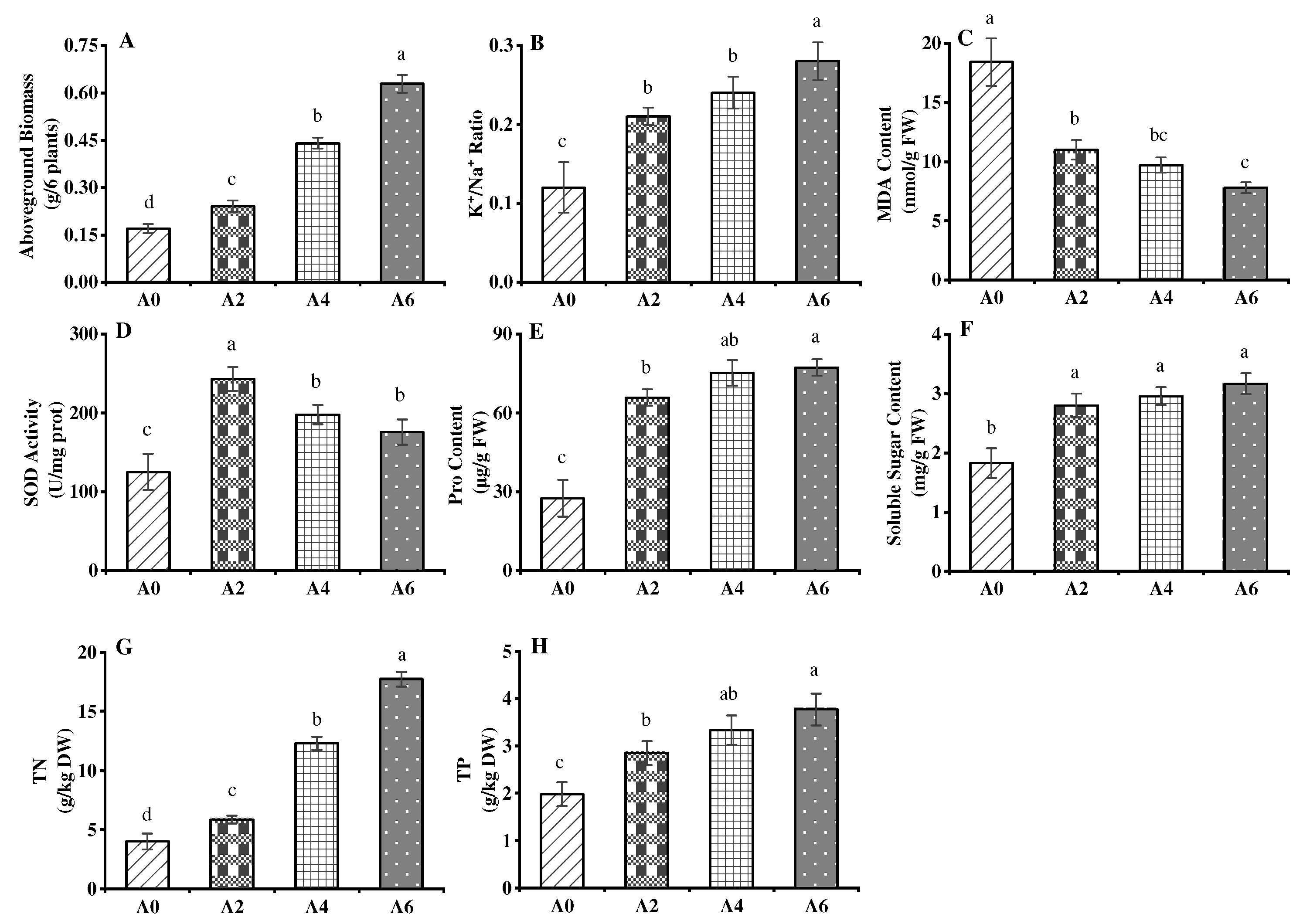
3.4. Physiological Properties of Rice Seedlings Grown in SMSHC-Amended Soil
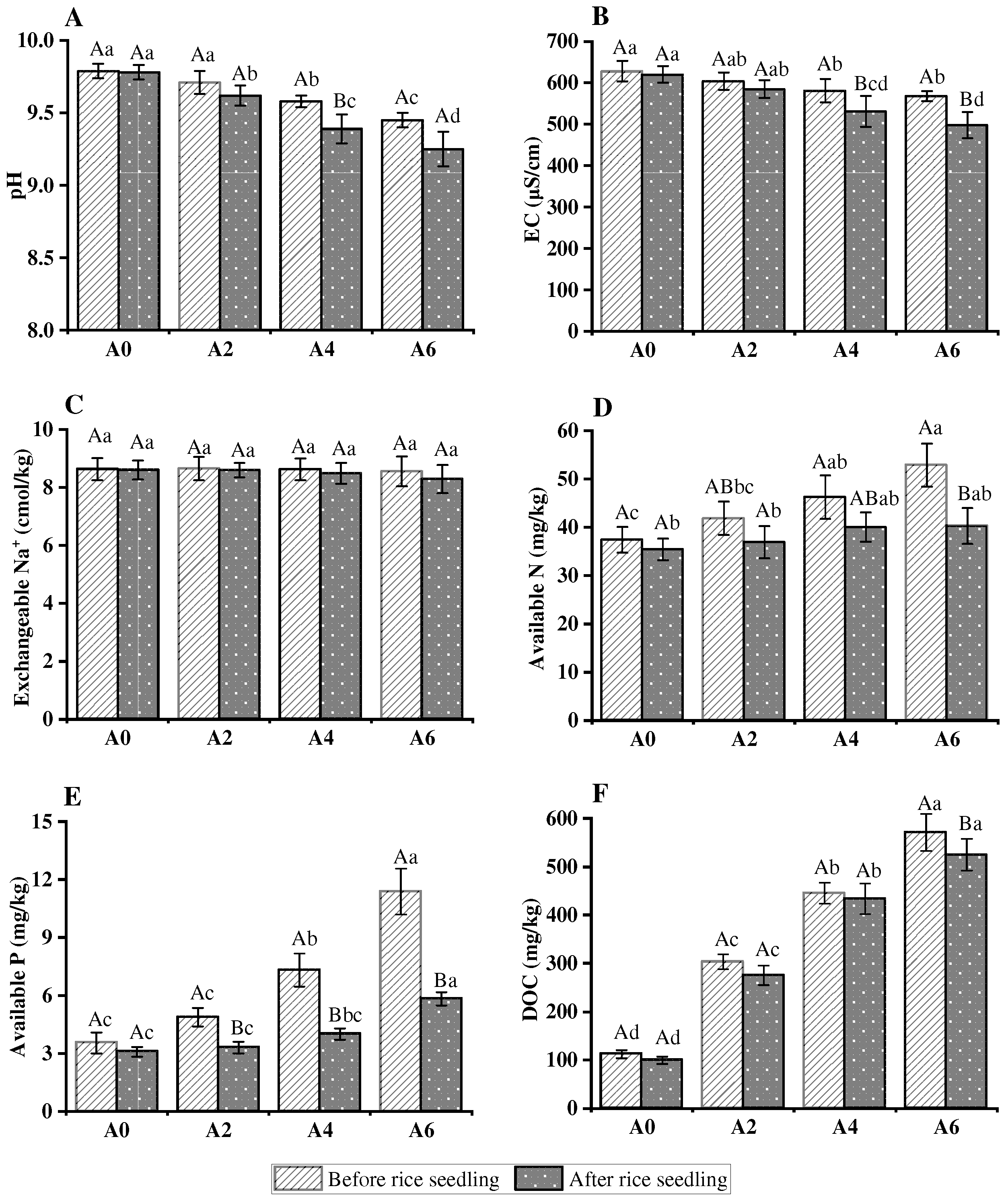
3.5. Analysis of Rhizosphere Soil of Rice Seedling
4. Discussion
4.1. Soil pH and EC
4.2. ESP and Exchangeable Na+, Ca2+, Mg2+
4.3. Soil Nutrients
4.4. Physiological Properties of Rice Seedlings
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atallah, E.; Zeaiter, J.; Ahmad, M.N.; Leahy, J.J.; Kwapinski, W. Hydrothermal carbonization of spent mushroom compost waste compared against torrefaction and pyrolysis. Fuel Process. Technol. 2021, 216, 106795. [Google Scholar] [CrossRef]
- Hanafi, F.H.M.; Rezania, S.; Taib, S.M.; Din, M.F.M.; Yamauchi, M.; Sakamoto, M.; Hara, H.; Park, J.; Ebrahimi, S.S. Environmentally sustainable applications of agro-based spent mushroom substrate (SMS): An overview. J. Mater. Cycles Waste Manag. 2018, 20, 1383–1396. [Google Scholar] [CrossRef]
- Noonsong, V.; Puttakun, N.; Tinsirisuk, M.; Seephueak, P. Recycling of spent Pleurotus compost for production of the Agrocybe cylindracea. Mycosphere 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Frutos, I.; Garcia-Delgado, C.; Garate, A.; Eymar, E. Biosorption of heavy metals by organic carbon from spent mushroom substrates and their raw materials. Int. J. Environ. Sci. Technol. 2016, 13, 2713–2720. [Google Scholar] [CrossRef]
- Miranda, T.; Nogales, S.; Román, S.; Montero, I.; Arranz, J.I.; Sepúlveda, F.J. Control of Several Emissions during Olive Pomace Thermal Degradation. Int. J. Mol. Sci. 2014, 15, 18349–18361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G.; Zhang, X.; Qi, H.; Ma, J.; Xiao, Y.; et al. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 15999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sewu, D.D.; Boakye, P.; Jung, H.; Woo, S.H. Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresour. Technol. 2017, 244, 1142–1149. [Google Scholar] [CrossRef]
- Lou, Z.; Sun, Y.; Bian, S.; Ali Baig, S.; Hu, B.; Xu, X. Nutrient conservation during spent mushroom compost application using spent mushroom substrate derived biochar. Chemosphere 2017, 169, 23–31. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.; Li, D.; Qi, C.; Han, L.; Chen, M.; Fu, F.; Yuan, J.; Li, G. Effects of calcium magnesium phosphate fertilizer, biochar and spent mushroom substrate on compost maturity and gaseous emissions during pig manure composting. J. Environ. Manag. 2020, 267, 110649. [Google Scholar] [CrossRef]
- Lam, S.S.; Lee, X.Y.; Nam, W.L.; Phang, X.Y.; Liew, R.K.; Yek, P.N.; Ho, Y.L.; Ma, N.L.; Rosli, M.H. Microwave vacuum pyrolysis conversion of waste mushroom substrate into biochar for use as growth medium in mushroom cultivation. J. Chem. Technol. Biotechnol. 2019, 94, 1406–1415. [Google Scholar] [CrossRef]
- Mau, V.; Arye, G.; Gross, A. Poultry litter hydrochar as an amendment for sandy soils. J. Environ. Manag. 2020, 271, 110959. [Google Scholar] [CrossRef]
- Kalderis, D.; Papameletiou, G.; Kayan, B. Assessment of Orange Peel Hydrochar as a Soil Amendment: Impact on Clay Soil Physical Properties and Potential Phytotoxicity. Waste Biomass Valorization 2019, 10, 3471–3484. [Google Scholar] [CrossRef]
- Hou, P.F.; Feng, Y.F.; Wang, N.; Petropoulos, E.; Li, D.T.; Yu, S.; Xue, L.H.; Yang, L.Z. Win-win: Application of sawdust-derived hydrochar in low fertility soil improves rice yield and reduces greenhouse gas emissions from agricultural ecosystems. Sci. Total Environ. 2020, 748, 142457. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Cao, X.; Ro, K.S.; Chappell, M.; Li, Y.; Mao, J. Chemical Structures of Swine-Manure Chars Produced under Different Carbonization Conditions Investigated by Advanced Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy. Energy Fuels 2011, 25, 388–397. [Google Scholar] [CrossRef]
- Fang, J.; Zhan, L.; Ok, Y.S.; Gao, B. Minireview of potential applications of hydrochar derived from hydrothermal carbonization of biomass. J. Ind. Eng. Chem. 2018, 57, 15–21. [Google Scholar] [CrossRef]
- Sun, R.X.; Zheng, H.; Yin, S.J.; Zhang, X.; You, X.W.; Wu, H.Y.; Suo, F.Y.; Han, K.X.; Cheng, Y.D.; Zhang, C.S.; et al. Comparative study of pyrochar and hydrochar on peanut seedling growth in a coastal salt-affected soil of Yellow River Delta, China. Sci. Total Environ. 2022, 833, 155183. [Google Scholar] [CrossRef] [PubMed]
- Chávez-García, E.; Siebe, C. Rehabilitation of a highly saline-sodic soil using a rubble barrier and organic amendments. Soil Tillage Res. 2019, 189, 176–188. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, S.; Li, Y.; Liu, J.; Zhuo, Y.; Chen, H.; Wang, J.; Xu, L.; Sun, Z. Extensive reclamation of saline-sodic soils with flue gas desulfurization gypsum on the Songnen Plain, Northeast China. Geoderma 2018, 321, 52–60. [Google Scholar] [CrossRef]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Singh, R.; Mavi, M.S.; Choudhary, O.P. Saline Soils Can Be Ameliorated by Adding Biochar Generated from Rice-Residue Waste. CLEAN Soil Air Water 2019, 47, 1700656. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Wang, X.; Huang, J. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Acetic Acid and Sodium Hydroxide-Aided Hydrothermal Carbonization of Woody Biomass for Enhanced Pelletization and Fuel Properties. Energy Fuels 2017, 31, 12200–12208. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Gan, X.; Zheng, L.; Peng, C.; Wang, B.; Li, C.; Zeng, G. Evaluation of the clean characteristics and combustion behavior of hydrochar derived from food waste towards solid biofuel production. Bioresour. Technol. 2018, 266, 275–283. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zheng, H.; Li, F.; Ngo, H.H.; Guo, W.; Liu, C.; Chen, L.; Xing, B. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Hinsinger, P.; Plassard, C.; Tang, C.X.; Jaillard, B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: A review. Plant Soil 2003, 248, 43–59. [Google Scholar] [CrossRef]
- Yuan, J.; Shengzhe, E.; Che, Z. Base cation-enhancing role of corn straw biochar in an acidic soil. Soil Use Manag. 2022, 38, 1322–1336. [Google Scholar] [CrossRef]
- Qadir, M.; Oster, J.D. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci. Total Environ. 2004, 323, 1–19. [Google Scholar] [CrossRef]
- Song, C.F.; Shan, S.D.; Yang, C.; Zhang, C.; Zhou, X.Q.; Ma, Q.; Yrjala, K.; Zheng, H.B.; Cao, Y.C. The comparison of dissolved organic matter in hydrochars and biochars from pig manure. Sci. Total Environ. 2020, 720, 137423. [Google Scholar] [CrossRef] [PubMed]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Chang, K.L.; Chen, X.M.; Sun, J.; Liu, J.Y.; Sun, S.Y.; Yang, Z.Y.; Wang, Y. Spent mushroom substrate biochar as a potential amendment in pig manure and rice straw composting processes. Environ. Technol. 2017, 38, 1765–1769. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1997, 114, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. [Google Scholar] [CrossRef]
- Ibrahim, M.E.H.; Ali, A.Y.A.; Zhou, G.S.; Elsiddig, A.M.I.; Zhu, G.L.; Nimir, N.E.A.; Ahmad, I. Biochar application affects forage sorghum under salinity stress. Chil. J. Agric. Res. 2020, 80, 317–325. [Google Scholar] [CrossRef]
- Nehela, Y.; Mazrou, Y.S.A.; Alshaal, T.; Rady, A.M.S.; El-Sherif, A.M.A.; Omara, A.E.; Abd El-Monem, A.M.; Hafez, E.M. The Integrated Amendment of Sodic-Saline Soils Using Biochar and Plant Growth-Promoting Rhizobacteria Enhances Maize (Zea mays L.) Resilience to Water Salinity. Plants 2021, 10, 1960. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.; Sahin, O.; Taskin, M.B.; Kaya, E.C. Impacts of biochar and processed poultry manure, applied to a calcareous soil, on the growth of bean and maize. Soil Use Manag. 2015, 31, 106–113. [Google Scholar] [CrossRef]
| SMS | SMSHC | SMSBC | |
|---|---|---|---|
| pH | 5.80 | 4.08 | 10.05 |
| EC (mS/cm) | 0.88 | 0.98 | 3.54 |
| Na (%) | 0.19 | 0.21 | 1.11 |
| K (%) | 0.85 | <0.10 | 1.19 |
| Mg (%) | 0.30 | 0.19 | 0.81 |
| Ca (%) | 5.15 | 5.19 | 9.69 |
| ABET (m2/g) | 2.56 | 5.08 | 7.75 |
| O/C ratio | 0.68 | 0.61 | 0.05 |
| Ash content (%) | 7.85 | 9.90 | 21.3 |
| Yield (%) | / | 51.5 | 34.3 |
| Soil | SMSHC (%) | pH | EC (µS/cm) | ESP (%) |
|---|---|---|---|---|
| A | 0 | 9.79 ± 0.05 a | 628 ± 25 a | 45.5 ± 0.8 a |
| 2 | 9.71 ± 0.08 a | 604 ± 21 ab | 45.1 ± 0.5 a | |
| 4 | 9.58 ± 0.04 b | 581 ± 28 b | 44.8 ± 0.2 a | |
| 6 | 9.45 ± 0.05 c | 568 ± 12 b | 44.5 ± 0.6 a | |
| B | 0 | 9.03 ± 0.05 a | 373 ± 12 a | 37.0 ± 0.7 a |
| 2 | 8.88 ± 0.10 b | 370 ± 14 a | 35.8 ± 0.4 b | |
| 4 | 8.62 ± 0.03 c | 372 ± 18 a | 33.3 ± 0.6 c | |
| 6 | 8.40 ± 0.05 d | 365 ± 15 a | 30.5 ± 0.4 d | |
| C | 0 | 8.24 ± 0.05 a | 265 ± 14 b | 31.6 ± 0.3 a |
| 2 | 8.01 ± 0.08 b | 278 ± 12 b | 27.7 ± 0.5 b | |
| 4 | 7.75 ± 0.06 c | 294 ± 15 b | 23.9 ± 0.6 c | |
| 6 | 7.49 ± 0.05 d | 329 ± 21 a | 18.6 ± 0.3 d |
| Ion Content (cmol/kg) | A6 | B6 | C6 | |||
|---|---|---|---|---|---|---|
| Day 0 | Day 60 | Day 0 | Day 60 | Day 0 | Day 60 | |
| CEC | 18.97 ± 0.87 | 19.21 ± 1.15 | 14.85 ± 0.55 | 17.69 ± 0.88 | 12.56 ± 0.56 | 22.31 ± 2.89 |
| Exchangeable Na+ | 8.63 ± 0.58 | 8.55 ± 0.38 | 5.42 ± 0.29 | 5.45 ± 0.29 | 3.97 ± 0.11 | 4.15 ± 0.15 |
| Exchangeable K+ | 0.30 ± 0.02 | 0.31 ± 0.02 | 0.22 ± 0.02 | 0.25 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 |
| Exchangeable Mg2+ | 5.13 ± 0.51 | 5.24 ± 0.50 | 4.99 ± 0.32 | 6.12 ± 0.55 | 4.89 ± 0.30 | 10.63 ± 1.33 |
| Exchangeable Ca2+ | 3.44 ± 0.11 | 3.50 ± 0.18 | 2.95 ± 0.12 | 4.17 ± 0.40 | 2.44 ± 0.18 | 5.31 ± 0.65 |
| 1.11 ± 0.04 | 0.68 ± 0.05 | 0.62 ± 0.02 | 0.26 ± 0.02 | 0.10 ± 0.01 | 0 | |
| 5.30 ± 0.23 | 5.16 ± 0.25 | 2.48 ± 0.08 | 1.62 ± 0.10 | 0.75 ± 0.04 | 0.15 ± 0.01 | |
| Nutrients | SMSHC | Soil A | Soil B | Soil C |
|---|---|---|---|---|
| Available N (mg/kg) | 166.35 | 36.92 | 61.38 | 81.89 |
| Available P (mg/kg) | 7.75 | 3.41 | 6.28 | 12.95 |
| DOC (g/kg) | 11.53 | 0.12 | 0.22 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Sun, Z.; Su, Y.; Yang, J.; Li, M.; Hong, B.; Chen, G. Hydrochar Derived from Spent Mushroom Substrate Ameliorates Soil Properties and Nutrient Levels in Saline–Sodic Soil: An Incubation Study. Sustainability 2022, 14, 12958. https://doi.org/10.3390/su142012958
Chen Y, Sun Z, Su Y, Yang J, Li M, Hong B, Chen G. Hydrochar Derived from Spent Mushroom Substrate Ameliorates Soil Properties and Nutrient Levels in Saline–Sodic Soil: An Incubation Study. Sustainability. 2022; 14(20):12958. https://doi.org/10.3390/su142012958
Chicago/Turabian StyleChen, Yuanhui, Zhengxiao Sun, Yingjie Su, Jinxia Yang, Mingtang Li, Bo Hong, and Guang Chen. 2022. "Hydrochar Derived from Spent Mushroom Substrate Ameliorates Soil Properties and Nutrient Levels in Saline–Sodic Soil: An Incubation Study" Sustainability 14, no. 20: 12958. https://doi.org/10.3390/su142012958
APA StyleChen, Y., Sun, Z., Su, Y., Yang, J., Li, M., Hong, B., & Chen, G. (2022). Hydrochar Derived from Spent Mushroom Substrate Ameliorates Soil Properties and Nutrient Levels in Saline–Sodic Soil: An Incubation Study. Sustainability, 14(20), 12958. https://doi.org/10.3390/su142012958







